Introduction
Breast cancer is the most common type of malignant
tumor in females (1–3). Despite major advances in breast
cancer screening, early diagnosis and treatment modalities, it
remains the predominant cause of cancer-associated mortality in
females worldwide (4). Existing
treatment strategies include surgery, chemotherapy, endocrine
therapy, and molecular-targeted therapy, which are limited by tumor
recurrence and drug resistance (5–7).
Therefore, novel approaches to enhance the effects of therapeutic
agents and improve the existing standards of care are urgently
required.
It is well-known that the phosphoinositide 3-kinase
(PI3K)/Akt/mammalian target of rapamycin (mTOR) and the
mitogen-activated protein kinase (MAPK)/MAPK kinase (MEK) signaling
pathways have important roles in tumor progression and drug
resistance in various types of cancer (4). Previous studies indicate that
activation of the PI3K/Akt/mTOR signaling pathway is closely
associated with the poor outcome of patients with breast cancer
undergoing endocrine therapy (8,9). The
activation of this signaling pathway had been identified as an
important mechanism of tamoxifen resistance (10–13).
The MEK/MAPK signaling pathway has also been associated with
tamoxifen resistance and chemoresistance (14,15).
In addition, the activation of the MEK/MAPK signaling pathway is
involved in resistance to epidermal growth factor receptor (EGFR)
tyrosine kinase inhibitor, gefitinib, in breast cancer cells
(16). Therefore, compounds
targeting these signaling pathways are likely to be promising
agents against endocrine therapy resistance in breast cancer.
Natural products are widely administered to prevent
cancer in multi-stage carcinogenesis in humans, and have been the
subject of intensive research in recent years (17). Isorhamnetin is a flavonoid that is
abundantly present in fruits, vegetables and tea, as well as in
herbs that are used as traditional medicine, such as Ginkgo
biloba extract and Persicaria thunbergii H (18,19).
These two herbs are administered for the treatment of rheumatism,
hemorrhage and cancer in traditional medicine (20–22).
Isorhamnetin is the active compound of these herbal medicinal
plants, and is an immediate metabolite of quercetin, also termed
3′-O-methylquercetin, which has been shown to inhibit various types
of cancer, including esophageal (23) and gastric cancer (24), leukemia (25,26),
skin (27), colon (28) and lung cancer (29). However, to the best of our
knowledge, no study to date has focused on the inhibitory effects
of isorhamnetin on breast cancer, and the molecular mechanisms
underlying its effects remain unclear.
To better understand the mechanism underlying the
effects of isorhamnetin on breast cancer, the present study
examined the inhibition of isorhamnetin and the proliferation of
various breast cancer cell lines, and explored the cell signaling
pathways involved in its pharmacological effects.
Materials and methods
Cell lines
MCF7, T47D, BT474, BT-549, MDA-MB-231 and MDA-MB-468
breast cancer cell lines, as well as a MCF10A normal breast
epithelial cell line (control) were purchased from the American
Type Culture Collection (Manassas, VA, USA). The cells were
routinely cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (Invitrogen Life
Technologies, Carlsbad, CA, USA) at 37°C in a 5% CO2
incubator. MCF7, T47D and BT474 are estrogen receptor (ER) and
progestogen receptor (PR)-positive cells, and human epidermal
growth factor (HER)2-negative cells. BT-549, MDA-MB-231 and
MDA-MB-468 are ER-, PR- and HER2-positive cells.
Reagents
Isorhamnetin was purchased from Shanghai Tongtian
Biotechnology Co., Ltd. (Shanghai, China). Perifosine, PD184352 and
JSH-23 were purchased from Selleck Chemicals (Houston, TX, USA).
EGF was purchased from Sigma-Aldrich (St. Louis, MO, USA). The
antibodies for mouse monoclonal β-actin (cat. no. 3700; 1:1,000),
rabbit polyclonal phosphorylated (p)-EGFR immunoglobulin (Ig)G
(cat. no. 2234; 1:1,000), rabbit monoclonal EGFR (cat. no. 4405;
1:1,000), rabbit monoclonal PI3K (cat. no. 4249; 1:1,000), rabbit
monoclonal p-Akt (S473; cat. no. 4060; 1:500), mouse monoclonal Akt
(cat. no. 2920; 1:1,000), mouse monoclonal p-ERK1/2 (cat. no. 9106;
1:500), rabbit monoclonal ERK1/2 (cat. no. 4695; 1:1,000) and
rabbit monoclonal cleaved caspase-3 (cat. no. 9664; 1:500) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA),
and antibodies for rabbit monoclonal B cell lymphoma 2 (Bcl-2; cat.
no. ab117115; 1:1,000), rabbit monoclonal Bcl-2-associated X
protein (Bax; cat. no. ab32503; 1:1,000), rabbit monoclonal
Bcl-extra large (xL; cat. no. ab32370; 1:2,000), rabbit monoclonal
IκB (cat. no. ab32518; 1:1,000), rabbit monoclonal anti-NF-κB P65
antibody (cat. no. ab32536; 1:2,000) and rabbit polyclonal H3 (cat.
no. ab1791; 1:2,000) were purchased from Abcam (Cambridge, MA,
USA).
Cell Counting kit-8 (CCK-8) assay
The cells were seeded into 96-well plates at a
density of 5×103 cells/well in 100 µl DMEM and
placed in cell incubator for 12 h at 37°C in an atmosphere
containing 5% CO2. The cells were then treated with
various concentrations of isorhamnetin (100, 33.3, 11.1, 3.7, 1.2,
0.4 and 0 µM) for 48 h, and cell proliferation rates were
determined by adding 10 µl CCK-8 solution (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) prior to incubation at 37°C
for 2 h. The absorbance was measured at a wavelength of 450 nm
using a SpectraMax 190 Microplate Reader (Molecular Devices, LLC,
Sunnyvale, CA, USA). For each assay, four parallel wells were
included, and the half maximal inhibitory concentration
(IC50) was measured using the inhibition curve and
presented as the mean of three independent experiments.
Western blot analysis
The cells were washed twice with ice-cold
phosphate-buffered saline (PBS) and harvested using
radioimmunoprecipitation (RIPA) buffer (Beyotime Institute of
Biotechnology; 50 mM Tris, 150 mM NaCl, 1% Triton X-100, 0.1% SDS,
and 1% sodium deoxycholate, with protease and phosphatase
inhibitors). The cell lysate was placed in RIPA for 30 min and
centrifuged at a speed of 12,000 × g for 15 min, and the
supernatant was then collected. Each aliquot of protein (10
µg) was separated by 12% SDS-PAGE and transferred to
Hybond-C nitrocellulose membranes (GE Healthcare Biosciences,
Pittsburg, PA, USA) in transfer buffer (192 mM glycine, 25 mM Tris,
2.5 mM SDS, and 10% methanol) (Sangon Biotech Co., Ltd., Shanghai,
China). The membranes were blocked with 5% non-fat milk in
Tris-buffered saline with 1% Tween-20 (TBST; Sangon Biotech Co.,
Ltd.) for 1 h at room temperature, incubated with the previously
mentioned primary antibodies at 4°C overnight, then washed three
times with TBST for 10 min prior to incubation with secondary
horseradish peroxidase-conjugated anti-rabbit (cat. no. 7074; Cell
Signaling Technology, Inc.) or anti-mouse IgG (cat. no. 7076; Cell
Signaling Technology, Inc.) antibodies, and again washed three time
with TBST for 10 min. The blots were developed with an Enhanced
Chemiluminescence Plus Western Blotting Detection system (GE
Healthcare Biosciences). The total protein was determined using the
Bicinchoninic Acid method (Invitrogen Life Technologies; cat. no.
23235).
Flow cytometric assay
To determine the effects of isorhamnetin on cell
apoptosis, the MCF7 or MDA-MB-468 cells were seeded at a density of
2×105 cells/well in a 6-well plate, and incubated at
37°C overnight. The cells were treated with isorhamnetin or the
inhibitors (perifosine, PD184352 and JSH-23) for 48 h, prior to
being detached and washed with cooled PBS. The cells were collected
by trypsin (Invitrogen Life Technologies) digestion and
centrifugation at 300 × g for 3 min. The cells were then
resuspended in binding buffer containing Annexin-V and propidium
iodide (PI; Beyotime Institute of Biotechnology, Jiangsu, China)
and incubated for 15 min in the dark at room temperature. Analysis
was performed using a FACSCalibur analyzer (BD Biosciences, San
Jose, CA, USA).
Statistical analysis
Protein expression was quantified using Image J
software (version 1.31; Utrecht University, Utrecht, Netherlands)
and expressed as the relative expression levels to the control
group. P-values were calculated by comparison to the control group
using analysis of variance with SPSS 19 (IBM SPSS, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant result.
Results
Isorhamnetin inhibits proliferation and
induces apoptosis of breast cancer cells
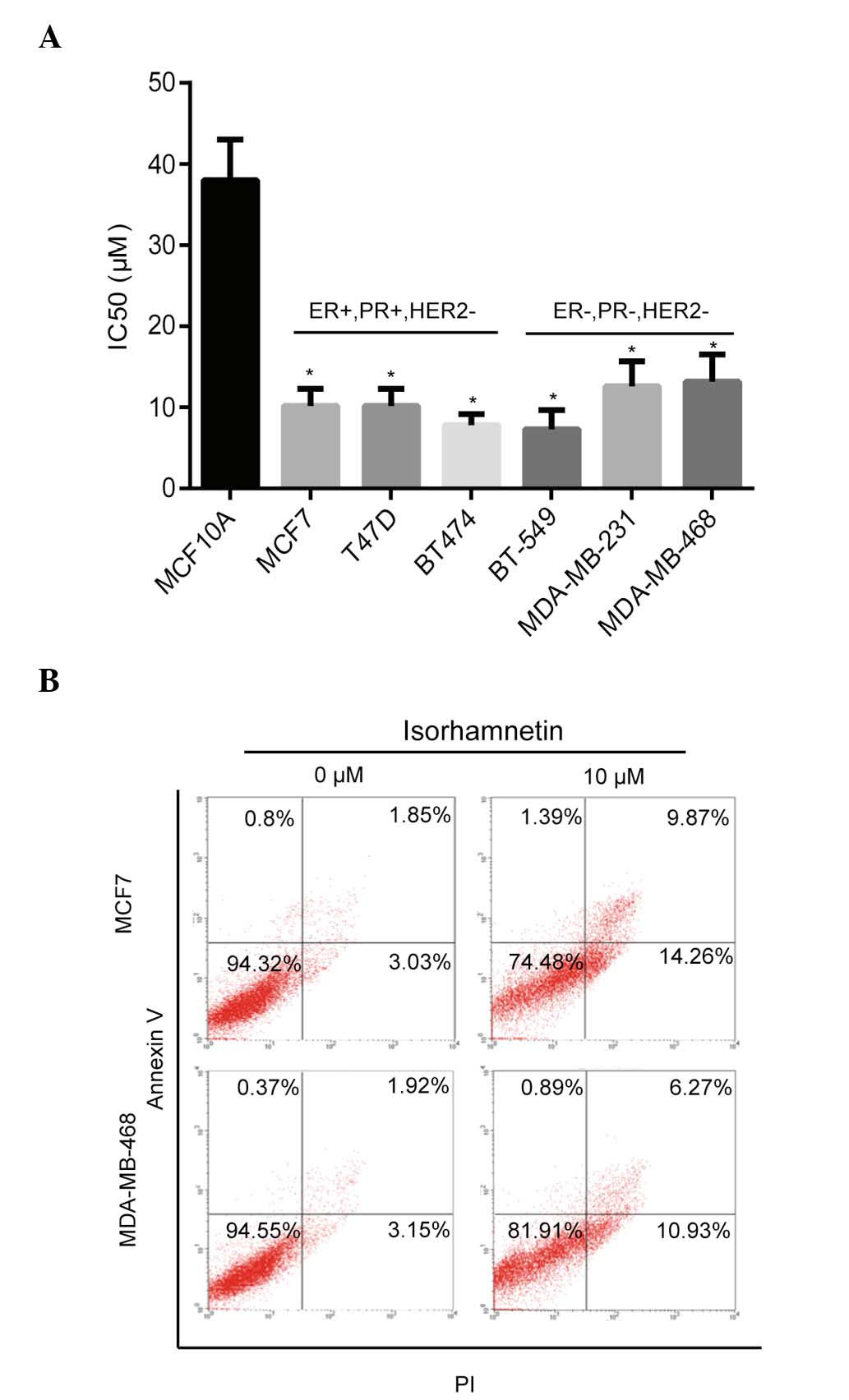
The inhibitory effects of isorhamnetin on breast
cancer cells were determined using the CCK-8 method. As shown in
Fig. 1A, isorhamnetin inhibited
the proliferation of numerous breast cancer cells (IC50,
~10 µM), including MCF7, T47D, BT474, BT-549, MDA-MB-231 and
MDA-MB-468, whereas less inhibitory activity was observed in the
MCF10A normal breast epithelial cell line (IC50, 38
µM). These results indicated that isorhamnetin induces
pronounced inhibitory effects on breast cancer cell lines
(P<0.05), as compared to normal breast epithelial cell lines,
which suggests that isorhamnetin may act on the activation pathway
of cancer cells. The effect of isorhamnetin on cell apoptosis was
subsequently determined using two breast cancer cell lines, MCF7
and MDA-MB-468. Isorhamnetin markedly promoted cell apoptosis of
the MCF7 and MDA-MB-468 cell lines (Fig. 1B) as shown by the sum of early and
late apoptotic cells, with increased apoptotic rates observed in
the MCF7 cells, as compared with the MDA-MD-468 cells; these
results were consistent with the IC50 values of
isorhamnetin that were determined for the two cell lines.
Isorhamnetin inhibited the Akt/mTOR and
MEK/ERK signaling pathways, and promoted the activity of the
mitochondrial apoptosis signaling pathway
In order to investigate the inhibitory mechanism
underlying the effects of isorhamnetin on breast cancer cells, the
PI3K/Akt/mTOR and MEK/ERK signaling pathways, which are closely
associated with cell proliferation and survival, were examined. As
shown in Fig. 2A, the
phosphorylation levels of Akt and mTOR were markedly decreased by
isorhamnetin, as well as the phosphorylation levels of MEK1/2 and
ERK1/2 both in MCF7 and MDA-MB-468 cells; however, those of their
upstream signaling molecules, such as phosphorylated EGFR and PI3K
(110α) remained unchanged. The expression of the components of the
cell apoptosis signaling pathway, including Bax, Bcl-2, Bcl-xL and
cleaved caspase-3 were also investigated. Bax expression was
induced by isorhamnetin, whereas the Bcl-2 expression level was
markedly downregulated, and a marginal decrease was observed in the
expression level of Bcl-xL (Fig.
2B). Cleaved caspase-3 expression increased, implying that cell
apoptosis was induced by isorhamnetin.
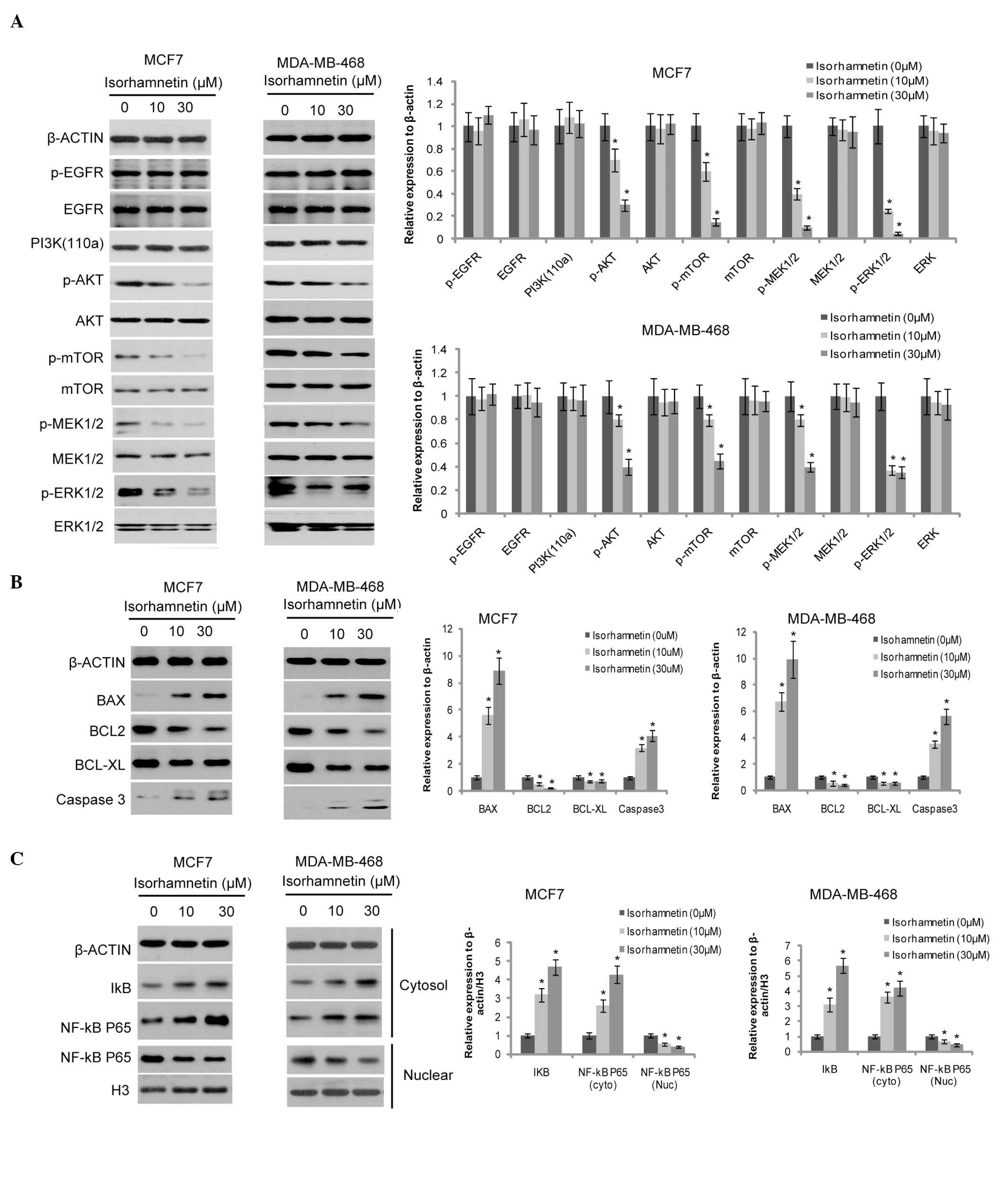 | Figure 2Effects of isorhamnetin on the cell
signaling cascade. (A) Isorhamnetin inhibited the phosphorylation
of Akt, mTOR, MEK1/2 and ERK1/2, but not of EGFR (12-h treatment).
The protein expression levels were quantified by Image J, and
presented as the relative expression levels to the control. (B)
Isorhamnetin increased the expression level of Bax and cleaved
caspase 3, and decreased the expression level of Bcl-2 and Bcl-xL
(12-h treatment); (C) Isorhamnetin decreased the nuclear
translocation of NF-κB (24-h treatment). p, phosphorylated; mTOR,
mammalian target of rapamycin; MEK, mitogen-activated protein
kinase kinase; ERK, extracellular signal-regulated kinase; EGFR,
epidermal growth factor receptor; PI3K, phosphoinositide 3-kinase;
Bcl-2, B cell lymphoma 2; Bax, Bcl-2-associated protein X;
Bcl-2-xL, Bcl-extra large; NF-κB, nuclear factor-κB.
*P<0.05, vs. the control group. |
As isorhamnetin exhibits anti-oxidant effects, its
impact on the downstream signaling molecule, NF-κB P65 was
investigated, in order to identify whether these effects
contributed to its anti-tumorigenic activity. As shown in Fig. 2C, the expression of cytosolic NF-κB
P65 and its inhibitor, IκB were increased by isorhamnetin, whereas
nuclear NF-κB P65 expression was decreased, suggesting that the
nuclear translocation of NF-κB P65 is inhibited by
isorhamnetin.
Inhibition of the Akt or MEK signaling
pathways attenuates the effects of isorhamnetin
Akt and MEK inhibitors (perifosine and PD184352)
were used to elucidate the mechanism of action of isorhamnetin
(whether it is via the Akt or MEK signaling pathways) on breast
cancer (Fig. 3). Perifosine is a
potent pan-Akt inhibitor, which was used to block the Akt signaling
pathway. Akt phosphorylation was inhibited by perifosine and partly
inhibited by isorhamnetin (10 µM; Fig. 3A,). Following pretreatment of the
MCF7 cells with perifosine prior to the addition of isorhamnetin,
induction of cell apoptosis was only marginally increased by
isorhamnetin, as determined by cleaved caspase-3 and Annexin-V/PI
dual staining (Fig. 3C). These
results demonstrate that blocking of Akt phosphorylation attenuated
the effects of isorhamnetin.
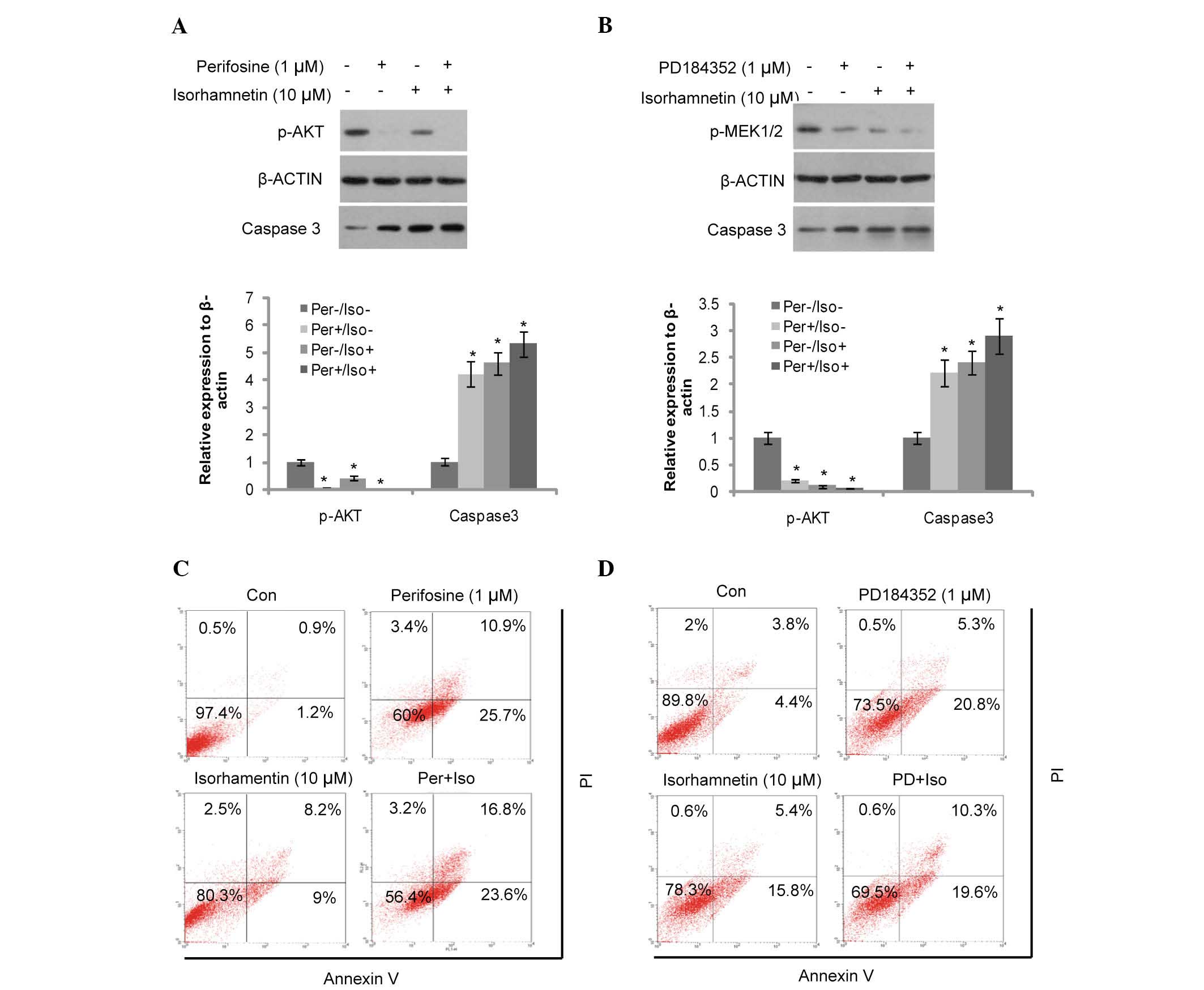 | Figure 3Induction of apoptosis was attenuated
by the Akt and MEK inhibitors in the MCF7 cells. (A) Induction of
caspase-3 cleavage was attenuated by the Akt inhibitor, perifosine
(12-h treatment). The protein expression levels were quantified by
Image J, and presented as the relative expression levels to the
control. (B) Induction of caspase-3 cleavage was attenuated by the
MEK inhibitor, PD184352 (12-h treatment). *P<0.05,
vs. the control group. (C) Induction of cell apoptosis was
attenuated by the Akt inhibitor, perifosine (48-h treatment). (D)
Induction of cell apoptosis was attenuated by the MEK inhibitor,
PD184352 (48-h treatment). MEK, mitogen-activated protein kinase
kinase; Con, control; PI, propidium iodide; PD, PD184352; Per,
perifosine; Iso, isorhamnetin. The upper right quadrant shows the
early apoptotic cells, the lower right the late apoptotic cells,
the upper left quadrant indicates cell debris and the lower left
the viable cells. |
Similarly, the MEK and ERK inhibitor, PD184352 was
used to block the corresponding signaling pathways. As shown in
Fig. 3B, PD184352 (1 µM)
and isorhamnetin (10 µM) inhibited MEK1/2 phosphorylation
and induced cell apoptosis. This was also determined by increased
levels of cleaved caspase-3 expression and Annexin-V/PI dual
staining (Fig. 3D). MCF7 cells
were pre-treated with PD184352 prior to the addition of
isorhamnetin, and the induction of cell apoptosis was only
marginally increased following treatment with isorhamnetin. These
results demonstrate that inhibition of the MEK signaling pathway
also attenuates the effects of isorhamnetin.
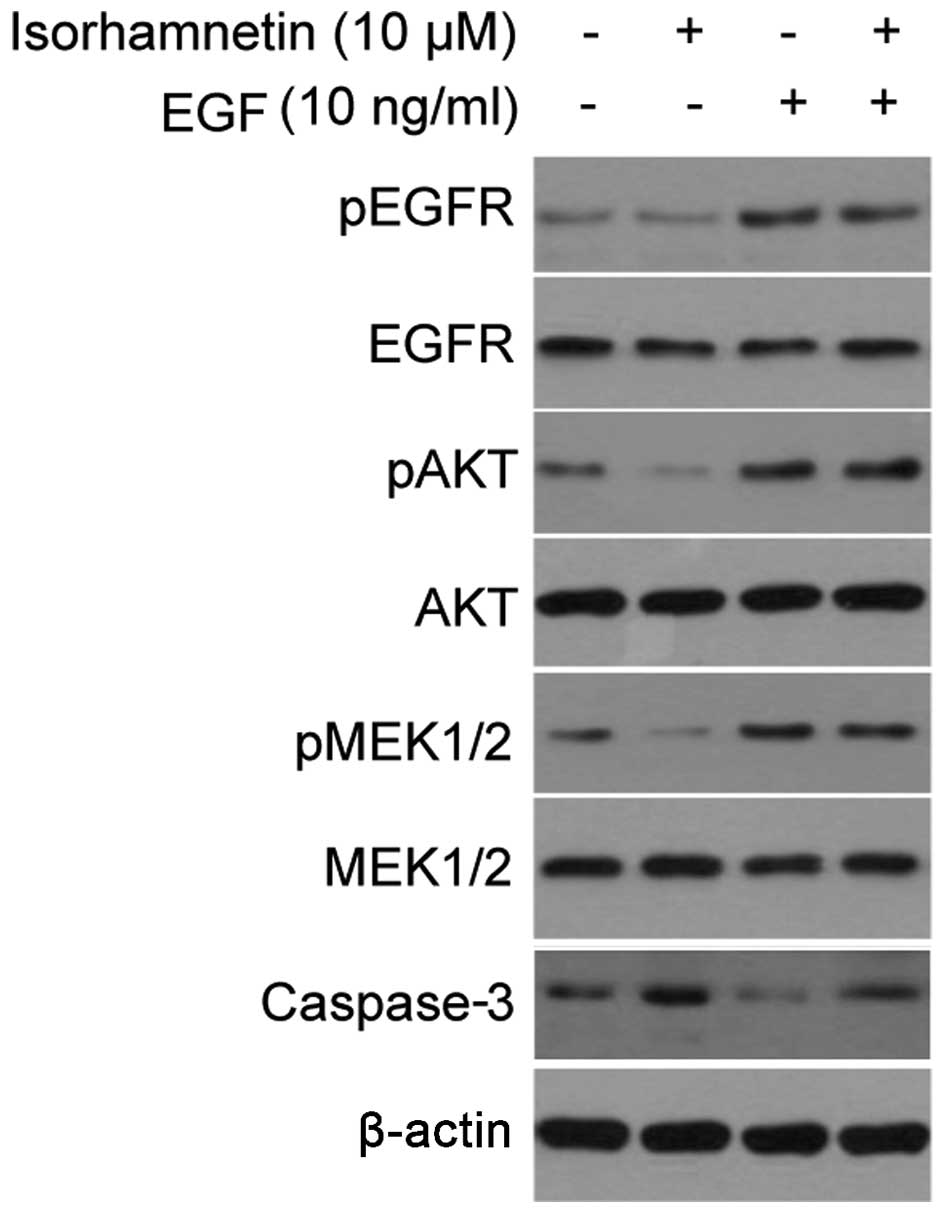
EGF reversed the inhibitory effects of
isorhamnetin
EGF is a growth factor that binds to EGFR in order
to induce the activation of downstream PI3K/Akt and MEK/ERK
signaling pathways. Although EGFR phosphorylation did not change
following treatment with isorhamnetin, the addition of EGF
activated the Akt and MEK signaling pathways, and may be used to
verify the effects of isorhamnetin. As shown in Fig. 4A, treatment with EGF markedly
increased the phosphorylation levels of EGFR, Akt and MEK1/2, and
these effects overrode the inhibitory effects of isorhamnetin on
Akt and MEK1/2, thus inhibiting the cell apoptosis induced by
isorhamnetin, as determined by decreased levels of cleaved
caspase-3 expression.
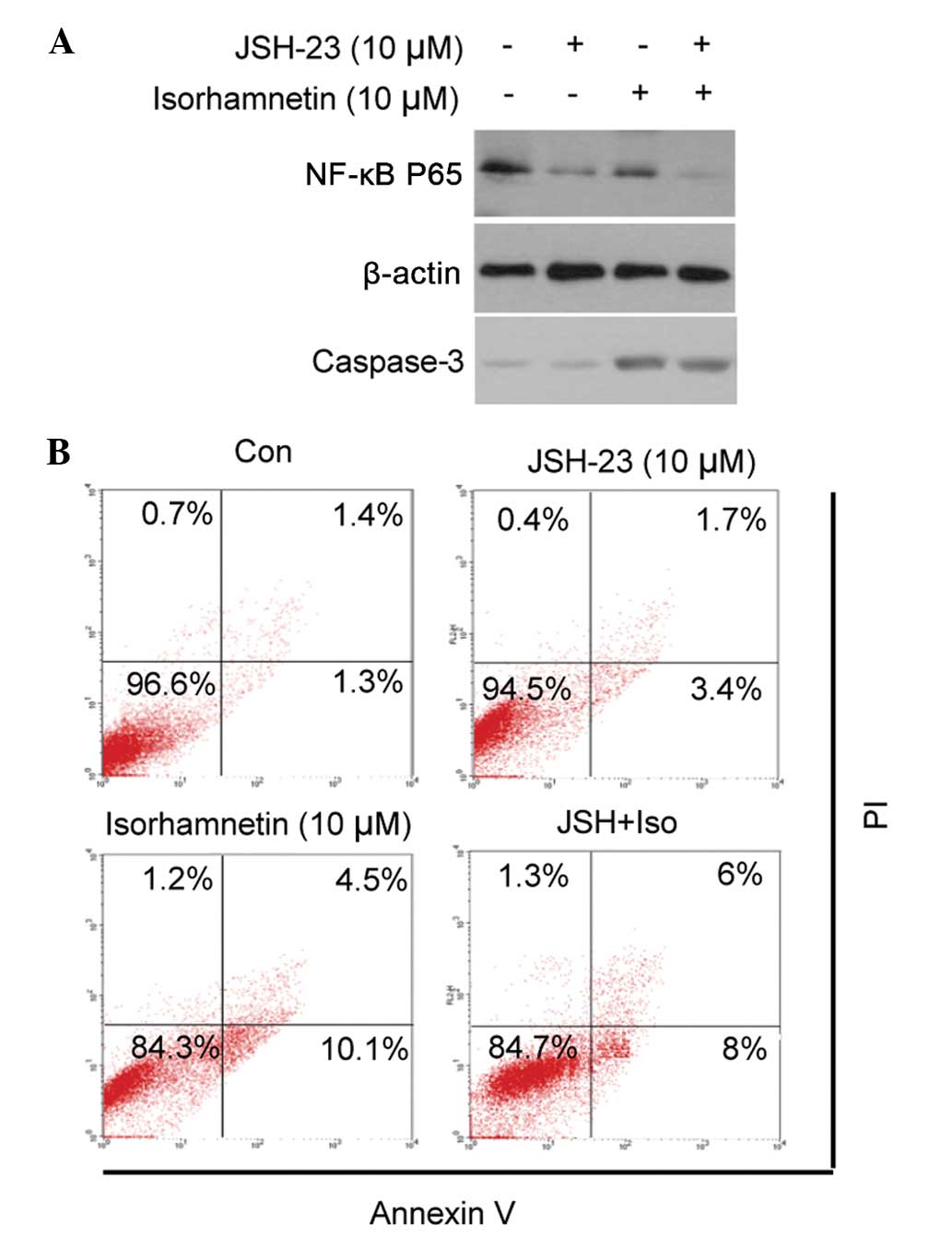
NF-κB does not contribute to the
induction of cell apoptosis by isorhamnetin
The present study investigated whether the
inhibition of NF-κB translocation contributed to cell apoptosis. An
NF-κB inhibitor, JSH-23, was used to block the nuclear
translocation of NF-κB, prior to treatment with isorhamnetin. As
shown in Fig. 5, treatment with
JSH-23 inhibited NF-κB translocation, but did not result in the
apoptosis of MCF7 cells, either alone or in combination with
isorhamnetin (as demonstrated by cleaved caspase-3 and Annexin-V/PI
dual staining). These results suggested that inhibition of the
NF-κB signaling pathway does not contribute to the induction of
cell apoptosis by isorhamnetin.
Discussion
The present study examined the inhibitory effects of
isorhamnetin on breast cancer cell lines, and demonstrated that
isorhamnetin inhibits the proliferation of various types of breast
cancer cell, in ER-positive PR-positive HER2-negative cells (MCF7,
T47D, BT474) and ER-PR-HER2-cells (BT-549, MDA-MB-231, MDA-MB-468),
but exhibited low inhibitory activity levels against normal MCF10A
breast epithelial cells, suggesting that isorhamnetin inhibited
breast cancer cells independently of ER and PR, and relies instead
on activated cancer signaling pathways. Isorhamnetin also induced
apoptosis in breast cancer cell lines. These results indicate that
isorhamnetin may act as a novel natural compound for the prevention
or treatment of breast cancer.
The molecular mechanisms underlying the observed
anticancer effects of isorhamnetin were also investigated. Since
the Akt/mTOR and MEK/ERK signaling pathways have important roles in
breast cancer, they are closely associated with endocrine therapy
resistance. Therefore, the effects of isorhamnetin on these
signaling pathways were analyzed, demonstrating that isorhamnetin
decreased the phosphorylation levels of Akt, mTOR, MEK1 and ERK1/2,
but not those of EGFR. The results indicate that isorhamnetin may
act on the Akt/mTOR and MEK/ERK signaling cascades, resulting in
cell apoptosis, as demonstrated by the increase in levels of Bax,
Bcl-2 and cleaved caspase-3 expression. The results of the present
study are concordant with those of a previous study that
demonstrated that isorhamnetin induces apoptosis via the inhibition
of PI3K and MEK1 (27,30). A decrease in the phosphorylation
levels of Akt and MEK1 was observed in the present study following
pretreatment with their respective inhibitors (perifosine and
PD184352). Furthermore, the inhibition of Akt and MEK was decreased
by EGF, which induced activation of the PI3K/Akt/mTOR and MEK/ERK
signaling pathways.
A previous study attributed the major
chemopreventive mechanism of isorhamnetin to its antioxidant
effects (31). Therefore, the
present study investigated whether NF-κB contributed to the
antitumor activity of isorhamnetin in breast cancer. The results
indicate that NF-κB did not contribute to the effects of
isorhamnetin on cell apoptosis and, therefore, the proposed
antioxidant activity may not explain the apoptotic effects of
isorhamnetin on breast cancer, which may be associated instead with
other effects, such as anti-inflammatory action. The antitumor
activity of isorhamnetin may be different in various cancer types,
such as in colon cancer, in which isorhamnetin has been shown to
inhibit c-Src activation and β-catenin nuclear translocation
(32). In gastric cancer,
isorhamnetin inhibits cell proliferation and invasion, and induces
apoptosis through the modulation of the peroxisome
proliferator-activated receptor γ activation signaling pathway
(24).
In conclusion, the results of the present study
demonstrate that the antiproliferative and pro-apoptotic effects of
isorhamnetin in breast cancer are mediated via inhibition of the
Akt/mTOR and MEK/ERK signaling pathways, and provide a basis for
pursuing the therapeutic significance and chemopreventive
capabilities of isorhamnetin in breast cancer. Furthermore,
isorhamnetin may be administered either alone or in combination
with existing therapeutic strategies to enhance the treatment
efficacy for breast cancer. However, the effects of isorhamnetin
have yet to be investigated in humans, and hence further studies in
humans are required prior to its clinical application to breast
cancer treatment.
Acknowledgments
The present study was supported by a grant from the
Science and Technology Bureau of Shaoxing (grant no.
2014B70079).
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 2013. American Cancer Society; Atlanta: 2013
|
|
2
|
Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu
L and He J: Report of incidence and mortality in China cancer
registries, 2009. Chin J Cancer Res. 25:10–21. 2013.PubMed/NCBI
|
|
3
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Massarweh S and Schiff R: Resistance to
endocrine therapy in breast cancer: Exploiting estrogen
receptor/growth factor signaling crosstalk. Endocr Relat Cancer.
13(Suppl 1): S15–S24. 2006. View Article : Google Scholar
|
|
6
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A,
Kaufman B, et al: Lapatinib plus capecitabine for HER2-positive
advanced breast cancer. N Engl J Med. 355:2733–2743. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guarneri V and Conte P: Metastatic breast
cancer: Therapeutic options according to molecular subtypes and
prior adjuvant therapy. Oncologist. 14:645–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fedele P, Calvani N, Marino A, Orlando L,
Schiavone P, Quaranta A and Cinieri S: Targeted agents to reverse
resistance to endocrine therapy in metastatic breast cancer: Where
are we now and where are we going? Crit Rev Oncol Hematol.
84:243–251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miller TW, Balko JM and Arteaga CL:
Phosphatidylinositol 3-kinase and antiestrogen resistance in breast
cancer. J Clin Oncol. 29:4452–4461. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pérez-Tenorio G and Stål O; Southeast
Sweden Breast Cancer Group: Activation of Akt/PKB in breast cancer
predicts a worse outcome among endocrine treated patients. Br J
Cancer. 86:540–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
deGraffenried LA, Friedrichs WE, Russell
DH, Donzis EJ, Middleton AK, Silva JM, Roth RA and Hidalgo M:
Inhibition of mTOR activity restores tamoxifen response in breast
cancer cells with aberrant Akt Activity. Clin Cancer Res.
10:8059–8067. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kirkegaard T, Witton CJ, McGlynn LM, Tovey
SM, Dunne B, Lyon A and Bartlett JM: Akt activation predicts
outcome in breast cancer patients treated with tamoxifen. J Pathol.
207:139–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tokunaga E, Kimura Y, Mashino K, Oki E,
Kataoka A, Ohno S, Morita M, Kakeji Y, Baba H and Maehara Y:
Activation of PI3K/Akt signaling and hormone resistance in breast
cancer. Breast Cancer. 13:137–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Donovan JC, Milic A and Slingerland JM:
Constitutive MEK/MAPK activation leads to p27(Kip1) deregulation
and anti-estrogen resistance in human breast cancer cells. J Biol
Chem. 276:40888–40895. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin W, Wu L, Liang K, Liu B, Lu Y and Fan
Z: Roles of the PI-3K and MEK pathways in Ras-mediated
chemoresistance in breast cancer cells. Br J Cancer. 89:185–191.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Normanno N, De Luca A, Maiello MR,
Campiglio M, Napolitano M, Mancino M, Carotenuto A, Viglietto G and
Menard S: The MEK/MAPK pathway is involved in the resistance of
breast cancer cells to the EGFR tyrosine kinase inhibitor
gefitinib. J Cell Physiol. 207:420–427. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao H, Xu W, Shi X and Zhang Z: Dietary
flavonoids as cancer prevention agents. J Environ Sci Health C
Environ Carcinog Ecotoxicol Rev. 29:1–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asensi M, Ortega A, Mena S, Feddi F and
Estrela JM: Natural polyphenols in cancer therapy. Crit Rev Clin
Lab Sci. 48:197–216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cushnie TP and Lamb AJ: Recent advances in
understanding the antibacterial properties of flavonoids. Int J
Antimicrob Agents. 38:99–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta SC, Kim JH, Prasad S and Aggarwal
BB: Regulation of survival, proliferation, invasion, angiogenesis
and metastasis of tumor cells through modulation of inflammatory
pathways by nutraceuticals. Cancer Metastasis Rev. 29:405–434.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma G, Yang C, Qu Y, Wei H, Zhang T and
Zhang N: The flavonoid component isorhamnetin in vitro inhibits
proliferation and induces apoptosis in Eca-109 cells. Chem Biol
Interact. 167:153–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suomela JP, Ahotupa M, Yang B, Vasankari T
and Kallio H: Absorption of flavonols derived from sea buckthorn
(Hippophaë rhamnoides L.) and their effect on emerging risk factors
for cardiovascular disease in humans. J Agric Food Chem.
54:7364–7369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi C, Fan LY, Cai Z, Liu YY and Yang CL:
Cellular stress response in Eca-109 cells inhibits apoptosis during
early exposure to isorhamnetin. Neoplasma. 59:361–369. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramachandran L, Manu KA, Shanmugam MK, Li
F, Siveen KS, Vali S, Kapoor S, Abbasi T, Surana R, Smoot DT, et
al: Isorhamnetin inhibits proliferation and invasion and induces
apoptosis through the modulation of peroxisome
proliferator-activated receptor γ activation pathway in gastric
cancer. J Biol Chem. 287:38028–38040. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boubaker J, Ben Sghaier M, Skandrani I,
Ghedira K and Chekir-Ghedira L: Isorhamnetin 3-O-robinobioside from
Nitraria retusa leaves enhance antioxidant and antigenotoxic
activity in human chronic myelogenous leukemia cell line K562. BMC
Complement Altern Med. 12:1352012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boubaker J, Bhouri W, Ben Sghaier M,
Ghedira K, Dijoux Franca MG and Chekir-Ghedira L: Ethyl acetate
extract and its major constituent, isorhamnetin 3-O-rutinoside,
from Nitraria retusa leaves, promote apoptosis of human myelogenous
erythroleukaemia cells. Cell Prolif. 44:453–461. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JE, Lee DE, Lee KW, Son JE, Seo SK, Li
J, Jung SK, Heo YS, Mottamal M, Bode AM, et al: Isorhamnetin
suppresses skin cancer through direct inhibition of MEK1 and PI3-K.
Cancer Prev Res (Phila). 4:582–591. 2011. View Article : Google Scholar
|
|
28
|
Jaramillo S, Lopez S, Varela LM,
Rodriguez-Arcos R, Jimenez A, Abia R, Guillen R and Muriana FJ: The
flavonol isorhamnetin exhibits cytotoxic effects on human colon
cancer cells. J Agric Food Chem. 58:10869–10875. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee HJ, Lee HJ, Lee EO, Ko SG, Bae HS, Kim
CH, Ahn KS, Lu J and Kim SH: Mitochondria-cytochrome C-caspase-9
cascade mediates isorhamnetin-induced apoptosis. Cancer Lett.
270:342–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li C, Yang X, Chen C, Cai S and Hu J:
Isorhamnetin suppresses colon cancer cell growth through the
PI3K-Akt-m TOR pathway. Mol Med Rep. 9:935–940. 2014.PubMed/NCBI
|
|
31
|
Di Carlo G, Mascolo N, Izzo AA and Capasso
F: Flavonoids: Old and new aspects of a class of natural
therapeutic drugs. Life Sci. 65:337–353. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saud SM, Young MR, Jones-Hall YL, Ileva L,
Evbuomwan MO, Wise J, Colburn NH, Kim YS and Bobe G:
Chemopreventive activity of plant flavonoid isorhamnetin in
colorectal cancer is mediated by oncogenic Src and β-catenin.
Cancer Res. 73:5473–5484. 2013. View Article : Google Scholar : PubMed/NCBI
|