Introduction
Kidney cancer accounts for ~3% of all novel cancer
diagnoses worldwide. It is the urologic malignancy with the lowest
rate of survival and has an estimated 5-year survival rate of
50–60% (1). Nearly 30% of patients
with renal cell carcinoma (RCC) develop distant metastasis at
initial presentation and up to 30% of patients with RCC who
received traditional surgery experienced recurrence during
subsequent follow-up (2). Clear
cell RCC (ccRCC) is the most common (80–90%) type of kidney cancer
(3). However, the numerous tumor
suppressor genes and oncogenes that are mutated and result in ccRCC
remain to be elucidated (4).
Global studies of copy number, gene sequencing, gene expression and
miRNA expression in primary RCC may aid in identifying these genes
(5).
MicroRNAs (miRNAs) are a class of noncoding RNAs,
typically 20–23 nucleotides in length. They have been confirmed to
be one of the most abundant groups of regulatory genes in
multicellular organisms, and are important in a number of
fundamental cellular processes (6). Numerous studies have used
high-throughput microarrays to identify cancer-specific miRNA
fingerprints in certain types of cancer (7–9),
including ccRCC (10). These
studies indicate that alterations in miRNAs are critical in the
carcinogenesis of numerous, and perhaps all types of human cancer
(11). Certain miRNAs may be
directly involved in cancer development by controlling cell
differentiation and apoptosis, while others may be involved by
targeting cancer oncogenes and/or tumor suppressor genes (12). Understanding of the function of
miRNAs is providing novel insights into the molecular basis of
cancer, and biomarkers for cancer diagnoses and therapy (13).
As a member of the miR-200 family, miR-429 has been
confirmed to be dysregulated in various types of human cancer,
including colorectal cancer (14),
gastric cancer (15) and
esophageal carcinoma (16). In
addition, aberrant expression of miR-429 can serve as a biomarker
for the early detection and prognosis of colorectal cancer
(14), non-small cell lung cancer
(17) and serous ovarian carcinoma
(18). However the role of miR-429
in ccRCC remains to be identified. Thus, the present study aimed to
determine the function and possible molecular mechanisms of miR-429
in ccRCC.
Materials and methods
ccRCC clinical specimens and cancer cell
lines
A total of 40 paired ccRCC specimens and adjacent
normal tissues were obtained from Peking University Shenzhen
Hospital (Shenzen, China) and Anhui Medical University First
Affiliated Hospital (Hebei, China). The samples were collected
during nephrectomy between August 2011 and July 2013, and written
informed consent was obtained from the patients. Collection was in
accordance with the IRB-approved protocol for human specimen
collection, and for the use of these materials and associated
clinical information for research purposes (19). All samples were processed and
stored at −80°C in RNAlater (Qiagen, Valencia, CA, USA) until RNA
isolation. The clinical and pathological information of all the
patients is summarized in Table I.
The present study was reviewed and approved by the Ethics Committee
of Peking University Shenzhen Hospital.
 | Table IClinical and pathological features of
40 patients. |
Table I
Clinical and pathological features of
40 patients.
| Variable | Number of cases |
|---|
| Total | 40 |
| Age (years) |
| ≥52 | 23 |
| <52 | 17 |
| Gender |
| Male | 25 |
| Female | 15 |
| PT-stage |
| T1 | 21 |
| T2 | 17 |
| T3 and T4 | 2 |
| AJCC clinical
stage |
| I | 21 |
| II | 16 |
| III+IV | 3 |
ACHN and 786-O human RCC cell lines, and the HeLa
cervical cancer cell line were used in the present study. HeLa
cells were used in the present study, as they are easily
transfected and often used for luciferase reporter assay The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum and incubated at 37°C in 5% carbon dioxide.
Total RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA of human specimens and cells, including
miRNA was extracted by TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
instructions. To quantify the expression of miR-429, cDNA templates
obtained by miScript Reverse Transcription (Qiagen, Hilden,
Germany) was used for RT-qPCR with U6 serving as an internal
control. While gene expression was quantified using cDNA templates
obtained by the Revert Aid First Strand cDNA Synthesis kit (MBI
Fermentas, Burlington, ON, Canada). SYBR Premix Ex Taq II (Tli
RNaseH Plus; Takara Biotechnology, Inc., Otsu, Japan) was used with
human GAPDH serving as an endogenous control. All primers used in
the present study are presented in Table II and qPCR was performed in the
LightCycler 480 Real-Time PCR system (Roche, Basel, Switzerland),
according to the manufacturer's instructions. PCR amplification was
performed using 1 µl cDNA in a 20 µl reaction system,
containing 10 µl QuantiTect SYBR Green PCR Master mix
(Qiagen, Valencia, CA, USA), 2 µl miScript Universal Primer
(Qiagen), 0.5 µl specific microRNA primer (Invitrogen;
Thermo Fisher Scientific, Inc.) and 6.5 µl RNase-free water.
PCR amplification conditions were set as: 95°C for 2 min, 40 cycles
of 95°C for 15 sec, 58°C for 30 sec and 72°C for 30 sec.
 | Table IIPrimers for reverse
transcription-quantitative polymerase chain reaction. |
Table II
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| miR-429 |
TAATACTGTCTGGTAAAACCGT | Provided by the
miScript SYBR Green PCR kit |
| U6 |
CTCGCTTCGGCAGCACA |
ACGCTTCACGAATTTGCGT |
| VEGF |
CACCATTGAAACCACTAGTT |
GAGAGATTTAGTATGTAGAATTCTC |
| c-MYC |
GGAAAAGTAAGGAAAACGATTC |
AAGATTTGGCTCAATGATATATTTG |
| GAPDH |
AGAAGGCTGGGGCTCATTTG |
AGGGGCCATCCACAGTCTTC |
Cell transfection
For upregulation of miR-429, the cancer cell lines
were transfected with Lipofectamine RNAiMAX transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and Opti-MEM
(Invitrogen; Thermo Fisher Scientific, Inc.) with synthetic miR-429
mimics (Shanghai GenePharma Co., Ltd., Shanghai, China). Cells were
seeded in 6-well plates for apoptosis assays and RNA isolation
(30×104 cells per well), in 12-well plates for wound
scratch assays (25×104 cells per well), in 24-well
plates for luciferase reporter assays (10×104 cells per
well), and in 96-well plates for cell proliferation assays (~5,000
cells per well). A normal control (NC), which simulated the
structure of the miR mimics, exerted no effect on cells following
transfection. The experiment was repeated a minimum of three times
and the expression ratio of miR-429 transfected with mimics versus
miR-429 transfected with NC was calculated.
Cell proliferation and migration
assay
To determine the effect of miR-429 on cell
proliferation, a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich) was used according to the manufacturer's
protocols. At 0, 24, 48 or 72 h after transfection for 6 h, the
cancer cells were incubated with 20 µl MTT (5 µg/ml)
for 4 h, followed by the addition of 150 µl dimethyl
sulfoxide (Sigma-Aldrich) and agitating for ~15 min at room
temperature. The optical density (OD) was determined using a
microplate reader (model 680; Bio-Rad, Hercules, CA, USA) at a dual
wavelength of 490/630 nm.
Wound scratch assay
Cells were seeded in 12-well plates and a scratch
was made with a P-20 micropipette tip. The initial length (0 h) and
the residual gap length 24 h after the scratch were calculated from
photomicrographs using MIAS-2000 software (Leica Microsystems GmbH,
Wetzlar, Germany). All experiments were performed in triplicate and
repeated at least three times.
Apoptosis assay
Flow cytometry (Beckman Coulter, Miami, FL, USA) was
performed to evaluate the apoptosis rate of cancer cells following
transfection. Cancer cells including floating cells were harvested
48 h after transfection, washed twice with cold phosphate-buffered
saline (PBS) and resus-pended in 100 µl 1X binding buffer
(Invitrogen; Thermo Fisher Scientific, Inc.), followed by the
addition of 5 µl of Annexin V-fluorescein isothiocyanate
(Invitrogen; Thermo Fisher Scientific, Inc.) and 5 µl
propidium iodide (Invitrogen; Thermo Fisher Scientific, Inc.). The
fluorescence of stained cells was then analyzed by flow cytometry
(Beckman Coulter, Brea, CA, USA) using 488 nm excitation within 30
min after staining, according to the manufacturer's
instructions.
Bioinformatics
The potential targets of miR-429 were predicted by
combining results from TargetScan (http://www.targetscan.org/), PicTar (http://pictar.mdc-berlin.de/), miRanda (http://www.targetscan.org/) and miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/).
Putative genes predicted by all the four algorithms were accepted
and candidates were selected based on the gene function.
Plasmid construction and luciferase
reporter assay
The miRNA target sequences were inserted between the
XhoI-NotI restriction sites in the 3′-untranslated
region (UTR) of the target gene in the psiCHECK-2 vector (Promega
Corporation, Madison, WI, USA), generating the wide type
psiCHECK2-3′UTR (Wt). The mutant type (Mt) was generated by
changing the putative binding site to 5′-AATACTG-3′ in the
complementary site for the seed region of miR-429. All constructed
plasmids were sequence-verified by DNA sequencing analysis.
HeLa cells were transfected with 200 ng vector, 40
pmol miRNA and 2.5 µl Lipofectamine (Invitrogen; Thermo
Fisher Scientific, Inc.) in 100 µl opti-MEM (Invitrogen;
Thermo Fisher Scientific, Inc.) in 24-well plates. Luciferase
assays were performed using a luciferase assay kit (Promega
Corporation) according to the manufacturer's protocol. The
activities of firefly and Renilla luciferase in the cell
lysates were determined with a dual-luciferase assay system.
Statistical analysis
All statistical analysis was conducted with SPSS
17.0 statistical software package (SPSS Inc., Chicago, IL, USA).
The difference in expression of miR-429 in ccRCC and paired normal
samples was analyzed by a paired t-test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Downregulation of miR-429 in ccRCC
quantified by RT-qPCR
miR-429 has been confirmed to be dysregulated in
numerous types of cancer (14–16);
however, its expression in RCC remains unclear. In the present
study, RT-qPCR was used to quantify the expression of miR-429 in 40
paired ccRCC specimens and normal specimens. The results showed
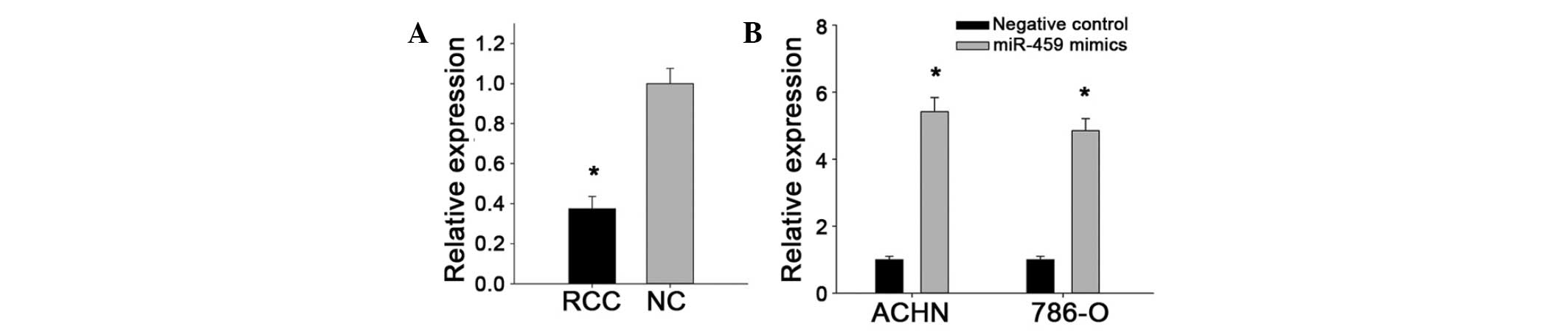
that miR-429 was downregulated in 33/40 ccRCC specimens, with an
average of 0.3737-fold reduction in expression (Fig. 1A). The decreased expression of
miR-429 was concordant with the results of a recent miRNA
expression profile study of ccRCC (10).
To analyze the effect of miR-429 on renal cancer
cells, synthetic miR-429 mimics were transfected into ACHN and
786-O cancer cell lines for the gain-of-function experiments. The
fold change of miR-429 expression in ACHN and 786-O cells after
transfection were 5.1627 and 4.8768 quantified by RT-qPCR,
respectively, as presented in Fig.
1B
Restoration of miR-429 inhibits cell
proliferation and migration
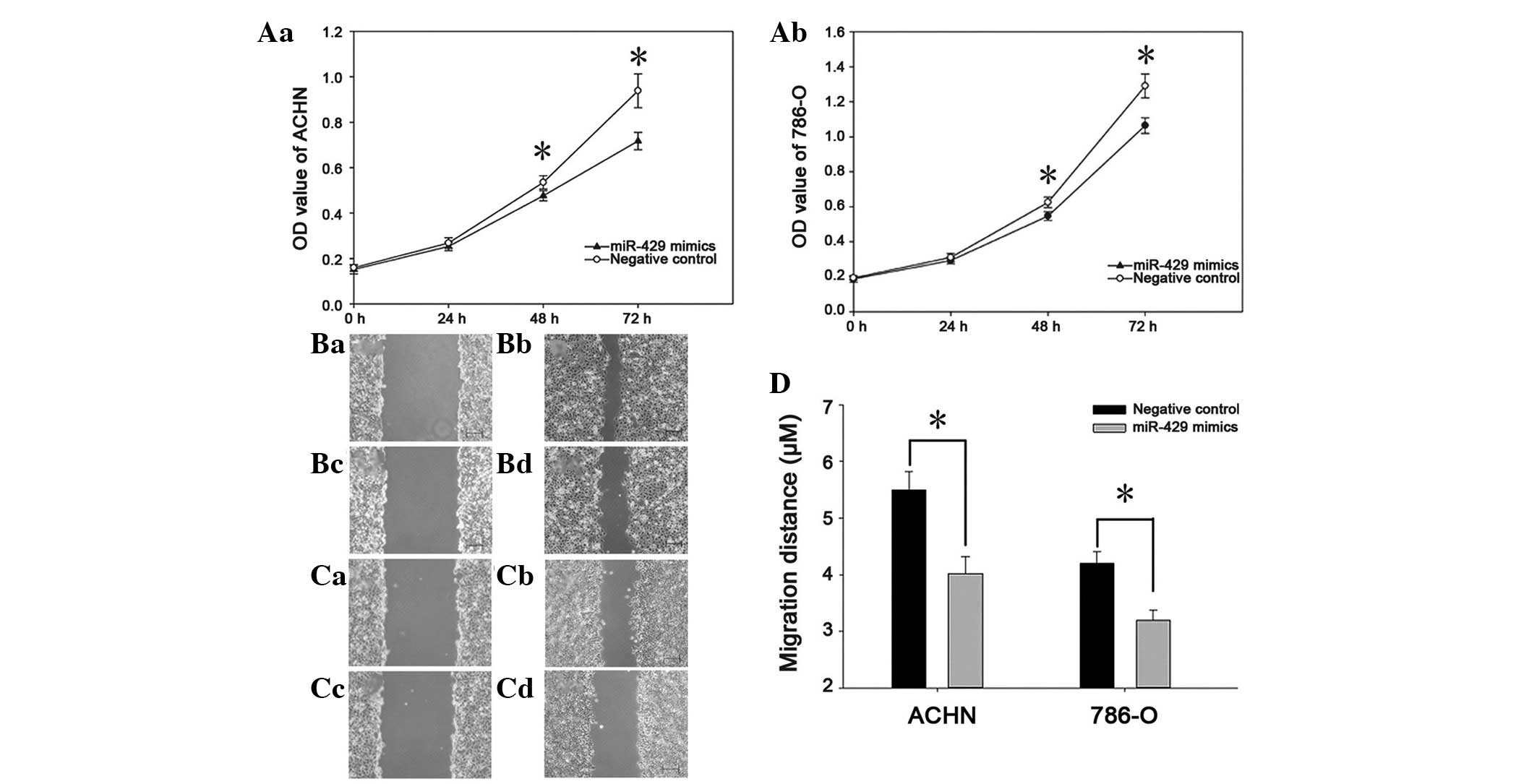
The impact of miR-429 on cell proliferation was
determined by an MTT assay, the OD value of the two groups (miR-429
mimics and negative control) was measured 0, 24, 48 and 72 h after
transfection. The results showed that the proliferation of ACHN
cells decreased by 5.76, 10.48 and 20.86% (P<0.05), while the
proliferation of 786-O cells decreased by 6.02, 11.65 and 26.12%
(P<0.05; Fig. 2A). Wound
scratch assays were used to evaluate the migration ability of
cancer cells. As presented in Fig.
2, the wound width in the group transfected with miR-429 mimics
was greater than that of the negative control group (P<0.05).
These results indicate that miR-429 can restrain the proliferation
and migration of renal cancer cells.
miR-429 mimics induce cell apoptosis
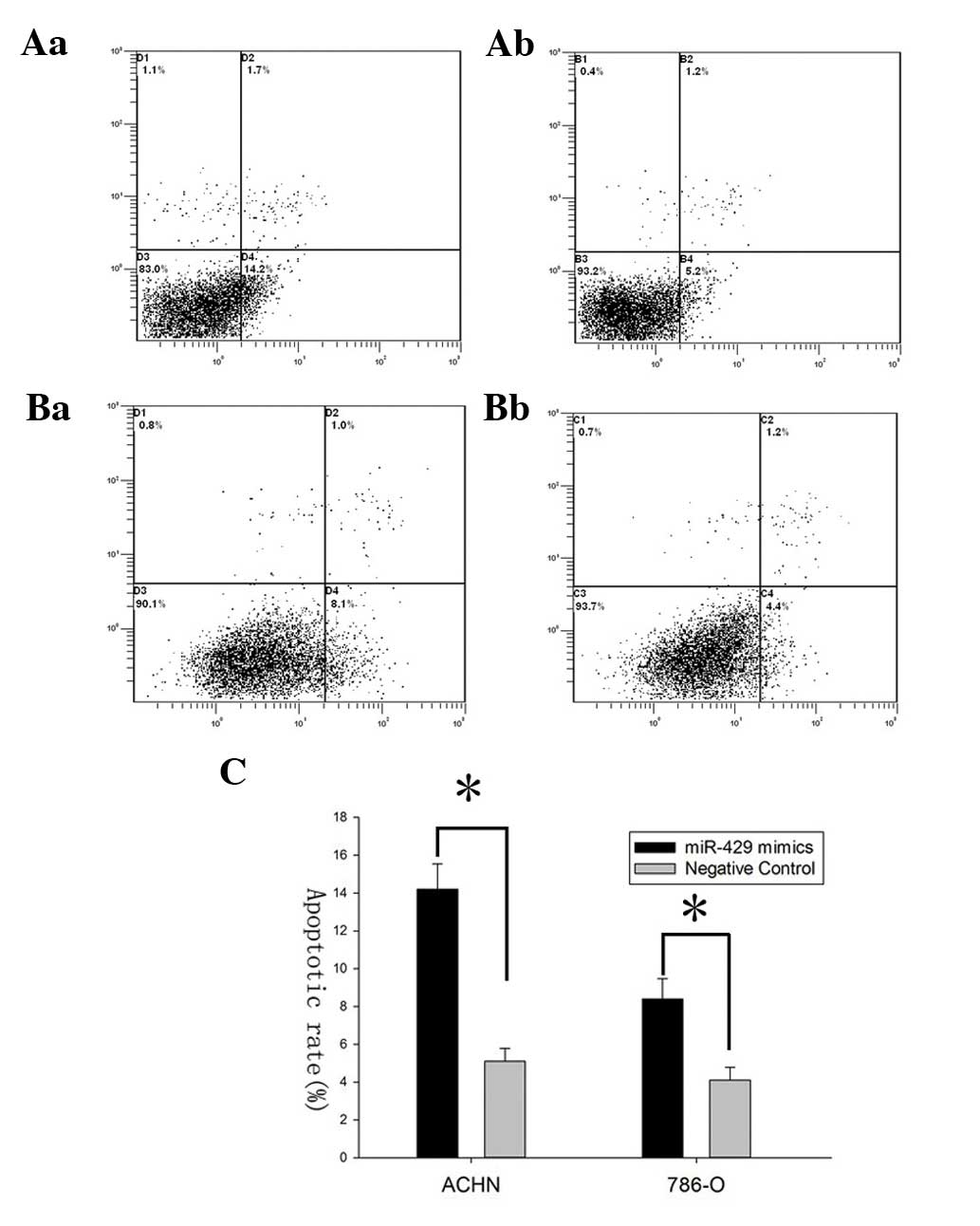
To demonstrate the effect of miR-429 on cell
apoptosis, flow cytometry was performed to detect the apoptosis
rates of ACHN and 786-O cells after transfection. The results
revealed that apoptosis rates of ACHN cells transfected with
miR-429 mimics and those in the negative control group were 14.2
vs. 5.2% while the apoptosis rates of 786-O cells were 8.1 vs. 4.4%
(*P<0.05), suggesting that miR-429 could induce the
apoptosis of renal cancer cells (Fig.
3).
miR-429 targets vascular endothelial
growth factor (VEGF) in ccRCC
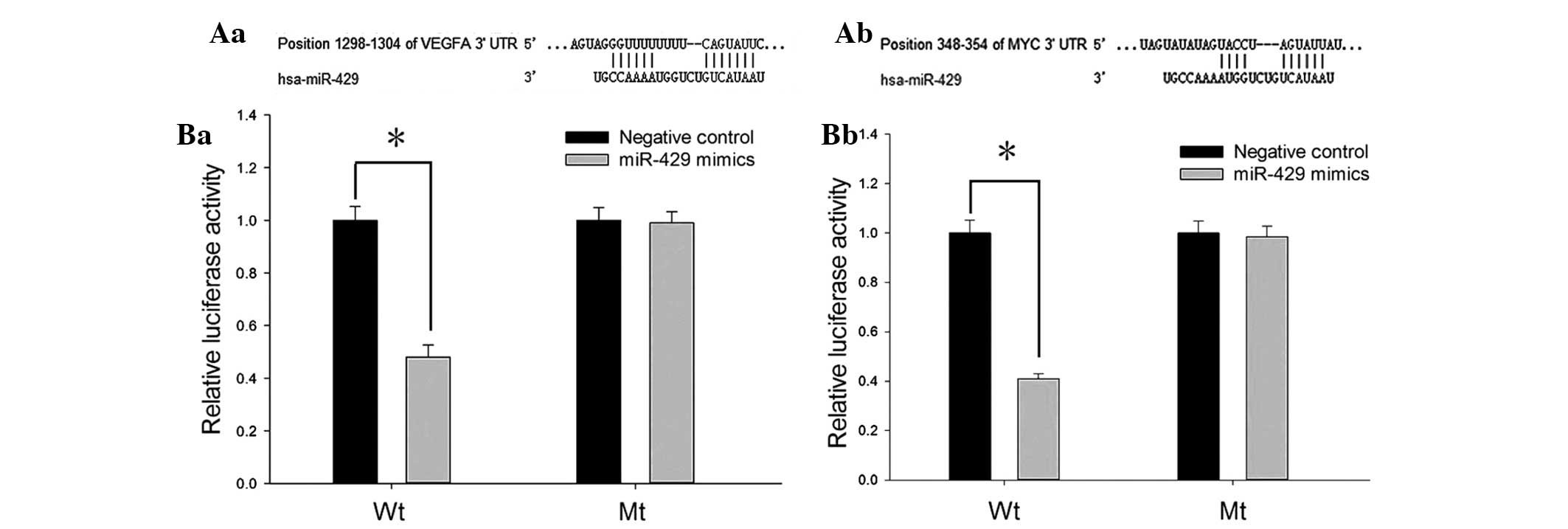
To investigate the potential target genes of
miR-429, TargetScan, PicTar, miRanda and miRWalk were combined to
predict the putative downstream genes. VEGF and c-MYC were two of
the target genes predicted by all four algorithms. The 3′UTR of the
two genes contained a complementary site for the seed sequences of
miR-429 (Fig. 4A).
To determine whether VEGF and c-MYC were directly
regulated by miR-429, the 3′UTR of the two genes containing the
putative binding site or mutant binding site were cloned into
psiCHECK-2 to construct recombinant plasmids (Wt and Mt), and the
luciferase reporter assay was performed in HeLa cells. As presented
in Fig. 4B, the relative
luciferase activity of Wt recombined plasmids (containing 3′UTR of
VEGF or c-MYC) was significantly decreased when transfected with
miR-429 mimics (P<0.05), while no notable reduction was observed
in the mutant groups.
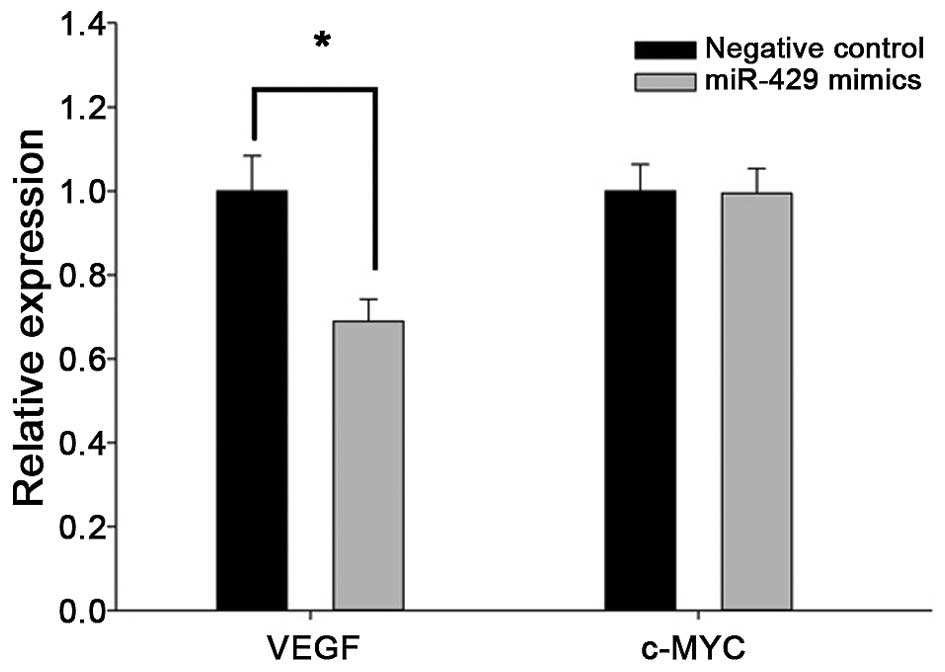
In addition, RT-qPCR analysis of cancer cells
transfected with miR-429 mimics showed decreased expression of VEGF
(Fig. 5), but not c-MYC. These
findings strongly indicate that VEGF is a direct target gene of
miR-429 in renal caner, but whether c-MYC is also a target of
miR-429 requires further investigation.
Discussion
MicroRNAs (miRNAs) are small endogenous non-coding
RNAs that exhibit important regulatory roles via the
RNA-interference pathway by targeting mRNAs for cleavage or
translational repression (20);
hence, decreasing the expression of the resulting protein (21). A number of experiments have shown
that miRNAs could function as important regulators in the
carcinogenesis of renal cancer. For example, miR-133b inhibited
cell proliferation, migration and invasion by targeting matrix
metallopeptidase-9 (22), and
downregulating miR-501-5p induced increases in caspase-3 activity
and p53 expression, as well as decreasing mTOR activation, which
leads to the stimulation of the apoptotic pathway (23). In addition, miRNAs may also be
promising biomarkers for RCC diagnosis. Vergho et al
(24) confirmed the combination of
expression levels of miR-21 and miR-126 is associated with
cancer-specific survival in ccRCC, and downregulation of
microRNA501-5p promotes a good prognosis (23). However, the expression and function
of miR-429 in ccRCC remains to be determined.
In the present study, RT-qPCR was used to quantify
the expression of miR-429 in 48 paired ccRCC specimens and normal
specimens, and the function of miR-429 on cellular proliferation,
migration and apoptosis were analyzed by an MTT assay, a wound
scratch assay and an apoptosis assay, respectively. The results
demonstrated that miR-429 was downregulated in ccRCC. Upregulation
of miR-429 by synthetic mimics restrained cellular proliferation
and migration, and induced apoptosis, indicating that miR-429 may
act as a tumor suppressor in ccRCC. To explore the potential target
genes of miR-429, TargetScan, PicTar, miRanda and miRWalk were
combined to predict the putative downstream genes, and VEGF and
c-MYC were selected. Furthermore, miR-429 decreased the 3′UTR
luciferase activity of VEGF and c-MYC. In addition, RT-qPCR
analysis of cancer cells transfected with miR-429 mimics showed
decreased expression of VEGF, but not c-MYC. All these findings
strongly indicated that VEGF was a direct target gene of miR-429 in
renal caner, but whether c-MYC was also the target of miR-429
remains to be explored.
ccRCC is the predominant and most aggressive subtype
of kidney cancer, which is associated with a high rate of
recurrence and mortality. Inactivation of the von Hippel-Lindau
(VHL) gene leads to increased levels of hypoxia-inducible factor
(HIF) and overexpression of HIF target genes, such as VEGF, CCND1,
ANGPTL4 and GLUT1 (25), which
exhibit an important role in the carcinogenesis of ccRCC (26). Advances in the knowledge of the
role of VEGF in tumor angiogenesis, growth and progression have
permitted development of approaches for the treatment of metastatic
RCC (mRCC), including several agents that target VEGF and VEGF
receptors (27). Currently,
available oral VEGF tyrosine kinase inhibitors approved for the
treatment of mRCC include sorafenib, sunitinib, pazopanib and
axitinib (28). In addition, the
MYC pathway was demonstrated to be activated in ccRCC and essential
for the proliferation of ccRCC cells (29). Anti-VEGF therapy has been widely
used in the treatment of mRCC, as VEGF can be downregulated by
miR-429. Therefore, targeting miRs may present as a novel
therapeutic option for mRCC treatment, via the downregulation of
VEGF expression.
As described above, miRNAs regulate gene expression
predominantly by translational repression, and partly, by causing
target gene mRNA cleavage. As shown in the current study, decreased
expression (~30% decrease) of VEGF was observed following the
transfection of miR-429 mimics, and no change in c-MYC expression
was identified. This may be because miR-429 could target VEGF mRNA
for cleavage and translational repression simultaneously, but could
only inhibit the translation of c-MYC mRNA. Further investigation,
such as gene function experiments and western blot analysis are
required in order to demonstrate the association between miR-429
and VEGF and c-MYC.
In conclusion, to the best of our knowledge, the
present study was the first to reveal that downregulation of
miR-429 was tumor suppressive by restraining cellular proliferation
and migration, inducing apoptosis, and targeting VEGF in ccRCC. The
correlation between miR-429 and c-MYC, and the potential use of
miR-429 in mRCC target therapy requires further investigation.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101922), the
Science and Technology Development Fund Project of Shenzhen (grant
nos. JCYJ20130402114702124 and JCYJ20150403091443329) and the
Guangdong Key Medical Subject fund.
References
|
1
|
Wulfken LM, Moritz R, Ohlmann C,
Holdenrieder S, Jung V, Becker F, Herrmann E, Walgenbach-Brünagel
G, von Ruecker A, Müller SC and Ellinger J: MicroRNAs in renal cell
carcinoma: Diagnostic implications of serum miR-1233 levels. PLoS
One. 6:e257872011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cho E, Adami HO and Lindblad P:
Epidemiology of renal cell cancer. Hematol Oncol Clin North Am.
25:651–665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen HT and McGovern FJ: Renal-cell
carcinoma. N Engl J Med. 353:2477–2490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keefe SM, Nathanson KL and Rathmell WK:
The molecular biology of renal cell carcinoma. Semin Oncol.
40:421–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cairns P: Renal cell carcinoma. Cancer
Biomark. 9:461–473. 2010.
|
|
6
|
Koscianska E, Baev V, Skreka K, Oikonomaki
K, Rusinov V, Tabler M and Kalantidis K: Prediction and preliminary
validation of oncogene regulation by miRNAs. BMC Mol Biol.
8:792007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmidt U and Begley CG: Cancer diagnosis
and microarrays. Int J Biochem Cell Biol. 35:119–124. 2003.
View Article : Google Scholar
|
|
8
|
Yu X, Zhang X, Bi T, Ding Y, Zhao J, Wang
C, Jia T, Han D, Guo G, Wang B, et al: MiRNA expression signature
for potentially predicting the prognosis of ovarian serous
carcinoma. Tumour Biol. 34:3501–3508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walter BA, Valera VA, Pinto PA and Merino
MJ: Comprehensive microRNA profiling of prostate cancer. J Cancer.
4:350–357. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osanto S, Qin Y, Buermans HP, Berkers J,
Lerut E, Goeman JJ and van Poppel H: Genome-wide microRNA
expression analysis of clear cell renal cell carcinoma by next
generation deep sequencing. PLoS One. 7:e382982012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aqeilan RI, Calin GA and Croce CM: miR-15a
and miR-16-1 in cancer: Discovery, function and future
perspectives. Cell Death Differ. 17:215–220. 2010. View Article : Google Scholar
|
|
12
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
14
|
Sun Y, Shen S, Tang H, Xiang J, Peng Y,
Tang A, Li N, Zhou W, Wang Z, Zhang D, Xiang B, et al: miR-429
identified by dynamic transcriptome analysis is a new candidate
biomarker for colorectal cancer prognosis. OMICS. 18:54–64. 2014.
View Article : Google Scholar :
|
|
15
|
Sun T, Wang C, Xing J and Wu D: miR-429
modulates the expression of c-myc in human gastric carcinoma cells.
Eur J Cancer. 47:2552–2559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Li M, Zang W, Ma Y, Wang N, Li P,
Wang T and Zhao G: MiR-429 up-regulation induces apoptosis and
suppresses invasion by targeting Bcl-2 and SP-1 in esophageal
carcinoma. Cell Oncol (Dordr). 36:385–394. 2013. View Article : Google Scholar
|
|
17
|
Zhu W, He J, Chen D, Zhang B, Xu L, Ma H,
Liu X, Zhang Y and Le H: Expression of miR-29c, miR-93, and miR-429
as potential biomarkers for detection of early stage non-small lung
cancer. PLoS One. 9:e877802014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim
JH, Kim JW and Kim S: MicroRNA expression profiles in serous
ovarian carcinoma. Clin Cancer Res. 14:2690–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine RJ: An IRB-approved protocol on the
use of human fetal tissue. IRB. 11:7–8. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoon S and De Micheli G: Prediction of
regulatory modules comprising microRNAs and target genes.
Bioinformatics. 21(Suppl 2): ii93–ii100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Gu J, Roth JA, Hildebrandt MA,
Lippman SM, Ye Y, Minna JD and Wu X: Pathway-based serum microRNA
profiling and survival in patients with advanced stage non-small
cell lung cancer. Cancer Res. 73:4801–4809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu D, Pan H, Zhou Y, Zhou J, Fan Y and Qu
P: microRNA-133b downregulation and inhibition of cell
proliferation, migration and invasion by targeting matrix
metallopeptidase-9 in renal cell carcinoma. Mol Med Rep.
9:2491–2498. 2014.PubMed/NCBI
|
|
23
|
Mangolini A, Bonon A, Volinia S, Lanza G,
Gambari R, Pinton P, Russo GR, Del Senno L, Dell'Atti L and Aguiari
G: Differential expression of microRNA501-5p affects the
aggressiveness of clear cell renal carcinoma. FEBS Open Bio.
4:952–965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vergho D, Kneitz S, Rosenwald A, Scherer
C, Spahn M, Burger M, Riedmiller H and Kneitz B: Combination of
expression levels of miR-21 and miR-126 is associated with
cancer-specific survival in clear-cell renal cell carcinoma. BMC
Cancer. 14:252014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang T, Niu X, Liao L, Cho EA and Yang H:
The contributions of HIF-target genes to tumor growth in RCC. PLoS
One. 8:e805442013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choueiri TK, Fay AP, Gagnon R, Lin Y,
Bahamon B, Brown V, Rosenberg JE, Hutson TE, Baker-Neblett KL,
Carpenter C, et al: The role of aberrant VHL/HIF pathway elements
in predicting clinical outcome to pazopanib therapy in patients
with metastatic clear-cell renal cell carcinoma. Clin Cancer Res.
19:5218–5226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Conti A, Santoni M, Amantini C, Burattini
L, Berardi R, Santoni G, Cascinu S and Muzzonigro G: Progress of
molecular targeted therapies for advanced renal cell carcinoma.
Biomed Res Int. 2013:4191762013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta S and Spiess PE: The prospects of
pazopanib in advanced renal cell carcinoma. Ther Adv Urol.
5:223–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang SW, Chang WH, Su YC, Chen YC, Lai YH,
Wu PT, Hsu CI, Lin WC, Lai MK and Lin JY: MYC pathway is activated
in clear cell renal cell carcinoma and essential for proliferation
of clear cell renal cell carcinoma cells. Cancer Lett. 273:35–43.
2009. View Article : Google Scholar
|