Introduction
The roots of Polygala tenuifolia Willd are
used widely as a traditional medicine in China and Korea for the
treatment of neurasthenia, amnesia and depression (1–3).
Previous studies have reported that oligosaccharide esters,
including tenuifolisid A (TFSA) and 3,6-disinapoyl sucrose are the
predominant active components of Polygala (4), and Liu et al demonstrated that
it exerted a neuroprotective effect on corticosterone-induced
neuron damage in SH-SY5Y cells (3). However, the effect of TFSA on the
promotion of neurite outgrowth remains to be elucidated.
PC12 cells, which are widely used as a model for
investigating neurite extension, are from a cell line derived from
the rat pheochromocytoma of the adrenal medulla (5). Upon treatment with nerve growth
factor (NGF), PC12 cell neurite extension occurs, differentiating
into morphologically and functionally neuron-like cells that
express neuronal specific genes, including 43 kD growth-associated
protein (GAP-43), Tuj1 and synapsin I. This process is associated
with the activation of the tropomyosin-related kinase
(TrkA)-dependent mitogen-activated protein kinase (MAPK) and
phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways
(6–8). The present study aimed to investigate
the effect of TFSA on neurite outgrowth in PC12 cells, and to
determine the potential involvement of the MAPK kinase
(MEK)/extracellular-signal regulated kinase (ERK)/cyclic AMP
response element-binding protein (CREB) and PI3K/Akt signaling
pathways. Thus, the neuritogenic effects of TFSA may suggest that
TFSA could be used as a potential treatment for CNS injury,
including spinal cord injury.
Materials and methods
Cell culture
Rat PC12 cells were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China) and
maintained in Roswell Park Memorial Institute 1640 medium
(Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% heat-inactivated horse serum (Invitrogen Life Technologies), 5%
fetal bovine serum (FBS; Invitrogen Life Technologies and 100 U/ml
penicillin/streptomycin (Invitrogen Life Technologies) under 5%
CO2 at 37°C. The medium was replaced every 3 days. For
the differentiation experiments, the PC12 cells were grown in
Dulbecco's modified Eagle's medium (DMEM)/F-12 media (Invitrogen
Life Technologies) in the presence of 1% FBS and 100 ng/ml NGF
(Sigma-Aldrich, St. Louis, MO, USA).
Measurement of neurite outgrowth
The PC12 cells (8×103) were plated in
ploy-l-lysine coated 24-well plates. Neurite outgrowth was examined
48 h following treatment with NGF (100 ng/ml) or TFSA (10
µM). In certain experiments, the cells were cultured with
PD98059 (10 µM; Sigma-Aldrich) or LY294002 (10 µM;
Sigma-Aldrich). In each well, images were captured of 10
randomly-selected fields, each containing ~100 cells using an
inverted phase contrast microscope (Nikon, Tokyo, Japan). The
cells, which exhibited extension of at least one neurite with a
length that was longer than the diameter of the cell body were
identified as neurite-bearing cells. The neurite lengths of all
neurite-bearing cells were measured using ImageJ software version
1.44p (National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
The cells were lysed in radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Haimen, China)
containing protease inhibitor cocktails and phosphatase inhibitor
cocktails (Sigma-Aldrich). The protein contents were measured using
a Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific,
Waltham, MA, USA). The extracted protein samples (20 µg)
were separated by 10% SDS-PAGE (KeyGen Biotech, Co., Ltd., Nanjing,
China) and transferred onto polyvinylidene difluoride membranes
(Merck Millipore, Darmstadt, Germany). Following blocking in 5%
bovine serum albumin (Sigma-Aldrich) in phosphate-buffered saline
for 2 h at room temperature, the membranes were incubated with
primary antibody at 4°C overnight, and were subsequently incubated
with horse anti-mouse (cat. no. 7076) or goat anti-rabbit (cat. no.
7074) IgG horseradish peroxidase-conjugated secondary antibodies
(1:2,000, Cell Signaling Technology, Inc., Danvers, MA, USA) for 2
h at room temperature. The primary antibodies were as follows:
Rabbit anti-rat total-ERK1/2 (monoclonal, 1:1,000; cat. no. 4695;
Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit anti-rat
total-Akt (monoclonal, 1:1,000; cat. no. 4685; Cell Signaling
Technology, Inc.), rabbit anti-rat total-CREB (monoclonal, 1:1,000;
cat. no. ab32515; Abcam, Cambridge, UK), rabbit anti-rat
phosphorlyated (p-) ERK1/2 (monoclonal, 1:1,000; cat. no. 4377;
Cell Signaling Technology, Inc.), mouse anti-rat p-Akt (monoclonal,
1:1,000; cat. no. 4058; Cell Signaling Technology, Inc.), rabbit
anti-rat p-CREB (monoclonal, 1:1,000; cat. no. ab32096; Abcam),
mouse anti-rat β-actin (monoclonal, 1:2,000; cat. no. 4970; Cell
Signaling Technology, Inc.) and rabbit anti-rat GAP43 (monoclonal,
1:1,000; cat. no. ab75810; Abcam). Finally, the bands were measured
using an enhanced chemiluminescent system (ECL Plus; Thermo Fisher
Scientific).
Statistical analysis
All numerical data are presented as the mean ±
standard deviation. Statistical differences were determined using
one-way analysis of variance, combined with Scheffe's test for
multiple comparisons. Data were analyzed using SPSS software
version 11 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference. Each experiment
was replicated at least three times.
Results
TFSA induces neurite outgrowth in PC12
cells
To investigate the effect of TFSA on neurite
outgrowth in PC12 cells, the cells, which were maintained in
low-serum medium, were treated with either vehicle (0.1% DMSO), NGF
(100 ng/ml) or TFSA (10 µM). As shown in Fig. 1A, TFSA treatment led to the
promotion of neurite extension of the PC12 cells 24 h
post-treatment. The percentage of neurite-bearing cells reached
25.4+5.01% following treatment with 10 µM TFSA, which was
significantly higher than percentage observed in the cells treated
with the vehicle.
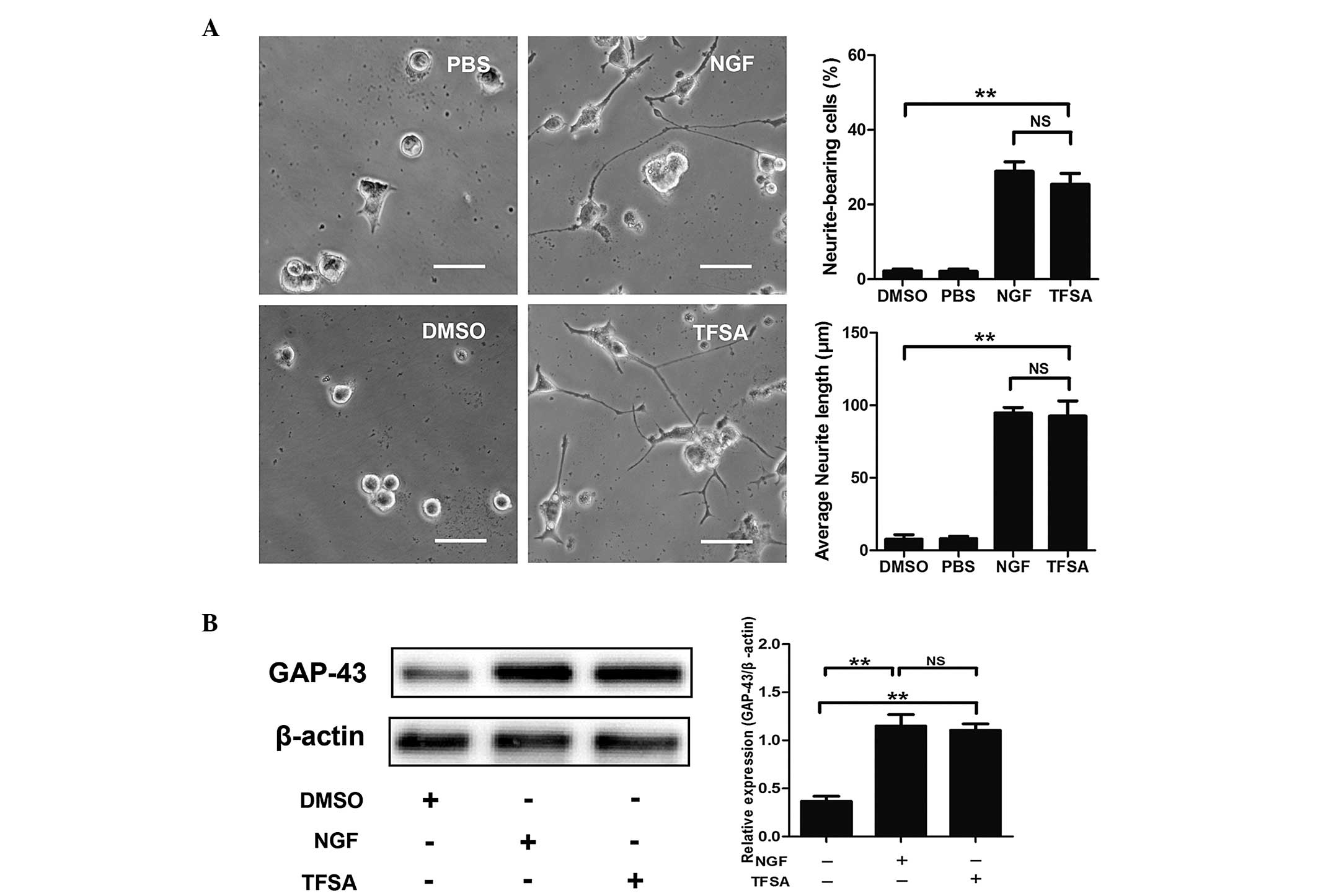 | Figure 1Effects of TFSA on neurite extension
in PC12 cells. (A) Percentage of neurite-bearing cells and average
neurite lengths in PC12 cells treated with PBS, DMSO, TFSA or NGF
for 24 h. Scale bar=50 µm. (B) Relative expression levels of
GAP-43 in PC12 cells treated with DMSO, TFSA or NGF. The data are
expressed as the mean ± standard devosatopn from three independent
experiments (**P<0.01). TFSA, tenuifoliside A; NGF,
nerve growth factor, DMSO, dimethyl sulfoxide; PBS,
phosphate-buffered saline; GAP-43, growth-associated protein 43;
NS, non-significant. |
GAP-43, a marker of neurite outgrowth, is expressed
in PC12 cells on differentiation towards a neuronal phenotype and
exhibits increased synthesis and axonal transport during axonal
regeneration (9). The present
study investigated whether TFSA affected the expression of GAP43 in
the PC12 cells. At 24 h post-treatment, the results of the western
blot analysis demonstrated that TFSA induced a higher expression
level of GAP-43, compared with that observed in the control group
(Fig. 1B).
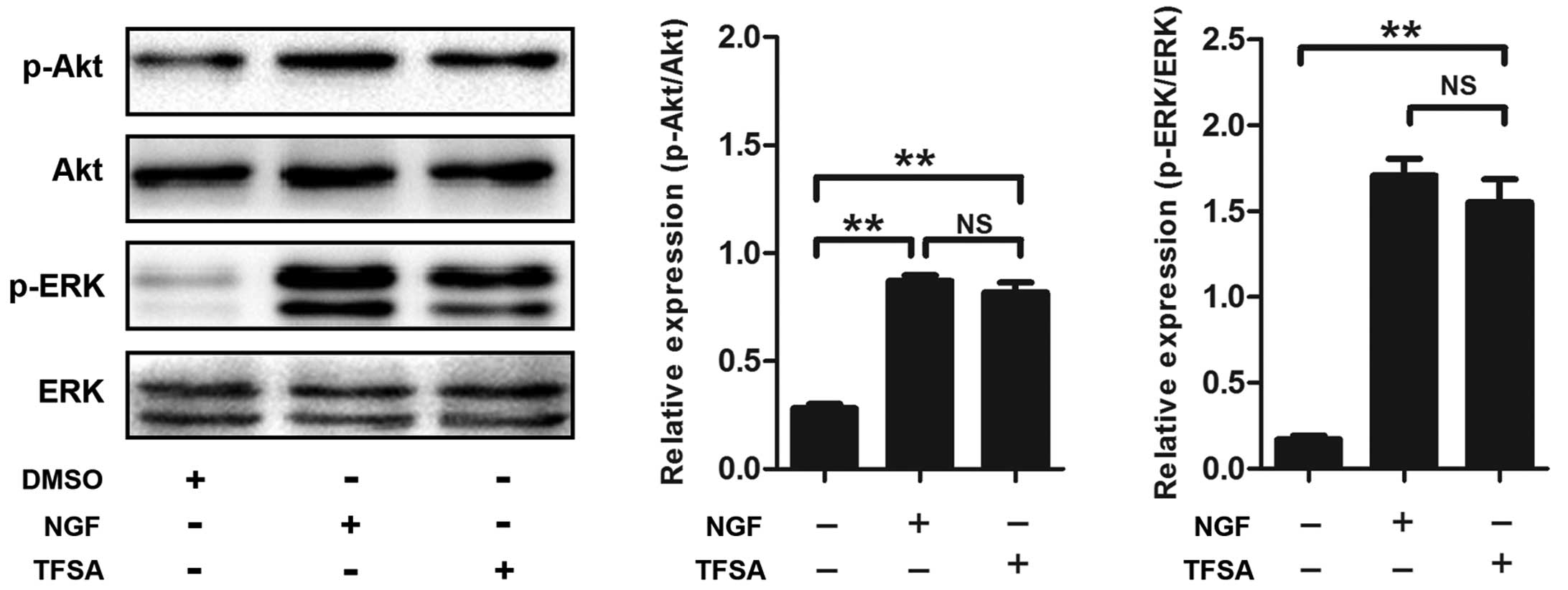
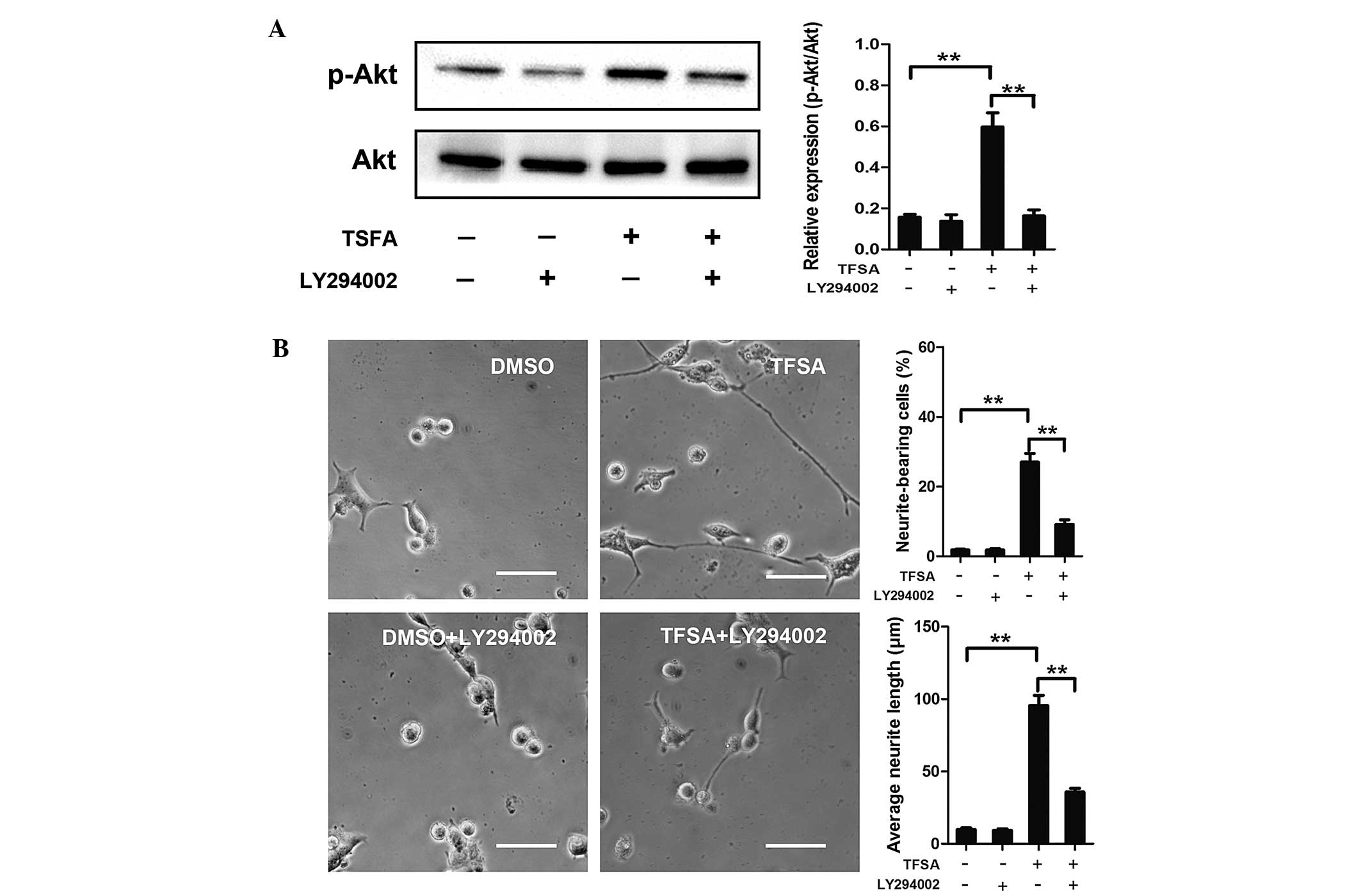
Activation of PI3K/Akt signalling with
TFSA
Subsequently, the present study investigated the
signaling pathway responsible for TFSA-induced neurite outgrowth.
Previous studies have demonstrated the role of the activated
PI3K/Akt pathway in inducing neural differentiation in PC12 cells.
In the present study, the TFSA-treated cells demonstrated a high
level of phosphorlyation (p-Akt-S473), compared with the control
cells (Fig. 2). To confirm the
role of the PI3K/Akt signaling pathway in the neurite outgrowth
induced by TFSA in the present study, LY294002., a specific
pharmacological inhibitor of PI3K, was used. As shown in Fig. 3, LY294002 significantly inhibited
TFSA-induced Akt phosphorylation and neurite outgrowth.
TFSA activates the MEK/ERK pathway
Previous studies have reported that signaling
through MAPK classes is important in the neural differentiation of
PC12 cells induced by NGF. The present study investigated whether
the MEK/ERK pathway was involved in TFSA-induced neurite outgrowth
in the PC12 cells. The results of the western blot analysis
indicated that the phosphorylation of ERK1/2 was significantly
increased in the PC12 cells, which were treated with TFSA (Fig. 2). In addition, the cells treated
with PD98059, which is an MAPK/ERK kinase inhibitor, significantly
inhibited TFSA-induced neurite formation (Fig. 4A and B).
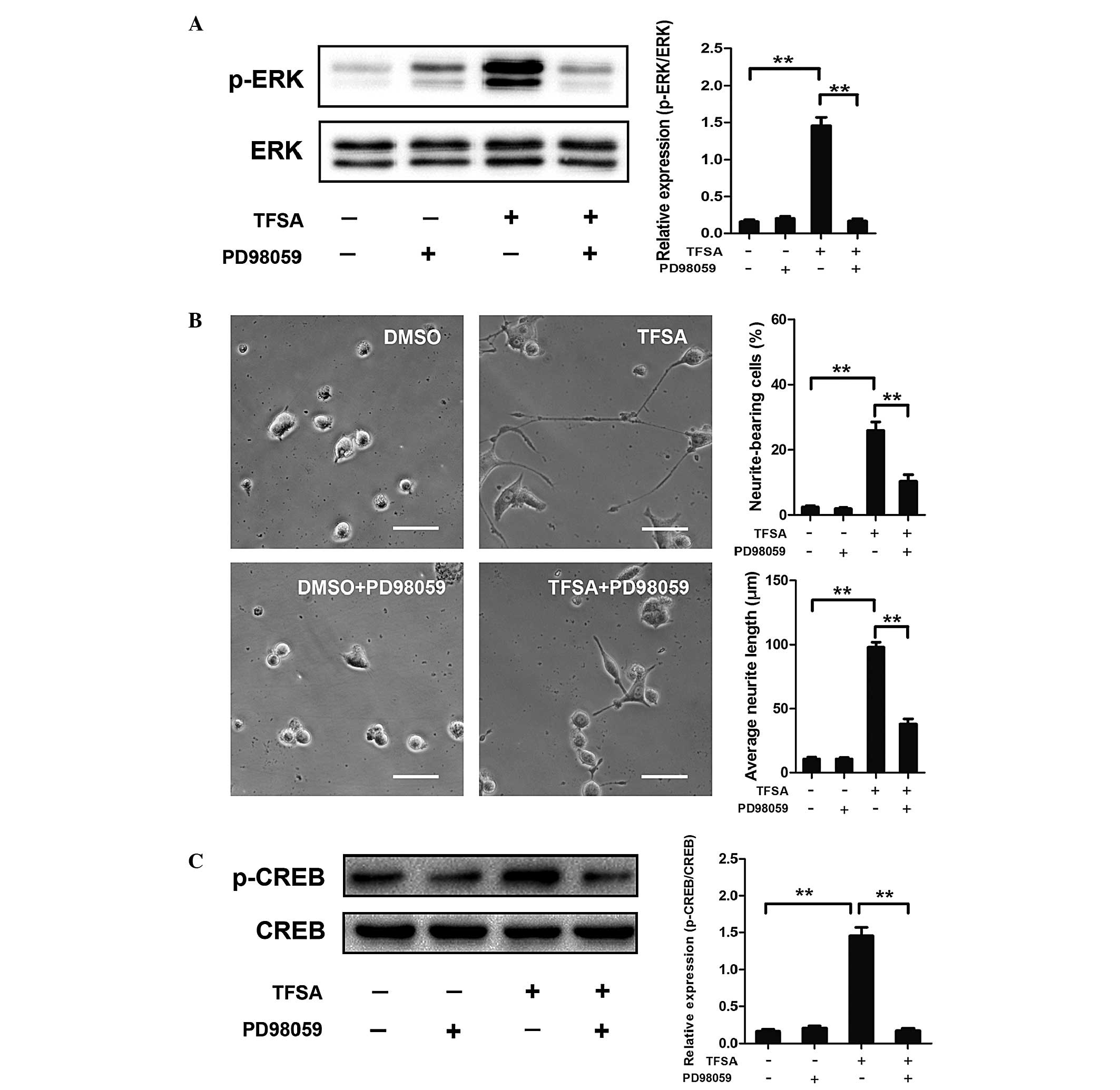 | Figure 4Effect of TFSA on MEK/ERK/CREB
activation and MEK/ERK/CREB-mediated neurite outgrowth. (A)
Phosphorylation of ERK in PC12 cells treated with DMSO,
DMSO+PD98059, TFSA or TFSA+PD98059. Scale bar=50 µm. (B)
Proportion of neurite-bearing cells and average neurite lengths in
PC12 cells treated with DMSO, DMSO+PD98059, TFSA or TFSA+PD98059.
(C) Phosphorylation of CREB in PC12 cells treated with DMSO,
DMSO+PD98059, TFSA or TFSA+PD98059. The data are expressed as the
mean ± standard deviation from three independent experiments
(**P<0.01). TFSA, tenuifoliside A; ERK, extracellular
signal-regulated kinase; p-ERK, phosphorylated ERK; MEK,
mitogen-activated protein kinase kinase; CREB; cyclic AMP response
element-binding protein; p-CREB, phosphorlyated CREB; DMSO,
dimethyl sulfoxide; NS, non-significant. |
TFSA promotes the phosphorylation of CREB
by activating MEK/ERK
Activation of the MEK/ERK pathway in PC12 cells
leads to the phosphorylation of CREB, which is critical in
neurogenesis (10,11). Therefore, the present study
investigated the effect of TFSA on the phosphorylation of CREB. As
shown in Fig. 4C, western blot
analysis revealed that the phosphorylation level of CREB was
significantly increased in the TFSA treated-group of cells. To
further investigate the dependence of CREB phosphorylation on the
activation of the MEK/ERK pathway, the effects of PD98059 on
TFSA-induced phosphorylation of CREB was detected. The results
indicated that the phosphorylation of CREB was almost completely
inhibited following treatment with PD98059, suggesting that the
TFSA-induced phosphorylation of CREB was MEK/ERK dependent
(Fig. 4C).
Discussion
TFSA is an oligosaccharide ester in Polygala.
As a widely used traditional Chinese medicine, Polygala can
be used to treat diseases, including amnesia, neurasthenia,
palpitation and insomnia (3,4). In
the present study, the effect of TFSA on neuritogenesis in PC12
cells was investigated. The results revealed that treatment with
TFSA induced neuronal differentiation and increased the expression
of the neuronal marker, GAP43, in PC12 cells. Furthermore, the
results demonstrated that the neurite formation induced by TFSA was
associated with the activation of the PI3K/AKT and MEK/ERK/CREB
signaling pathways.
PC12 cells are a cell line derived from the rat
pheochromocytoma of the adrenal medulla. These cells have been
extensively used as a neural cell model of neurite outgrowth
(12–14). Undifferentiated PC12 cells do not
produce neurites (<1%), as reported in a previous study
(15). The results of the present
study demonstrated that TFSA induced neurite outgrowth in the PC12
cells. In addition, no significant difference was observed between
TFSA and NGF in the promotion of neurite outgrowth. To further
investigate the neurite outgrowth-inducing effect of TFSA, the
present study examined the expression of GAP43 in the PC12 cells.
Western blot analysis revealed that treatment of the PC12 cells
with TFSA was associated with significant increases in the
expression levels of GAP-43. GAP-43 is a major protein kinase C
substrate of growing axons, which has been demonstrated to be
important in growth cone formation and neurite outgrowth (16,17).
The present study also investigated the potential
signaling pathway responsible for TFSA-induced neurite outgrowth in
the PC12 cells. Previous studies have demonstrated that NGF
activates different extracellular and intracellular signaling
pathways leading to neurite outgrowth, including the PI3K/Akt and
MEK/ERK pathways (18–21). In the present study, treatment with
TFSA led to a significant increase in the phosphorylation of Akt at
Ser473. In addition, the LY294002 PI3K inhibitor suppressed
TFSA-induced neurite outgrowth, indicating the involvement of the
PI3K/Akt pathway in the neuritogenic effect of TFSA. It is also
known that the binding of NGF to its receptor tyrosine kinase,
TrkA, activates the ERK1/2 pathway, and activation of the ERK1/2
pathway has been demonstrated to be to critical in the
differentiation of PC12 cells, as the PD98059 ERK inhibitor
inhibits NGF-induced neuronal differentiation (22,23).
To further investigate whether the TFSA-induced neurite outgrowth
involved the activation of ERK1/2 MAPK, the present study assessed
the phosphorylation state of ERK1/2 in the PC12 cells. The results
of the western blot analysis revealed that the degree of ERK1/2
phosphorylation increased significantly, compared with that
observed in the control group, and was comparable to that detected
following treatment with NGF. In addition, the outgrowth of
neurites induced by TFSA was significantly inhibited by application
of the PD98059 MEK inhibitor (10 µM).
As previously demonstrated, activation of the
MEK/ERK pathway, induced by NGF, leads to the phosphorylation of
CREB, which in turn stimulates the ability of CREB to activate
transcription in NGF-treated cells (24–26).
In the present study, TFSA was observed to have a similar effect.
Incubation of PC23 cells with TFSA resulted in an increase in the
phosphorylation of CREB, which was comparable to that induced by
NGF. To elucidate whether the phosphorylation of CREB resulted from
MEK/ERK activation, the PD98059 MEK inhibitor was used, which
almost completely inhibited the phosphorylation of CREB induced by
TFSA. These results suggested that the activation of CREB may occur
via the MER/ERK pathway.
In conclusion, the present study demonstrated that
TFSA promoted neurite outgrowth in the PC12 cells via the
activation of pathways involved in NGF-induced neuritogenesis.
Treatment with TFSA activated the MEK/ERK and PI3K/Akt pathways and
triggered the phosphorylation of CREB, followed by MEK/ERK
activation, in the PC12 cells.
References
|
1
|
Jiang Y, Zhang W, Tu P and Xu X: Xanthone
glycosides from Polygala tenuifolia and their conformational
analyses. J Nat Prod. 68:875–879. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li C, Yang J, Yu S, Chen N, Xue W, Hu J
and Zhang D: Triterpenoid saponins with neuroprotective effects
from the roots of Polygala tenuifoli a. Planta Med. 74:133–141.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu P, Hu Y, Guo DH, Wang DX, Tu HH, Ma L,
Xie TT and Kong LY: Potential antidepressant properties of Radix
polygalae (Yuan Zhi). Phytomedicine. 17:794–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Y, Liao HB, Dai-Hong G, Liu P, Wang YY
and Rahman K: Antidepressant-like effects of 3,6′-disinapoyl
sucrose on hippocampal neuronal plasticity and neurotrophic signal
pathway in chronically mild stressed rats. Neurochem Int.
56:461–465. 2010. View Article : Google Scholar
|
|
5
|
Greene LA and Tischler AS: Establishment
of a noradrenergic clonal line of rat adrenal pheochromocytoma
cells which respond to nerve growth factor. Proc Natl Acad Sci USA.
73:2424–2428. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kao S, Jaiswal RK, Kolch W and Landreth
GE: Identification of the mechanisms regulating the differential
activation of the mapk cascade by epidermal growth factor and nerve
growth factor in PC12 cells. J Biol Chem. 276:18169–18177. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Markus A, Zhong J and Snider WD: Raf and
akt mediate distinct aspects of sensory axon growth. Neuron.
35:65–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaudry D, Stork PJ, Lazarovici P and Eiden
LE: Signaling pathways for PC12 cell differentiation: Making the
right connections. Science. 296:1648–1649. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frey D, Laux T, Xu L, Schneider C and
Caroni P: Shared and unique roles of CAP23 and GAP43 in actin
regulation, neurite outgrowth, and anatomical plasticity. J Cell
Biol. 149:1443–1454. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakagawa S, Kim JE, Lee R, Malberg JE,
Chen J, Steffen C, Zhang YJ, Nestler EJ and Duman RS: Regulation of
neurogenesis in adult mouse hippocampus by cAMP and the cAMP
response element-binding protein. J Neurosci. 22:3673–3682.
2002.PubMed/NCBI
|
|
11
|
Xing J, Kornhauser JM, Xia Z, Thiele EA
and Greenberg ME: Nerve growth factor activates extracellular
signal-regulated kinase and p38 mitogen-activated protein kinase
pathways to stimulate CREB serine 133 phosphorylation. Mol Cell
Biol. 18:1946–1955. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greene LA: Nerve growth factor prevents
the death and stimulates the neuronal differentiation of clonal
PC12 pheochromocytoma cells in serum-free medium. J Cell Biol.
78:747–755. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao CF, Liu Y, Ni YL, Yang JW, Hui HD,
Sun ZB and Liu SJ: SCIRR39 promotes neurite extension via RhoA in
NGF-induced PC12 cells. Dev Neurosci. 35:373–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Duan X, Ren Y, Liu Y, Huang M, Liu
P, Wang R, Gao G, Zhou L, Feng Z and Zheng W: FoxO3a negatively
regulates nerve growth factor-induced neuronal differentiation
through inhibiting the expression of neurochondrin in PC12 cells.
Mol Neurobiol. 47:24–36. 2013. View Article : Google Scholar
|
|
15
|
Chang YJ, Hsu CM, Lin CH, Lu MS and Chen
L: Electrical stimulation promotes nerve growth factor-induced
neurite outgrowth and signaling. Biochim Biophys Acta.
1830:4130–4136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aigner L and Caroni P: Depletion of 43-kD
growth-associated protein in primary sensory neurons leads to
diminished formation and spreading of growth cones. J Cell Biol.
123:417–429. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lalli G and Hall A: Ral GTPases regulate
neurite branching through GAP-43 and the exocyst complex. J Cell
Biol. 171:857–869. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Read DE and Gorman AM: Involvement of Akt
in neurite outgrowth. Cell Mol Life Sci. 66:2975–2984. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reddy EM, Chettiar ST, Kaur N, Shepal V
and Shiras A: Dlxin-1, a MAGE family protein, induces accelerated
neurite outgrowth and cell survival by enhanced and early
activation of MEK and Akt signalling pathways in PC12 cells. Exp
Cell Res. 316:2220–2236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hafner A, Obermajer N and Kos J: γ-Enolase
C-terminal peptide promotes cell survival and neurite outgrowth by
activation of the PI3K/Akt and MAPK/ERK signalling pathways.
Biochem J. 443:439–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cui H, Shao C, Liu Q, Yu W, Fang J, Yu W,
Ali A and Ding K: Heparanase enhances nerve-growth-factor-induced
PC12 cell neuritogenesis via the p38 MAPK pathway. Biochem J.
440:273–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sarina, Yagi Y, Nakano O, Hashimoto T,
Kimura K, Asakawa Y, Zhong M, Narimatsu S and Gohda E: Induction of
neurite outgrowth in PC12 cells by artemisinin through activation
of ERK and p38 MAPK signaling pathways. Brain Res. 1490:61–71.
2013. View Article : Google Scholar
|
|
23
|
Poplawski GH, Tranziska AK, Leshchyns'ka
I, Meier ID, Streichert T, Sytnyk V and Schachner M: L1CAM
increases MAP2 expression via the MAPK pathway to promote neurite
outgrowth. Mol Cell Neurosci. 50:169–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ginty DD, Bonni A and Greenberg ME: Nerve
growth factor activates a Ras-dependent protein kinase that
stimulates c-fos transcription via phosphorylation of CREB. Cell.
77:713–725. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mullenbrock S, Shah J and Cooper GM:
Global expression analysis identified a preferentially nerve growth
factor-induced transcriptional program regulated by sustained
mitogen-activated protein kinase/extracellular signal-regulated
kinase (ERK) and AP-1 protein activation during PC12 cell
differentiation. J Biol Chem. 286:45131–45145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Andreassi C, Zimmermann C, Mitter R, Fusco
S, De Vita S, Saiardi A and Riccio A: An NGF-responsive element
targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron
axons. Nat Neurosci. 13:291–301. 2010. View
Article : Google Scholar : PubMed/NCBI
|