Introduction
Chronic renal diseases (CKDs), irrespective of the
means of the initial insult, relentlessly progress to end-stage
kidney disease, with an irreversible loss of renal architecture and
function (1). Renal
tubulointerstitial injury is considered to be the hallmark of
progressive kidney disease, and the extent of the injury is
inversely correlated with renal function. Renal tubulointerstitial
injury is histopathologically characterized by an accumulation of
myofibroblasts, the marked deposition of extracellular matrix and
tubular atrophy (2). Although the
molecular mechanisms underlying interstitial injury have been
partly elucidated (3), only a few
therapies are currently used clinically. Therefore, specific
treatments to target interstitial injury are urgently required.
Among the signaling pathways associated with renal
tubulointerstitial injury, the Smad-mediated transforming growth
factor (TGF)-β1 signaling pathway occupies a central role in this
process (4). In experimental
models and human kidney diseases, TGF-β1 is markedly upregulated,
and Smad2/3 is highly activated in the fibrotic kidney (5).
The underlying mechanisms by which TGF-β1 mediate
renal injury are as follows: i) TGF-β1 markedly induces the
proliferation and activation of renal fibroblasts during renal
fibrosis (5,6); ii) TGF-β1 elicits the production of
the extracellular matrix (ECM) through Smad-dependent mechanisms
(7); iii) TGF-β1 inhibits ECM
degradation by suppressing matrix metalloproteinases and inducing
the tissue inhibitor of metalloproteinase (8); and iv) TGF-β1 is involved in tubular
deletion (9,10).
Lefty is a member of the TGF-β superfamily with two
variants, which are designated as leftyA and leftyB in humans
(11), and lefty1 and lefty2 in
mice (12). Initially, a study by
Meno et al (13) revealed
that lefty was involved in the formation of embryonic lateral
patterning. Ulloa et al (14) demonstrated that leftyA inhibited
the phosphorylation of Smad2/3, inhibited the heterodimerization of
R-Smads with Smad4, and also inhibited the nuclear translocation of
the Smad complex (14). In
addition, it was demonstrated that leftyA markedly reduced the
quantity of collagen deposition, and promoted collagenolysis
(15). It was also demonstrated
that leftyA or lefty1 attenuated the epithelial-mesenchymal
transition (EMT) by inhibiting TGF-β1 signaling in the mouse HK2
tubular epithelial (16), or the
rat NRK52E tubular epithelial (17) cell lines, respectively. Due to the
active involvement of the TGF-β1 signaling pathway in renal injury,
it was hypothesized that lefty1 may alleviate renal interstitial
injury.
Using a mouse model of unilateral ureteral
obstruction (UUO), a well-established experimental model of renal
injury characterized by progressive tubulointerstitial fibrosis and
tubular atrophy, the present study aimed to assess the ability of
lefty1 to protect against renal injury, and to determine the
possible underlying mechanisms.
Materials and methods
Experimental design
Male C57BL/6 mice (weighing, ~20 g) were obtained
from the Experimental Animal Center of Wuhan University (Wuhan,
China). The mice were granted free access to water and chow, and
were kept under a 12-h light/dark cycle, at 24°C and 50% humidity.
The UUO was performed using a previously described method (18). Briefly, under sodium pentobarbital
anesthesia (60 mg/kg body weight; Merck, Darmstadt, Germany), a
complete ureteral obstruction was performed by ligating the left
ureter with 4-0 silk (Tianhou Medical Co., Jinan, China).
Sham-operated mice had their ureters exposed and manipulated,
although they were not ligated. To study the correlation between
the expression of lefty1 and the degree of interstitial fibrosis,
20 mice were randomly assigned to four groups: i) Sham-operation;
ii) UUO for 3 days; iii) UUO for 5 days; iv) UO for 7 days
(n=5/group). The mice were subsequently sacrificed on days 3, 5 and
7 under anesthesia and were fixed by perfusion through the left
ventricle. To assess the effects of lefty1 on renal
tubulointerstitial injury, 18 mice were divided into three groups:
i) Sham-operation; ii) UUO plus vehicle treatment; iii) UUO plus
lefty1 treatment. On the day following surgery, the mice were
administered recombinant mouse lefty1 (R&D Systems, Inc.,
Minneapolis, MN, USA) through a tail-vein injection at a dose of
300 µg/kg body weight, and subsequently on every other day
for up to 6 days. The control mice received an injection of an
identical volume of vehicle (0.9% saline solution; 100 µl).
On day 7 following surgery, the mice were sacrificed, and the
kidneys were removed. One half of each of the kidneys was fixed in
4% buffered paraformaldehyde for 24 h for histological studies; the
other half was snap-frozen in liquid nitrogen, and stored at −80°C
prior to protein or mRNA extraction. All experiments involving the
mice were performed according to institutional guidelines and the
protocol of the present study was approved by The Animal Research
Ethics Committee of Wuhan University (Wuhan, China).
Histological studies
Kidney sections from the paraffin-embedded tissues
were prepared at a thickness of 5 µm. The sections were
stained with periodic acid - Schiff reagent (Biyuntian, Wuhan,
China) to assess the extent of tubular atrophy. Picrosirius red
(PSR; Biyuntian) staining was used to identify the interstitial
collagen. Rabbit polyclonal anti-human lefty1 (cat. no. ab22569;
1:200), rabbit polyclonal anti-human collagen I (cat. no. ab34710;
1:100), rabbit polyclonal anti-human fibronectin (cat. no. ab2413;
1:400) and rabbit polyclonal anti-human anti-kidney injury
molecular-1 (Kim-1; cat. no. ab47635; 1:100) antibodies were
purchased from Abcam (Cambridge, UK). Rabbit polyclonal anti-human
α-smooth muscle actin (SMA) antibody (cat. no. BM0002; 1:50) was
purchased from Boshide Bioengineering Co., Ltd. (Wuhan, China). The
primary antibodies were incubated for 1 h at room temperature, or
at 4°C overnight. Goat polyclonal anti-rabbit IgG (cat. no. BA1055;
1:200; Boshide Bioengineering Co., Ltd.) was used as the secondary
antibody. The antigen-specific, positive cells were visualized
using streptavidin peroxidase reagents purchased from Boshide
Bioengineering Co., Ltd. Brown staining taken up by the cells was
indicative of a positive result.
Semi-quantitative analysis for the
histological studies
A total of 20 random, non-overlapping fields
(magnification, ×400) from each animal was selected for assessing
PSR staining. These were quantified by assessing the size of the
positive area as a percentage of the total area. A total of 10
random, non-overlapping fields (magnification, ×400) of each animal
were assessed for evaluating tubular Kim-1 staining. These were
quantified by counting the positive tubules as a percentage of the
total. A total of 10 random, non-overlapping fields (magnification,
×200) of each animal were selected for evaluating the tubular
atrophy. Tubular atrophy was scored on a scale between 0 and 3, as
previously reported (19),
although with certain modifications: 0, absent (no atrophy); 1,
mild (percentage of atrophied tubules, 1–15%); 2, moderate
(percentage of atrophied tubules, 16–30%); 3, severe (percentage of
atrophied tubules, >30%).
Western blot analysis
The kidney tissues were homogenized in a lysis
buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X
100, sodium pyrophosphate, β-glycerophosphate, EDTA,
Na3VO4 and leupeptin (Biyuntian, Wuhan,
China) on ice. The lysates were centrifuged at 12,000 × g at 4°C
for 20 min and the protein concentration was determined using a
bicinchoninic acid (BCA) protein assay kit. A total of 40 µg
protein was separated by 12 or 8% SDS-PAGE (Boshide, Wuhan, China).
The proteins were electrotransferred onto a nitrocellulose membrane
(Merck-Millipore, Billerica, MA, USA). Non-specific binding to the
membrane was blocked for 1 h at room temperature using 5% non-fat
milk or bovine serum albumin (Boshide). The membranes were
subsequently incubated overnight at 4°C with the primary antibodies
in blocking buffer. Antibodies against TGF-β1 (cat. no. sc-146,
1:500), phosphorylated (p-)Smad2/3 (cat. no. sc-11769; 1:400) and
β-actin (cat. no. sc-1619; 1:200) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Anti-collagen I (cat. no.
bs7158R; 1:100) antibody was purchased from Beijing Boao Sen
Biotechnology Co., Ltd. (Beijing, China). The sources of the other
antibodies used in the western blot analysis [anti-lefty1 (1:400),
anti-fibronectin (1:400) and anti-α-SMA (1:200) antibodies] were as
described above. All antibodies were rabbit polyclonal anti-human
antibodies. Subsequently, blots were incubated with the following
secondary antibodies: Horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin (Ig)G (cat no. sc-2004; 1:5,000
dilution; Santa Cruz Biotechnology, Inc.) or IRDye®
infrared dye-conjugated anti-rabbit IgG (cat no. 611-1202; 1:5,000
dilution (Rockland Immunochemicals, Inc., Gilbertsville, PA, USA).
The bands were visualized using an enhanced chemiluminescence
detection kit (GE Healthcare, Little Chalfont, UK) and the
Odyssey® imaging system (Li-Cor Biosciences, Lincoln,
NE, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed to assess the transcription
levels of lefty1. The total RNA was extracted using TRIzol
(Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) and 1
µg aliquots of RNA were used in the RT reaction with M-MuLV
reverse transcriptase (cat. no. K1621; Thermo Fisher Scientific).
The resulting cDNA was used as a template for qPCR analysis. The
primers were obtained from Sangon Biological Engineering Technology
and Services, Co., Ltd. (Shanghai, China), and the specific primers
were designed as follows: Lefty1, sense:
5′-ACTCAAGACCCTTTCAGGACAC-3′ and antisense:
5′-CAGCAGAGCCACAGGAATG-3′; for glyceraldehyde-3-phosphate
dehydrogenase, sense: 5′-GGTGAAGGTCGGTGTGAACG-3′ and antisense:
5′-CTCGCTCCTGGAAGATGGTG-3′. A 20 µl sample of PCR reaction
solution, which included SYBR® Green PCR Master mix
(cat. no. RR420A; Takara Bio, Inc., Dalian, China), was amplified
according to the manufacturer's instructions with the following
thermocycling conditions: Initial denaturation at 94°C for 5 min,
followed by 40 cycles of denaturation at 94°C for 20 sec, primer
annealing at 58°C for 30 sec and elongation at 72°C for 45 sec. The
reactions were performed on an ABI PRISM® 7500 Sequence
Detection system (Applied Biosystems Life Technologies, Beijing,
China). The calculations of the relative changes in the mRNA
expression levels were performed using the 2−ΔΔCt method
(20).
Statistical analysis
The data are expressed as the mean ± standard
deviation. One-way analysis of variance, followed by the
Student-Newman-Keuls test, was used for the quantitative data,
whereas the Kruskal-Wallis test was used for non-normally
distributed data. Statistical analyses of the data were performed
using GraphPad Prism (version 5.0; GraphPad Software, Inc., La
Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of lefty1 is inversely
correlated with renal fibrosis
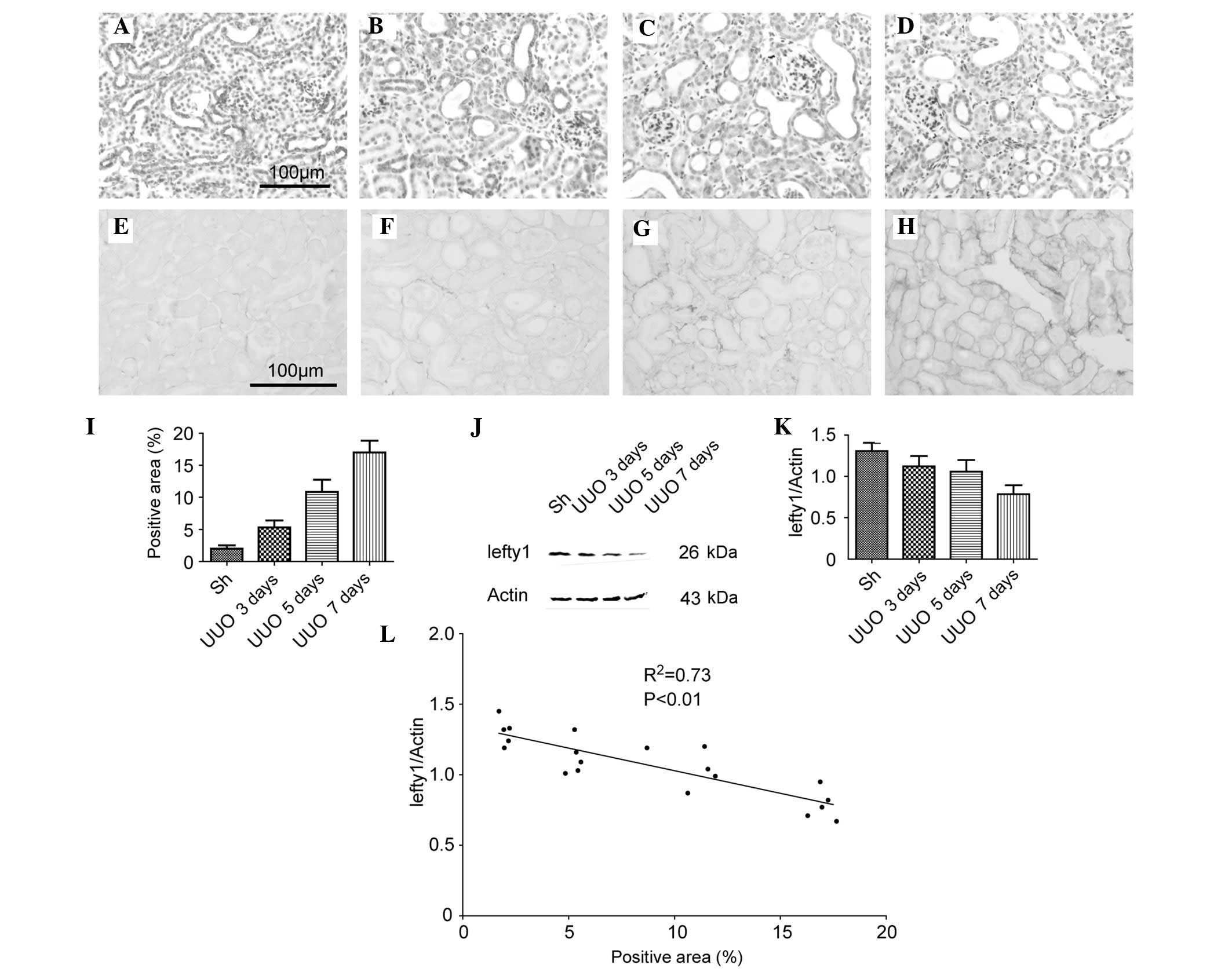
The protein expression of lefty1 in the kidneys of
mice with a UUO were investigated. As shown in Fig. 1A–D, the immunohistochemical (IHC)
staining demonstrated that lefty1 was predominantly localized in
the tubular epithelial cells and that the expression levels of
lefty1 were gradually decreased in the obstructed kidneys in a
time-dependent manner (up to 7 days following the UUO)..
Furthermore, during the first week of the UUO, the degree of the
renal interstitial fibrosis increased, as assessed by the PSR
staining experiments (Fig. 1E–I).
Western blotting was used to corroborate these results (Fig. 1J). A semi-quantitative analysis of
the western blotting results revealed that the protein expression
of lefty1 in the kidneys of the mice in the sham-operation and UUO
for 3, 5 or 7 day groups (normalized against β-actin) were
1.31±0.10, 1.12±0.13, 1.06±0.14 and 0.78±0.11, respectively
(Fig. 1K). Subsequently, the
correlation between the protein expression of lefty1 and the degree
of renal fibrosis was examined. The percentage of positive area in
the kidneys of mice in the sham-operation and UUO for 3, 5 or 7 day
groups were 1.99±0.51, 5.30±.08, 10.85±1.90 and 17.01±1.85%,
respectively (Fig. 1L). A linear
regression plot revealed a close correlation
(R2=0.73; P<0.01) between the protein
expression of lefty1 and the degree of renal fibrosis (Fig. 1K). These results indicated that the
protein expression of lefty1 was inversely correlated with the
degree of renal interstitial fibrosis.
Lefty1 attenuates the degree of
interstitial fibrosis
Due to the inverse correlation between the protein
expression of lefty1 and the extent of interstitial fibrosis,
exogenous recombinant lefty1 was administered to mice with a UUO to
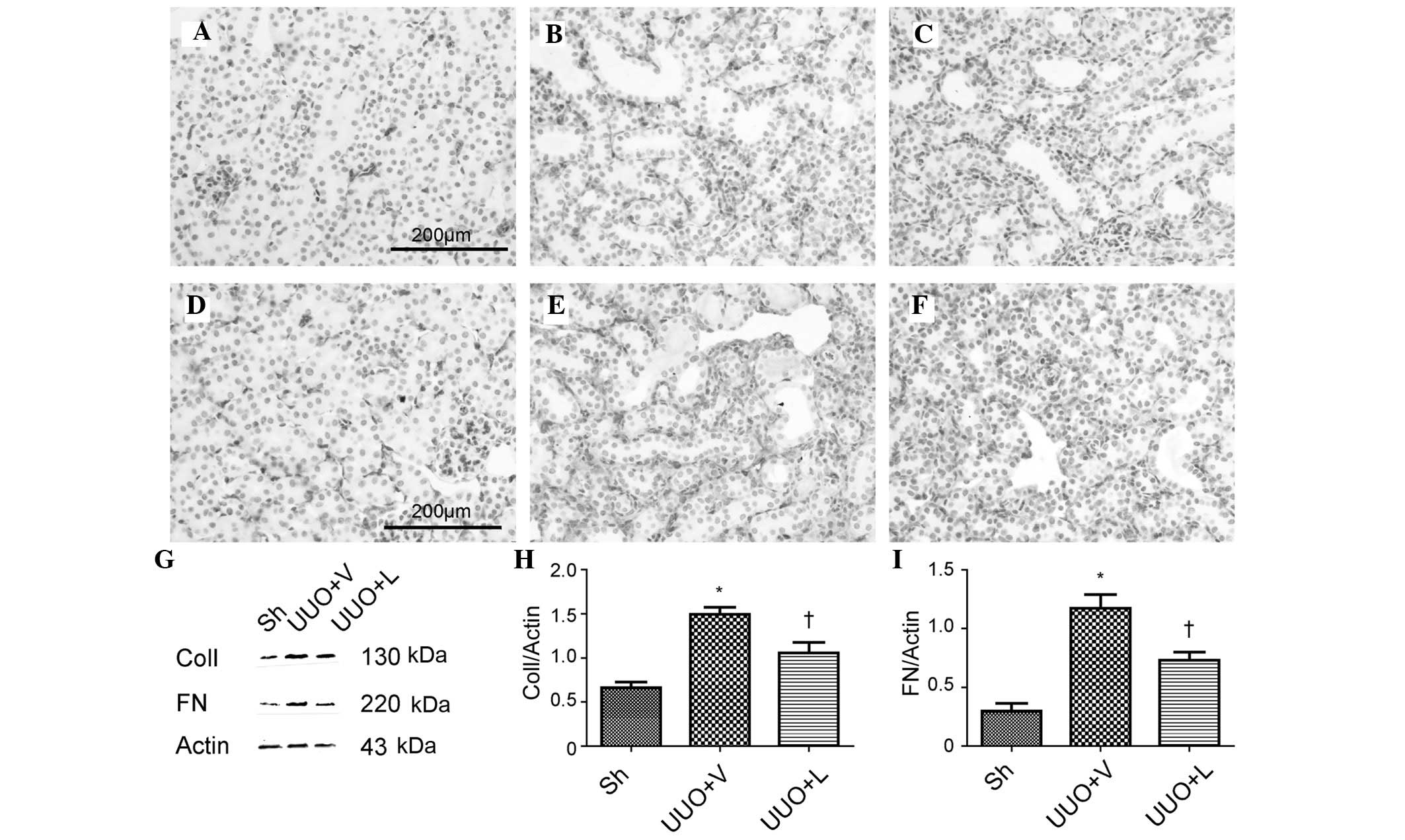
examine whether lefty1 attenuates interstitial fibrosis. The IHC
staining patterns for collagen I and fibronectin are shown in
Fig. 2A–C and Fig. 2D–F, respectively. In comparison
with the sham-operated group, the protein expression levels of
collagen I and fibronectin were significantly increased in mice on
treatment with vehicle, which decreased following treatment with
lefty1. These findings were corroborated by western blot analysis.
Compared with the sham-operated group, the protein expression
levels of collagen I were significantly increased (1.49±0.09, vs.
0.67±0.07; P<0.05), which were subsequently diminished upon
treatment with lefty1 (1.06±0.11, vs. 1.49±0.09; P<0.05).
Furthermore, the UUO led to an increased protein expression of
fibronectin (1.17±0.12, vs. 0.30±0.07; P<0.05), which was
reduced following administration of lefty1 (0.73±0.07, vs.
1.17±0.12; P<0.05). These results indicated that lefty1
attenuated renal interstitial fibrosis in mice with a UUO.
Lefty1 suppresses the accumulation of
myofibroblasts
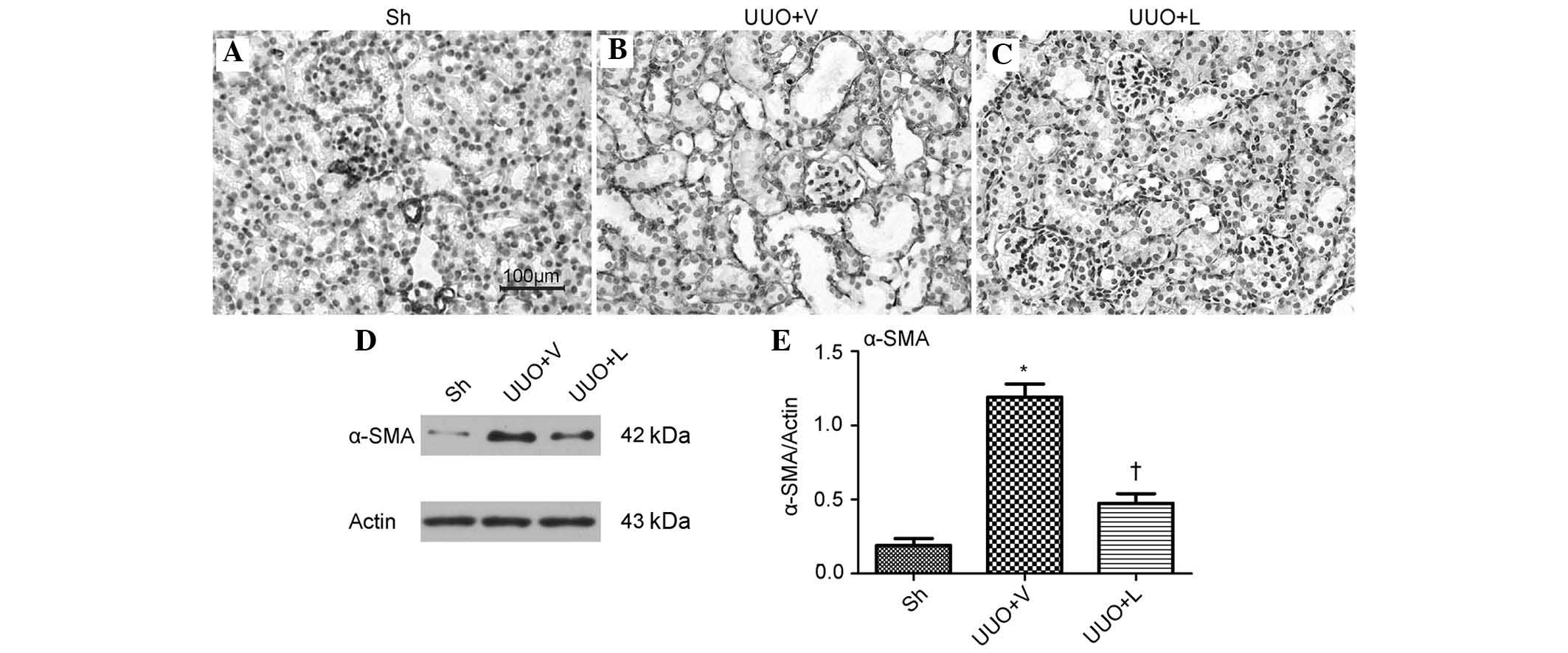
α-SMA is a marker for myofibroblasts, which are the
major class of ECM-producing cells. Therefore it was hypothesized
that treatment with lefty1 may suppress the accumulation of
myofibroblasts. As shown in Fig.
3A–C, IHC staining demonstrated that α-SMA was localized in the
vascular smooth muscle cells in the normal mouse kidney. Compared
with the sham-operated group, a large quantity of α-SMA-positive
cells were localized in the peritubular interstitial space in the
UUO mice, which were treated with vehicle, and the subsequent
treatment with lefty1 decreased the protein expression of α-SMA.
Western blot analysis also revealed a markedly increased expression
of α-SMA in the UUO mice, which were treated with vehicle, compared
with the sham-operated mice (1.19±0.09, vs. 0.19±0.05; P<0.05;
Fig. 3D and E). Treatment with
lefty1 resulted in a significant reduction in the protein
expression levels of α-SMA compared with the UUO plus vehicle
treatment group (0.47±0.07, vs. 1.19±0.09; P<0.05). These
results indicated that lefty1 suppressed the accumulation of
myofibroblasts.
Lefty1 ameliorates tubular injury and
atrophy
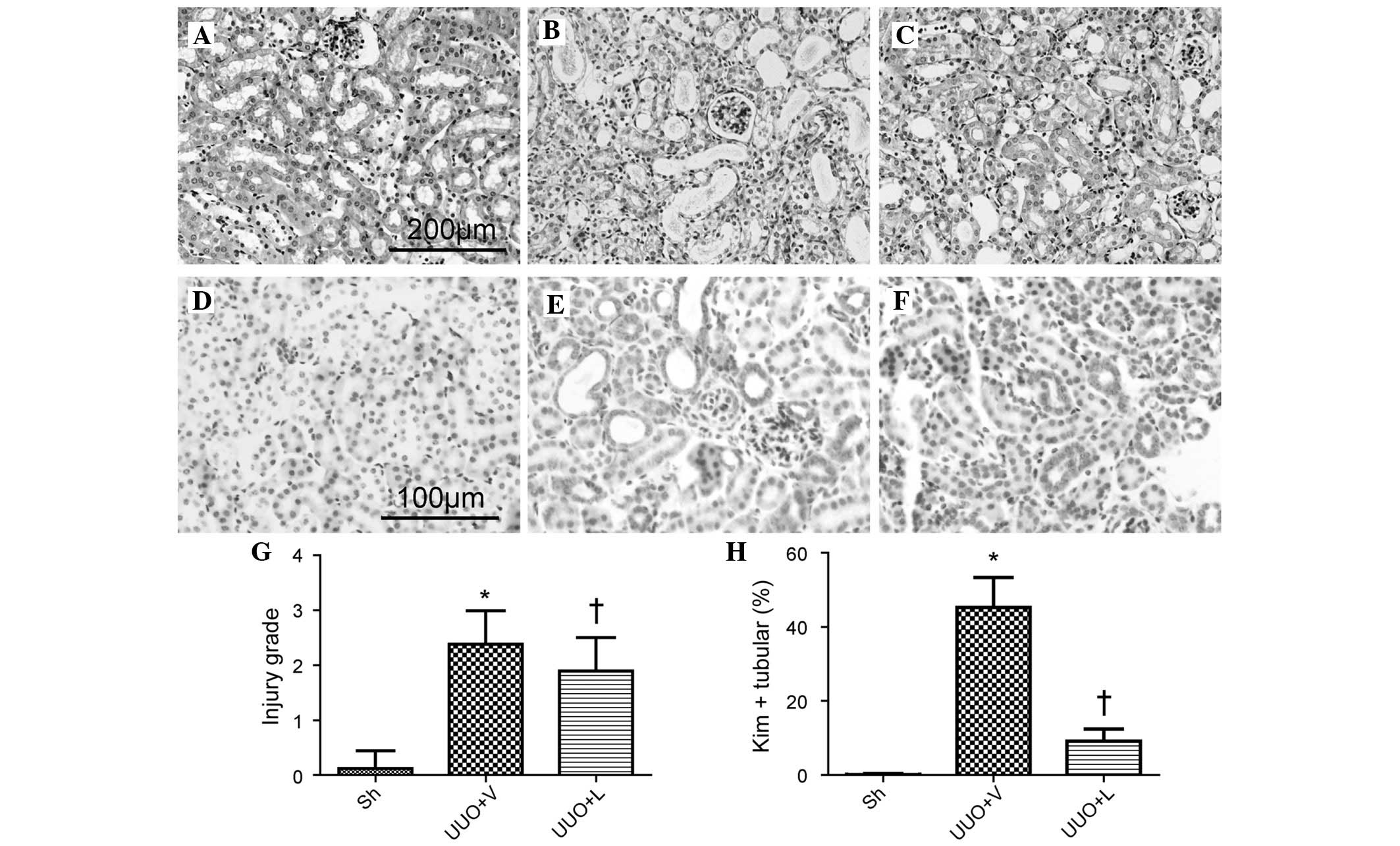
Tubular atrophy is a prominent feature in
tubulointerstitial injury. In comparison with the sham-operated
group, the UUO plus vehicle treatment group exhibited severe
tubular atrophy (2.38±0.16, vs. 0.12±0.32%; P<0.05). However,
lefty1 suppressed tubular atrophy (1.90±0.60, vs. 2.38±0.16%;
P<0.05; Fig. 4A–C) in the
obstructed kidney. Since tubular atrophy is a histopathological
manifestation of tubular injury, it was hypothesized whether lefty1
inhibited tubular damage, and staining with Kim-1, an indicator of
tubular damage, was used to assess this. In comparison with the
sham-operated group, the UUO plus vehicle treatment group exhibited
more Kim-1-positive tubules (45.27±8.12, vs. 0.12±0.32%;
P<0.05). Lefty1 significantly suppressed tubular injury
(9.12±3.31, vs. 45.27±8.12%; P<0.05; Fig. 4D–F) in the obstructed kidney. These
results indicated that lefty1 ameliorated tubular atrophy and
injury.
Lefty1 inhibits the Smad-dependent TGF-β1
signaling pathway
Due to the evidence that the Smad-dependent TGF-β1
signaling pathway occupies a central role in renal injury, whether
treatment with lefty1 inhibited this pathway was subsequently
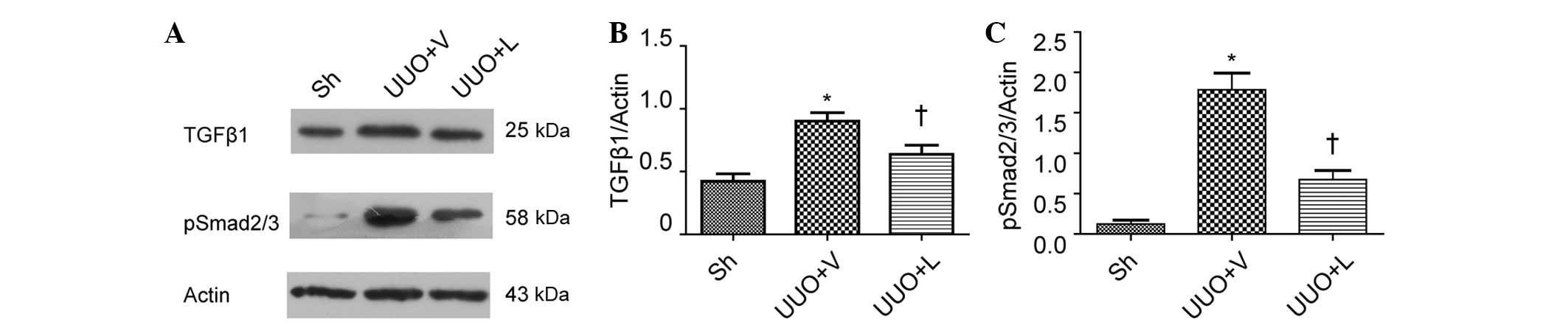
investigated. Western blot analysis revealed that the protein
expression levels of TGF-β1 in the obstructed kidney was
significantly increased compared with the sham group (0.90±0.07,
vs. 0.42±0.06; P<0.05; Fig. 5A and
B). Treatment with lefty1 reduced the protein expression of
TGF-β1 (0.64±0.07, vs. 0.90±0.07; P<0.05; Fig. 5B). In addition, the protein
expression of p-Smad2/3 in the obstructed kidney was considerably
increased compared with the sham group (1.78±0.21, vs. 0.12±0.05;
P<0.05), and the subsequent treatment with lefty1 also
diminished the protein expression of p-Smad2/3 (0.67±0.11, vs.
1.78±0.21; P<0.05; Fig. 5C).
These results indicated that lefty1 inhibited the Smad-dependent
TGF-β1 signaling pathway.
Exogenously administered lefty1 restores
the expression of endogenous lefty1
Finally, whether exogenously administered lefty1
restored the endogenous expression of lefty1 was investigated
(Fig. 5). Compared with the sham
group, the UUO plus vehicle treatment group demonstrated a reduced
protein expression of lefty1 (0.42±0.07, vs. 1.04±0.12; P<0.05;
Fig. 5B). However, lefty1
treatment augmented the expression of lefty1 (0.42±0.07, vs.
0.72±0.12; P<0.05). At the mRNA expression level, the UUO plus
vehicle treatment group exhibited a decreased mRNA expression of
lefty1 (0.29±0.11, vs. 1.00±0.03; P<0.05; Fig. 5C). Nevertheless, lefty1 treatment
restored lefty1 expression (0.55±0.15, vs. 0.29±0.11;
P<0.05).
Discussion
Due to the high level of homology between human
leftyA and B, and the mouse lefty1 and 2 proteins (11), it is conceivable that they may
share a similar function. An in vitro study, reported
previously by our laboratory, revealed that leftyA attenuated the
EMT in a tubular cell line by dampening TGF-β1-mediated signal
transduction (16). These results
were confirmed by another previous study (17). Despite its antagonism towards
TGF-β1 signaling in vitro, whether or not lefty exerted an
identical effect in vivo remained to be elucidated. The
present study demonstrated for the first time, to the best of our
knowledge, that lefty1 is a protective regulator during the UUO
process. In UUO-challenged mice, the lefty1 protein was
specifically downregulated by ureteral ligation, and treatment with
lefty1 restored the expression of endogenous lefty1 in the
obstructed kidney. Furthermore, treatment with lefty1 also
decreased the extent of tubular injury and interstitial fibrosis.
At the mechanistic level, the present study revealed that
TGF-β1-mediated signal transduction was mitigated by lefty1.
Lefty was initially characterized as a diffusible
morphogen, which is transiently expressed in the left half of
gastrulating mouse embryos (13).
In adult mice, lefty is expressed in the mouse endometrium during
the estrous cycle and the peri-implantation period (21). In the present study, the presence
of lefty1 in the kidney was demonstrated, although the biological
function of lefty1 in kidney diseases remains to be elucidated. The
present study demonstrated that lefty1 is specifically
downregulated upon induction by a UUO. Indeed, lefty1 may be
regulated by other types of signaling molecules under a variety of
conditions, including Smad2/3, FoxH1, forkhead activin signal
transducer 2, Oct3/4 and microRNAs (22). UUO manipulation generated a
diversity of signaling molecules, among which TGF-β1 may be ranked
as the most important. The in vitro study published
previously by our laboratory demonstrated that TGF-β1 inhibited
lefty expression in a dose-dependent manner (16). Therefore, the UUO-induced
downregulation of lefty1 may be partially caused by an
overproduction of this cytokine. Notably, in the present study, it
was observed that the exogenous administration of lefty1 restored
the expression of endogenous lefty1 at the transcriptional level.
The mechanism by which exogenous lefty1 regulates the endogenous
expression of lefty1 remains to be elucidated. Besser (23) demonstrated that the expression of
lefty in undifferentiated human embryonic stem cells required the
activation of Smad2/3. However, the present study revealed that
p-Smad2/3 was downregulated. This discrepancy may be explained by
the different cellular microenvironment, therefore, an
investigation into the mechanism underlying the regulation of
lefty1 following the induction of UUO is required in future
studies.
Lefty1 may attenuate tubulointerstitial injury, as
evidenced by the decreased deposition of the ECM, suppression of
the accumulation of the myofibroblasts and the amelioration of
tubular atrophy. The identification of lefty1 as a key protein in
the amelioration of interstitial injury permits an exploration of
the underlying mechanism. It is widely accepted that TGF-β1 is a
key modulator of renal interstitial injury in experimental model
and human kidney diseases (9). For
example, TGF-β1 is markedly upregulated in the fibrotic kidney,
regardless of the initial cause of the kidney disease. Furthermore,
the overexpression of TGF-β1 by renal tubular epithelial cells
resulted in tubulointerstitial fibrosis and tubular deletion in the
absence of any injury, revealing the functional importance of
TGF-β1 in CKDs (6). TGF-β1
activates several intracellular Smad signaling cascades to fulfil
its functions. The present study demonstrated that the upregulation
of TGF-β1 was induced in the obstructed kidney, whereas treatment
with lefty1 decreased the expression of TGF-β1. Furthermore, lefty1
also diminished the phosphorylation of Smad2/3. Indeed, the
injury-promoting effects of TGF-β1 were further confirmed by a
report which demonstrated that the complete inhibition of TGF-β1 by
a neutralizing TGF-β1 antibody markedly ameliorated renal damage
in vivo (24). Furthermore,
a deficiency of Smad3 attenuated renal tubular injury following a
UUO (10). These results
demonstrated that the inhibitory effect of lefty1 on the
Smad-dependent TGF-β1 signaling pathway was responsible for its
ability to protect against injury.
It is noteworthy that, in view of the various
different functions of lefty, its ability to inhibit renal injury
in the obstructed kidneys may be mediated by other mechanisms.
Previous studies demonstrated that lefty contributes to the
remodeling of the ECM by increasing the collagenolytic,
gelatinolytic, elastolytic and caseinolytic activities in
vivo (15). In human
endometrium, lefty also regulates the expression and activation of
matrix metalloproteinase 9 (25).
Therefore, increased activities of the matrix degradation enzymes
mediated by lefty may provide additional pathways that lead to the
suppression of renal fibrogenesis in vivo. In addition, it
remains to be determined whether the protective effect of lefty1 is
associated with the non-Smad-dependent TGF-β1 pathway, which is
also responsible for renal injury (26). In fact, it is possible that the
multiple pathways triggered by lefty1 from exogenous sources may
work in concert, culminating ultimately in the amelioration of
renal injury in the obstructed kidneys in vivo.
It should be noted that TGF-β1 is a pleiotropic
cytokine involved in a number of cell functions, including cell
growth, differentiation and the regulation of immune responses, in
addition to the control of the biosynthesis of extracellular
connective tissue (27). In the
present study, the mice receiving lefty1 treatment were active, and
there appeared to be no clear side effects. However, in the
clinical setting, it remains to be elucidated whether patients
exposed to a prolonged treatment with lefty1 experience any adverse
reactions, including the impairment of immune function or delayed
wound healing. Nevertheless, the findings of the present study may
have important implications for developing clinically relevant
therapeutic strategies for renal injury. The administration of
lefty may provide a novel and effective treatment for CKDs by
specifically inhibiting the activation of the TGF-β1 signaling
pathway. Although the protective effect of lefty in vivo
remains to be confirmed in other animal model systems and in
patients with CKDs, the administration of exogenous lefty protein
appears to have the potential to hinder the progression of CKDs,
devastating conditions which are incurable at present.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81170710) and the Hospital
Foundation for Doctors (no. YB15B01).
References
|
1
|
Meguid El, Nahas A and Bello AK: Chronic
kidney disease: The global challenge. Lancet. 365:331–340. 2005.
View Article : Google Scholar
|
|
2
|
Barnes JL and Glass WF II: Renal
interstitial fibrosis: A critical evaluation of the origin of
myofibroblasts. Contrib Nephrol. 169:73–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chuang PY, Menon MC and He JC: Molecular
targets for treatment of kidney fibrosis. J Mol Med (Berl).
91:549–559. 2013. View Article : Google Scholar
|
|
4
|
López-Hernández FJ and López-Novoa JM:
Role of TGF-β in chronic kidney disease: An integration of tubular,
glomerular and vascular effects. Cell Tissue Res. 347:141–154.
2012. View Article : Google Scholar
|
|
5
|
Gewin L and Zent R: How does TGF-β mediate
tubulointerstitial fibrosis? Semin Nephrol. 32:228–235. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koesters R, Kaissling B, Lehir M, Picard
N, Theilig F, Gebhardt R, Glick AB, Hähnel B, Hosser H, Gröne HJ
and Kriz W: Tubular overexpression of transforming growth
factor-beta1 induces autophagy and fibrosis but not mesenchymal
transition of renal epithelial cells. Am J Pathol. 177:632–643.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moon JA, Kim HT, Cho IS, Sheen YY and Kim
DK: IN-1130, a novel transforming growth factor-beta type I
receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in
obstructive nephropathy. Kidney Int. 70:1234–1243. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Douthwaite JA, Johnson TS, Haylor JL,
Watson P and El Nahas AM: Effects of transforming growth
factor-beta1 on renal extracellular matrix components and their
regulating proteins. J Am Soc Nephrol. 10:2109–2119.
1999.PubMed/NCBI
|
|
9
|
García-Sánchez O, López-Hernández FJ and
López-Novoa JM: An integrative view on the role of TGF-beta in the
progressive tubular deletion associated with chronic kidney
disease. Kidney Int. 77:950–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inazaki K, Kanamaru Y, Kojima Y, Sueyoshi
N, Okumura K, Kaneko K, Yamashiro Y, Ogawa H and Nakao A: Smad3
deficiency attenuates renal fibrosis, inflammation, and apoptosis
after unilateral ureteral obstruction. Kidney Int. 66:597–604.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kosaki K, Bassi MT, Kosaki R, Lewin M,
Belmont J, Schauer G and Casey B: Characterization and mutation
analysis of human LEFTY A and LEFTY B, homologues of murine genes
implicated in left-right axis development. Am J Hum Genet.
64:712–721. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meno C, Ito Y, Saijoh Y, Matsuda Y,
Tashiro K, Kuhara S and Hamada H: Two closely-related left-right
asymmetrically expressed genes, lefty-1 and lefty-2: Their distinct
expression domains, chromosomal linkage and direct neuralizing
activity in Xenopus embryos. Genes Cells. 2:513–524. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meno C, Saijoh Y, Fujii H, Ikeda M,
Yokoyama T, Yokoyama M, Toyoda Y and Hamada H: Left-right
asymmetric expression of the TGF beta-family member lefty in mouse
embryos. Nature. 381:151–155. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ulloa L and Tabibzadeh S: Lefty inhibits
receptor-regulated Smad phosphorylation induced by the activated
transforming growth factor-beta receptor. J Biol Chem.
276:21397–21404. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mason JM, Xu HP, Rao SK, Leask A, Barcia
M, Shan J, Stephenson R and Tabibzadeh S: Lefty contributes to the
remodeling of extracellular matrix by inhibition of connective
tissue growth factor and collagen mRNA expression and increased
proteolytic activity in a fibrosarcoma model. J Biol Chem.
277:407–415. 2002. View Article : Google Scholar
|
|
16
|
Li Y, Zhang J, Fang L, Luo P, Peng J and
Du X: Lefty A attenuates the TGF-beta1-induced epithelial to
mesenchymal transition of human renal proximal epithelial tubular
cells. Mol Cell Biochem. 339:263–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mariasegaram M, Tesch GH, Verhardt S,
Hurst L, Lan HY and Nikolic-Paterson DJ: Lefty antagonises
TGF-beta1 induced epithelial-mesenchymal transition in tubular
epithelial cells. Biochem Biophys Res Commun. 393:855–859. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sorensen I, Susnik N, Inhester T, Degen
JL, Melk A, Haller H and Schmitt R: Fibrinogen, acting as a mitogen
for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney
Int. 80:1035–1044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li L, Zepeda-Orozco D, Black R and Lin F:
Autophagy is a component of epithelial cell fate in obstructive
uropathy. Am J Pathol. 176:1767–1778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Tang M, Xu Y, Julian J, Carson D and
Tabibzadeh S: Lefty is expressed in mouse endometrium in estrous
cycle and peri-implantation period. Hum Reprod. 20:872–880. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tabibzadeh S and Hemmati-Brivanlou A:
Lefty at the crossroads of ʻstemness' and differentiative events.
Stem Cells. 24:1998–2006. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Besser D: Expression of nodal, lefty-a and
lefty-B in undifferentiated human embryonic stem cells requires
activation of Smad2/3. J Biol Chem. 279:45076–45084. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyajima A, Chen J, Lawrence C, Ledbetter
S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, et al:
Antibody to transforming growth factor-beta ameliorates tubular
apoptosis in unilateral ureteral obstruction. Kidney Int.
58:2301–2313. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cornet PB, Galant C, Eeckhout Y, Courtoy
PJ, Marbaix E and Henriet P: Regulation of matrix
metallopro-teinase-9/gelatinase B expression and activation by
ovarian steroids and LEFTY-A/endometrial bleeding-associated factor
in the human endometrium. J Clin Endocrinol Metab. 90:1001–1011.
2005. View Article : Google Scholar
|
|
26
|
Zhang YE: Non-Smad pathways in TGF-beta
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar :
|
|
27
|
Lan HY: Diverse roles of TGF-β/Smads in
renal fibrosis and inflammation. Int J Biol Sci. 7:1056–1067. 2011.
View Article : Google Scholar
|