Introduction
Triptolide (TPL), the predominant active ingredient
of the thunder duke vine, Tripterygium wilfordii, is used in
traditional Chinese medicine (1).
It is a double terpenoid. Previous studies confirmed that TPL
exerts a role in a number of biological processes, including a
range of immunosuppressive, anti-inflammatory and antitumor effects
(1). In particular, TPL has
potential as an antitumor drug in clinical applications. For
example, the use of TPL in treating leukemia has entered phase I
clinical trials (2,3), and its antitumor potential for the
treatment of ovarian cancer was demonstrated by the induction of
cell apoptosis (4).
As a first-line treatment for non-small cell lung
cancer chemotherapy, paclitaxel (or Taxol®) resistance
is an important factor, which influences the effect of
chemotherapy. In vitro and animal experiments revealed that
TPL exerts a marked inhibitory effect on the growth of lung cancer
and other solid tumor types (5).
Previous studies revealed that TPL exerts an
antitumor effect primarily through the inhibition of nuclear factor
(NF)-κB, heat shock factor 1, activator protein-1 and other
transcription factors, and the NF-κB pathway is one of the most
critical targets of the antitumor effect (6,7). The
NF-κB/inhibitor of NF-κB (IκB) pathway is associated with tumor
cell survival, apoptosis and metastasis (8,9). In
addition, the genes which regulate the NF-κB signaling pathway,
particularly apoptosis-associated genes, provide an important basis
for the occurrence of multi-drug resistance (9,10).
For example, the upregulation of Bcl-2 and the downregulation of
Bax, proteins which are involved in apoptosis, are associated with
a strengthening of resistance to chemotherapy, and the caspase
inhibitors, FLICE-like inhibitory protein (FLIP) and X-linked
inhibitor of apoptosis protein (XIAP), may be directly involved in
the regulation of chemosensitivity (11). Therefore, the inhibition of the
NF-κB signaling pathway, particularly regarding the transcription
and expression of antiapoptotic genes, may provide the underlying
molecular mechanism of chemoresistance reversal. Previous studies
indicate that TPL may exert a drug-resistance-reversal effect
(11,12). Chen et al (12) demonstrated that TPL inhibited the
expression of multidrug resistance protein and enhanced the
antitumor effects on KB cells mediated by 5-fluorouracil. Li et
al (11) revealed that TPL
improved the sensitivity of K562/A02 cells to adriamycin by
inhibiting the expression of microRNA-21, in addition to
upregulating the expression level of phosphatase and tensin
homolog. Although the anti-tumor activity of TPL is recognized and
it may exert potential drug-resistance reversal effects, the
underlying molecular mechanism remains to be fully elucidated.
In the present study, a Taxol-resistant strain of
lung adenocarcinoma cell line (A549/Taxol) was established with an
aim to investigate the impact of TPL on A549/Taxol cell
proliferation, apoptosis and the cell cycle. The present study also
aimed to investigate whether TPL may exert a resistance reversal
effect in lung adenocarcinoma Taxol-resistance, and the underlying
mechanism of its action. The results of the present study may be of
important clinical significance in consideration of whether TPL may
be applied as a resistance reversal agent, in combination
chemotherapy with Taxol/paclitaxel.
Materials and methods
Establishment of the Taxol-resistant lung
adenocarcinoma cell line, A549/Taxol
The A549 lung adenocarcinoma cell line was purchased
from the Typical Culture Preservation Commission Cell Bank, Chinese
Academy of Sciences (Shanghai, China). The Taxol-resistant lung
adenocarcinoma cell line, A549/Taxol, was established by the method
of increasing the drug concentration gradient. Human lung
adenocarcinoma A549 cells in the logarithmic growth phase were
cultured in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA) medium
supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin
(Sigma-Aldrich) and 100 µg/ml streptomycin (Sigma-Aldrich)
at 37°C in an atmosphere containing 5% CO2.
Subsequently, Taxol (Qilu Pharmaceutical Co., Ltd., Jinan, China)
was added to the culture medium at a minimum concentration of 20
ng/ml. The cells were cultured for 24 h. Sensitive cells died as
the drug induced apoptosis. The surviving cells were cultivated to
the next logarithmic phase in the culture medium without Taxol. In
the next cycle, the cells were cultured and induced by Taxol using
the same process, however, the concentration of Taxol was increased
from 20 ng/l to 400 ng/l (the concentration gradient was 20, 40,
60, 80, 100, 120, 200, 30 and 400 ng/ml). The process was repeated
until the A549 cells grew steadily in the medium with 400 ng/l
Taxol. These cells were Taxol-resistant lung adenocarcinoma cells
(A549/Taxol). On completion of the initial stage of the assessment
of the cells, the resistance index of the cell line was calculated,
according to the half maximal inhibitory concentration
(IC50) of A549/Taxol / IC50 of A549, to be
51.87.
Effect of TPL on A549/Taxol cell
apoptosis
Annexin-V fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double staining (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for the detection of apoptosis.
A549/Taxol cells in the logarithmic growth phase were inoculated
into 6-well plates at a density of 1×105
cells/cm2 and cultured for 24 h. Once the cells had
adhered, different concentrations of TPL (0.03, 0.3 or 3
µmol/l) were added to the cells, and the negative control
group was established. The cells were incubated for 2, 4, 6 or 12
h, and were subsequently trypsinized and collected using 0.25%
trypsin (excluding EDTA; Sigma-Aldrich). The cells were washed
twice with phosphate-buffered saline (PBS), prior to centrifugation
at 870 × g for 5 min, and 5×105 cells were collected.
Subsequently, 500 µl binding buffer suspension (Invitrogen;
Thermo Fisher Scientific, Inc.) and 5 µl annexin V-FITC were
added to the cells prior to mixing, and then 5 µl PI was
added and mixed. The incubation was performed for 5–15 min at room
temperature in the dark. The extent of apoptosis was assessed using
a flow cytometer (XL/XL-MCL; Beckman Coulter, Fullerton, CA, USA;
excitatory wavelength (λex) 488 nm; emission wavelength,
(λem) 530 nm). The data were analyzed using FlowJo 7.1
(TreeStar, San Carlos, CA, USA) and ModFit LT 3.1 (Verity Software
House Inc., Topsham, ME, USA).
Effect of TPL on the A549/Taxol cell
cycle
PI staining was used to detect the cell cycle.
A549/Taxol cells in the logarithmic growth phase were inoculated
into 6-well plates at a density of 1×105
cells/cm2, prior to culture for 24 h. Once the cells had
adhered, different concentrations of TPL (0.03, 0.3 or 3
µmol/l) were added to the cells for 2, 4, 6 or 12 h. The
cells were subsequently harvested with 0.25% trypsin (excluding
EDTA) and washed with PBS, prior to centrifugation at 870 × g for 5
min, and 5×105 cells were collected. Single cell
suspensions were fixed with the volume fraction of 70% ethanol
overnight, preserved at 4°C, and subsequently washed with PBS
fixative prior to staining. An aliquot of 100 µl RNase A was
added and the cells were incubated in a water bath for 30 min at
37°C. Subsequently, 400 µl PI was added to stain the cells
prior to blending, and the cells were maintained in the dark for 30
min at 4°C. The quantity of red fluorescence at an excitation
wavelength of 488 nm was assessed using a flow cytometer (Beckman
Coulter).
Western blotting
The protein expression levels of NF-κB-mediated
drug-resistant genes were determined by western blotting. The
extracted protein concentration was measured using a conventional
bicinchoninic acid method. Samples of 40 µg protein were
cooled on ice following an incubation at 95–100°C for 5 min, and
the samples were subsequently electrophoresed using 8% SDS-PAGE
(Beyotime Institute of Biotechnology, Jiangsu, China). For the
electrophoresis, a stacking gel was used at a constant voltage (80
V) for 20 min, followed by a separating gel at 100 V for ~80 min.
The gel was removed and placed in transfer buffer (Beyotime
Institute of Biotechnology) to equilibrate for 15 min. The filter
paper and the polyvinylidene fluoride (PVDF) membrane (EMD
Millipore, Billerica, MA, USA) were prepared and placed in transfer
buffer and deionized water, respectively. For the wet
electrotransfer stage, the bottom electrode (anode) was laid flat,
with the filter paper, PVDF membrane, the gel and a filter paper
placed on top. The top electrode (cathode) was placed on the
interlayer following the exclusion of air bubbles. For the
electrotransfer, the apparatus was powered by a constant current
(200 mA) for 1 h. The PVDF membranes were blocked with 5% skimmed
milk blocking buffer (incubated at room temperature for 1 h), and
subsequently the blocking solution was discarded. The primary
antibody, rabbit anti-CK19 (1:5,000; cat. no. ab133496; Abcam,
Cambridge, UK) and a rabbit anti-β-actin antibody (1:4,000; cat.
no. ab8227; Abcam) were added (~0.1 ml/cm2), followed by
an incubation with agitation at 4°C overnight. The membrane was
rinsed with PBS with Tween® 20 (PBST) four times, each
time for 5 min. The membrane and secondary goat anti-rabbit
immunoglobulin G antibody conjugated with HRP (horseradish
peroxidase-labeled antibody and secondary antibody in blocking
buffer; 1:5,000; cat. no. CW0114; Beijing ComWin Biotech Co., Ltd.,
Beijing, China) were incubated with agitation at room temperature
for 1–2 h. The membrane was subsequently washed with PBST, and
rinsed five times (5 min each time). The calculated quantity of
developer (EMD Millipore) was 0.1 ml/cm2, and the
developer was applied to the PVDF membrane and placed at room
temperature for 1 min. The PVDF membrane was wrapped in plastic in
order to avoid air bubbles. The membrane proteins were attached to
the X-ray film for quick exposure in a darkroom and developed. The
exposure time was adjusted to enable the best development of the
protein bands to occur.
Immunofluorescence staining
The effect of TPL on the A549/Taxol cellular
localization was determined using immunofluorescence. The
A549/Taxol cells were treated with TPL (3 µM) for 12 h. The
cells on the slides were subsequently fixed with 4%
paraformaldehyde. A total of two drops of 3%
H2O2/methanol solution was added to each
slice at room temperature (15–25°C) for 10 min, prior to immersion
in PBS three times. Aliquots of 50–100 µl ready-to-use goat
serum were added dropwise and the cells were incubated at room
temperature for 20 min. Subsequently, 50–100 µl primary
rabbit anti-CK19 antibody (1:200 dilution) was added. The cells
were incubated in a wet box for 2 h at 37°C and were subsequently
immersed in PBS three times. Subsequently, 50–100 µl FITC
tetramethylrhodamine (1:200 dilution) secondary antibody was added,
and the cells were incubated in the dark for 1 h at 37°C, prior to
immersion in PBS three times. Aliquots of 50–100 µl
formulated dye, 4′,6-diamidino-2-phenylindole, was added to each
slice, which was subsequently placed in the dark at room
temperature for 5 min. The expression of the proteins was observed
under a fluorescent microscope (BX51TF; Olympus Corporation, Tokyo,
Japan), and images were captured at three areas where high levels
of expression had occurred.
Statistical analysis
The data are presented as the mean ± standard
deviation of three independent experiments. Statistical analyses
were conducted using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA).
Statistical significance was determined using analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TPL induces cell apoptosis in the
Taxol-resistant A549/Taxol human lung adenocarcinoma cell line in a
dose- and time-dependent manner
Initially, following the establishment of the
Taxol-resistant human lung adenocarcinoma cell line, A549/Taxol,
the cells were treated with increasing concentrations of TPL (0.03,
0.3 or 3 µmol/l) for 2, 4, 6 or 12 h, and the negative
control group was established. Subsequently, the apoptotic cells
were stained using annexin V-FITC/PI double staining and detected
using flow cytometry (λex, 488 nm; λem, 530
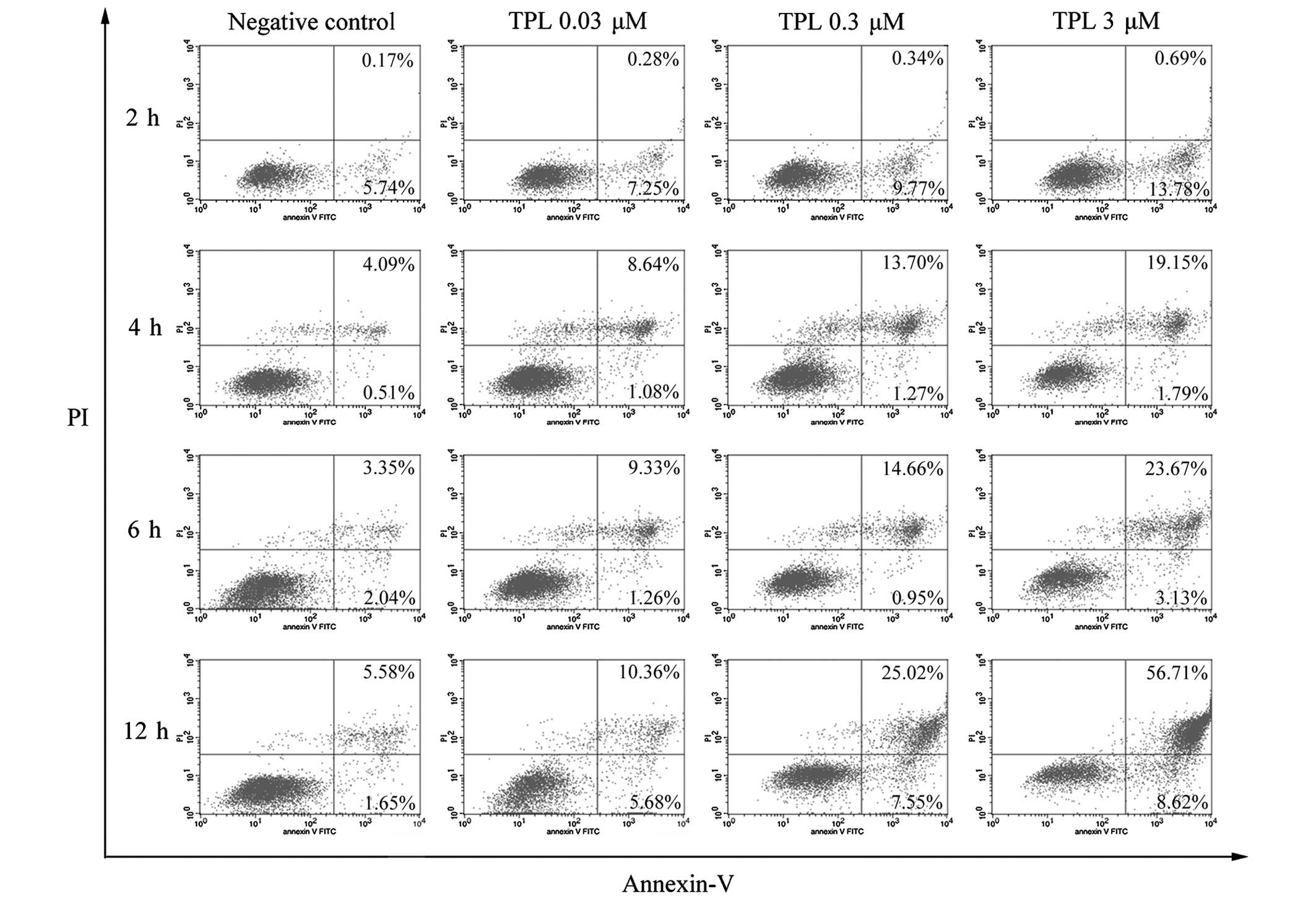
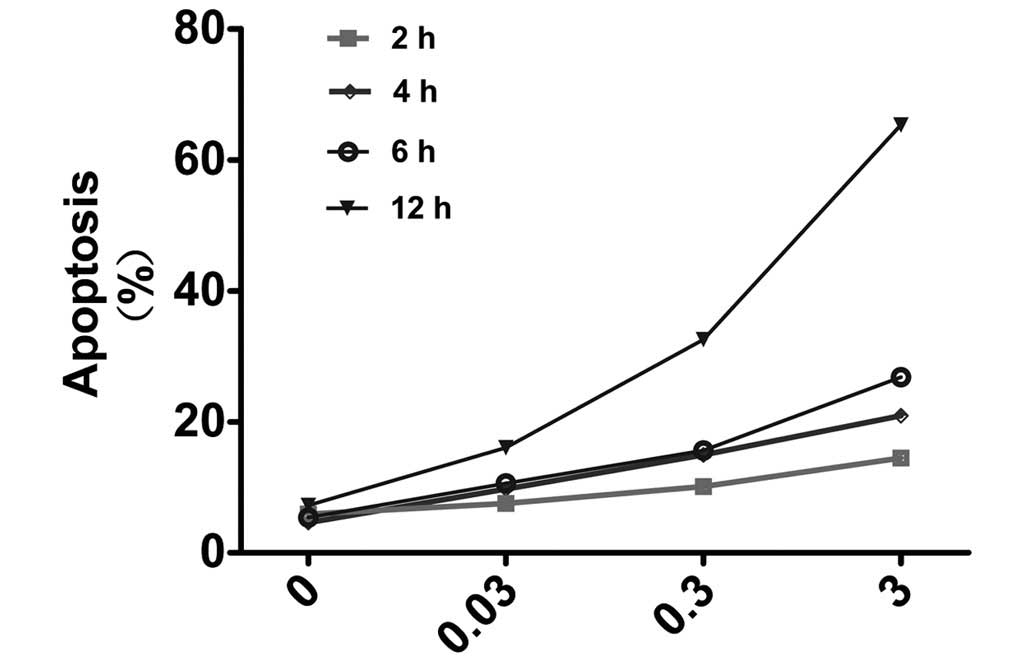
nm). The experimental results are shown in Fig. 1. TPL promoted A549/Taxol cell
apoptosis. The cells in the upper right quadrant were annexin
V-FITC and PI double positive, denoting the quantity of late
apoptotic or necrotic cells present. The lower right quadrant shows
the cells, which were annexin V-FITC positive (the early apoptotic
cells). The proportion of apoptotic cells increased with an
increase of the concentration of TPL at different treatment
durations (Figs. 1 and 2). On exposure to 3 µM TPL for 2,
4, 6 and 12 h, the extent of cell apoptosis observed markedly
increased. The inhibitory effect reached a maximum with 3 µM
TPL at the 12 h time point (cell apoptotic rate, 65.33%), whereas
the apoptotic rate of the control group was 7.23% at 12 h. In
addition, the cell apoptotic rate increased with an increase in the
treatment time at an identical concentration (Fig. 2). These results confirmed that TPL
may induce cell apoptosis in the A549/Taxol lung adenocarcinoma
Taxol-resistant strain, and this effect occurred in a dose- and
time-dependent manner.
TPL induces cell cycle arrest at the
synthesis (S) phase in A549/Taxol cells
The present study next investigated whether the
apoptosis induced by TPL was associated with the cell cycle. The
A549/Taxol cells were treated with different concentrations of TPL
(0.03, 0.3 or 3 µmol/l) for 2, 4, 6 and 12 h, and a negative
control group was established. The PI staining method was used for
the detection of the cell cycle. A total of two groups of
experimental data were analyzed, the first with 3 µM TPL
treatment for 2, 4, 6 and 12 h, and the second with 0, 0.03, 0.3
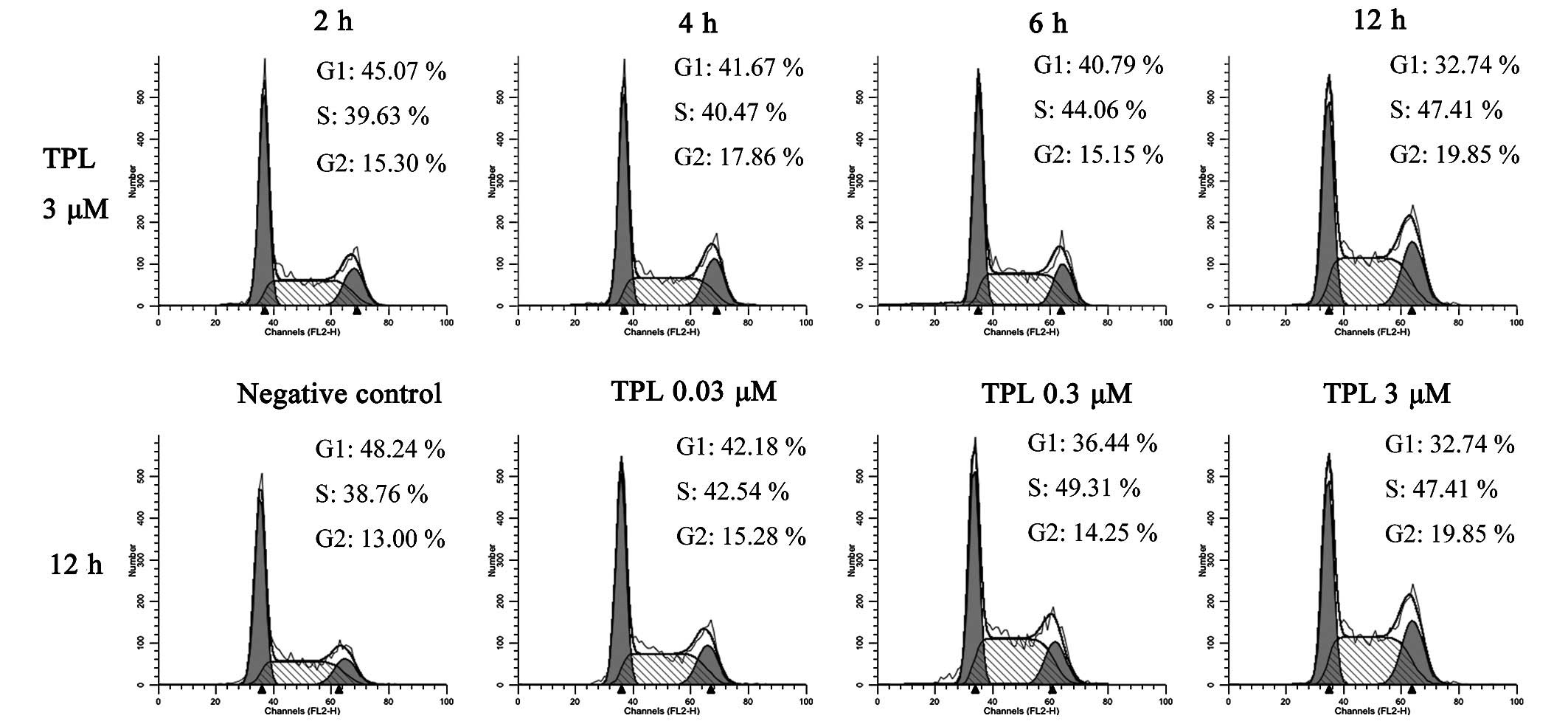
and 3 µM TPL treatment for 12 h (Fig. 3). When the A549/Taxol cells were
treated with 3 µM TPL for 2, 4, 6 and 12 h, the percentage
of cells in the G1 phase decreased from 45.07 to 32.74%, and the
percentage of cells in the S phase increased from 39.63 to 47.41%.
The cells in the G2 phase increased, although a linear trend was
not observed. With the second experimental group, where the cells
were treated with 0, 0.03, 0.3 and 3 µM TPL for 12 h, the
percentage of cells in the G1 phase decreased from 48.24 to 32.74%,
whereas the percentage of cells in the S phase increased from 38.76
to 49.31% when the dose was increased from 0 to 0.3 µM. This
effect was not evident at 3 µM TPL, possibly due to the
occurrence of a small level of apoptosis in the A549/Taxol cells.
These data revealed that a dose- and time-dependent association
existed between the A549/Taxol cells and their treatment with TPL,
with TPL inducing the arrest of the A549/Taxol cell cycle in the S
phase, followed by the promotion of cell apoptosis.
TPL inhibits NF-κB nuclear transfer and
protein expression in the A549/Taxol cells
Cell apoptosis may be regulated by the NF-κB
signaling pathway. Whether NF-κB was inhibited by TPL in the
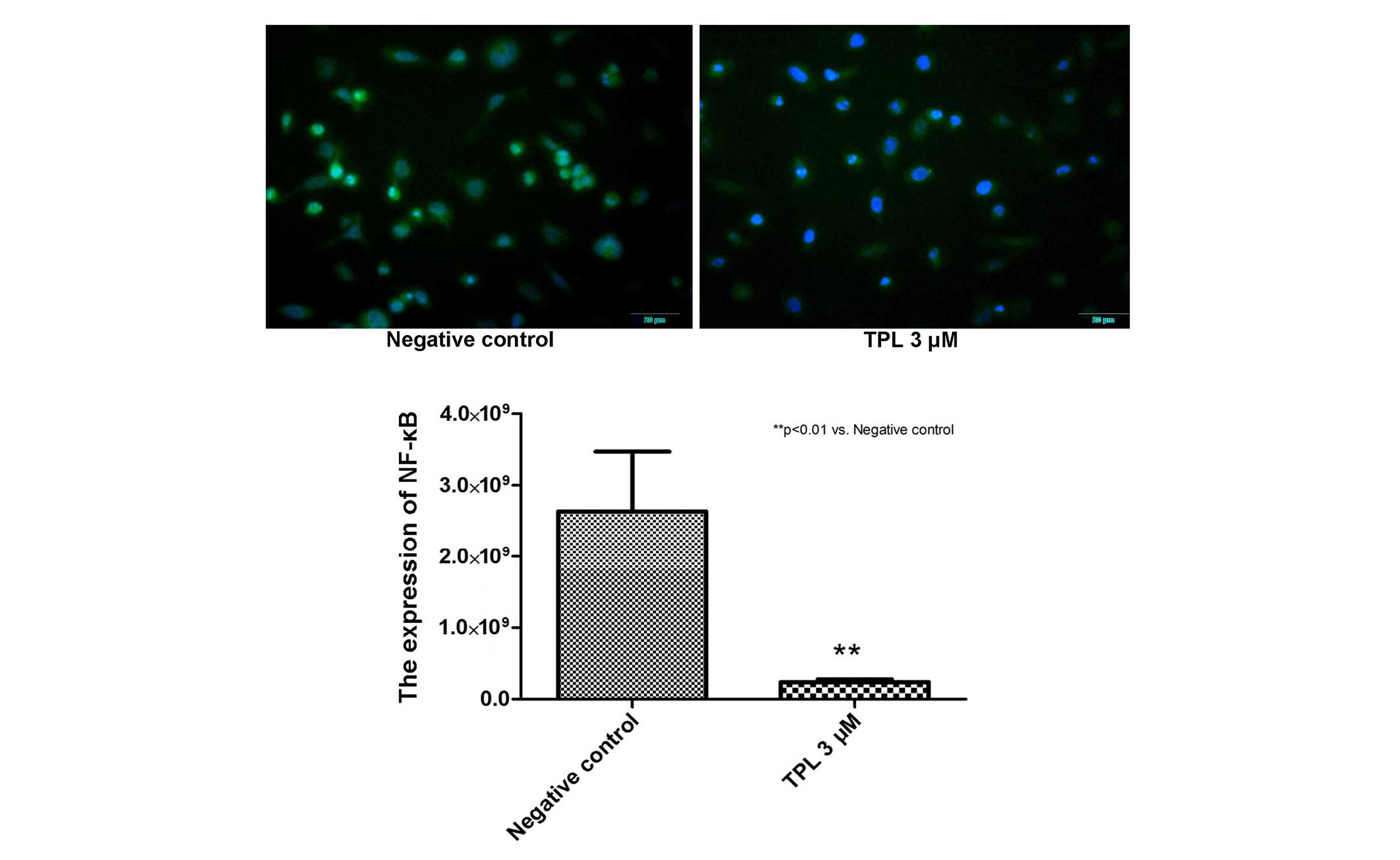
A549/Taxol cells was subsequently investigated. On treatment of the
A549/Taxol cells with 3 µM TPL for 12 h, images were
captured using immunofluorescence analysis, and inhibition of NF-κB
nuclear transfer was observed. In the images, the negative control
registered 46,230.33±8,407.43 intensity data, and the TPL 3
µM group registered a reading of 13,106.33±680.65 units
(Fig. 4). These data indicated
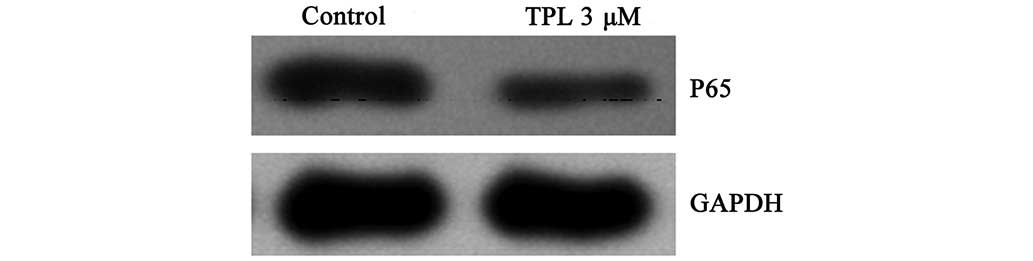
that the expression levels of NF-κB were inhibited. Furthermore,
the p65 protein was extracted from the TPL 3 µM treatment
and the control groups and analyzed using western blotting
(Fig. 5), in order to compared the
expression level. The protein bands were quantified, using
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal
reference protein for standardization. The ratio of p65/GAPDH was
0.36 in the negative control group, whereas it was 0.17 in the
treatment group, suggesting that the expression of p65 in
A549/Taxol cells was inhibited.
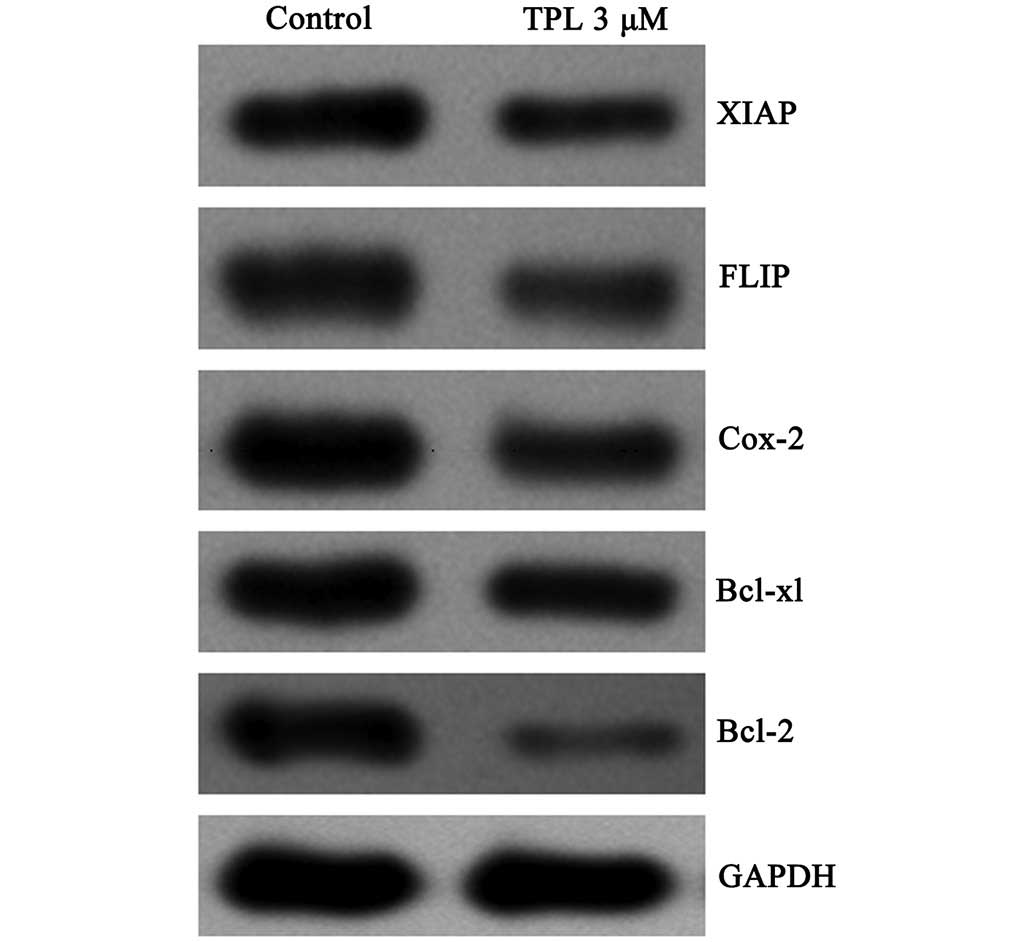
TPL inhibits the expression of FLIP,
XIAP, Bcl-2, Bcl-xL and cyclo-oxygenase 2 (COX-2) regulated by
NF-κB
FLIP, XIAP, Bcl-2, Bcl-xL and COX-2 are a number of
drug-resistant genes, which are regulated by NF-κB. The protein
expression levels in the A549/Taxol cells treated with TPL (3
µM, 12 h) were assessed by western blotting. To compare the
protein expression levels, GAPDH was used as a standard internal
reference protein. The inhibition of the protein expression levels
occurred to differing degrees (Fig.
6), although the inhibition of Bcl-2 was the most marked. The
ratio of the intensity of the Bcl-2GAPDH protein bands was 0.04 in
the treatment group, compared with 0.20 in the control group. These
experiments confirmed that TPL inhibits the expression of multidrug
resistance-associated genes regulated by NF-κB, including FLIP,
XIAP, Bcl-2, Bcl-xL and COX-2.
Discussion
Paclitaxel (or Taxol®) is the current
first-line treatment for non-small cell lung cancer chemotherapy
(13). It exerts its antitumor
effects predominantly through the induction of mitotic arrest.
Previous studies revealed that intratumoral concentrations of Taxol
caused cell death due to chromosomal missegregation on multipolar
spindles (14), however, Taxol
resistance is an important factor, which affects the effectiveness
of the chemotherapy (15).
In vitro and animal experiments revealed that
the administration of TPL markedly inhibits growth in a variety of
solid tumor types (5,16). In the present study, it was
initially confirmed that TPL exerts a clear resistance-reversal
effect on the A549/Taxol lung adenocarcinoma Taxol-resistant cell
line. Subsequently, the levels of apoptosis and the cell cycle,
which are the two major regulatory mechanisms associated with cell
death, were monitored. The A549/Taxol cells were treated with
different concentrations of TPL for different durations and
apoptosis was subsequently detected using flow cytometric analysis.
The results of these experiments revealed that the apoptotic rate
of the A549/Taxol cells increased concomitantly with the
concentration or the treatment duration. When the A549/Taxol cells
were incubated with 3 µM TPL for 12 h, the level of
apoptosis occurring was determined to be 65.33%, whereas it was
7.23% in the negative control group. These results suggested that
TPL promoted A549/Taxol cell apoptosis, exerting a drug-resistant
reversal effect, and that the effect was dose- and time-dependent.
The cell cycle was assessed using PI staining and it was observed
that the cells were arrested in the S phase in the TPL treatment
group. With an increase in the concentration or the treatment time,
TPL reduced the percentage of A549/Taxol cells in the G1 phase,
increased the proportion of cells in the S phase, and a greater
number of cells were arrested in the S phase. These results
suggested that the cells arrested in the G1 phase would return to
the G0 phase or re-differentiate, and that the cells would die by
apoptosis if they were arrested at any checkpoint, with the
exception of the G1 phase (17).
In the present study, the proportion of cells in the S phase
increased in the treatment group. These results supported the
hypothesis that TPL induced A549/Taxol cell death by arresting the
cell cycle, and suggested that TPL exerts a marked drug-resistant
reversal effect on the A549/Taxol cell line.
The antitumor activity of chemotherapeutic drugs is
mediated through a direct induction of DNA damage and cell death by
apoptosis, a process which also requires NF-κB (18,19).
The present study proposed that TPL-induced A549/Taxol apoptosis
may be associated with the NF-κB signaling pathway. The
transcription factor NF-κB is an antiapoptotic transcription
factor, which has an important role in cell survival signaling. The
interaction between IκB and NF-κB is balanced in the cytoplasm,
where they form a dimer. NF-κB may be activated by a variety of
signals, including pro-inflammatory and stress factors, which cause
phosphorylation of the IκB inhibitory proteins by the IκB-kinase
complex (20). NF-κB-activated
transcription induces the expression of several antiapoptotic
proteins, including the Bcl-2 family members, and the inhibition of
the NF-κB signaling pathway promotes apoptosis (21). p65 is an important constituent
subunit of NF-κB (22). In the
present study, western blotting was used to detect the protein
expression levels of p65 in the TPL treatment and the negative
control groups. When the protein levels were compared between the
two groups, the ratio of p65/GAPDH was determined to be 0.17 for
the TPL treatment group (3 µM, 12 h), whereas in the control
group, the ratio for p65/GAPDH was 0.36. These results indicated
that the expression of NF-κB in the experimental group of the
A549/Taxol cells was inhibited. By immunofluorescence, the results
of the present study revealed that NF-κB nuclear transfer in the
A549/Taxol cell line was inhibited in the TPL treatment group (3
µM, 12 h). These results suggested that the NF-κB signaling
pathway was inhibited during the A549/Taxol resistance-reversal
process elicited by TPL treatment.
FLIP and XIAP are caspase inhibitors, which inhibit
the expression of caspase, prompting the survival of tumor cells,
and subsequently causing resistance of the tumors to develop
(23). The antiapoptotic protein,
Bcl-2, interacts with the pro-apoptotic protein, Bax, in tumor
cells. Apoptosis may be reduced by upregulating the expression of
Bcl-2 or downregulating the expression of Bax, and COX-2 exerts a
role in tumor development and progression by promoting cell
proliferation, inhibiting apoptosis, promoting angiogenesis and
inhibiting the immune function mechanism (24). In the present study, it was
hypothesized that the drug-resistant genes mediated by NF-κB,
including FLIP, XIAP, Bcl-2, Bcl-xL and COX-2, are associated with
the occurrence of tumor drug resistance. The protein levels of
these drug-resistant genes were compared in the treatment and the
negative control groups using western blot analysis. It was
revealed that these resistance genes were inhibited to different
degrees, with Bcl-2 being inhibited the most markedly. The
interaction between the Bcl-2 and Bax proteins is crucial in
determining whether the cells survive or die. Therefore, the onset
of apoptosis is associated with the inhibition of the expression of
the antiapoptotic protein, Bcl-2, and an increase in the expression
of the proapoptotic protein, Bax. In the present study, it was
revealed that TPL markedly inhibited the expression of Bcl-2 and
other drug-resistant proteins, including FLIP, XIAP, Bcl-xL and
COX-2, although to differing degrees. These results indicated that
the expression of NF-κB-regulated drug-resistant genes was
suppressed in the resistance-reversal process elicited upon
treatment with TPL.
In conclusion, the present study confirmed that TPL
may induce cellular apoptosis in the Taxol-resistant A549/Taxol
lung adenocarcinoma cell line, indicating that TPL exerts a reverse
effect on the Taxol-resistant lung adenocarcinoma in a dose- and
time-dependent manner. The inhibition of the NF-κB signaling
pathway, and in particular, the inhibition of expression of the
NF-κB-regulated drug-resistant-associated genes, provided the
predominant molecular mechanism to account for the TPL
resistant-reversal effect. Therefore, these results indicated that
TPL may be used in the treatment of non-small cell lung cancer as a
drug-resistant reversal agent, in combination with paclitaxel.
However, further research in this field is required.
Acknowledgments
The authors would like to thank Dr Edward C Mignot
(Shandong University) for offering linguistic advice during the
preparation of this manuscript.
References
|
1
|
Qiu D and Kao PN: Immunosuppressive and
anti-inflammatory mechanisms of triptolide, the principal active
diterpenoid from the Chinese medicinal herb Tripterygium wilfordii
Hook. f. Drugs R D. 4:1–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corson TW and Crews CM: Molecular
understanding and modern application of traditional medicines:
Triumphs and trials. Cell. 130:769–774. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Efferth T, Li PC, Konkimalla VS and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Westfall SD, Nilsson EE and Skinner MK:
Role of triptolide as an adjunct chemotherapy for ovarian cancer.
Chemotherapy. 54:67–76. 2008. View Article : Google Scholar
|
|
5
|
Chen L, Liu Q, Huang Z, Wu F, Li Z, Chen X
and Lin T: Tripchlorolide induces cell death in lung cancer cells
by autophagy. Int J Oncol. 40:1066–1070. 2012.
|
|
6
|
Jang BC, Lim KJ, Choi IH, Suh MH, Park JG,
Mun KC, Bae JH, Shin DH and Suh SI: Triptolide suppresses
interleukin-1beta-induced human beta-defensin-2 mRNA expression
through inhibition of transcriptional activation of NF-kappaB in
A549 cells. Int J Mol Med. 19:757–763. 2007.PubMed/NCBI
|
|
7
|
Westerheide SD, Kawahara TL, Orton K and
Morimoto RI: Triptolide, an inhibitor of the human heat shock
response that enhances stress-induced cell death. J Biol Chem.
281:9616–9622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: From innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oya M, Takayanagi A, Horiguchi A, Mizuno
R, Ohtsubo M, Marumo K, Shimizu N and Murai M: Increased nuclear
factor-kappa B activation is related to the tumor development of
renal cell carcinoma. Carcinogenesis. 24:377–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Hui L, Xu W, et al: Triptolide
modulates the sensitivity of K562/A02 cells to adriamycin by
regulating miR-21 expression. Pharm Biol. 50:1233–1240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YW, Lin GJ, Chuang YP, Shen H, Chen
Q, Long L and Zhu X: Triptolide circumvents drug-resistant effect
and enhances 5-fluorouracil antitumor effect on KB cells.
Anticancer Drugs. 21:502–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edelman MJ: Novel taxane formulations and
microtubule-binding agents in non-small-cell lung cancer. Clin Lung
Cancer. 10(Suppl 1): S30–S34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kastl L, Brown I and Schofield AC: Altered
DNA methylation is associated with docetaxel resistance in human
breast cancer cells. Int J Oncol. 36:1235–1241. 2010.PubMed/NCBI
|
|
16
|
Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei
XF, Yang J, Underhill CB and Zhang L: Triptolide inhibits the
growth and metastasis of solid tumors. Mol Cancer Ther. 2:65–72.
2003.PubMed/NCBI
|
|
17
|
Pietenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology. 181–182. 475–481. 2002.
|
|
18
|
Morales-Cano D, Calviño E, Rubio V,
Herráez A, Sancho P, Tejedor MC and Diez JC: Apoptosis induced by
paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4
human leukaemia cells is not modulated by ERK inhibition. Exp
Toxicol Pathol. 65:1101–1108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ryan KM, Ernst MK, Rice NR and Vousden KH:
Role of NF-kappaB in p53-mediated programmed cell death. Nature.
404:892–897. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gilmore TD, Koedood M, Piffat KA and White
DW: RelNF-kappaBIkappaB proteins and cancer. Oncogene.
13:1367–1378. 1996.PubMed/NCBI
|
|
23
|
Deveraux QL, Roy N, Stennicke HR, Van
Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS and Reed
JC: IAPs block apoptotic events induced by caspase-8 and cytochrome
c by direct inhibition of distinct caspases. EMBO J. 17:2215–2223.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|