Introduction
Glioblastoma multiforme (GBM) is the most common and
aggressive type of glioma with an extremely poor prognosis.
Patients with GBM have an average survival time of 1–2 years after
treatment with the available therapies (1). This poor prognosis is presumed to be
due to its invasive nature, as the majority of patients with GBM
die from local recurrence, which usually occurs within 2–3 cm of
the resection margin (2). Thus,
understanding the phenotypic characteristics and biochemical
features of invasive tumor cells may be critical in the development
of a potential treatment for this incurable condition.
Numerous experimental animal models, which share the
characteristics of human GBM have been developed via the
intracranial implantation of malignant glioma cell lines into
rodents. Among these glioma models, the C6 rat glioma cell line
represents an important and widely used model, which is easily
handled and maintained. Although different implantation protocols
(3,4) or rodent hosts (5,6) used
to develop the model may affect the overall period of tumor
progression, the key steps in tumorigenesis, including tumor
growth, angiogenesis and invasion, are generally similar (7).
Following implantation of C6 cells in rodent hosts,
C6 cells initially grow as a tumor spheroid and then certain C6
cells aggressively invade into the normal brain tissue along white
matter tracts or blood vessels (8,9).
Furthermore, tumor cells targeting the pre-existing vessels of the
host, a mechanism termed vascular co-option (10), has been observed to be important in
brain metastasis (11) and glioma
formation (12). Winkler et
al (13) reported a real-time
observation of glioma cells in living experimental animals. The
authors concluded that perivascular glioma cells were able to move
significantly faster than non-perivascular glioma cells, indicating
that glioma cells utilize the perivascular space of the host as an
avenue for migration. Furthermore, Farin et al (14) revealed that glioma cells were able
to migrate along the perivascular space rapidly in all directions
and proliferate en route when they met vascular branch points in a
brain slice model. However, whether these perivascular mitotic
cells form secondary tumor structures was not investigated in their
study.
A growing body of evidence has suggested that
invading tumor cells have distinct characteristics from those in
tumor spheroids (15). For
example, the invading tumor cells have a considerably lower
proliferative rate than those in the tumor spheroids (15–17)
and are more resistant to chemotherapy (18). Previously, Chicoine and Silbergeld
(19) demonstrated that invading
C6 cells isolated from the contralateral hemisphere in a rodent
model were able to form tumor spheroids following re-implantation.
On the basis of tumor recurrence in human glioblastoma, invading
tumor cells dispersed in normal parenchyma may have the potential
to further undergo phenotypic changes, leading to the formation of
secondary tumor masses (20). Thus
far, despite evidence that multiple C6 glioma models have been
delineated in previous decades, how and when invading cells
re-enter mitosis and form secondary tumor masses has not at present
been characterized in rodent models (6,21)
In the present study, the features of various
tumorigenic stages were re-examined, and the phenotypic alterations
of implanted C6 cells in a C6 rat glioma model was investigated. In
addition, the morphological and phenotypic variations of C6 cells
in various regions of the tumor, specifically invading cells and
cells in other regions of the tumor were characterized under high
magnification. The biochemical features of the C6 cells were also
characterized by immunofluorescence staining.
Materials and methods
Animals
A total of 46 adult male Sprague-Dawley (SD) rats
weighing between 360 and 400 g were obtained from Charles River
Laboratories (BioLASCO, Taipei, Taiwan). For western blot analysis,
two groups of 5 rats were injected with C6 cells or
phosphate-buffered saline (PBS), respectively. The remaining 36
rats were implanted with C6 cells and divided into six subgroups.
Subgroups of rats were sacrificed at 3, 5, 7, 9, 11 or 15 days
post-implantation. All animal experimental protocols were approved
by the Institutional Animal Care and Use Committee of the
Chang-Gung Memorial Hospital (Chiayi, Taiwan) and performed
according to the guidelines of the National Institutes of Health
(Bethesda, MD, USA) for the care and use of laboratory animals.
Cell culture and brain tumor
xenograft
The C6 rat glioma cell line was obtained from the
American Type Culture Collection (CLL-107, Rockville, MD, USA). The
cells were cultured in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum (Mediatech, Herndon, VA,
USA) and 1% penicillin-streptomycin (Mediatech) at 37°C in a
humidified 5% CO2 incubator. All implantation procedures
were performed according to the methods used in our previous study
(22). Briefly, each rat was
anesthetized with ketamine hydrochloride (50 mg/ml; Pfizer, Inc.,
New York, NY, USA) and then placed in a stereotactic frame
(Stoelting, Wood Dale, IL, USA). Following standard aseptic
preparation, the animals were implanted intracranially with
1×105 C6 glioma cells. The rats were then allowed to
recover from the anesthesia on a heat pad to avoid post-operative
hypothermia.
Western blot analysis
The rats were decapitated following anesthesia and
C6 gliomas were harvested with the aid of an operating microscope
(Leica M650; Leica Microsystems GmbH, Wetzlar, Germany). Normal rat
brain tissues were also obtained from the right putamen region.
Equal quantities of whole cellular protein (50 µg) from the
C6 gliomas, the normal brain tissues and the C6 cell lysates were
separated on 12% sodium dodecyl sulfate-polyacrylamide gels
(Sigma-Aldrich, St. Louis, MO, USA) and then transferred onto
polyvinylidene difluoride membranes (Sigma-Aldrich). The membranes
were blocked with 8% non-fat dry milk for 2 h at room temperature
and then incubated with the primary antibodies overnight at 4°C.
Antibodies to hypoxia-inducible factor 1-α (HIF1α; rabbit
monoclonal; 1:200; cat. no. ab51608; Abcam, Cambridge, UK), TWIST
(rabbit polyclonal; 1:200; cat. no. sc-15393; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), matrix metalloproteinase
(MMP)-9 (monoclonal mouse; 1:400; cat. no. ab119906; Abcam),
vascular endothelial growth factor (VEGF; polyclonal rabbit; 1:200;
cat. no. sc-48835; Santa Cruz Biotechnology, Inc.), vascular
endothelial growth factor receptor (VEGFR) 2 (monoclonal rabbit;
1:100; cat. no. sc-6251; Santa Cruz Biotechnology, Inc.), nerve
growth factor (NGF; polyclonal rabbit; 1:500; cat. no. sc-548;
Santa Cruz Biotechnology, Inc.), p75 neurotrophin receptor (p75;
polyclonal rabbit; 1:500; cat no. ab8874; Abcam), neurotrophic
tyrosine kinase receptor type 1 (TrkA; rabbit polyclonal; 1:50:
cat. no. ab8871; Abcam) and β-actin (rabbit polyclonal; 1:100; cat.
no. a2066; Sigma-Aldrich) were used. Following a brief rinse with
Tris-buffered saline containing Tween-20, the membranes were
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibodies. Chemiluminescence reactions were developed using
SuperSignal West Pico chemiluminescent substrate (Pierce
Biotechnology, Inc., Rockford, IL, USA).
Hematoxylin and eosin (H&E)
staining
The rats with glioma were decapitated on the 3rd,
5th, 7th, 9th, 11th and 15th day post-implantation (n=6 in each
group). The rat brains were harvested and fixed in 10% formaldehyde
(Sigma-Aldrich), or preserved in optimal cutting temperature
compound and stored at -80°C. The brain sections (10-µm)
were stained with H&E for the histological studies. The mitotic
index at the center (core), periphery and tumor cluster was
determined as the ratio of mitotic cells in 10 randomly selected
high-power fields (magnification, ×400) of brain sections from at
least 3 rat gliomas harvested at 15 days post-implantation
(DPI).
Immunohistochemical and immunofluorescent
staining
For immunohistochemical analysis, brain sections
were incubated with an antibody against MMP-2 (monoclonal mouse;
1:200; cat. no. sc-6840; Santa Cruz Biotechnology, Inc.). The
sections were washed with PBS and then incubated with the
HRP-conjugated secondary antibody (Superpicture HRP conjugate;
Zymed, San Francisco, CA, USA). 3,3′-Diaminobenzidine
tetrahydrochloride (Sigma-Aldrich) was used for chromogenic
detection. For immunofluorescence analysis, the tissue sections
were fixed in a 1:1 mixture of acetone-methanol for 15 min at room
temperature and then blocked with Odyssey blocking buffer (Rockland
Inc., Limerick, PA, USA). The sections were stained with antibodies
against α-smooth muscle actin (α-SMA; rabbit polyclonal; 1:100;
cat. no. ab5694; Abcam), CD31 (mouse monoclonal; 1:50; cat. no.
ab24590; Abcam), Ki67 (rabbit polyclonal; 1:200; cat. no. ab66155;
Abcam), HIF1α (mouse monoclonal; 1:200; Novus, Saint Charles, MO,
USA), VEGF (Santa Cruz Biotechnology, Inc.), receptors for vascular
endothelial growth factor (VEGFR2; goat polyclonal; 1:100; ab10972;
Abcam), TWIST (rabbit polyclonal; 1:100; cat. no. ab50581; Abcam),
Vimentin (mouse monoclonal; 1:200; cat. no. sc-6260; Santa Cruz
Biotechnology, Inc.), p75 (rabbit polyclonal; 1:50; cat. no.
ab38335; Abcam) and TrkA (rabbit polyclonal; 1:50; cat. no. ab8871;
Abcam). The sections were incubated with secondary antibodies
(Invitrogen Life Technologies) for 1 h at room temperature. The
nuclei were stained with DAPI (Invitrogen Life Technologies) for 30
sec at 37°C. The expression levels of other proteins detected by
immunohistochemical or immunofluorescence staining were evaluated
using a semi-quantitative method (23). The scoring system was defined as
follows: −, <1% positive cells; +, 2–25% positive cells; ++,
26–50% positive cells; +++, 51–75% positive cells; ++++, >75%
positive cells. The Ki67 index at the center, periphery and tumor
cluster were calculated as the ratio of Ki67-positive cells in 10
randomly selected high-power fields (magnification, ×400) of brain
sections from at least three rat gliomas harvested at 15 DPI.
Experimental analysis
Images of H&E, immunohistochemical and
immunofluorescence staining were captured and analyzed using a CCD
camera (Eclipse E600, Nikon, Tokyo, Japan) and Image ProPlus 3.0
software (Media Cybernetics, Sarasota, FL, USA).
Statistical analysis
The mitotic rate and Ki67 index were compared by
performing a one-way analysis of variance. P<0.05 was considered
to indicate a statistically significant difference. All statistical
tests were two-tailed and performed using SPSS, version 12.0 (SPSS,
Inc., Chicago, IL, USA).
Results
Formation of C6 glioma in SD rats and
gene expression in C6-derived tumors
C6 cells (1×105) were stereotactically
injected into the right putamen region of male adult SD rats to
establish a C6 glioma model. The SD rats implanted with C6 cells
consistently succumbed to glioma at 15–18 DPI in our established
system (22). As the tumor grew,
the C6 glioma protruded from the surface of rat brain with a
whitish, soft, and well-circumscribed nodular appearance (Fig. 1A). From a cross-sectional view, the
tumor mass formed at 15 DPI, and exhibited small hemorrhagic spots,
distorted structures and necrotic portions (Fig. 1B).
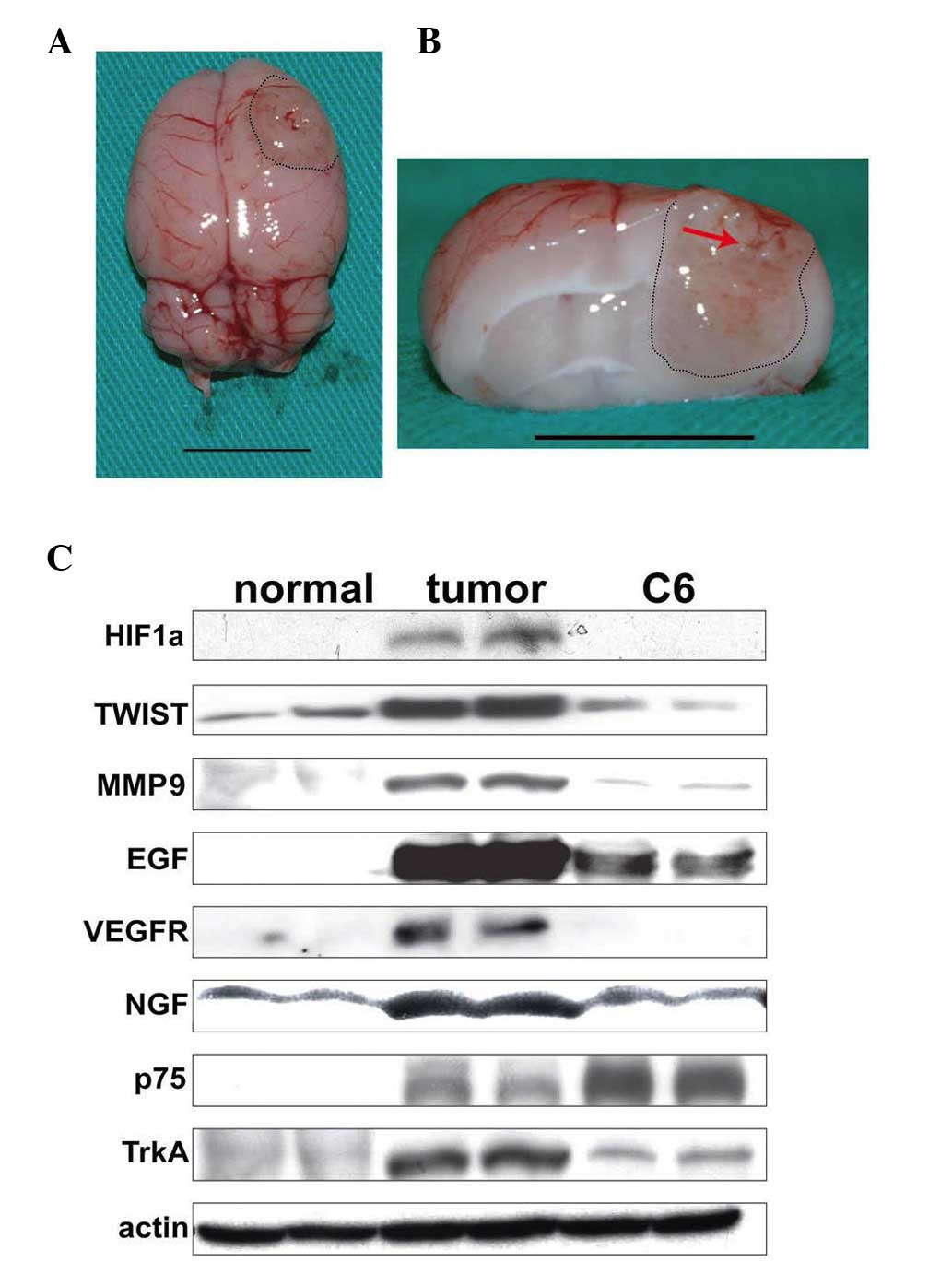 | Figure 1Relative expression level of
tumor-associated genes in normal brain tissues (normal), C6 glioma
tissues (tumor) and cultured C6 cells (C6). (A) Superior view of a
rat brain with C6 glioma (scale bar, 1 cm). (B) Cross section of a
rat brain with C6 glioma. The tumor formed at the right hemisphere
revealed a blurred margin (black dotted line) with petechial
hemorrhage in the central region (red arrow; scale bar, 1 cm). (C)
Expression of tumor-associated genes in normal brain tissues
(normal), C6 glioma tissues (tumor) and cultured C6 cells (C6).
HIF1α, hypoxia-inducible factor 1-α, MMP, matrix metalloproteinase;
VEGF, vascular endothelial growth factor; VEGFR, vascular
endothelial growth factor receptor; NGF, nerve growth factor; p75,
p75 neurotrophin receptor; TrkA, neurotrophic tyrosine kinase
receptor type 1. |
To examine the differential gene expression among
normal brains, C6 gliomas and the cultured C6 cells, several known
tumor-associated genes were detected in these samples. As shown in
Fig. 1C, the samples from the
mature C6 gliomas exhibited a higher expression of HIF1α, TWIST
(23), MMP-9, VEGF, VEGFR, NGF
(24) and TrkA (25), when compared with those from normal
brain tissue and the cultured C6 cells. These results indicated the
successful establishment of a C6 glioma model and implied that the
neural micro-environment in SD rats is able to further modulate the
expression of specific tumor-associated genes in C6 cells.
Formation of the primary tumor spheroids
and neovascularization during the early stages of
tumorigenesis
To further characterize the morphological or
functional changes of tumor cells during the progression of C6
glioma, brain specimens were harvested during the period between
3–15 DPI. At 3 DPI, C6 cells initially formed a small tumor
spheroid at the site of injection (Fig. 2A). The C6 cells were densely packed
in the tumor spheroid with a demarcated border. These tumor cells
in the spheroid were round or oval and contained a pleomorphic
nucleus (Fig. 2B). A minor
hemorrhage caused by the injection of the C6 cells was noted on the
wall of the injection tract.
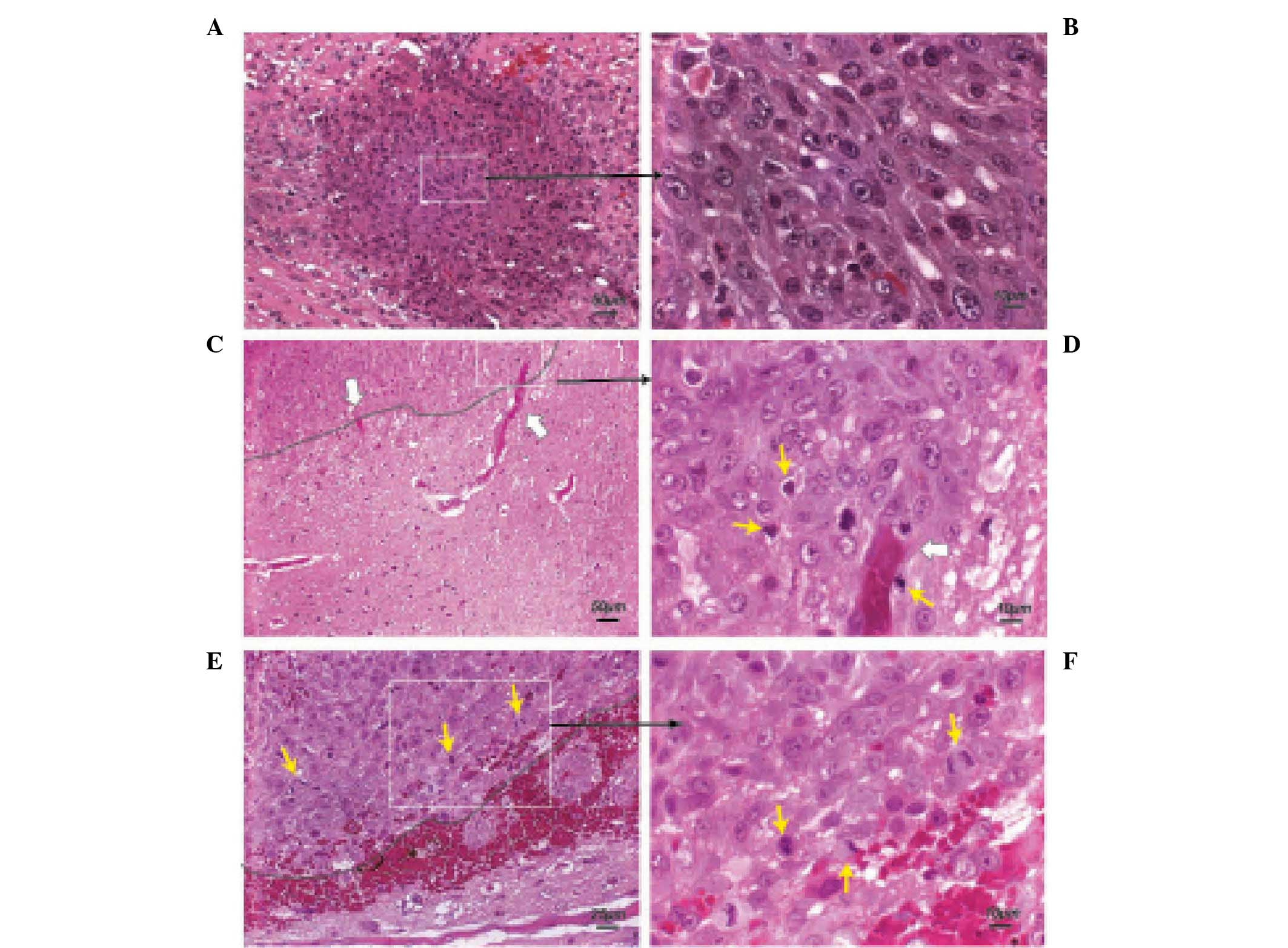 | Figure 2Morphological features occurring in
early stages (3–5 DPI) of a C6 glioma. (A) Small tumor spheroids
were detected at 3 DPI. The paraffin-embedded section was stained
with H&E (scale bar, 50 µm). (B) C6 cells were densely
compacted at the injection site and exhibited nuclear pleomorphism
at this stage (H&E; scale bar, 10 µm). (C) Prominent
neovascularization was observed at the tumor margin (black dash
line) at 5 DPI (H&E; scale bar, 50 µm). The white arrows
indicate blood vessels. (D) Magnified view (H&E; scale bar, 10
µm) of the white dashed rectangle from (C). White arrows
indicate blood vessels and yellow arrows indicate mitotic cells.
(E) Tumor margins remained smooth (black dash line, tumor margin;
yellow arrow, cell mitosis) at 5 DPI. (H&E; scale bar, 25
µm). (F) Magnified view (H&E; scale bar, 10 µm)
of the white dashed rectangle of panel (E). Yellow arrows indicate
mitotic cells. DPI, days post-implantation; H&E, hematoxylin
and eosin. |
At 5 DPI, active neovascularization was observed in
the tumor and the parenchyma near the tumor margin (Fig. 2C and D). Features at this stage
included the presence of actively dividing cells (Fig. 2E and F) and the formation of new
blood vessels at the tumor margin.
Tumor cell invasion at 7–9 DPI
At 7–9 DPI, tumor cells underwent prolific spreading
and invasion, which resulted in the formation of a diffuse and
irregular front zone (thin region outside visual boundary layer of
tumor) surrounding the tumor (Fig.
3A and 3B). The invading tumor
cells were able to migrate far from the tumor margin and aggregate
along the host blood vessels outside the tumor periphery (Fig. 3C). Under a high magnification view
(magnification, ×1,000), active mitosis of the perivascular tumor
cells was detected (Fig. 3D,
yellow arrow). It was noted that tumor cells at different locations
exhibited different morphological characteristics at this stage.
Tumor cells within the tumor core were generally round to oval, and
densely packed (Fig. 3E). Mitotic
cells could be observed in the tumor core (Fig. 3F, yellow arrow). By contrast, the
tumor cells near the tumor margin (periphery) exhibited a larger
cell size and larger nuclei with irregularly marginated chromatin
(Fig. 3G). Outside the tumor
margin, certain migrating tumor cells were clustered around the
blood vessels of the host (Fig.
3H). These perivascular tumor cells exhibited a significant
mitotic count, and partially corresponded with the intensive
staining of H&E similar to those in the center of the tumor
(Fig. 3F, yellow arrow).
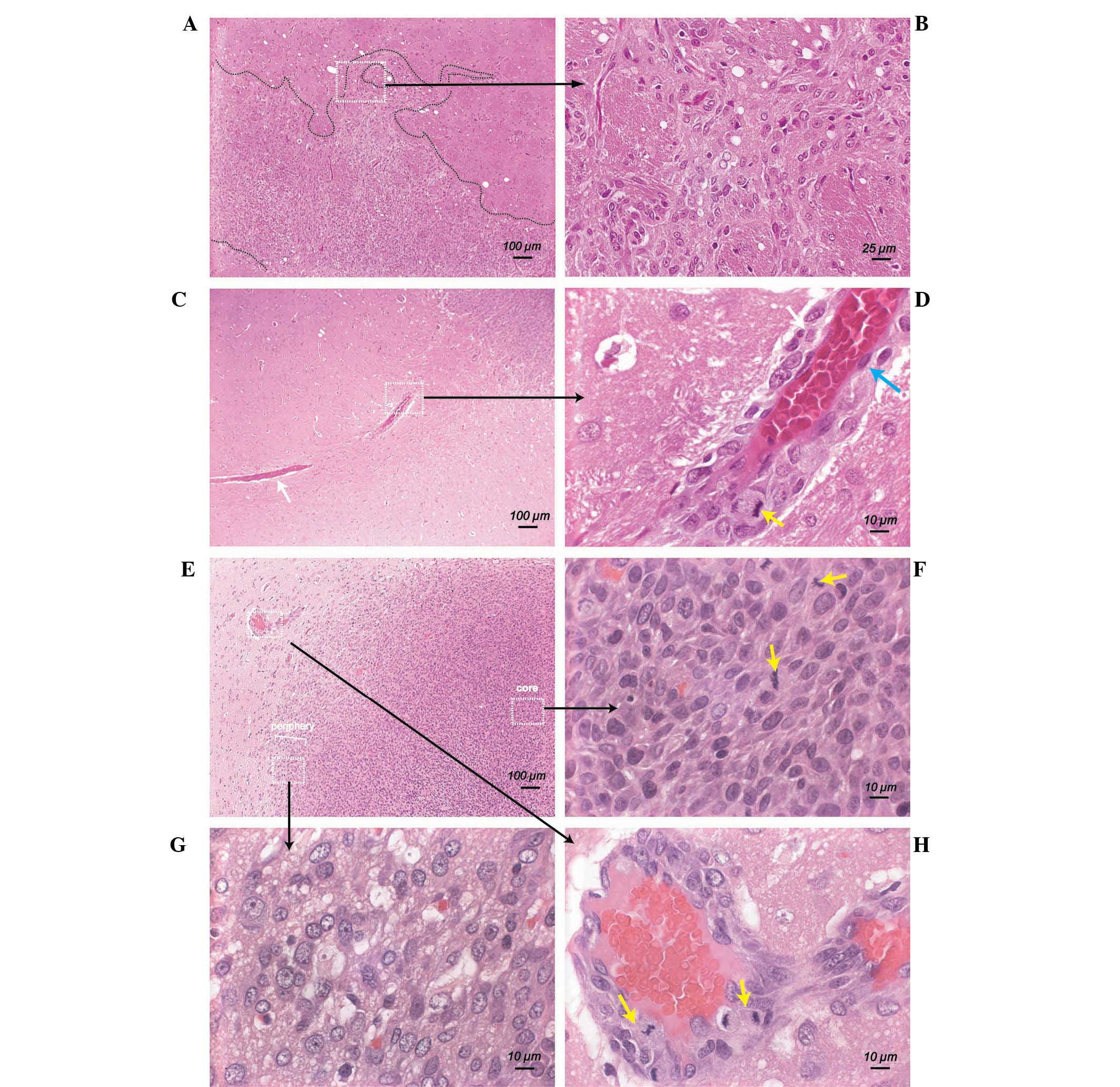 | Figure 3Active invasion of C6 cells at 7–9
DPI. (A) Formation of unusual tumor margins following extensive
invasion of tumor cells into normal parenchyma (H&E; scale bar,
100 µm) (B) Magnified view (H&E; scale bar, 25
µm) of the white rectangle from (A). Tumor cells grew in a
disorganized manner. (C) Migration of tumor cells along an adjacent
blood vessel (white rectangle) (H&E; scale bar, 100 µm).
(D) Microscopic view (H&E; scale bar, 10 µm) of the
white rectangle from (C). Ongoing mitosis (yellow arrow) was
observed in perivascular tumor cells (H&E; scale bar, 10
µm). The blue arrow indicates an endothelial cell. (E)
Photomicrograph of tumor core and tumor periphery (H&E; scale
bar, 100 µm). (F) High magnification view of the right
rectangle (H&E; scale bar, 10 µm) from (E). The yellow
arrows indicate cells undergoing mitosis. (G) Magnified view of the
bottom rectangle (H&E; scale bar, 10 µm) from (E). (H)
Magnified view of the left rectangle (H&E; scale bar, 10
µm) from (E). Mitotic tumor cells (yellow arrow) were
present in the perivascular space of a blood vessel. H&E,
hematoxylin and eosin; DPI, days post-implantation. |
Occurrence of tumor necrosis and
formation of tumor cell multilayer clusters at 11–15 DPI
At 11–15 DPI, the C6 glioma revealed typical
features of a malignant glioma, including hemorrhage, necrosis
(Fig. 4A), and pseudopalisades
(26) (Fig. 4B). Similar to the rat gliomas at
7–9 DPI, the morphology of tumor cells near the tumor margin
(Fig. 4C) was significantly
different from the tumor cells in the tumor core (Fig. 4D). Notably, tumor cell multi-layer
clusters distant from the primary tumor mass could be detected at
this stage (Fig. 4E and F). Serial
sample sections were examined to exclude the possibility that these
clusters were extensions of the primary tumor mass. Specifically,
all these multi-layer cell clusters were formed surrounding a host
blood vessel, which was confirmed by staining with α-SMA (Fig. 4G) and CD31 (Fig. 4H). Based on the diameter (20–40
µm) of the blood vessels detected by α-SMA staining
(Fig. 4G), it was suggested that
these blood vessels may represent small to medium size arterioles
(27,28).
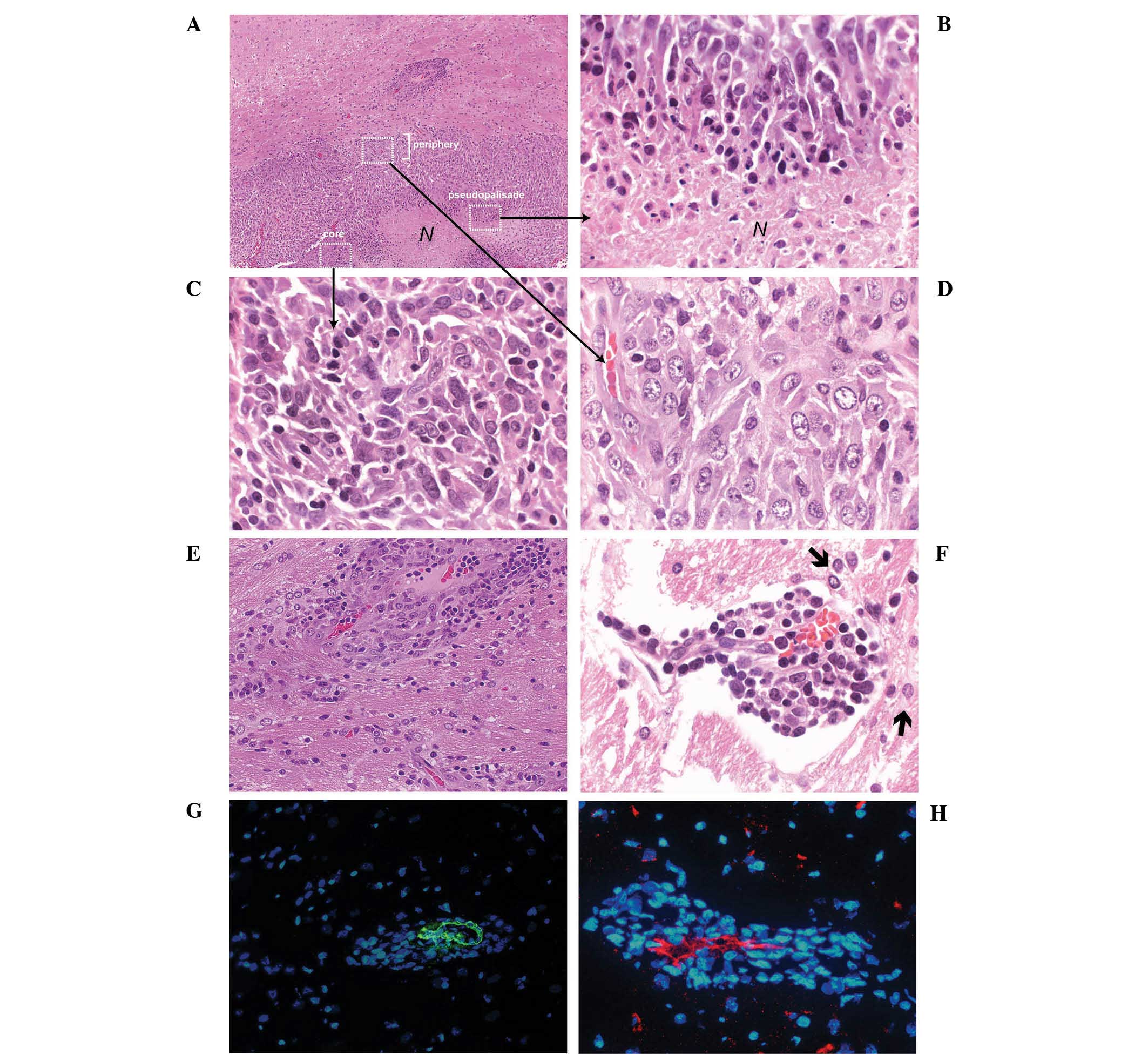 | Figure 4Occurrence of central necrosis and
formation of highly proliferative tumor cell clusters at 11–15 DPI.
(A) Malignant C6 glioma at 11 DPI (H&E; scale bar, 100
µm). N represents necrosis, C indicates a small tumor cell
cluster and white rectangles represent the region of the tumor
core, periphery and pseudopalisades. (B) At the perinecrotic
region, pseudopalisading C6 cells with condensed nuclei and an
elongated cytoplasm were observed in rows (H&E; scale bar, 10
µm). (C) Tumor cells carrying compact hyperchromatic nuclei
were detected in the tumor core (H&E; scale bar, 10 µm).
(D) Larger, loosely arranged tumor cells were identified at the
periphery of the tumor. (H&E; scale bar, 10 µm). (E and
F) Multi-layer tumor cell clusters were formed around small blood
vessels. As noted, the endothelium of the blood vessel exhibited
regression in the distal section (H&E; scale bar, 10
µm). Black arrow indicates a migrating cell. (G and H)
Immunofluorescence staining with anti-α-smooth muscle actin and
anti-CD31 antibodies (Scale bar, 25 µm). H&E,
hematoxylin and eosin; DPI, days post-implantation. |
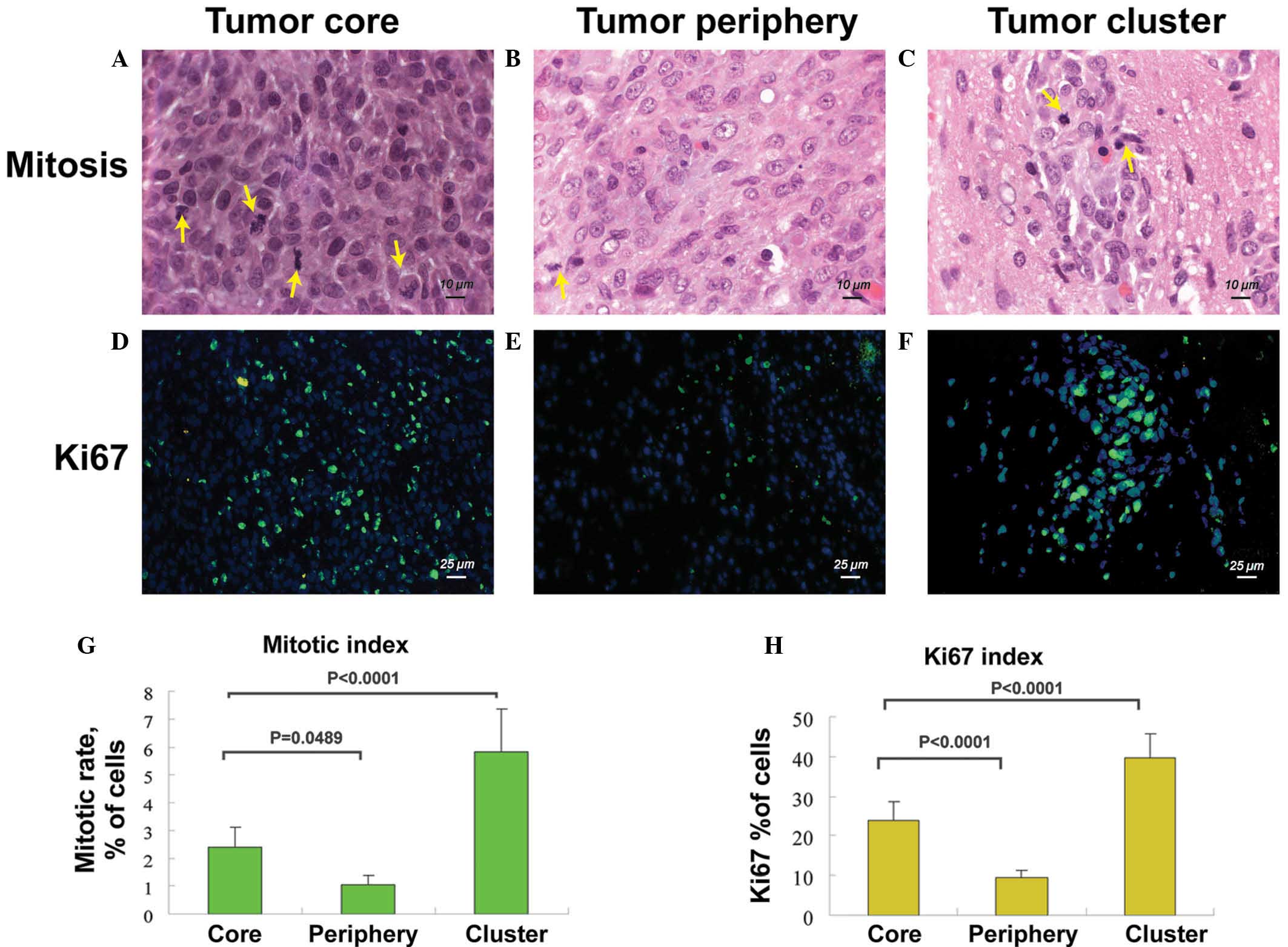
Differential cell proliferation rate in
different tumor areas
To investigate the proliferative rate of tumor cells
in the tumor core, tumor periphery and tumor cell clusters, the
mitotic index and Ki67 index were determined using H&E and Ki67
staining of tumor sections. Although there is no strict border
between the tumor core and tumor periphery, the tumor core was
defined as the region in the central section of the tumor or just
next to regions of tumor necrosis, while the tumor periphery was
defined as region in the extreme perimeter of the tumor margin.
As shown in Fig. 5,
the average mitotic index was 2.39±0.7% in the tumor core,
1.0±0.35% in the tumor periphery and 5.84±1.5% in the secondary
tumor clusters (Fig. 5A, B, C and
G). The average Ki67 index was 23.9±4.7% in the tumor core,
9.6±1.7% in the tumor periphery and 39.5±6.2% in the newly formed
clusters (Fig. 5D, E, F and H).
These results indicate that tumor cells in the secondary tumor
clusters have the highest cell proliferation rate, while tumor
cells proliferate poorly in the tumor periphery.
Differential expression of
tumor-associated genes in different areas of C6 glioma
To characterize gene expression patterns in
different areas of a mature glioma at 11–15 DPI, the expression of
tumor-associated genes, including MMP-2, HIF1α, VEGF, VEGFR2,
TWIST, Vimentin, p75 (29) and
TrkA, were analyzed using immunohistochemistry or
immunofluorescence as summarized in Table I. As shown in Fig. 6, MMP-2 expression was predominantly
detected in the periphery of the glioma. By contrast, HIF1α was
highly expressed in the tumor core, particularly in the
perinecrotic region. Notably, HIF1α, VEGF and VEGFR2 were
abundantly expressed in the tumor core and the tumor clusters, but
not in the periphery of the glioma. Despite different tumor cell
densities in different areas of the glioma, the positive staining
for TWIST, Vimentin and TrkA appeared to be distributed evenly in
the different tumor areas. In addition, p75 was abundantly
expressed in the secondary tumor clusters when compared with the
tumor core or periphery.
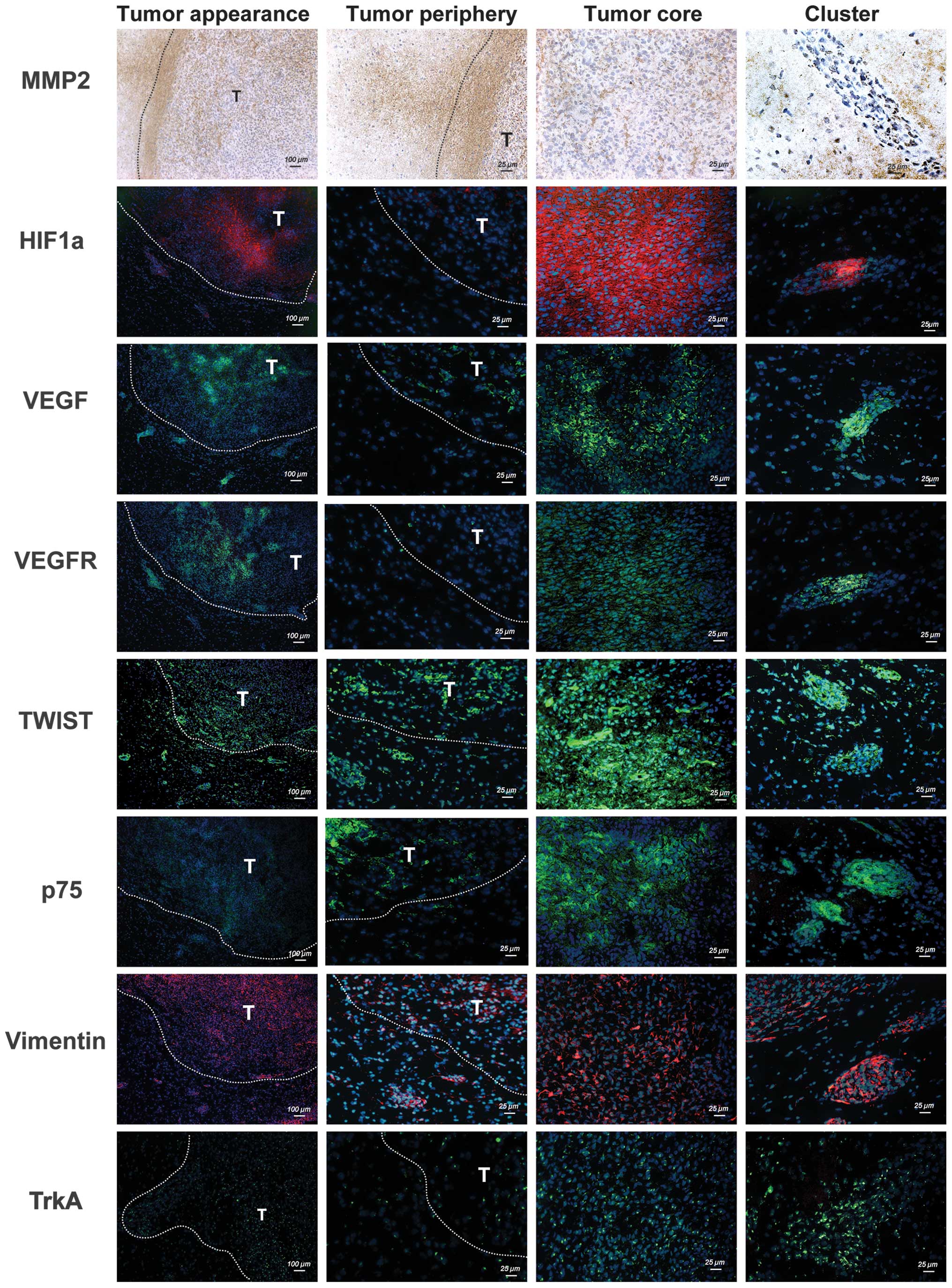 | Figure 6Spatial expression profiles of MMP-2,
HIF1α, VEGF, VEGFR2, TWIST, Vimentin, p75 and TrkA in C6 glioma.
The expression of the indicated tumor-associated genes in tissue
sections of C6 glioma was detected by immunohistochemistry or
immunofluorescence. The white dotted line indicates the tumor
margin and T indicates the tumor. HIF1α, hypoxia-inducible factor
1-α, MMP, matrix metalloproteinase; VEGF, vascular endothelial
growth factor; VEGFR, vascular endothelial growth factor receptor;
NGF, nerve growth factor; p75, p75 neurotrophin receptor; TrkA,
neurotrophic tyrosine kinase receptor type 1. |
 | Table ISpatial expression profiles of MMP-2,
HIF1α, VEGF, VEGFR2, TWIST, Vimentin, p75 and TrkA in C6
glioma. |
Table I
Spatial expression profiles of MMP-2,
HIF1α, VEGF, VEGFR2, TWIST, Vimentin, p75 and TrkA in C6
glioma.
| Gene | Tumor
periphery | Tumor core | Cluster |
|---|
| MMP-2 | +++ | + | + |
| HIF1α | − | +++ | ++ |
| VEGF | + | +++ | +++ |
| VEGFR | + | +++ | +++ |
| TWIST | ++ | +++ | +++ |
| p75 | + | ++ | ++++ |
| Vimentin | ++ | +++ | +++ |
| trkA | ++ | +++ | +++ |
Discussion
The C6 glioma model is a well-established malignant
brain tumor model, which shares a number of histopathological
features with human GBM (30).
Although the cytological features of C6 glioma progression have
been described in previous decades, the potential phenotypic switch
of C6 cells in rodent hosts remains to be elucidated. Thus,
re-examination of the phenotypic changes of C6 cells in the growing
tumors remains important to verify or support certain newly
developing hypotheses. In the present study, it was demonstrated
that a number of tumor-associated genes in C6 cells may be further
upregulated following implantation (Fig. 1C), suggesting that the neural
micro-environment in SD rats stimulates the expression of these
tumor-associated genes. These genes include HIF-1α, TWIST, MMP-9,
VEGF, VEGFR, NGF, and TrkA. Additionally, the present results
provided evidence of the phenotypic changes of invading tumor cells
and the formation of secondary tumor clusters during the late
tumorigenic stages.
In the present study, significant morphological and
phenotypic differences were identified between the tumor cells in
the tumor core and those in the tumor periphery during tumor
growth. Tumor cells with densely stained nuclei were closely packed
in the tumor core, in which mitotic cells were frequently
identified (Fig. 3F). By contrast,
invading tumor cells at the periphery were loosely arranged and
exhibited marginated chromatin (Fig.
3G). As suggested previously, the morphological and phenotypic
differences between invading tumor cells and those in the tumor
core may be due to the particular local environment (16). Tumor cells responding to different
supplies of nutrients, oxygen or other regulatory factors may
produce differential characteristics during the development of a
tumor (31). In addition to the
morphological changes, it was also demonstrated that invading tumor
cells have a relatively lower proliferation rate (Fig. 5) than those in the tumor core and
may be more resistant to apoptosis (15,18),
which contributes to their invasive nature (17,32).
These observations are consistent with the previous studies
concerning the dichotomy of cancer cell migration and invasion
(16,17). Notably, when the invading tumor
cells dock at blood vessels and form tumor clusters outside the
primary tumor mass, these tumor cells exhibited a high cell
proliferation rate. This suggests that invading cells may have the
potential to undergo another phenotypic change and continue to grow
into a secondary tumor mass under appropriate conditions.
The infiltration of tumor cells has been known to
form the structures termed 'secondary structures of Scherer' in
human GBM (33). The infiltration
of tumor cells is also regarded as the origin of multifocal or
multicentric human glioblastomas and is associated with a poor
prognosis (20). However, it has
rarely been investigated when or how the invading glioma cells
re-enter mitosis and form the secondary tumors in animal models
(6,21). Previously, Zhao et al
(34) established a xenograft
mouse model using an MMP-9-expressing U1242 MG glioma cell line
revealing an extensive invasion pattern. Large numbers of
individual tumor cells and cell clusters outside the implanted site
were observed in their model, and silencing of MMP-9 expression in
the U1242 MG cells significantly inhibited the motility of tumor
cells and the formation of tumor clusters. By contrast, treatment
of the xenograft rodent model with anti-VEGF antibodies was able to
reduce tumor blood flow and volume, which increased hypoxia in the
tumor core and resulted in the enhancement of tumor cell invasion
(35,36). Furthermore, the invading tumor
cells in these two models (MMP overexpression and anti-VEGF
treatment) exhibit significant perivascular invasion and formation
of multiple tumor clusters by co-option of the host vasculature at
the tumor periphery. Although cell clusters or satellite tumors may
be detected in these specific models, it remains to be elucidated
whether the formation of cell clusters or satellite tumors is a
common event during glioma development in rodent models.
Furthermore, the characteristics of these cell clusters or
satellite tumors remain elusive. In the present study, it was
demonstrated that the formation of cell clusters or satellite
tumors may be observed at the later stages of the C6 glioma model
and it may represent a common event in rodent models. Evidence was
also provided to demonstrate that tumor cell clusters are formed by
vascular co-option as proposed by Holash et al (12). On the basis of their hypothesis,
the tumor formation (of the primary tumor) undergoes blood vessel
co-option, blood vessel regression and tumor growth. In addition,
the growth of the secondary tumor clusters appears to follow a
similar path (Figs. 3 and 4).
Several specific features have been identified for
these tumor cell clusters, including multilayers (>3 layers) of
tumor cells surrounding a blood vessel (20–40 µm) up to
several millimeters away from the primary tumor mass, a high
proliferation rate comparable to that in the tumor core, and
similar gene expression patterns to that in the tumor core
(Fig. 6). Notably, not all
microvessels near the primary tumor were observed to support the
formation of tumor cell clusters. Therefore, tumor cell clusters
may only be formed when the invading cells identify a hospitable
perivascular space as suggested by the 'seed and soil' hypothesis
(37,38). As the small tumor cell clusters may
potentially be the origin of the secondary tumors, the
characterization of these cell clusters may be useful for
elucidating the micro-environmental regulation of tumor-host
interaction.
Immunohistochemical and immunofluorescent staining
were performed to compare the expression of specific
tumor-associated markers between different tumor regions, including
the tumor core, tumor periphery and secondary tumor clusters. The
present findings indicated that tumor cells in the tumor clusters
express similar biomarkers to those in the tumor core, but not to
those in the tumor periphery. The tumor cells in the secondary
tumor clusters, although derived from the aggregation and mitosis
of perivascular invading cells, exhibited higher expression levels
of HIF1α, VEGF and VEGFR, but not MMP-2, than the migrating tumor
cells from the tumor periphery (Table
I). These findings imply that the secondary tumor clusters
possess an enhanced capacity to induce angiogenesis. A
significantly higher expression of p75 was also observed in tumor
clusters as compared with that in the tumor periphery or in the
tumor core, suggesting p75 may be critical in tumor re-initiation
at different sites.
In conclusion, the progression of C6 glioma in SD
rats was re-characterized and evidence for the existence of the
perivascular tumor clusters surrounding the tumor margin was
provided, which may serve as the origin for the secondary tumors.
These findings clearly suggest that the phenotypic switch of
invasive tumor cells and the specific surrounding microenvironment
contribute to the initiation of the secondary tumor formation.
Acknowledgments
The present study was supported by grants from the
Chang-Gung Memorial Hospital (grant nos. CMRP680401, CMRPG680402
and CMRPD680403). The authors would like to express their sincere
appreciation to Miss I-Gin Lin for her extensive technical
assistance.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor LP: Diagnosis, treatment, and
prognosis of glioma: Five new things. Neurology. 75(Suppl 1):
S28–S32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagano N, Sasaki H, Aoyagi M and Hirakawa
K: Invasion of experimental rat brain tumor: Early morphological
changes following microinjection of C6 glioma cells. Acta
Neuropathol. 86:117–125. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vajkoczy P, Farhadi M, Gaumann A,
Heidenreich R, Erber R, Wunder A, Tonn JC, Menger MD and Breier G:
Microtumor growth initiates angiogenic sprouting with simultaneous
expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin
Invest. 109:777–785. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karmakar S, Olive MF, Banik NL and Ray SK:
Intracranial stereotaxic cannulation for development of orthotopic
glioblastoma allograft in Sprague-Dawley rats and
histoimmunopathological characterization of the brain tumor.
Neurochem Res. 32:2235–2242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vince GH, Bendszus M, Schweitzer T,
Goldbrunner RH, Hildebrandt S, Tilgner J, Klein R, Solymosi L,
Christian Tonn J and Roosen K: Spontaneous regression of
experimental gliomas - an immunohistochemical and MRI study of the
C6 glioma spheroid implantation model. Exp Neurol. 190:478–485.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sibenaller ZA, Etame AB, Ali MM, Barua M,
Braun TA, Casavant TL and Ryken TC: Genetic characterization of
commonly used glioma cell lines in the rat animal model system.
Neurosurg Focus. 19:E12005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bernstein JJ, Laws ER Jr, Levine KV, Wood
LR, Tadvalkar G and Goldberg WJ: C6 glioma-astrocytoma cell and
fetal astrocyte migration into artificial basement membrane: A
permissive substrate for neural tumors but not fetal astrocytes.
Neurosurgery. 28:652–658. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pedersen PH, Marienhagen K, Mørk S and
Bjerkvig R: Migratory pattern of fetal rat brain cells and human
glioma cells in the adult rat brain. Cancer Res. 53:5158–5165.
1993.PubMed/NCBI
|
|
10
|
Carbonell WS, Ansorge O, Sibson N and
Muschel R: The vascular basement membrane as 'soil' in brain
metastasis. PLoS One. 4:e58572009. View Article : Google Scholar
|
|
11
|
Kienast Y, von Baumgarten L, Fuhrmann M,
Klinkert WE, Goldbrunner R, Herms J and Winkler F: Real-time
imaging reveals the single steps of brain metastasis formation. Nat
Med. 16:116–122. 2010. View
Article : Google Scholar
|
|
12
|
Holash J, Maisonpierre PC, Compton D,
Boland P, Alexander CR, Zagzag D, Yancopoulos GD and Wiegand SJ:
Vessel cooption, regression, and growth in tumors mediated by
angiopoietins and VEGF. Science. 284:1994–1998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Winkler F, Kienast Y, Fuhrmann M, Von
Baumgarten L, Burgold S, Mitteregger G, Kretzschmar H and Herms J:
Imaging glioma cell invasion in vivo reveals mechanisms of
dissemination and peritumoral angiogenesis. Glia. 57:1306–1315.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farin A, Suzuki SO, Weiker M, Goldman JE,
Bruce JN and Canoll P: Transplanted glioma cells migrate and
proliferate on host brain vasculature: A dynamic analysis. Glia.
53:799–808. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: Invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mariani L, Beaudry C, McDonough WS,
Hoelzinger DB, Kaczmarek E, Ponce F, Coons SW, Giese A, Seiler RW
and Berens ME: Death-associated protein 3 (Dap-3) is overexpressed
in invasive glioblastoma cells in vivo and in glioma cell lines
with induced motility phenotype in vitro. Clin Cancer Res.
7:2480–2489. 2001.PubMed/NCBI
|
|
17
|
Giese A, Loo MA, Tran N, Haskett D, Coons
SW and Berens ME: Dichotomy of astrocytoma migration and
proliferation. Int J Cancer. 67:275–282. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saito R, Bringas J, Mirek H, Berger MS and
Bankiewicz KS: Invasive phenotype observed in
1,3-bis(2-chloroethyl)-1-nitrosourea-resistant sublines of 9L rat
glioma cells: A tumor model mimicking a recurrent malignant glioma.
J Neurosurg. 101:826–831. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chicoine MR and Silbergeld DL: Invading C6
glioma cells maintaining tumorigenicity. J Neurosurg. 83:665–671.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hassaneen W, Levine NB, Suki D, Salaskar
AL, de Moura Lima A, McCutcheon IE, Prabhu SS, Lang FF, DeMonte F,
Rao G, et al: Multiple craniotomies in the management of multifocal
and multicentric glioblastoma. Clinical article. J Neurosurg.
114:576–584. 2011. View Article : Google Scholar
|
|
21
|
Grobben B, De Deyn PP and Slegers H: Rat
C6 glioma as experimental model system for the study of
glioblastoma growth and invasion. Cell Tissue Res. 310:257–270.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang TC, Hsiao IT, Cheng YK, Wey SP, Yen
TC and Lin KJ: Noninvasive monitoring of tumor growth in a rat
glioma model: Comparison between neurological assessment and animal
imaging. J Neurooncol. 104:669–678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mikheeva SA, Mikheev AM, Petit A, Beyer R,
Oxford RG, Khorasani L, Maxwell JP, Glackin CA, Wakimoto H,
González-Herrero I, et al: TWIST1 promotes invasion through
mesenchymal change in human glioblastoma. Mol Cancer. 9(194)2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Walsh EM, Kim R, Del Valle L, Weaver M,
Sheffield J, Lazarovici P and Marcinkiewicz C: Importance of
interaction between nerve growth factor and α9β1 integrin in glial
tumor angiogenesis. Neuro-oncol. 14:890–901. 2012. View Article : Google Scholar
|
|
25
|
Assimakopoulou M, Kondyli M, Gatzounis G,
Maraziotis T and Varakis J: Neurotrophin receptors expression and
JNK pathway activation in human astrocytomas. BMC Cancer.
7(202)2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brat DJ and Van Meir EG: Vaso-occlusive
and prothrombotic mechanisms associated with tumor hypoxia,
necrosis, and accelerated growth in glioblastoma. Lab Invest.
84:397–405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCaslin AF, Chen BR, Radosevich AJ, Cauli
B and Hillman EM: In vivo 3D morphology of astrocyte-vasculature
interactions in the somatosensory cortex: Implications for
neurovascular coupling. J Cereb Blood Flow Metab. 31:795–806. 2011.
View Article : Google Scholar :
|
|
28
|
Seylaz J, Charbonné R, Nanri K, Von Euw D,
Borredon J, Kacem K, Méric P and Pinard E: Dynamic in vivo
measurement of erythrocyte velocity and flow in capillaries and of
microvessel diameter in the rat brain by confocal laser microscopy.
J Cereb Blood Flow Metab. 19:863–870. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Johnston AL, Lun X, Rahn JJ, Liacini A,
Wang L, Hamilton MG, Parney IF, Hempstead BL, Robbins SM, Forsyth
PA, et al: The p75 neurotrophin receptor is a central regulator of
glioma invasion. PLoS Biol. 5:e2122007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacobs VL, Valdes PA, Hickey WF and De Leo
JA: Current review of in vivo GBM rodent models: Emphasis on the
CNS-1 tumour model. ASN Neuro. 3:e000632011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gorin F, Harley W, Schnier J, Lyeth B and
Jue T: Perinecrotic glioma proliferation and metabolic profile
within an intrace-rebral tumor xenograft. Acta Neuropathol.
107:235–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hoelzinger DB, Mariani L, Weis J, Woyke T,
Berens TJ, McDonough WS, Sloan A, Coons SW and Berens ME: Gene
expression profile of glioblastoma multiforme invasive phenotype
points to new therapeutic targets. Neoplasia. 7:7–16. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zagzag D, Lukyanov Y, Lan L, Ali MA,
Esencay M, Mendez O, Yee H, Voura EB and Newcomb EW:
Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in
glioblastoma: Implications for angiogenesis and glioma cell
invasion. Lab Invest. 86:1221–1232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao Y, Xiao A, diPierro CG, Carpenter JE,
Abdel-Fattah R, Redpath GT, Lopes MB and Hussaini IM: An extensive
invasive intracranial human glioblastoma xenograft model: Role of
high level matrix metalloproteinase 9. Am J Pathol. 176:3032–3049.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rubenstein JL, Kim J, Ozawa T, Zhang M,
Westphal M, Deen DF and Shuman MA: Anti-VEGF antibody treatment of
glioblastoma prolongs survival but results in increased vascular
cooption. Neoplasia. 2:306–314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Keunen O, Johansson M, Oudin A, Sanzey M,
Rahim SA, Fack F, Thorsen F, Taxt T, Bartos M, Jirik R, et al:
Anti-VEGF treatment reduces blood supply and increases tumor cell
invasion in glioblastoma. Proc Natl Acad Sci USA. 108:3749–3754.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fidler IJ: The pathogenesis of cancer
metastasis: The 'seed and soil' hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fidler IJ and Poste G: The 'seed and soil'
hypothesis revisited. Lancet Oncol. 9(808)2008. View Article : Google Scholar
|