Introduction
Parkinson's disease (PD), the second most common
chronic neurodegenerative disorder following Alzheimer's disease,
affects individuals >60 years old, and has a prevalence of
~5,000,000 individuals worldwide (1). PD is characterized primarily by the
progressive degeneration of dopaminergic (DA) neurons in the
substantia nigra pars compacta (2)
and the appearance of cytoplasmic Lewy body inclusions in the
surviving neurons (3–5). Due to a reduction in the
concentration of striatal dopamine, patients diagnosed with PD
eventually lead to the development of an array of motor disorders,
including bradykinesia, resting tremor, rigidity and postural
instability (6). Furthermore, PD
progressively affects multiple neuronal systems centrally and
peripherally, leading to numerous non-motor symptoms, including
olfactory deficits, anxiety and affective disorders, autonomic and
digestive dysfunction and, in particular, memory impairments
(7,8). Several risk factors have been
suggested to be associated with the etiology of the disease,
including age, genetics, oxidative stress, mitochondrial
dysfunction and environmental factors (9). However, increasing evidence has
demonstrated that abnormal autophagy is a fundamental processes
contributing to neuronal death in DA neurons, and the inhibition of
autophagy through pharmacological or genetic methods may be a
critical therapeutic strategy for PD.
NogoA is a myelin-associated protein, which is
important in inhibiting axonal fiber growth and in the regeneration
that occurs following injury to the mammalian central nervous
system (10). It is expressed at
high levels by oligodendrocytes and subpopulations of neurons
during early neuronal development, and is downregulated during
adulthood in the majority of regions, with the exclusion of the
hippocampus (11–13). Previous genetic studies have
reported that NogoA is involved in schizophrenia (14,15)
and synaptic plasticity (16,17),
and is a negative regulator of central nervous system angiogenesis
(18). However, NogoA knockout or
deficiency mice leads to the exhibition of a variety of behavioral
alterations, including decreased anxiety, behavioral inflexibility
and impairments in short-term memory (19,20).
These symptoms are similar to those in patients with PD, and these
results suggest that NogoA is key in the process of PD. However,
the physiological role of neuronal NogoA in patients with PD
remains to be elucidated. NogoA has an A-amino-acid-residue
extracellular domain and an axon growth inhibiting domain, which is
important in the inhibition of neurite outgrowth in the central
nervous system through binding to the NogoA receptor (NgR)
expressed on the neuron (21–23).
To further investigate the function of NogoA for PD,
PC12 cells were treated with 1-methyl-4-phenylpyridinium
(MPP+) as a model of PD in vitro, and NogoA was
found to be upregulated following MPP+ treatment. To
further investigate the effect and regulatory mechanisms of NogoA,
the PC12 cells were exposed to MPP+ following
pretreatment with NogoA small interfering (si)RNA or negative
control siRNA. The results demonstrated that NogoA knockdown
reduced MPP+-induced neurotoxicity and inhibited the
expression levels of mTOR and STAT3 in the PC12 cells. Furthermore,
NogoA overexpression had similar effects on the PC12 cells as
MPP+ treatment. Treatment with rapamycin, an inhibitor
of the mTOR/STAT3 signaling pathway, had similar effects in the
MPP+ treated PC12 cells as those observed following
NogoA siRNA transfection. The results suggested that NogoA may
regulate MPP+-induced neurotoxicity in PC12 cells via
the mTOR/STAT3 signaling pathway. Our results further demonstrated
that NogoA may be a potential target protein for PD treatment. In
addition, our results provided a novel explanation regarding the
mechanism of NogoA on the regulation of PD.
Materials and methods
Cell culture and PD model
PC12 rat pheochromocytoma cell line was obtained
from American Type Culture Collection (Manassas, VA, USA). The PC12
cells were cultured in a type I collagen-coated culture flask (BD
Biosciences, Franklin Lakes, NJ, USA) in RPMI-1640 medium
supplemented with 5% heat-inactivated fetal bovine serum, 10%
heat-inactivated horse serum (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 mg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). The
cells were maintained at 37°C in a humidified incubator supplied
with 5% CO2. To establish the PD model, 1 mM
MPP+ (Sigma-Aldrich, St. Louis, MO, USA) was added to
the medium for 24 h.
siRNA and NogoA overexpression
The siRNA sequence used for NogoA was
5′-GAGGCAGAUUAUGUUACAATT-3′ (Ribobio, Guangzhou, China). The
full-length of NogoA was cloned and inserted into the plasmid
expression vector, pcDNA3.1 (Promega, Madison, WI, USA). The
transfection of siRNAs and plasmids was performed using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol.
Treatment groups
The PC12 cells were divided into four groups, as
follows: i) negative control (NC) group, which was pretreated with
200 nmol/l NC siRNA for 24 h prior to exposure to 1 mM
MPP+ for 24 h; ii) siRNA group, which was pretreated
with 200 nmol/l NogoA siRNA for 24 h prior to exposure to 1 mM
MPP+ for 24 h; iii) overexpression group, which was
transfected with NogoA overexpression vector for 48 h; and iv)
rapamycin group, which was pretreated with mTOR inhibitor,
rapamycin (25 mmol/l; Sigma-Aldrich) for 24 h prior to exposure to
1 mM MPP+ for 24 h. The PC12 cells were seeded at
1×103/well in a 96-well plate for the cell proliferation
assay, 4×105/well in a 6-well plate for flow cytometric
analysis and western blotting. Then cells were treated according to
the experimental design. Cells were harvested for each assay.
Cell proliferation assay
Cell proliferation was monitored using a Cell
Counting Kit-8 (CCK8; Beyotime Institute of Biotechnology, Haimen,
China), according to the manufacturer's protocol. The PC12 cells
were seeded at 1×103 per well in a 96-well plate and
treated according to the experimental design. CCK8 reagent (10
µl) was added to each well and the plate was incubated for 4
h at 37°C. The absorbance was measured at a wavelength of 490 nm
using a Vmax Kinetic ELISA micro-plate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA). Each sample was assayed in
triplicate.
Cell apoptosis assay
The PC12 cells were harvested and washed twice with
phosphate-buffered saline (PBS; Hyclone, Logan, UT, USA). According
to the manufacturer's protocol, 5×105 cells were
collected and 500 µl binding buffer from a KGA107 kit
(Nanjing KeyGen Biotech Co., Ltd, Nanjing, China) was added to
suspend the cells. Annexin V-fluorescein isothiocyanate (5
µl) was added, followed by 5 µl of propidium iodide.
Following incubation for 10 min at room temperature in the dark,
the cells were analyzed immediately using a BD FACSCalibur™ (BD
Accuri C6; BD Biosciences).
Assessment of changes in mitochondrial
membrane potential (ΔΨm)
Changes in the ΔΨm were determined quantitatively
using flow cytometry, using Molecular Probes ™ JC-1 dye (200
nmol/l; Invitrogen; Thermo Fisher Scientfic, Inc.) according to the
manufacturer's protocol. PC12 cells (5×104) were seeded
in 6-well plates. Following treatment, the cells were collected,
washed twice with PBS, and resuspended in 500 ml JC-1 working
solution for 15–20 min. Cells were centrifuged at 1,000 rpm for 5
min at room temperature and the supernatant was removed. The cells
were resuspended in 500 µl 1X incubation buffer and changes
in fluorescence were detected by flow cytometry (Ex / Em =488
nm/530 nm. The JC-1 dye has an excitation of 480 nm and an emission
of 580/535 nm) and forms aggregates, which result in a red emission
in normal polarized mitochondria, whereas it forms monomers
emitting green fluorescence on the depolarized mitochondrial
membrane. The ΔΨm depolarization is an early characteristic of
apoptosis (24). The percentages
of cells with green fluorescence (JC-1 monomers), which indicate
depolarized cells, were measured.
Western blot analysis
The PC12 cells (2×106) were collected and
washed twice with ice-cold PBS. The cell pellets were suspended in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) for 40 min on ice and the lysates were centrifuged
at 12,000 × g at 4°C for 15 min. Total protein concentration was
determined using a BCA Protein Assay kit (Beyotime Institute of
Biotechnology). Equal quantities (30 µg) of protein in the
supernatant were resolved by 10% SDS polyacrylamide gel
electrophoresis and transferred onto a polyvinylidene fluoride
membrane (Pall Life Sciences, Port Washington, NY, USA). The
membranes were blocked for 1.5 h at 37°C with 5% non-fat milk,
prior to incubation with rabbit anti-rat NogoA polyclonal antibody
(1:1,000, cat. no. ab62024), rabbit anti-rat NgR polyclonal
antibody (1:1,000, cat. no. ab189792), mouse anti-rat mTOR
monoclonal antibody (1:200, cat. no. ab87540), mouse anti-rat STAT3
monoclonal antibody (1:5,000), or rabbit anti-rat polyclonal Beclin
1 antibody (1:100, cat. no. ab55878; all primary antibodies
purchased from Abcam, Cambridge, MA, USA). Following washing with
Tris-buffered saline with 0.5% Tween-20 (TBST), the membrane was
incubated with horseradish peroxidase-conjugated goat anti-rabbit
IgG (1:20,000, cat. no. BA1055) or goat anti-mouse IgG (1:25,000,
cat. no. BA1050; all secondary antibody purchased from BosterBio,
Wuhan, China) at 37°C for 40 min. Following further washing with
TBST, the membrane was assayed using enhanced chemiluminescence
(EMD Millipore, Billerica, MA, USA) and recorded on X-ray films.
The protein bands were quantified by densitometry using QuantityOne
software (Bio-Rad, Hercules, CA, USA), and the values were
expressed relative to GAPDH.
Statistical analysis
Statistical analyses were performed using the SPSS
19.0 software package (IBM SPSS, Armonk, NY, USA). All numerical
data were analyzed using Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
MPP+ treatment increases the
expression of NogoA and activates the mTOR/STAT3 signaling
pathway
To investigate the role of NogoA in the
MPP+-induced PD model, PC12 cells were treated with 1 mM
MPP+ for 24 h. The protein expression levels of NogoA
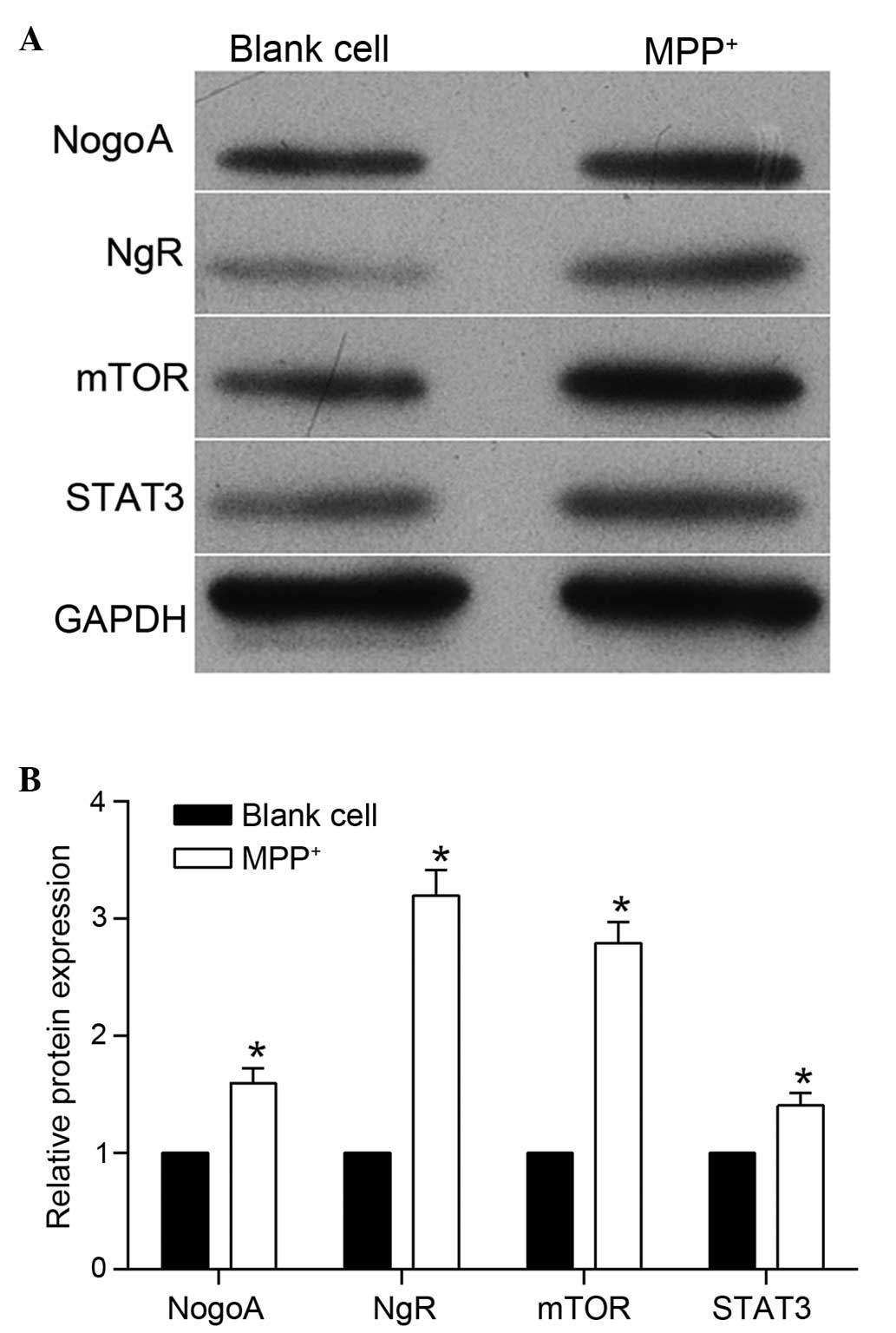
were detected and, as shown in Fig.
1, the protein expression level of NogoA was markedly
upregulated following MPP+ treatment. The expression
levels of NgR were also detected, and western blotting revealed
that the protein expression level of NgR was also increased
following MPP+ treatment. These results indicated that
NogoA may be involved in MPP+-induced neurotoxicity in
PC12 cells. To further investigate the possible regulatory
mechanism of NogoA in MPP+-induced PD model, the
expression levels of mTOR and STAT3, which are key proteins in of
the mTOR/STAT3 signaling pathway, were also detected. As shown in
Fig. 1, the protein expression
levels of mTOR and STAT3 were markedly upregulated following
MPP+ treatment. This result indicated that the
mTOR/STAT3 signaling pathway may be involved in
MPP+-induced neurotoxicity in PC12 cells.
Knockdown of NogoA inhibits the decrease
in cell viability induced by MPP+ in PC12 cells
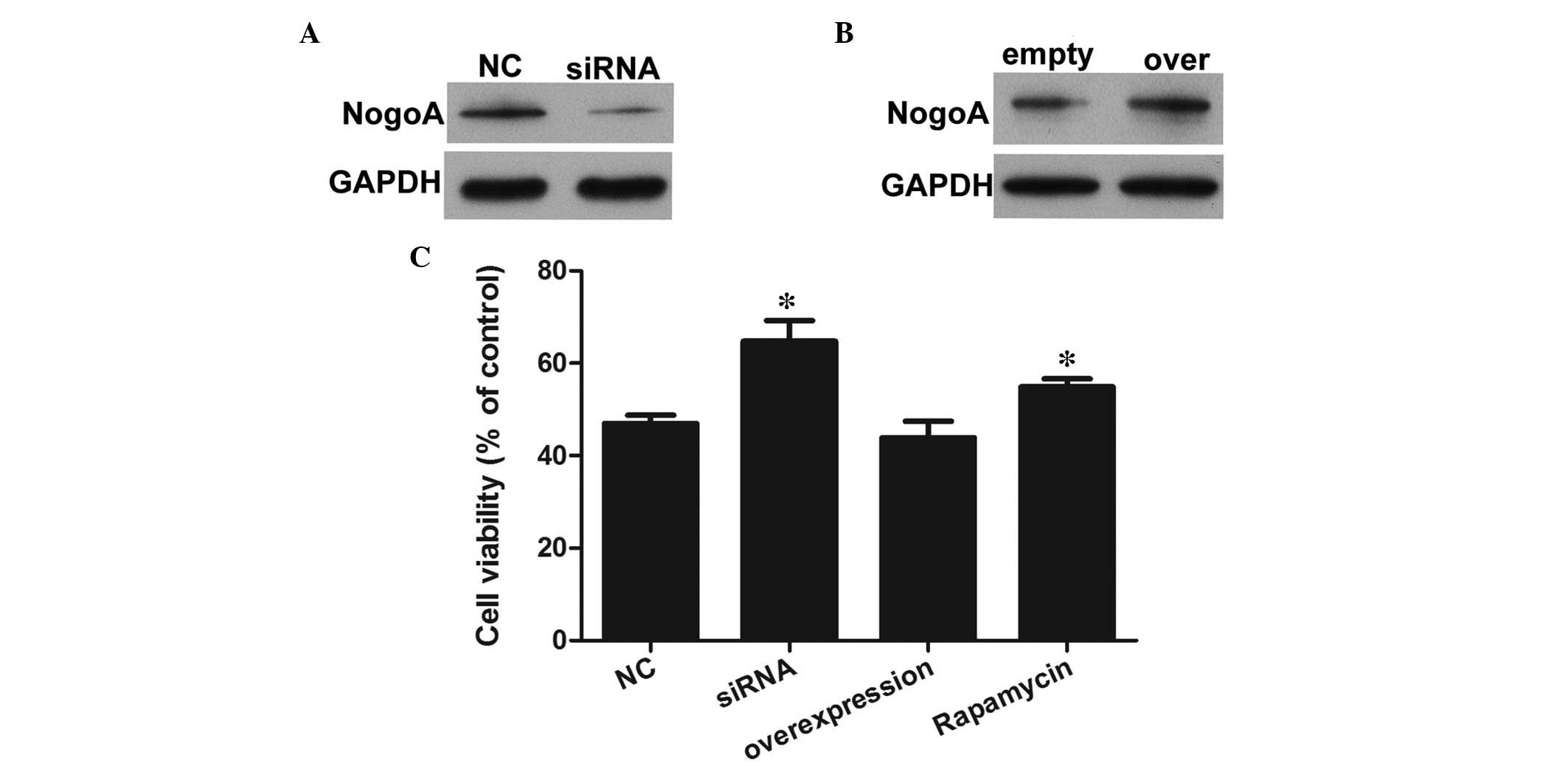
To evaluate the role of NogoA, NogoA knockdown and
overexpression models were established via transfection with NogoA
siRNA or a NogoA overexpression vectors. As shown in Fig. 2A and B, the expression of NogoA was
successfully decreased and overexpressed in the knockdown and
overexpression models, respectively. A CCK8 assay was performed to
evaluate the effects of NogoA against the MPP+-induced
significant decrease in cell viability (Fig. 2C). The results indicated that 1 mM
MPP+ significantly decreased the viability of the PC12
cells in the NC group. However, the viabilities of the PC12 cells
pretreated with NogoA siRNA for 24 h were markedly increased,
compared with the NC group. The cell viability in the NogoA
overexpression group was similar to that in the NC group, whereas
the cell viability in the rapamycin treated group was markedly
increased, compared with the NC group. Therefore, pretreatment with
either the NogoA siRNA or mTOR/STAT3 signaling pathway inhibitor
had neuroprotective effects against MPP+-induced
cytotoxicity.
Knockdown of NogoA inhibits the decrease
in cell autophagy induced by MPP+ in PC12 cells
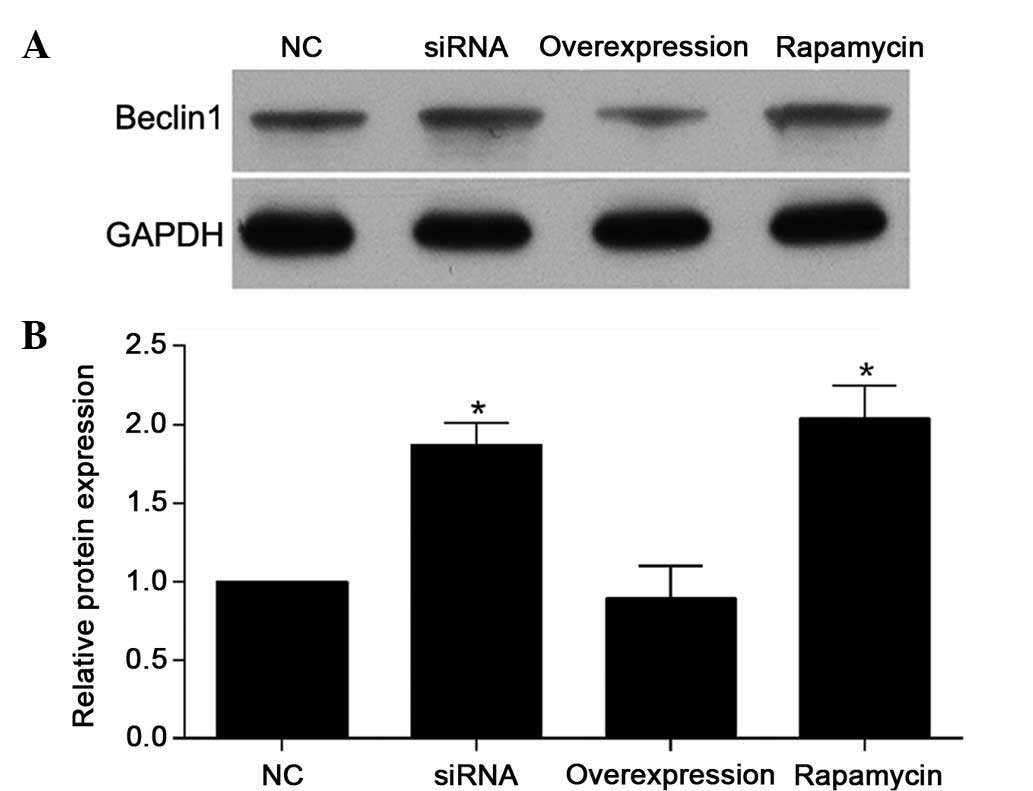
To examine the effect of NogoA and rapamycin
treatment on MPP+-induced autophagy, the expression
level of Beclin-1 was also detected (Fig. 4). The results demonstrated that the
expression levels of Beclin-1 were increased in the NogoA siRNA
group and rapamycin group, compared with the NC group. In addition,
the effect of NogoA overexpression on the expression of Beclin-1
was similar to the effect observed in the NC group, which was
pretreated with 200 nmol/l NC siRNA for 24 h prior to exposure to 1
mM MPP+ for 24 h.
NogoA silencing and rapamycin treatment
decrease the expression levels of NgR, mTOR and STAT3
To investigate the regulatory mechanism of NogoA on
PC12 cells treated with MPP+, the protein expression
levels of NgR, mTOR and STAT3 were detected using western blotting.
As shown in Fig. 5, the expression
levels of NgR, mTOR and STAT3 were decreased in the NogoA siRNA and
rapamycin groups, compared with the NC group. In addition, the
effect of NogoA overexpression was similar to the effect observed
in the NC group, which was pretreated with 200 nmol/l NC siRNA for
24 h prior to exposure to 1 mM MPP+ for 24 h.
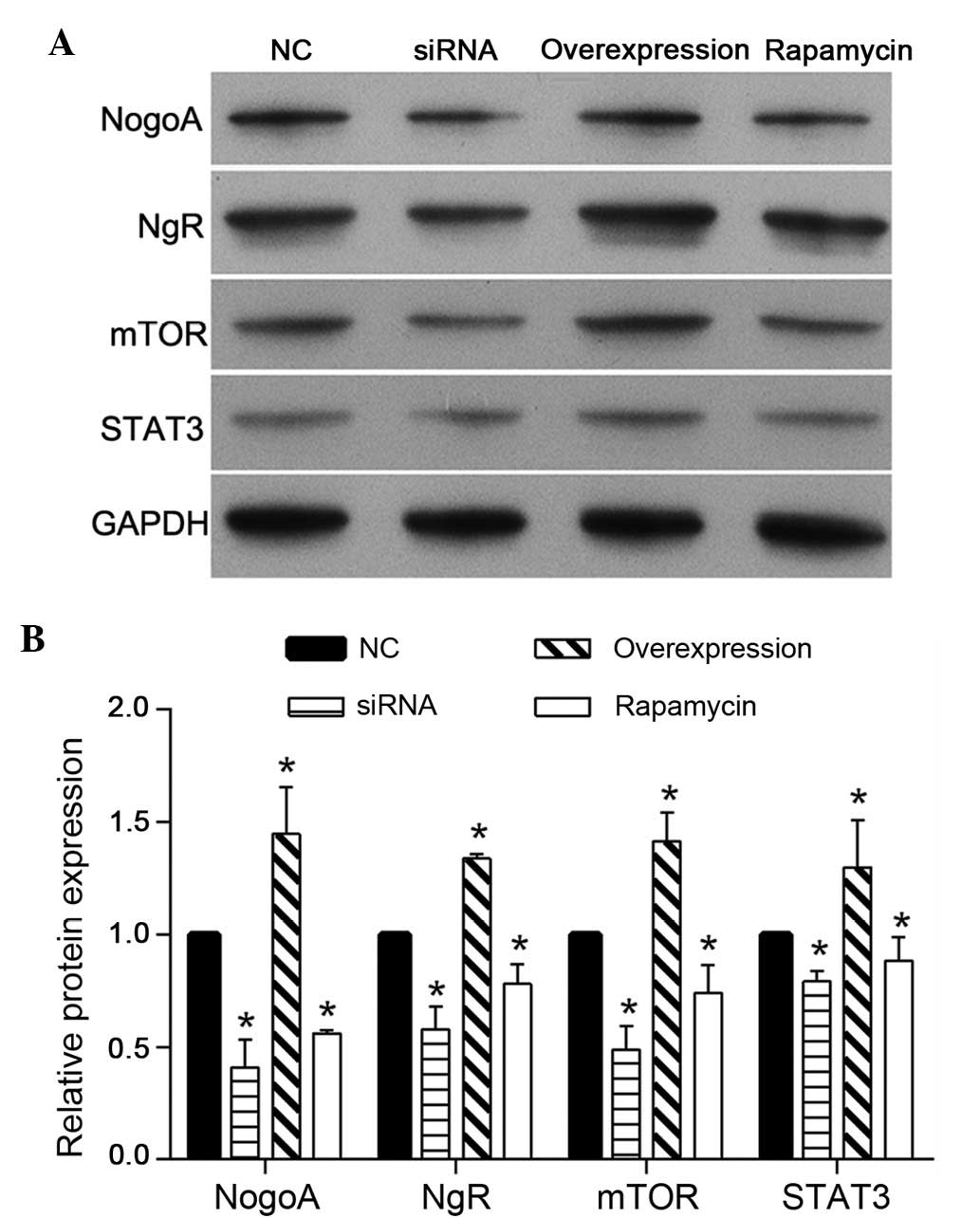 | Figure 5Protein expression levels of NogoA,
NgR, mTOR and STAT3 in each group of PC12 cells. (A) Representative
western blotting of NogoA, NgR, mTOR and STAT3 protein expression.
(B) Quantification of the expression levels of NogoA, NgR, mTOR and
STAT3 in each group, presented as the fold-increase. The NC group
was pretreated with 200 nmol/l NC siRNA for 24 h, prior to exposure
to 1 mM MPP+ for 24 h. The siRNA group was pretreated
with 200 nmol/l NogoA siRNA for 24 h, prior to exposure to 1 mM
MPP+ for 24 h. The overexpression group was transfected
with the NogoA overexpression vector for 48 h and the rapamycin
group was pretreated with rapamycin for 24 h, prior to exposure to
1 mM MPP+ for 24 h. *P<0.05, vs. NC group.
NC, negative control; siRNA, small interfering RNA;
MPP+, 1-methyl-4-phenylpyridinium. |
Discussion
In the present study, NogoA was found to be
upregulated by MPP+ treatment, which suggested that
NogoA may be involved in MPP+-induced neurotoxicity in
PC12 cells. The present study subsequently investigated the effect
of NogoA knockdown on MPP+-induced cell proliferation,
autophagy and apoptosis in the PC12 cells. The results demonstrated
that NogoA knockdown inhibited MPP+-induced apoptosis,
and decreases cell viability and autophagy. These results suggested
that a reduction in NogoA levels may prevent
MPP+-induced neurotoxicity in PC12 cells.
NogoA, particularly neuronal NogoA, regulates the
modulation of neuronal and synaptic plasticity, and adult
neurogenesis in the adult brain (25–27).
A previous study suggested that the Nogo signaling pathway is
important in neuropsychiatric diseases of neurodevelopmental
origin, notably schizophrenia and bipolar disorder (28). Based on these results, NogoA
appears to be an important regulator in the nervous system. PC12
cells treated with MPP+ have been frequently used as a
PD model to investigate the molecular mechanisms of PD in
vitro (29,30). The results of the present study
indicated that the knockdown of NogoA prevented
MPP+-induced neurotoxicity in the PC12 cells, and it was
hypothesized that NogoA may be involved in regulating the
development of PD and may assist in further elucidating the role of
NogoA in regulating PD.
mTOR is a serine-threonine protein kinase, which
regulates multiple intracellular processes in response to
extracellular signals, nutrient availability, the energy status of
the cell and stress (31). In the
nervous system, mTOR regulates the differentiation, survival and
development of neurons (31). The
mTOR-dependent signaling pathway is regulated by various types of
protein in the neuronal cell membrane, however, the regulatory
protein in patients with PD remains to be elucidated. In the
present study, MPP+ treatment was found to activate the
mTOR/STAT3 signaling pathway, and that this activation was
inhibited following a decrease in the expression of NogoA.
Furthermore, the effect of NogoA siRNA on the mTOR/STAT3 signaling
pathway was similar to that of rapamycin. Therefore, it was
hypothesized that NogoA is a regulatory protein of the mTOR/STAT3
signaling pathway on the cell membrane in PC12 cells treated by
MPP+.
The mTOR signaling pathway also regulates the
cellular processes of autophagy and apoptosis (32,33).
Autophagy and apoptosis are two important cellular processes with
complex and associated protein networks (34). In the PC12 cells, the level of
autophagy, indicated by the protein expression of Beclin-1,
increased following NogoA siRNA or rapamycin treatment, whereas the
rate of apoptosis decreased. These results indicated that NogoA
hass dual functions in regulating autophagy and apoptosis via the
mTOR/STAT3 signaling pathway.
In conclusion, the present study demonstrated that
NogoA was upregulated by MPP+ treatment, and that NogoA
knockdown inhibited the MPP+-induced apoptosis, and
decrease in cell viability and autophagy. In addition, the
mTOR/STAT3 signaling pathway was involved in the regulation of
NogoA on MPP+-induced PC12 cells. Therefore, NogoA may
regulate MPP+-induced neurotoxicity in PC12 cells via
the mTOR/STAT3 signaling pathway. The present results further
demonstrated that NogoA may be a potential target protein for PD
treatment. In addition, these results provided a novel mechanism
regarding NogoA on regulating the process of PD.
Acknowledgments
The present study was supported by the Natural
Science Foundation of Guangdong Province (grant no.
S2012010010242).
References
|
1
|
de Lau LM, Koudstaal PJ, Hofman A and
Breteler MM: Subjective complaints precede Parkinson disease: The
rotterdam study. Arch Neurol. 63:362–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schapira AH and Olanow CW: Neuroprotection
in Parkinson disease: Mysteries, myths, and misconceptions. JAMA.
291:358–364. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirsch E, Graybiel AM and Agid YA:
Melanized dopaminergic neurons are differentially susceptible to
degeneration in Parkinson's disease. Nature. 334:345–348. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jellinger KA: The pathology of Parkinson's
disease. Adv Neurol. 86:55–72. 2001.PubMed/NCBI
|
|
5
|
Braak H, Del Tredici K, Rüb U, de Vos RA,
Jansen Steur EN and Braak E: Staging of brain pathology related to
sporadic Parkinson's disease. Neurobiol Aging. 24:197–211. 2003.
View Article : Google Scholar
|
|
6
|
Fahn S and Sulzer D: Neurodegeneration and
neuroprotection in Parkinson disease. NeuroRx. 1:139–154. 2004.
View Article : Google Scholar
|
|
7
|
Braak H, Ghebremedhin E, Rüb U, Bratzke H
and Del Tredici K: Stages in the development of Parkinson's
disease-related pathology. Cell Tissue Res. 318:121–134. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chaudhuri KR and Odin P: The challenge of
non-motor symptoms in Parkinson's disease. Prog Brain Res.
184:325–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou RR, Chen JZ, Chen H, Kang XG, Li MG
and Wang BR: Neuroprotective effects of
(−)-epigallocatechin-3-gallate (EGCG) on paraquat-induced apoptosis
in PC12 cells. Cell Biol Int. 32:22–30. 2008. View Article : Google Scholar
|
|
10
|
Schwab ME: Functions of Nogo proteins and
their receptors in the nervous system. Nat Rev Neurosci.
11:799–811. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zagrebelsky M, Schweigreiter R, Bandtlow
CE, Schwab ME and Korte M: Nogo-A stabilizes the architecture of
hippocampal neurons. J Neurosci. 30:13220–13234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kempf A and Schwab ME: Nogo-A represses
anatomical and synaptic plasticity in the central nervous system.
Physiology (Bethesda). 28:151–163. 2013. View Article : Google Scholar
|
|
13
|
Mironova YA and Giger RJ: Where no
synapses go: Gatekeepers of circuit remodeling and synaptic
strength. Trends Neurosci. 36:363–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jitoku D, Hattori E, Iwayama Y, Yamada K,
Toyota T, Kikuchi M, Maekawa M, Nishikawa T and Yoshikawa T:
Association study of Nogo-related genes with schizophrenia in a
Japanese case-control sample. Am J Med Genet B Neuropsychiatr
Genet. 156B:581–592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krištofiková Z, Vrajová M, Sirová J, Valeš
K, Petrásek T, Schönig K, Tews B, Schwab M, Bartsch D, Stuchlík A
and Rípová D: N-Methyl-d-Aspartate receptor - Nitric oxide synthase
pathway in the cortex of Nogo-A-Deficient rats in relation to brain
laterality and schizophrenia. Front Behav Neurosci. 7:902013.
View Article : Google Scholar
|
|
16
|
Delekate A, Zagrebelsky M, Kramer S,
Schwab ME and Korte M: NogoA restricts synaptic plasticity in the
adult hippocampus on a fast time scale. Proc Natl Acad Sci USA.
108:2569–2574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zemmar A, Weinmann O, Kellner Y, Yu X,
Vicente R, Gullo M, Kasper H, Lussi K, Ristic Z, Luft AR, et al:
Neutralization of Nogo-A enhances synaptic plasticity in the rodent
motor cortex and improves motor learning in vivo. J Neurosci.
34:8685–8698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wälchli T, Pernet V, Weinmann O, Shiu JY,
Guzik-Kornacka A, Decrey G, Yüksel D, Schneider H, Vogel J, Ingber
DE, et al: Nogo-A is a negative regulator of CNS angiogenesis. Proc
Natl Acad Sci USA. 110:E1943–E1952. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Willi R, Weinmann O, Winter C, Klein J,
Sohr R, Schnell L, Yee BK, Feldon J and Schwab ME: Constitutive
genetic deletion of the growth regulator Nogo-A induces
schizophrenia-related endophenotypes. J Neurosci. 30:556–567. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tews B, Schönig K, Arzt ME, Clementi S,
Rioult-Pedotti MS, Zemmar A, Berger SM, Schneider M, Enkel T,
Weinmann O, et al: Synthetic microRNA-mediated downregulation of
Nogo-A in transgenic rats reveals its role as regulator of synaptic
plasticity and cognitive function. Proc Natl Acad Sci USA.
110:6583–6588. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
GrandPré T, Li S and Strittmatter SM:
Nogo-66 receptor antagonist peptide promotes axonal regeneration.
Nature. 417:547–551. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Shen J, Xiong N, Zhao H and Chen
Y: Protein kinase B is involved in Nogo-66 inhibiting neurite
outgrowth in PC12 cells. Neuroreport. 22:733–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan J, Zhou X, Guo JJ, Mao L, Wang YJ, Sun
J, Sun LX, Zhang LY, Zhou XF and Liao H: Nogo-66 inhibits adhesion
and migration of microglia via GTPase Rho pathway in vitro. J
Neurochem. 120:721–731. 2012. View Article : Google Scholar
|
|
24
|
Cossarizza A, Baccarani-Contri M,
Kalashnikova G and Franceschi C: A new method for the
cytofluorimetric analysis of mitochondrial membrane potential using
the J-aggregate forming lipophilic cation
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolcarbocyanine
iodide (JC-1). Biochem Biophys Res Commun. 197:40–45. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Akbik F, Cafferty WB and Strittmatter SM:
Myelin associated inhibitors: A link between injury-induced and
experience-dependent plasticity. Exp Neurol. 235:43–52. 2012.
View Article : Google Scholar
|
|
26
|
Pernet V and Schwab ME: The role of Nogo-A
in axonal plasticity, regrowth and repair. Cell Tissue Res.
349:97–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rolando C, Parolisi R, Boda E, Schwab ME,
Rossi F and Buffo A: Distinct roles of Nogo-a and Nogo receptor 1
in the homeostatic regulation of adult neural stem cell function
and neuroblast migration. J Neurosci. 32:17788–17799. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Willi R and Schwab ME: Nogo and Nogo
receptor: Relevance to schizophrenia? Neurobiol Dis. 54:150–157.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deng Q and Yang X: Protective effects of
Gynostemma pentaphyllum polysaccharides on PC12 cells impaired by
MPP(+). Int J Biol Macromol. 69:171–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng B, Guo Y, Li C, Ji B, Pan Y, Chen J
and Bai B: Edaravone protected PC12 cells against MPP(+)-cytoxicity
via inhibiting oxidative stress and up-regulating heme oxygenase-1
expression. J Neurol Sci. 343:115–119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Swiech L, Perycz M, Malik A and Jaworski
J: Role of mTOR in physiology and pathology of the nervous system.
Biochim Biophys Acta. 1784:116–132. 2008. View Article : Google Scholar
|
|
32
|
Jiang LB, Cao L, Yin XF, Yasen M, Yishake
M, Dong J and Li XL: Activation of autophagy via Ca(2+)
dependent AMPK/mTOR pathway in rat notochordal cells is a cellular
adaptation under hyperosmotic stress. Cell Cycle. 14:867–879. 2015.
View Article : Google Scholar
|
|
33
|
Kumar S, Guru SK, Pathania AS, Manda S,
Kumar A, Bharate SB, Vishwakarma RA, Malik F and Bhushan S:
Fascaplysin induces caspase mediated crosstalk between apoptosis
and autophagy through the inhibition of PI3K/AKT/mTOR signaling
cascade in human leukemia HL-60 cells. J Cell Biochem. 116:985–997.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mukhopadhyay S, Panda PK, Sinha N, Das DN
and Bhutia SK: Autophagy and apoptosis: Where do they meet?
Apoptosis. 19:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|