Introduction
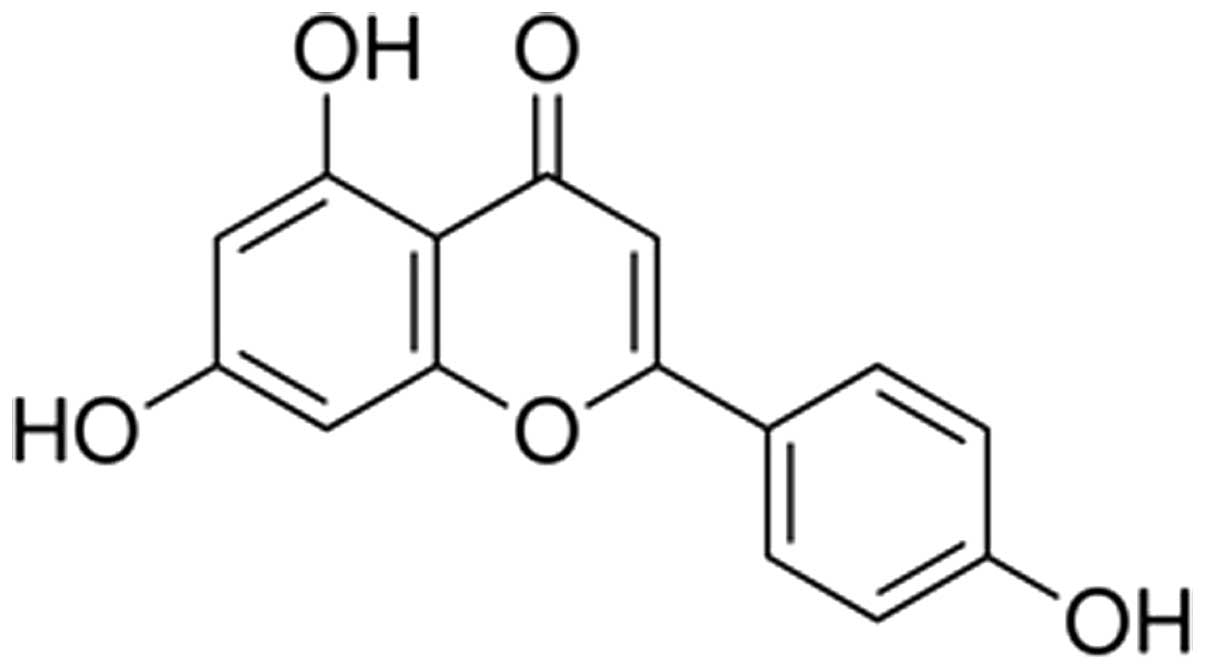
Apigenin (4,5,7-trihydroxyflavone or Api) is a
non-mutagenic flavone subclass of flavonoids exhibiting low levels
of toxicity, which is isolated from the leaves of Apium
graveolens L. var. dulce DC (a traditional Chinese
medicinal herb). Api is also present in a variety of shrubs,
vegetables, plants, fruits and herbs, a number of which are widely
marketed as dietary and herbal supplements (1–3).
Previous studies have demonstrated that Api possesses a wide range
of biological activities, including anti-carcinogenic, antiviral,
antibacterial, antioxidant and anti-inflammatory effects (4–8).
Following the ingestion of Api with food, Api becomes widely
distributed in various tissues and provides several protective
effects. A previous study demonstrated that Api protects the
endothelium-dependent relaxation of the rat aorta against oxidative
stress (9). In addition, the
intake of Api-rich foods can significantly increase the levels of
antioxidant enzymes in vivo (10), and Api is correlated with a reduced
incidence of cardiovascular disease (11). However, to the best of our
knowledge, no studies have been performed to determine whether Api
can inhibit I/R induced myocardial injury.
p38 mitogen-activated protein kinase (MAPK) is an
important signaling pathways, which regulates various pathological
conditions, including myocardial I/R (12). The activation of MAPK results in
increased I/R-induced myocardial injury (13) and impaired cardiac function
(14). In addition, Api reduces
the activation of p38 MAPK, having beneficial effects in various
tissues (15–17).
The present study aimed to investigate the
protective effects of Api following I/R injury and to explore the
mechanisms underlying these effects.
Materials and methods
Materials
Api (purity >98%; Fig. 1) was purchased from Xi'an XiaoCao
Botanical Development Co., Ltd. (Xi'an, China).
2,3,5-triphenyltetrazolium chloride (TTC) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). The superoxide dismutase (SOD),
malondialdehyde (MDA), creatine kinase (CK) and lactate
dehydrogenase (LDH) activity assay kits were purchased from Nanjing
Jiancheng Biochemical Reagent Co. (Nanjing, China). Anti-p38 MAPK
and anti-phosphorylated (p)-p38 MAPK antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All other
chemicals and reagents were commercially available and of standard
biochemical quality.
Animal model of I/R
Sprague-Dawley (SD) male rats weighing ~250–300 g
were obtained from The Animal Center of The General Hospital of
Lanzhou Command (Lanzhou, China) and were maintained on a 12 h
light/dark cycle and administered standard rodent chow at a
constant room temperature (22°C±1°C). The rats (n=22) were randomly
divided into three groups: i) Sham group (n=6), ii) I/R group
(n=8), iii) Api+I/R group (n=8). Pentobarbital sodium (30 g/l; 40
mg/kg) was used to anesthetize the rats. The carotid artery was
cannulated to monitor mean blood pressure (MBP) using a pressure
transducer, and a Lead-II electrocardiogram (ECG-300G; Shanghai Tai
Yi Medical Equipment Co., Ltd., Shanghai, China) was used to
monitor the heart rate (HR) using subcutaneous stainless steel
electrodes (Shanghai Tai Yi Medical Equipment Co., Ltd.). The HR
and MBP were recorded at different time-points. The rat limbs were
supinely fixed, and subcutaneous electrodes were connected to
monitor ECG changes. The chest was opened through the left border
of the sternum, and the heart was exposed by cutting the
pericardium. I/R was produced by passing a 5–0 silk suture
underneath the left anterior descending coronary artery (LAD) and
forming a ligature. Significant ECG changes, including widening of
the QRS complex and elevation of ST segment were considered to
determine successful coronary occlusion and reperfusion. The
present study was performed in accordance with the National
Institutes of Health Guidelines for the Use of Experimental
Animals, and approved by the Committee for the Ethical Use of
Experimental Animals at the General Hospital of Lanzhou Command.
All experiments were performed in adherence with the National
Institute of Health Guidelines for the Use of Experimental Animals
(National Institutes of Health, Bethesda, MA, USA) and all animal
protocols were approved by the Committee for the Ethical Use of
Experimental Animals at the General Hospital of Lanzhou Command
(Lanzhou, China).
Experimental protocol
The SD rats were randomly divided into three groups:
(1) A Sham group, in which silk
sutures were placed underneath the LAD but the LAD was not ligated;
(2) I/R group, in which the LAD
was ligated for 45 min and then allowed to reperfuse for 180 min
with intravenously administered 0.9% NaCl vehicle; (3) Api+I/R group, in which 5 mg/kg Api was
intravenously administered prior to ischemia. The experiments were
performed in a double-blinded manner.
Evaluation of CK and LDH
Following myocardial I/R, the myocardial enzyme
leakage levels of CK and LDH, which were collected from blood
samples (0.5 ml) from the ventricular chambers, were measured to
assess the level of myocardial injury. The levels of CK and LDH in
blood samples correlate positively with the extent of myocardial
injury (18), therefore, serum
levels of CK and LDH from each group were measured using
corresponding kits and analyzed using a DU640 spectrophotometer
(Beckman Coulter, Fullerton, CA, USA).
Determination of myocardial
infarction
Following a reperfusion period, the rat was
euthanized by overdose with pentobarbital sodium anesthesia. The
heart was then removed and washed using a Langendorff apparatus
(Radnoti Glass Technology Inc., Monrovia, CA, USA). Subsequently,
1% Evans blue dye (Sigma-Aldrich), which stains normal non-I/R
myocardium, the area not at risk (ANAR) and does not stain I/R
myocardium, the area at risk (AAR), was injected into the hearts,
and the hearts were frozen at −20°C for 3 h. Subsequently, 2–3
mm-thick slices of the frozen ventricle area were made and
incubated in 1% TTC solution (Sigma-Aldrich) in 0.1 M Tris buffer
(pH 7.8) for 15 min at 37°C. TTC can stain the viable areas of the
I/R myocardium a brick red color, whereas the infarct area is not
stained with TTC. Myocardial infarct size (INF) was calculated as
INF / AAR×100%.
Estimation of SOD and MDA
Oxidative stress was estimated by measuring the
levels of SOD and MDA in the damaged myocardium. At the end of
reperfusion, an SOD activity assay kit and MDA assay kit (Nanjing
Jiancheng Bioengineering Institute) were used to measure the
activity of SOD and the level of MDA in the cardiac tissues. For
biochemical analysis, cardiac tissues were washed twice with cold
saline solution and stored at −80°C until analysis. MDA was reacted
with thiobarbituric acid by incubating for 1 h at 95–100°C and the
fluorescence intensity was measured in the n-butanol phase via
fluorescence spectrophotometry (DU 640; Beckman Coulter), by
comparing with a standard solution of 1,1,3,3-tetramethoxypropane.
SOD activity was measured according to reduction of nitro-blue
tetrazolium via the xanthine-xanthine oxidase system.
Western blot analysis
As described previously (19), tissue samples, which were placed in
lysis buffer (20 mmol/l Tris-HCl, 150 mmol/l NaCl, 1 mmol/l Na2
EDTA, 1 mmol/l EDTA, 1% Triton, 0.1% SDS, 0.1% sodium deoxycholate,
pH 7.4), were mechanically minced. The homogenates were centrifuged
at 15,000×g for 20 min at 4°C. The Bradford method was used for
protein quantification. Proteins were extracted from the hearts
using standard tissue lysates, including a protease inhibitor
cocktail (P8340; Sigma-Aldrich) and phosphatase inhibitor cocktail
(P5726; Sigma-Aldrich). The protein content of the homogenates was
determined according to the Bradford method. Protein concentrations
were estimated by reference to absorbances at 595 nm obtained for a
series of standard protein dilutions, which are assayed alongside
the unknown samples. Homogenate samples representing 20 µg
of total protein were run on 12% SDS-polyacrylamide gels (Bio-Rad
Laboratories, Inc.). Following electrophoresis, proteins were
transferred onto a nitrocellulose membrane (Bio-Rad Laboratories,
Inc.). Nonspecific binding of antibodies was blocked by washing
with 5% fat-free milk for 1 h. The blot was then subjected to two
brief washes with Tris-buffered saline plus 0.1% Tween-20,
incubated with the primary antibody against p38 MAPKS (cat. no.
sc-535; 1:500; rabbit polyclonal IgG; Santa Cruz Biotechnology,
Inc.) and p-p38 MAPKS (cat. no. sc-17852-R; 1:500; rabbit
polyclonal IgG; Santa Cruz Biotechnology, Inc.) overnight at 4°C.
Samples were then incubated with horseradish peroxidase-conjugated
secondary antibodies (anti-rabbit IgG; cat. no. A0208; 1:1,000;
Beyotime Institute of Biotechnology, Haimen, China) at room
tempera-ture for 1 h. Chemiluminescence was used to detect the
bands (ECL; Bio-Rad ChemiDoc XRS, Bio-Rad Laboratories, Inc.). The
band densities were analyzed using the Quantity One v4.62 software
package (Bio-Rad Laboratories, Inc.).
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. Statistical comparisons were performed using Student's
t-test, and differences between multiple groups were
assessed using a two-way analysis of variance. Data analysis was
performed with a personal computer statistical software package
(Prism v5.0, GraphPad Software, La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Measurement of hemodynamics
As shown in Fig. 2,
the HR and MBP of the rats in all groups were determined. The HR in
the I/R group increased significantly following I/R, compared with
the Api+I/R group (P<0.05; Fig.
2). The MBP in the I/R group, monitored at the same time-point,
was significantly lower than that in the Sham group (P<0.05;
Fig.. 2). The MBP in the I/R group
at the same time-point was also lower, compared with that in the
Api+I/R group, however, no statistical significance was
observed.
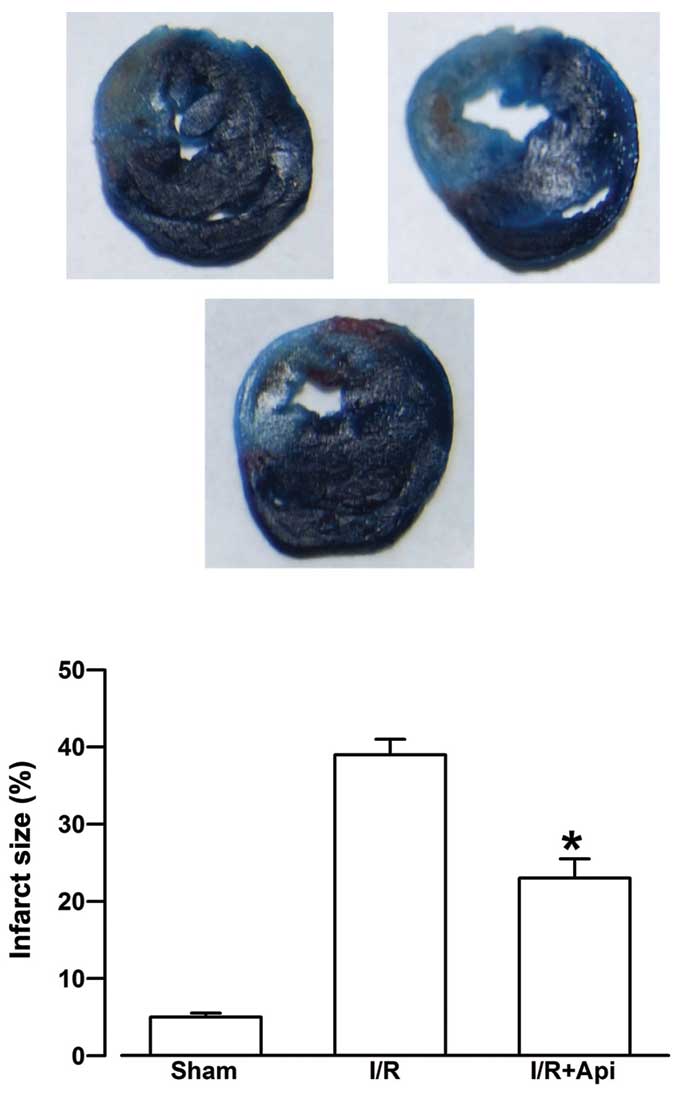
Effects of Api on myocardial infarct
size
Evans blue staining does not stain the AAR in
tissues, and the size of the AAR depends predominantly on the area
of blood supply of the blocked artery. As shown in Fig. 3, the infarct size in the I/R group
was 39.16±1.98%. Treatment with Api was observed to significantly
reduce myocardial infarction size (23.52±2.53%; P<0.05).
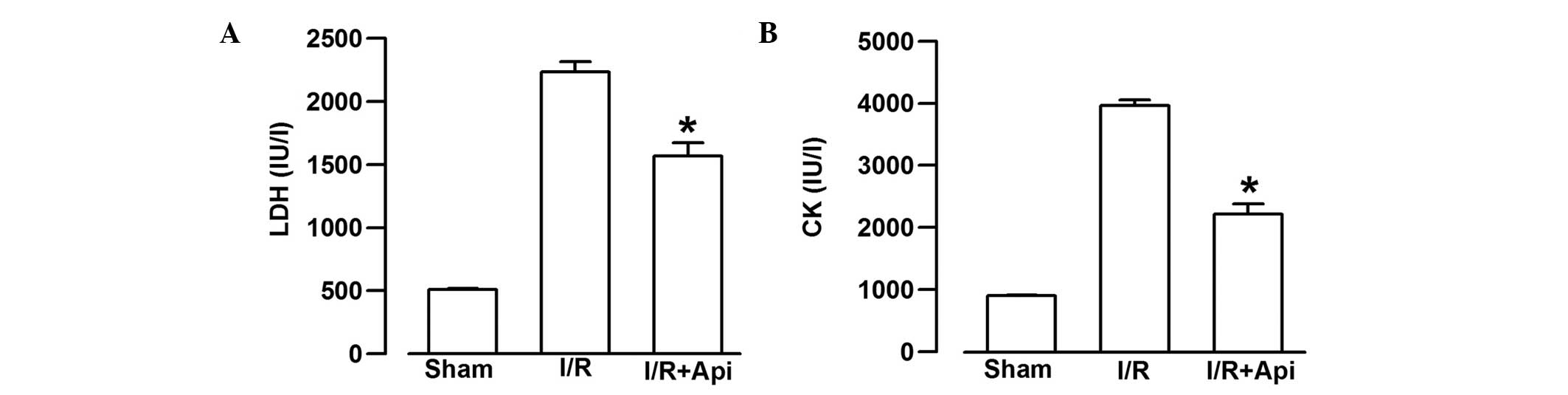
Effects of Api on LDH and CK release
To examine the effects of Api on I/R-induced injury,
the present study measured the extent of LDH and CK leakage. I/R
markedly increased the extent of CK and LDH leakage from the
myocardium (Fig. 4). As shown in
Fig. 4A, treatment of the rats
with Api significantly decreased the extent of LDH leakage,
compared with the I/R group (P<0.05). In addition, Api also
significantly decreased the extent of CK leakage, compared with the
I/R group (P<0.05, Fig.
4B).
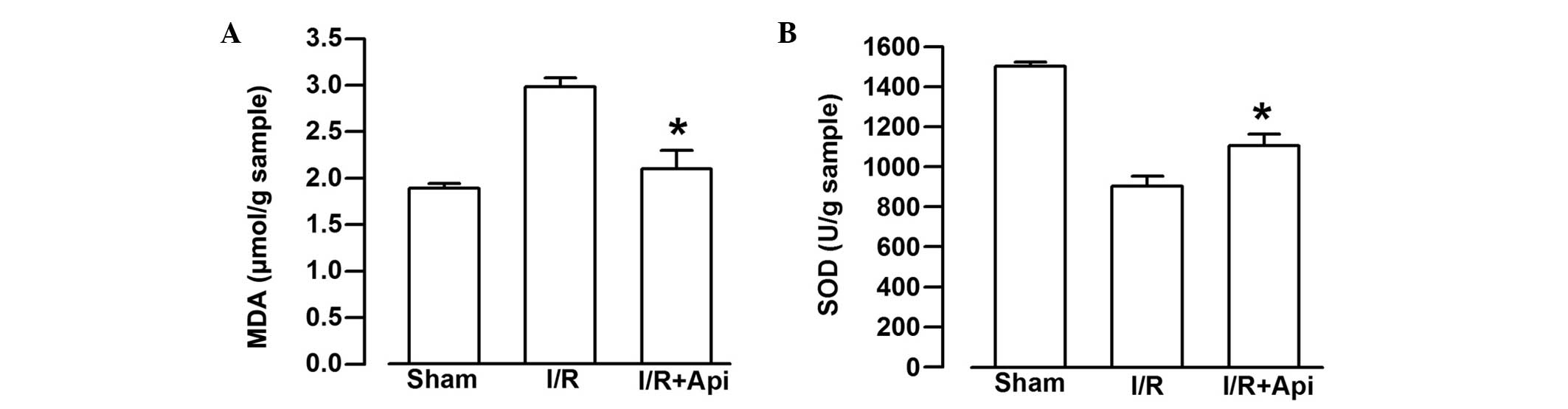
Effects of Api on SOD activity and MDA
content
To examine the effects of Api on I/R--induced
injury, the present study also measured the SOD activity and the
content of MDA. I/R markedly decreased the SOD activity and
significantly increased the content of MDA (Fig. 5). As shown in Fig. 5A, treatment of the rats with Api
significantly decreased the content of MDA, compared with the I/R
group (P<0.05). By contrast, Api markedly increased the activity
of SOD, compared with the I/R group (P<0.05; Fig. 5B).
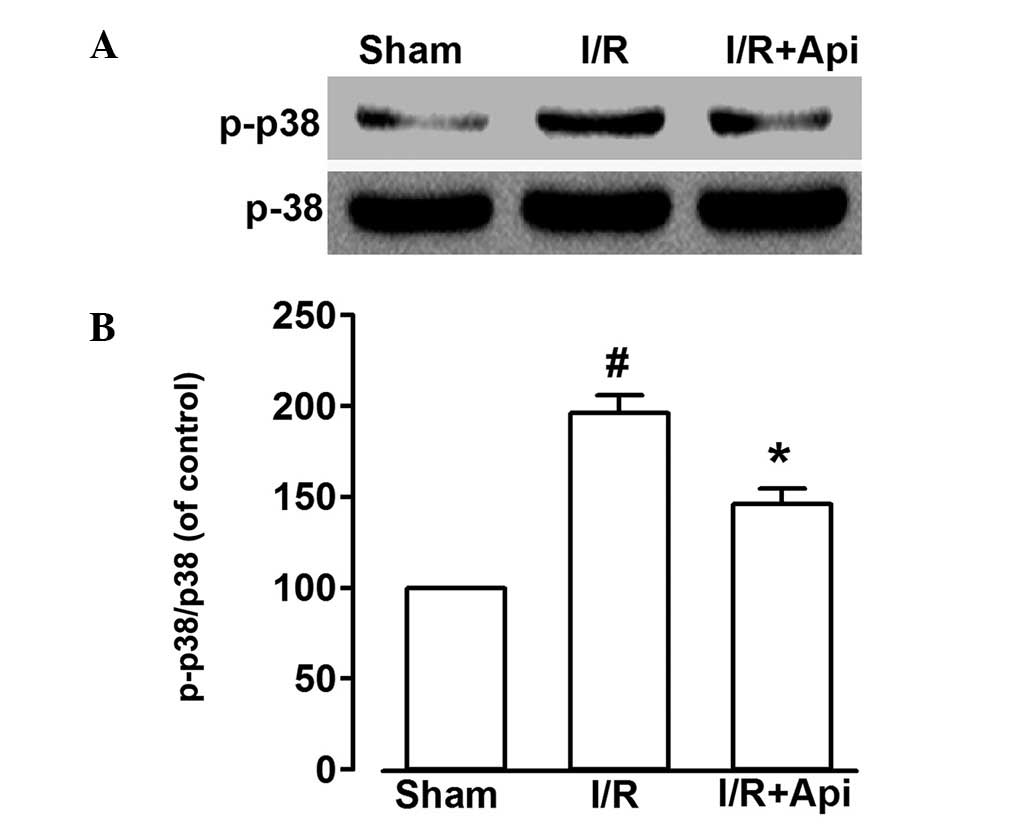
Effects of the inhibition of p38 MAPKS on
Api and protection against isolated myocardial I/R injury
As shown in Fig. 6,
I/R markedly increased the phosphorylation of p38 MAPKS. Treatment
with Api decreased the level of I/R-induced p38 MAPKS
phosphorylation (P<0.05). These findings indicated that Api may
protect the myocardium by suppressing the phosphorylation of p38
MAPKS.
Discussion
The results of the present study demonstrated that
Api protected the heart against I/R injury. The following novel
findings were revealed: Administration of Api attenuated myocardial
I/R injury, which was evidenced by a decrease in myocardial enzyme
leakage, a reduction in myocardial infarct size and an improvement
in cardiac function; Api pre-treatment induced endogenous
anti-oxidative enzyme activity and inhibited oxidative stress; and
Api prevented the cardiac dysfunction caused by I/R injury by
downregulating the expression of myocardial p-p38 MAPKS.
Api, a plant flavone predominantly isolated from
Apium graveolens L. var. dulce DC leaves, a traditional
Chinese medicinal herb, is of significant value due to its
health-promoting benefits, and, it is widely distributed in the
plant kingdom (1). Api has several
biological activities, including antiaggregatory, antibacterial,
antioxidant and anti-inflammatory effects. Consistent with lower
prevalence of cardiovascular diseases (11), the present study demonstrated that
Api exerted significant cardioprotective effects against I/R injury
in a rat heart model.
A previous study demonstrated that an increase in
LDH is observed following an increase in infarct size in the heart
(20). The increase in infarct
size is also accompanied by increased levels of CK (21). In the present study, treatment with
Api treatment resulted in a decrease in myocardial infarct size. In
all the Api-treated groups, the serum levels of LDH and CK were
reduced, which suggested that Api had cardioprotective effects.
The present study further confirmed the
anti-oxidative effect of Api treatment. Increased levels of
oxidants have been reported in I/R-induced mitochondrial injury, in
addition to reduced levels of oxidant-eliminating agents. Several
studies have reported that I/R-induced oxidative stress increases
the production of MDA and inhibits the activity of SOD in the heart
(4,6). In addition, studies have reported
that Api decreases the content of MDA and improves the activity of
SOD in brain and intestine following I/R (22,23).
The present study demonstrated that I/R upregulated the content of
MDA and downregulated the activity of SOD. However, pre-treatment
of the experimental animal with Api inhibited the production of MDA
and improved the activity of SOD in following I/R-induced
myocardial injury. These data suggested that Api may protect the
myocardium against I/R injury by inhibiting oxidative stress.
Oxidative stress and inflammatory cytokines, which
occur following myocardial I/R, can activate the p38 MAPKS pathway
(24). Increasing evidence has
demonstrated that p38 MAPKS is important in I/R-induced myocardial
injury, and its targeted inhibition can reduce myocardial I/R
injury (25,26).
The phosphorylation of p38 MAPKS, which is observed
with an increase in adhesion molecules and cytokines, contributes
to cell death in I/R injury (27).
To the best of our knowledge, the present study is the first to
report that Api exerted a cardioprotective effect against I/R
injury via inhibiting the p38 MAPKS pathway. Therefore, Api-induced
inactivation of p38 MAPKS may contribute to the cardioprotective
effect following I/R-induced injury. These findings indicated the
potential use of Api as a potential therapeutic agent to attenuate
myocardial I/R injury.
In conclusion, the present study suggested that Api
protects heart from I/R injury via inhibiting the p38 MAPKS
pathway. However, the investigations in the present study only
partially revealed the cardioprotective mechanisms of Api.
Therefore, further investigations are required to investigate the
cardioprotective mechanisms of Api.
Acknowledgments
Financial support was provided by the China
Postdoctoral Science Foundation (grant. no 2013M542442) and the
National Natural Science Foundation of China (grant. no.
81400276).
References
|
1
|
Sharma H, Kanwal R, Bhaskaran N and Gupta
S: Plant flavone apigenin binds to nucleic acid bases and reduces
oxidative DNA damage in prostate epithelial cells. PLoS One.
9:e915882014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu XY, Sun DL, Chen ZJ, Chen T, Li LP, Xu
ZH, Jiang HD and Zeng S: Relative contribution of small and large
intestine to deglycosylation and absorption of flavonoids from
Chrysanthemun morifolium extract. J Agric Food Chem.
58:10661–10667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beara IN, Lesjak MM, Jovin ED, Balog KJ,
Anackov GT, Orcić DZ and Mimica-Dukić NM: Plantain (Plantago L.)
species as novel sources of flavonoid antioxidants. J Agric Food
Chem. 57:9268–9273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanazawa K, Uehara M, Yanagitani H and
Hashimoto T: Bioavailable flavonoids to suppress the formation of
8-OHdG in HepG2 cells. Arch Biochem Biophys. 455:197–203. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang YH, Park YS, Kim TJ, Fang LH, Ahn
HY, Hong JT, Kim Y, Lee CK and Yun YP: Endothelium-dependent
vasorelaxant and antiproliferative effects of apigenin. Gen
Pharmacol. 35:341–347. 2000. View Article : Google Scholar
|
|
6
|
Basile A, Giordano S, López-Sáez JA and
Cobianchi RC: Antibacterial activity of pure flavonoids isolated
from mosses. Phytochemistry. 52:1479–1482. 1999. View Article : Google Scholar
|
|
7
|
Gupta S, Afaq F and Mukhtar H: Involvement
of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle
arrest and apoptosis by apigenin in human prostate carcinoma cells.
Oncogene. 21:3727–3738. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lindenmeyer F, Li H, Menashi S, Soria C
and Lu H: Apigenin acts on the tumor cell invasion process and
regulates protease production. Nutr Cancer. 39:139–147. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin BH, Qian LB, Chen S, Li J, Wang HP,
Bruce IC, Lin J and Xia Q: Apigenin protects endothelium-dependent
relaxation of rat aorta against oxidative stress. Eur J Pharmacol.
616:200–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meyer H, Bolarinwa A, Wolfram G and
Linseisen J: Bioavailability of apigenin from apiin-rich parsley in
humans. Ann Nutr Metab. 50:167–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bellosta S, Bogani P, Canavesi M, Galli C
and Visioli F: Mediterranean diet and cardioprotection: Wild
artichoke inhibits metalloproteinase 9. Mol Nutr Food Res.
52:1147–1152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeong CW, Yoo KY, Lee SH, Jeong HJ, Lee CS
and Kim SJ: Curcumin protects against regional myocardial
ischemia/reperfusion injury through activation of RISK/GSK-3β and
inhibition of p38 MAPK and JNK. J Cardiovasc Pharmacol Ther.
17:387–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang Y, Hu SJ, Li L and Chen GP:
Cardioprotection by polysaccharide sulfate against
ischemia/reperfusion injury in isolated rat hearts. Acta Pharmacol
Sin. 30:54–60. 2009. View Article : Google Scholar
|
|
14
|
Schwertz H, Carter JM, Abdudureheman M,
Russ M, Buerke U, Schlitt A, Müller-Werdan U, Prondzinsky R, Werdan
K and Buerke M: Myocardial ischemia/reperfusion causes VDAC
phosphorylation which is reduced by cardioprotection with a p38 MAP
kinase inhibitor. Proteomics. 7:4579–4588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin M, Lu SS, Wang AX, Qi XY, Zhao D, Wang
ZH, Man MQ and Tu CX: Apigenin attenuates dopamine-induced
apoptosis in melanocytes via oxidative stress-related p38, c-Jun
NH2-terminal kinase and Akt signaling. J Dermatol Sci. 63:10–16.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noh HJ, Sung EG, Kim JY, Lee TJ and Song
IH: Suppression of phorbol-12-myristate-13-acetate-induced tumor
cell invasion by apigenin via the inhibition of p38
mitogen-activated protein kinase-dependent matrix
metalloproteinase-9 expression. Oncol Rep. 24:277–283.
2010.PubMed/NCBI
|
|
17
|
Huang CH, Kuo PL, Hsu YL, Chang TT, Tseng
HI, Chu YT, Kuo CH, Chen HN and Hung CH: The natural flavonoid
apigenin suppresses Th1- and Th2-related chemokine production by
human monocyte THP-1 cells through mitogen-activated protein kinase
pathways. J Med Food. 13:391–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan X, Niu HT, Wang PL, et al:
Cardioprotective effect of Licochalcone D against myocardial
ischemia/reperfusion injury in Langendorff-perfused rat hearts.
PLoS One. 10:e01283752015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu J, Wang Z, Guo YY, Zhang XN, Xu ZH, Liu
SB, Guo HJ, Yang Q, Zhang FX, Sun XL and Zhao MG: A role of
periaqueductal grey NR2B-containing NMDA receptor in mediating
persistent inflammatory pain. Mol Pain. 5:712009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pagliaro P, Mancardi D, Rastaldo R, Penna
C, Gattullo D, Miranda KM, Feelisch M, Wink DA, Kass DA and
Paolocci N: Nitroxyl affords thiol-sensitive myocardial protective
effects akin to early preconditioning. Free Radic Biol Med.
34:33–43. 2003. View Article : Google Scholar
|
|
21
|
Chen C, Du P and Wang J: Paeoniflorin
ameliorates acute myocardial infarction of rats by inhibiting
inflammation and inducible nitric oxide synthase signaling
pathways. Mol Med Rep. 12:3937–3943. 2015.PubMed/NCBI
|
|
22
|
Xitao C, Jianping B, Huizhi Z and Kenming
Y: The protective effects of apigenin in myocardium of rats with
ischemia reperfusion injury. J Pharmacology and Clinics of Chinese
Materia Medica. 2:0192011.
|
|
23
|
Jeyabal PV, Syed MB, Venkataraman M,
Sambandham JK and Sakthisekaran D: Apigenin inhibits oxidative
stress-induced macromolecular damage in N-nitrosodiethylamine
(NDEA)-induced hepatocellular carcinogenesis in Wistar albino rats.
Mol carcinog. 44:11–20. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thomas CJ, Ng DC, Patsikatheodorou N,
Limengka Y, Lee MW, Darby IA, Woodman OL and May CN:
Cardioprotection from ischaemia-reperfusion injury by a novel
flavonol that reduces activation of p38 MAPK. Eur J Pharmacol.
658:160–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaiser RA, Bueno OF, Lips DJ, Doevendans
PA, Jones F, Kimball TF and Molkentin JD: Targeted inhibition of
p38 mitogen-activated protein kinase antagonizes cardiac injury and
cell death following ischemia-reperfusion in vivo. J Biol Chem.
279:15524–15530. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li G, Barrett EJ, Barrett MO, Cao W and
Liu Z: Tumor necrosis factor-alpha induces insulin resistance in
endothelial cells via a p38 mitogen-activated protein
kinase-dependent pathway. Endocrinology. 148:3356–3363. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bassi R, Heads R, Marber MS and Clark JE:
Targeting p38-MAPK in the ischaemic heart: Kill or cure? Curr Opin
Pharmacol. 8:141–146. 2008. View Article : Google Scholar : PubMed/NCBI
|