Introduction
Hepatocellular carcinoma (HCC) accounts for 90% of
all primary liver malignancies and is resistant to chemotherapy
(1,2). The survival rates of patients with
HCC is usually <1 year following diagnosis (3). Only 10–20% of patients with HCC are
suitable for resective surgery, and the five-year relapse-free
survival rate is no more than 20–30% (4). Developments in the techniques used to
treat HCC have improved the survival rates of patients with HCC
(5), however, this remains far
from satisfactory. Therefore, novel effective therapeutic
strategies are in high demand.
Epidemiological and clinical studies have shown that
the downregulation of vitamin D is associated with an increased
risk of various types of cancer; whereas a high level of
1,25(OH)2D3 decreases the risk of cancer
(6). Other investigations have
also demonstrated that 1,25(OH)2D3 can
modulate calcium and skeletal homeostasis, and exert a marked
effect on the growth and differentiation of various tissues
(7). Further evidence indicates
that 1,25(OH)2D3 modulates the bioactivity of
various immune cells (8). Of note,
protein modification, and the acetylation of histones and
non-histone proteins mediates gene expression levels and cell
signaling. The removal of acetyl groups from acetylated histones by
histone deacetylase 2 (HDAC2) reverses the bioactivity of histone
acetyltransferases and returns histones to a basal state, which is
followed by suppression of the activities of gene transcription.
There is evidence indicating that HDAC2 controls the functions of
key proteins associated with the cell cycle (9). Furthermore, the overexpression of
HDAC2 is found in various types of cancer, resulting in the
deregulation of uncontrolled proliferation (10). HDAC inhibitors have been reported
to exert anticancer activities in multiple types of solid and
hematologic malignancies (11,12).
The expression levels of HDAC2 are enhanced between non-tumor
tissues and histopathological grades of HCC (13). Thus,
1,25(OH)2D3 may exert antitumor activities in
HCC via mediating the levels of HDAC2. Aberrant regulation of HDAC2
deletion leads to the upregulation of p21(WAF1/Cip1) (14), which is one of the
well-characterized modulators critical in cell senescence and
apoptosis (15). Therefore, the
present study investigated the effects of
1,25(OH)2D3 on the expression levels of HDAC2
and p21(WAF1/Cip1). It is possible that
1,25(OH)2D3 exhibits antitumor properties
against HCC and may be a potential therapeutic agent for the
treatment of HCC.
Materials and methods
Cell lines and cell culture
The HpG2 hepatocellular carcinoma cell line was
obtained from China Center for Typical Culture Collection (Wuhan,
China) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Beijing Solarbio, Beijing, China) supplemented with 10%
heat-inactivated fetal bovine serum (GE Healthcare Life Sciences,
Logan, UT, USA; cat. no. F4135), 100 U/ml penicillin (Macgene Co.,
Ltd., Beijing, China), and 100 U/ml streptomycin (Macgene Co.,
Ltd.). During the experiments, 3×105 cells were plated
in culture flasks and grown in a humid atmosphere (37°C; 5%
CO2). The cells were cultured with different
concentrations (0, 0.001, 0.01, 0.1, 1 and 10 nM) of
1,25(OH)2D3 (Biofriendship, Inc., Beijing,
China) or vehicle (dimethyl-sulfoxide).
Cell viability
The viability of the HpG2 cells was measured using a
trypan blue uptake assay (Beyotime Institute of Biotechnology,
Beijing, China) and a [3H]thymidine incorporation assay
(Atom HighTech, Co., Ltd., Beijing, China) following treatment of
the cells with different concentrations of
1,25(OH)2D3 (0, 0.001, 0.01, 0.1, 1, 10, 100
and 1,000 nM) (Beyotime, Beijing, China) for 24 h. The numbers of
trypan blue-positive cells were calculated among 400 cells in two
separate microscopic fields using an optical microscope (CX41;
Olympus Corporation, Tokyo, Japan). To avoid bias caused by the
disappearance of nonviable cells, the total cell number was counted
at different times, with the results presented as the percentage of
viable cells, reflecting data collected from experiments, compared
with the fixed number of total cells. For the
[3H]thymidine uptake assay, the cells were counted
following exposure to different concentrations of
1,25(OH)2D3, and a 200 µ1 cell
suspension, containing 1.0×105 cells/ml for each
condition, were added to each well of 96-well plates (Falcon
Labware, Oxnard, CA, USA). Following culture for 24 h, the cells
were labeled with 1 Ci/well of [3H]thymidine (67
Ci/mmol) for 4 h, following which the samples were collected using
an automated sample harvester (MASH II; Microbiological Associates,
Inc., Rockville, MD, USA), dried and measured in 5 ml Aquassure
(New England Nuclear; PerkinElmer, Inc., Waltham, MA, USA), and
were measured using a Scintillation counter (4810TR; PerkinElmer,
Waltham, MA, USA)
Construction of the pcDNA3.1-HDAC2
plasmid
The HDAC2 gene (Accession no. CR541717; http://www.ncbi.nlm.nih.gov/nuccore/CR541717) was
amplified using the following primers (Takara Biotechnology Co.,
Ltd., Dalian, China): Forward 5′-AGT CCA TAT GGC GCA GAC GCA GGG
CAC C CG GAG-3′ and reverse 5′-CTA CGA ATT CTC AGG CCA ACT TGA CCT
CCT CCT TG-3′, generating a 1,449 bp product. The underline
highlights the NdeI and EcoRI, respectively The
polymerase chain reaction (PCR; 100 µl) contained 20
µl 5X Primer star PCR buffer, 8 µl 25 mM dNTP, 0.5
µl 100 µM fo rward primer, 0.5 µl 100
µM reverse primer, 1 µl template plasmid, 0.5
µl Primer star DNA polymerase (Takara Biotechnology Co.,
Ltd.) and 34.5 µl distilled water. The following PCR
thermocycling steps were performed on Eppendorf 5331 MasterCycler
Gradient Thermal Cycler (Hamburg, Germany): 94°C for 5 min;
followed by 30 cycles at 94°C for 30 sec, 55°C for 30 sec and 68°C
for 2 min; and a final step at 68°C for 10 min. The PCR product was
linked to the NheI-EcoRI sites (Takara Biotechnology
Co., Ltd.) of a pcDNA3.1 vector (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), which was termed
pcDNA3.1-HDAC2. The pcDNA3.1-HDAC2 plasmid was amplified in E.
coli TOP10 (Takara Biotechnology Co., Ltd.) and isolated using
a QIAprep Miniprep kit (Qiagen, Beijing, China), then confirmed
using DNA sequencing (Takara Biotechnology Co., Ltd.).
Short hairpin (sh)RNA constructs for
HDAC2 gene silencing
The pTZU6+1 plasmids were provided by Chongqing
Medical University (Chongqing, China). According to the principles
of shRNA design (16), and using
the HDAC2 cDNA sequence, 19–21 nt DNA fragments were designed,
which spanned the TTGG insertion sequence, with an identical
sequence to that of the HDAC2 gene. The HDAC2 coding sequence and
the reverse complementary sequence were designed as follows:
siHDAC2, forward 5′-GAC TGT CGA CTC GAC CCT CCT TGA CTG TAC GCC ATG
TTG GCA TGG CGT ACA GTC AAG GAG GTT TTT T-3′ and reverse 5′-AGT CTC
TAG ACT AGA AAA AAC CTC CTT GAC TGT ACG CCA TGC CAA CAT GGC GTA CAG
TCA AGG AGG G-3′. The SalI and XbaI (underlined;
Takara Biotechnology Co., Ltd.) restriction sites were added to
either end of the oligos for constructing the pTZU 6+1-HDAC2
vectors.
shRNA expression levels of HDAC2 and
HDAC2
The cells were transfected with 1 µg of the
HDAC2 or HDAC2-shRNA expression vector. Following 48 h
transfection, Geneticin G418 (Invitrogen; Thermo Fisher Scientific,
Inc.) was added to the DMEM (500 µg/ml), and the
G418-resistant colonies were selected. The medium was replaced
every 2 days. After 3 weeks, the G418-resistant cells were selected
and cultured separately.
Reverse transcription-quantitative
(RT-qPCR)
Total RNA was isolated from the cells using a Takara
MiniBEST Universal RNA Extraction kit (Takara Biotechnology Co.,
Ltd.), according to the manufacturer's protocol. cDNA was amplified
using a Transcriptor First Strand cDNA Synthesis kit (Takara
Biotechnology Co., Ltd). RT-qPCR was performed on a qTOWER2
(Analytik Jena AG, Jena, Germany), according to the manufacturer's
protocol. The following PCR primers were used: HDAC2 (NCBI
Reference Sequence: NM_001527.3), forward 5′-GGT GAT GGT GTT GAA
GAAGC-3′ and reverse 5′-GCA CTA GGT TGA TAC ATCTC-3′. The
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (NCBI
Reference Sequence: NM_001289746.1), forward 5′-GAT CCC TCC AAA ATC
AAGTG-3′ and reverse 5′-ATG ATC TTG AGG CTG TTGTC-3′) was used as a
loading control for normalizing data.
Two master mixes (HDAC2 and GAPDH) were used for
qPCR analysis. Each mix contained 10 µl SYBR Green (Bioline,
Ltd., London, UK), 0.5 µl of each of primer (100 pM), and 8
µl Aqua Dest distilled water/per well of a 96-well plate
(Frame Star Fast Plate 96; 4titude, Surrey, UK). A total of 19
µl master mix and 1 µl cDNA were used, and two
replicates for the expression levels of HDAC2 and GAPDH were used
for each individual. Consequently, 21 animals were measured per
plate, together with 12 non-template controls. These controls
contained RNAse-free water instead of cDNA. The plates were sealed
using q-stick adhesive for qPCR (4titude) and were centrifuged for
a few seconds to ensure all liquid was inside the well (MPS 1000;
Labnet, Edison, NJ, USA). Subsequently, the plates were incubated
at 95°C for 2 min and run on an Applied Biosystems 7500 Real-Time
PCR system (Invitrogen; Thermo Fisher Scientific, Inc.) in 45
cycles of 5 sec at 95°C, follwed by 30 sec at 60°C and 30 sec at
72°C. The mRNA expression levels were normalized against the levels
of GAPDH.
Western blot analysis
The cells were lysed using radioimmunoprecipitation
assay buffer (Shanghai Sigma High-Tech Co., Ltd., Shanghai, China),
containing 20 mM TrisHCl (pH 8.0), 100 mM NaCl, 1% NP-40, 1% sodium
deoxycholate, 0.1 % SDS, 0.1 mg/ml phenylmethylsulfonyl fluoride, 1
µg/ml aprotinin and 0.5% sodium orthovanadate.
Polyacrylamide gels (10%) for SDS-PAGE (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) were used for the separation of protein samples.
Following SDS-PAGE, the proteins were transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Following the blotting, the membranes were blocked with 5%
skim milk in Tris-buffered saline with Tween 20 (TBS-T) for 1 h at
room temperature. The membranes were incubated with rabbit
anti-human anti-HDAC2 antibody (1:1,000; cat. no. SC-7872; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) overnight at 4°C.
Following three washes with TBS-T, the membranes were incubated
with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG
antibody (1:10,000; cat. no. G21234; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. Signals were measured using
chemiluminescent HRP substrate (EMD Millipore). The relative
expression of HDAC2 was measured using Image J software (Windows
version 1.49; National Institutes of Health, Maryland, MD,
USA).
Cell growth assay
The HpG2 cells (1×104) were cultured in
24-well plates with DMEM, with or without
1,25-(OH)2D3. The number of viable cells were
examined by adding 2 mg/ml 3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide
(Sigma-Aldrich, St. Louis, MO, USA) in each well for 3 days
consecutively. The medium was discarded after 2, and the formazan
crystals were dissolved in 100 µl dimethyl-sulfoxide/per
well. The absorbance values were read at 590 nm using a microplate
reader (WD-2102A; Beijing Liuyi Instrument Factory, Beijing,
China).
Statistical analysis
All experiments were performed three times and all
samples were measured in triplicate. The results are presented as
the mean ± standard deviation). The statistical difference between
groups was examined using Student's t-test. Statistical comparisons
were made using a two-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference. All
data were analyzed using the SPSS 20.0 software package (IBM SPSS,
Armonk, NY, USA).
Results
Effects of
1,25-(OH)2D3 toxicity in HpG2 cells
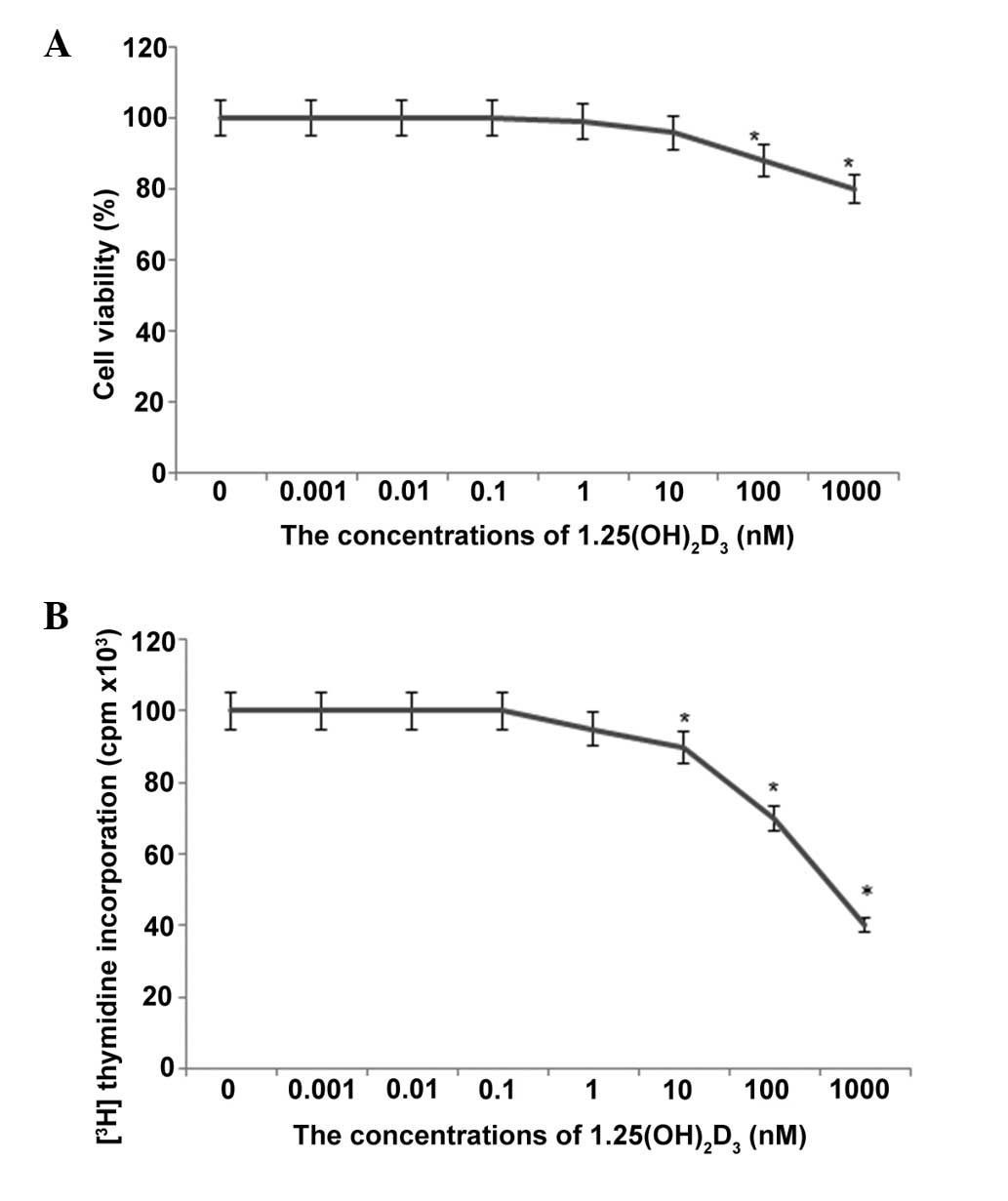
To avoid the presence of cytotoxic effects of
1,25-(OH)2D3 on the HCC cells, the
concentration of 1,25-(OH)2D3 was
investigated prior to performing experiments to assess its effects.
Exposure of the HpG2 cells to 1,25-(OH)2D3,
in the presence of >1,000 nM, resulted in loss of cell
viability, as observed by the uptake of trypan blue (P<0.05). At
concentrations <100 nM, the cell viability was >90% (Fig. 1). These effects of
1,25-(OH)2D3 were not noted immediately
following addition. The percentage viabilities of the cells in the
1,25-(OH)2D3 groups were 100, 98 and 96%,
compared with the controls, after 6, 12 and 24 h culture,
respectively (Fig. 1). Toxicity
was also evaluated by examining the ability of cells to incorporate
[3H]thymidine into DNA following exposure to different
concentrations of 1,25-(OH)2D3. As shown in
Fig. 1B, this method was a more
sensitive index of cell injury. A significant reduction in
[3H]thymidine uptake was measured at concentrations
>100 nM (P<0.05). Based on these results, the lower
concentrations (0.001, 0.01, 0.1, 1 and 10 nM) of
1,25-(OH)2D3 were used for the subsequent
culture of HpG2 cells.
Treatment with
1,25(OH)2D3 reduces the mRNA levels of HDAC2
and increase the levels of p21(WAF1/Cip1)
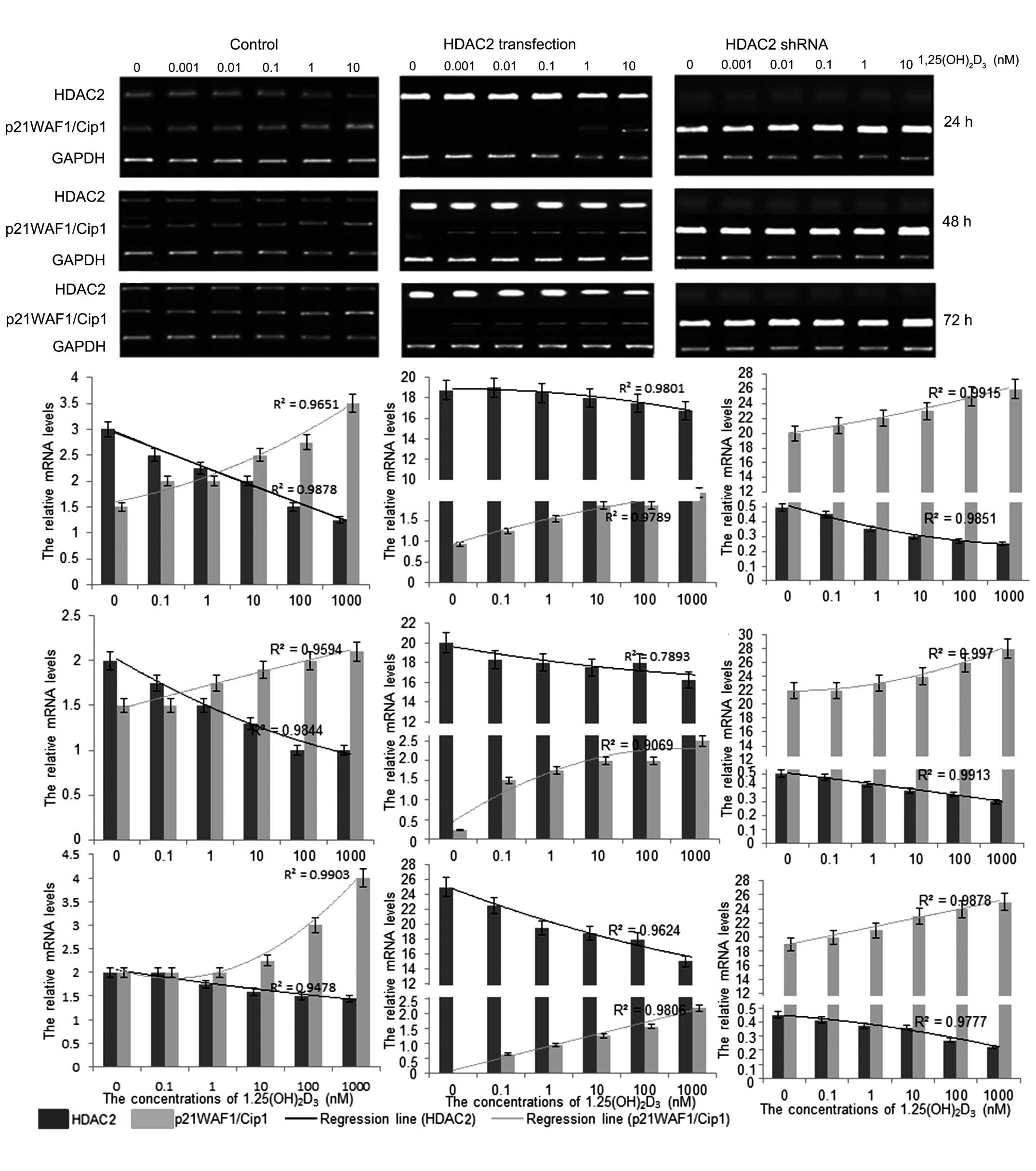
To understand the mechanism underlying the antitumor
activity of 1,25(OH)2D3 in vitro, the
mRNA levels of HDAC2 and p21(WAF1/Cip1) were investigated following
treatment with 1,25(OH)2D3. The results
showed that 1,25(OH)2D3 treatment reduced the
mRNA levels of HDAC2 and increased the levels of p21(WAF1/Cip1)
(Fig. 2). The mRNA level of HDAC2
was significantly enhanced when the HpG2 cells were transfected
with pCDNA3.1-HDAC2. In addition, the mRNA level of p21(WAF1/Cip1)
decreased significantly, compared with the cells without HDAC2
transfection. The changes in the levels of HDAC2 and p21(WAF1/Cip1)
were also marginally affected by 1,25(OH)2D3
in a dose-dependent manner, according to the logistic regression
(Fig. 2). By contrast, the mRNA
expression levels of HDAC2 were almost completely absent when the
HpG2 cells were transfected with HDAC2 shRNA, whereas the mRNA
expression levels of p21(WAF1/Cip1) were significantly increased,
compared with the cells without HDAC2 shRNA transfection. In
addition, the change in the expression levels of HDAC2 and
p21(WAF1/Cip1) were affected by 1,25(OH)2D3
in a dose-dependent manner, according to the logistic regression
(Fig. 2). These results suggested
that 1,25(OH)2D3 affected the development of
HCC, predominantly by regulating the mRNA levels of HDAC2, which
mediated the mRNA level of p21(WAF1/Cip1).
Treatment with
1,25(OH)2D3 reduces the protein levels of
HDAC2 and increases the protein levels of p21(WAF1/Cip1)
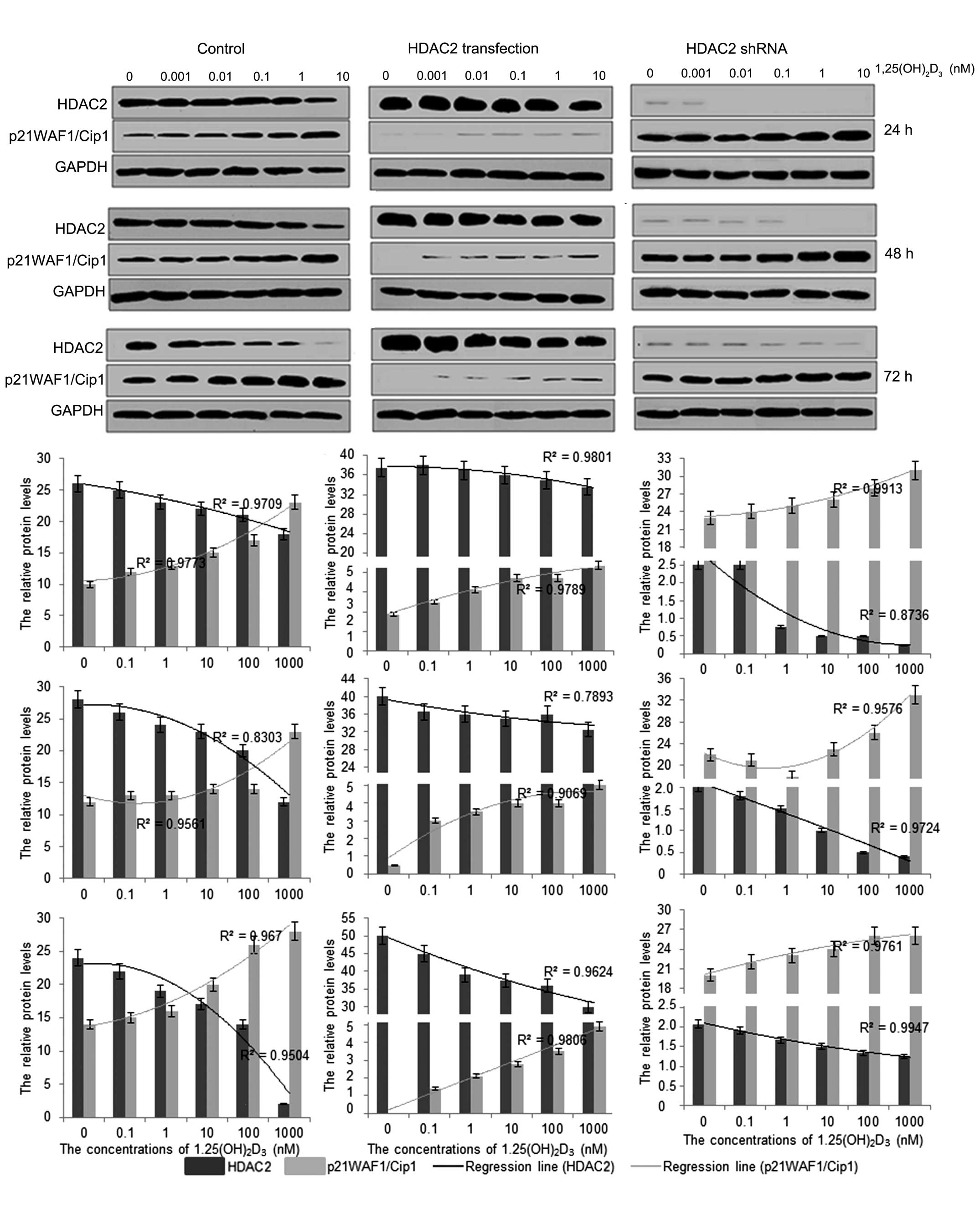
To further investigate the mechanism underlying the
antitumor activity of 1,25(OH)2D3 in
vitro, the protein expression levels of HDAC2 and
p21(WAF1/Cip1) were investigated following treatment with
1,25(OH)2D3. The results showed that
1,25(OH)2D3 treatment reduced the protein
levels of HDAC2 and increased the protein levels of p21(WAF1/Cip1;
Fig. 3). The protein expression of
HDAC2 was significantly enhanced when the HpG2 cells were
transfected with pCDNA3.1-HDAC2, whereas the protein expression of
p21(WAF1/Cip1) was decreased significantly, compared with that in
the cells without HDAC2 transfection. In addition, the changes in
the levels of HDAC2 and p21(WAF1/Cip1) were also marginally
affected by 1,25(OH)2D3 in a dose-dependent
manner, according to the logistic regression (Fig. 3). By contrast, the protein
expression of HDAC2 was almost completely eliminated when the HpG2
cells were transfected with HDAC2 shRNA. The protein levels of
p21(WAF1/Cip1) were also significantly increased, compared with
those in the cells without HDAC2 shRNA transfection, and the
changes in the levels of HDAC2 and p21(WAF1/Cip1) were affected by
1,25(OH)2D3 in a dose-dependent manner,
according to the logistic regression (Fig. 3). These results suggested that
1,25(OH)2D3 affected the development of HCC,
predominantly by regulating the protein levels of HDAC2, which
mediated the expression of p21(WAF1/Cip1).
1,25(OH)2D3
treatment significantly reduces the growth rate of HCC
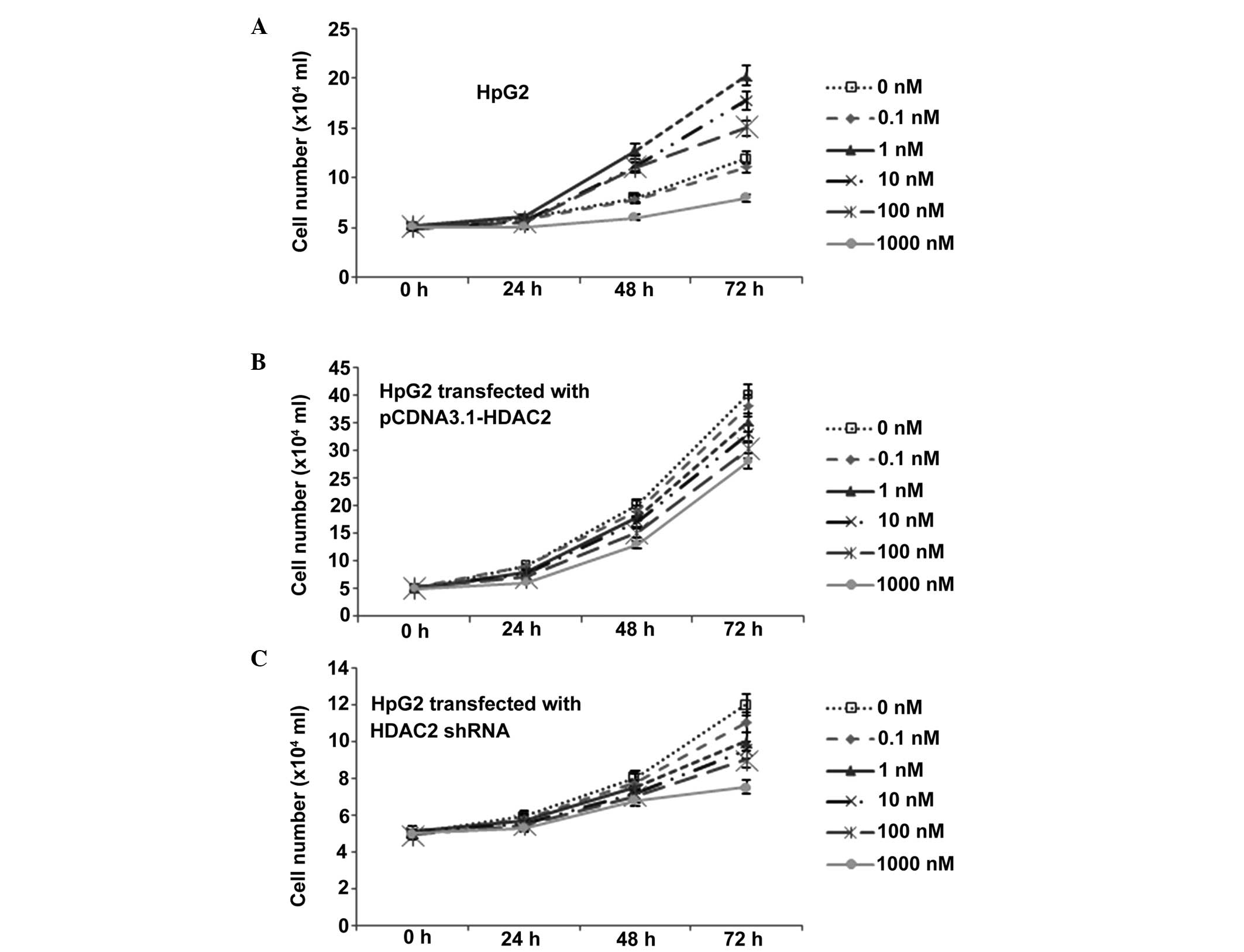
To examine the antitumor effects of
1,25(OH)2D3 in HCC, the present study
examined its antiproliferative effects in the human HpG2 HCC line.
The cells were incubated with different concentrations of
1,25(OH)2D3 for 3 days and the status of cell
growth was measured. Consistent with previous evidence (17), cell growth in the
1,25(OH)2D3 group was decreased in a
dose-dependent manner, compared with the control group. The growth
curve line (Fig. 4A) showed a
significant proliferation inhibitory effect of
1,25(OH)2D3, which occurred in a
dose-dependent manner.
The average growth rate of the
pCDNA3.1-HDAC2-transfected HpG2 cells was increased, compared with
the untransfected cells (Fig. 4).
Additionally, expression of HDAC2 in the HpG2 cells reversed the
inhibitory effect of 1,25(OH)2D3 (Fig. 4). The average growth rate was also
enhanced in the cells treated with a high concentration of
1,25(OH)2D3 (10 nM/day). Of note, there was
difference in growth rate between the cells treated with
1,25(OH)2D3 and those without treatment when
the expression of HDAC2 was induced by transfection (Fig. 4), although the inhibitory effects
of HDAC2 remained, according to the logistic regression. Following
shRNA transfection, the average growth rate of the HpG2 cells was
reduced. Additionally, HDAC2 shRNA transfection of the HpG2 cells
had a similar function as the inhibitory effect of
1,25(OH)2D3 (Fig. 4). The average growth rate was also
decreased, although the cells was not treated with high
concentration of 1,25(OH)2D3. Of note, there
was a difference in growth rate between the cells treated with
1,25(OH)2D3 and the untreated cells when
HDAC2 was silenced (Fig. 4),
although the inhibitory effects of HDAC2 remained, according to the
logistic regression.
Discussion
At present, epidemiological evidence indicates that
decreased concentrations of vitamin D are associated with an
enhanced risk of various types of cancer (18). Although previous studies have
indicated that 1,25(OH)2D3 inhibits the
proliferation of HCC in a dose dependent manner (19), the detailed molecular mechanism
underlying the function of 1,25(OH)2D3 in
preventing the progression of HCC remains to be fully
elucidated.
Prior to commencement of the present study, the
potential toxicity of high concentrations of
1,25(OH)2D3 on HCC cells were examined. As
observed inhibitory effects may be caused by toxicity, rather than
by other molecular mechanisms, the toxicity of
1,25(OH)2D3 was first measured using trypan
blue and [3H]thymidine incorporation assays. As
expected, the viability of the HCC cells was significantly affected
when the concentration of 1,25(OH)2D3 was
>100 nM (Fig. 1). Therefore,
the concentration used in the subsequent experiments was ≤10 nM.
This ensured that any inhibitory effects were not caused by
toxicity, but by other molecular mechanisms.
To investigate the molecular mechanism underlying
the inhibitory effect of 1,25(OH)2D3 in HCC,
the present study transfected HCC cells with HDAC2. The resulting
high expression levels of HDAC2 led to a significant reduction in
the inhibitory effects of 1,25(OH)2D3 on the
HCC cells (Fig. 4). In addition,
the expression of p21(WAF1/Cip1) was reduced. By contrast, when the
HCC cells were transfected with HDAC2 shRNA, leading to silencing
of the HDAC2 gene, similar inhibitory effects on HCC cell growth
were observed in the cells, which were not treated with
1,25(OH)2D3. Furthermore, exogenous
1,25(OH)2D3 treatment decreased the
expression levels of HDAC2 and enhanced the expression levels of
p21(WAF1/Cip1) in a dose-dependent manner. Therefore,
1,25(OH)2D3 inhibited the development of HCC
via the downregulation of HDAC2, which mediated the levels of
p21(WAF1/Cip1).
There is increasing evidence reinforcing the
hypothesis that excessively and chronically expressed HDAC2
contributes to the development of HCC (13,14).
HDAC2 is a major deacetylase, and its expression is inversely
associated with changes in the acetylation state of histone H4K5.
RNA interference-mediated gene silencing of HDAC2 leads to
hyperacetylation of histone H4 and a developmental delay, although
the levels of HDAC3 remain high (20,21).
Increased expression levels of p21(WAF1/Cip1) may contribute to the
observed developmental delay. HDAC2 regulates a number of
biological processes in cells. Although HDAC2 inhibits the growth
of the liver in aged mice, the levels of HDAC2 are also increased
in HCC (10). Increased levels of
HDAC2 lead to its interaction with p21(WAF1/Cip1) via the Sp1
binding sites of the p21(WAF1/Cip1) gene promoter, and inhibition
of acetylated histone H3 on these sites (22). Histone H3 is a conserved protein in
the nucleus, which can be readily modified post-translationally,
and the state of acetylation shows clinical diagnostic significance
in HepG2 cells. The levels of acetylated peptides are associated
with malignant transformation in the liver (23). The results of the present study
indicated that 1,25(OH)2D3 inhibited HDAC2,
and its downregulation was involved in the upregulation of
p21(WAF1/Cip1), which resulted in the inhibition of HCC
proliferation.
Although 1,25(OH)2D3 has
potent inhibitory effects in several liver diseases (24,25),
the molecular mechanism underlying the inhibitory effect of
1,25(OH)2D3 in HCC development remains to be
fully elucidated. The present study demonstrated the novel finding
that downregulating the expression of HDAC2 resulted in an increase
in the expression of p21(WAF1/Cip1), which eventually inhibited HCC
development. HDAC2 ablation suppressed HCC development and enhanced
the expression of p21(WAF1/Cip1), which was similar to the
inhibitory effects of 1,25(OH)2D3 on HCC
(Figs. 2Figure 3–4). In addition, elevated levels of HDAC2
promoted the development of HCC and decreased the activity of
p21(WAF1/Cip1), which reversed the inhibitory effects of
1,25(OH)2D3 on the development of HCC, even
when the cells were treated with a high dose of 1,
25(OH)2D3. Treatment with
1,25(OH)2D3 led to the downregulation in the
expression of HDAC2, which mediated the level of p21(WAF1/Cip1).
Further investigations of the effects of
1,25(OH)2D3 on hepatocarcinogenesis may
provide further insights into the detailed molecular mechanism
underlying vitamin D in antitumor activity. The results of the
present study support the potential development of
1,25(OH)2D3 as an effective drug for the
treatment of HCC.
Acknowledgments
The present study was supported by the joint fund of
the Science and Technology Department of Guizhou Province and
Guiyang Medical College (grant no. LH(2014)7114).
References
|
1
|
Tang B, Zhang Y, Liang R, Gao Z, Sun D and
Wang L: RNAi-mediated EZH2 depletion decreases MDR1 expression and
sensitizes multidrug-resistant hepatocellular carcinoma cells to
chemotherapy. Oncol Rep. 29:1037–1042. 2013.PubMed/NCBI
|
|
2
|
Kirikoshi H, Yoneda M, Mawatari H, Fujita
K, Imajo K, Kato S, Suzuki K, Kobayashi N, Kubota K, Maeda S, et
al: Is hepatic arterial infusion chemotherapy effective treatment
for advanced hepatocellular carcinoma resistant to transarterial
chemoembolization? World J Gastroenterol. 18:1933–1939. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Bustamante J, Castells A,
Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J and Bruix J: Natural
history of untreated nonsurgical hepatocellular carcinoma:
rationale for the design and evaluation of therapeutic trials.
Hepatology. 29:62–67. 1999. View Article : Google Scholar
|
|
4
|
Portolani N, Coniglio A, Ghidoni S,
Giovanelli M, Benetti A, Tiberio GA and Giulini SM: Early and late
recurrence after liver resection for hepatocellular carcinoma:
Prognostic and therapeutic implications. Ann Surg. 243:229–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu L, Sun HC, Zhang W, Chai ZT, Zhu XD,
Kong LQ, Wang WQ, Zhang KZ, Zhang YY, Zhang QB, et al: Aspirin
minimized the pro-metastasis effect of sorafenib and improved
survival by up-regulating HTATIP2 in hepatocellular carcinoma. PLoS
One. 8:e650232013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deeb KK, Trump DL and Johnson CS: Vitamin
D signalling pathways in cancer: Potential for anticancer
therapeutics. Nat Rev Cancer. 7:684–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gocek E and Studzinski GP: Vitamin D and
differentiation in cancer. Crit Rev Clin Lab Sci. 46:190–209. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhalla AK, Amento EP, Clemens TL, Holick
MF and Krane SM: Specific high-affinity receptors for
1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear
cells: Presence in monocytes and induction in T lymphocytes
following activation. J Clin Endocrinol Metab. 57:1308–1310. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung KH, Noh JH, Kim JK, Eun JW, Bae HJ,
Xie HJ, Chang YG, Kim MG, Park H, Lee JY and Nam SW: HDAC2
overexpression confers oncogenic potential to human lung cancer
cells by deregulating expression of apoptosis and cell cycle
proteins. J Cell Biochem. 113:2167–2177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim HS, Chang YG, Bae HJ, Eun JW, Shen Q,
Park SJ, Shin WC, Lee EK, Park S, Ahn YM, et al: Oncogenic
potential of CK2α and its regulatory role in EGF-induced HDAC2
expression in human liver cancer. FEBS J. 281:851–861. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimizu T, LoRusso PM, Papadopoulos KP,
Patnaik A, Beeram M, Smith LS, Rasco DW, Mays TA, Chambers G, Ma A,
et al: Phase I first-in-human study of CUDC-101, a multitargeted
inhibitor of HDACs, EGFR and HER2 in patients with advanced solid
tumors. Clin Cancer Res. 20:5032–5040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Zou X, Berger AD, Twiss C, Peng Y,
Li Y, Chiu J, Guo H, Satagopan J, Wilton A, et al: Increased
expression of histone deacetylaces (HDACs) and inhibition of
prostate cancer growth and invasion by HDAC inhibitor SAHA. Am J
Transl Res. 1:62–71. 2009.PubMed/NCBI
|
|
13
|
Noh JH, Bae HJ, Eun JW, Shen Q, Park SJ,
Kim HS, Nam B, Shin WC, Lee EK, Lee K, et al: HDAC2 provides a
critical support to malignant progression of hepatocellular
carcinoma through feedback control of mTORC1 and AKT. Cancer Res.
74:1728–1738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noh JH, Jung KH, Kim JK, Eun JW, Bae HJ,
Xie HJ, Chang YG, Kim MG, Park WS, Lee JY and Nam SW: Aberrant
regulation of HDAC2 mediates proliferation of hepatocellular
carcinoma cells by deregulating expression of G1/S cell cycle
proteins. PLoS One. 6:e281032011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parra E, Gutiérrez L and Ferreira J:
Increased expression of p21Waf1/Cip1 and JNK with costimulation of
prostate cancer cell activation by an siRNA Egr-1 inhibitor. Oncol
Rep. 30:911–916. 2013.PubMed/NCBI
|
|
16
|
Sachidanandam R: RNAi: design and
analysis. Current Protocols in Bioinformatics. 13:10–11. 2004.
|
|
17
|
Abe J, Nakano T, Nishii Y, Matsumoto T,
Ogata E and Ikeda K: A Novel Vitamin-D3 Analog,
22-Oxa-1,25-Dihydroxyvitamin-D3, Inhibits the Growth of Human
Breast-Cancer Invitro and Invivo without Causing Hypercalcemia.
Endocrinology. 129:832–837. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giovannucci E: Vitamin D and cancer
incidence in the harvard cohorts. Ann Epidemiol. 19:84–88. 2009.
View Article : Google Scholar
|
|
19
|
Pourgholami MH, Akhter J, Lu Y and Morris
DL: In vitro and in vivo inhibition of liver cancer cells by
1,25-dihydroxyvitamin D3. Cancer Lett. 151:97–102. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Methot JL, Hoffman DM, Witter DJ, Stanton
MG, Harrington P, Hamblett C, Siliphaivanh P, Wilson K, Hubbs J,
Heidebrecht R, et al: Delayed and prolonged histone
hyperacetylation with a selective HDAC1/HDAC2 inhibitor. ACS Med
Chem Lett. 5:340–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma P and Schultz RM: Histone deacetylase 2
(HDAC2) regulates chromosome segregation and kinetochore function
via H4K16 deacetylation during oocyte maturation in mouse. PLoS
Genet. 9:e10033772013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Di Padova M, Bruno T, De Nicola F, Iezzi
S, D'Angelo C, Gallo R, Nicosia D, Corbi N, Biroccio A, Floridi A,
et al: Che-1 arrests human colon carcinoma cell proliferation by
displacing HDAC1 from the p21WAF1/CIP1 promoter. J Biol Chem.
278:36496–36504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang W, Xu L, Kong J, Fan H and Yang P:
Quantitative research of histone H3 acetylation levels of human
hepatocellular carcinoma cells. Bioanalysis. 5:327–339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Küçükazman M, Ata N, Dal KA, Yeniova AÖ,
Kefeli A, Basyigit S, Aktas B, Akin KO, Ağladıoğlu K, Üre ÖS, et
al: The association of vitamin D deficiency with non-alcoholic
fatty liver disease. Clinics (Sao Paulo). 69:542–546. 2014.
View Article : Google Scholar
|
|
25
|
Mandorfer M, Payer BA, Schwabl P, Steiner
S, Ferlitsch A, Aichelburg MC, Stättermayer AF, Ferenci P,
Obermayer-Pietsch B, Grabmeier-Pfistershammer K, et al: Revisiting
liver disease progression in HIV/HCV-coinfected patients: The
influence of vitamin D, insulin resistance, immune status, IL28B
and PNPLA3. Liver Int. 35:876–885. 2014. View Article : Google Scholar : PubMed/NCBI
|