Introduction
Chronic obstructive pulmonary disease (COPD) is one
of the leading causes of morbidity and mortality worldwide,
characterized by poorly reversible airflow limitation (1,2). The
pathophysiology of COPD remains to be fully elucidated, which
limits the development of a more effective therapies and novel
drugs for COPD. Various methods have been used for diagnosing,
monitoring and evaluating COPD, predominantly comprising indirect
methods, including lung function tests (3) and imaging techniques (high resolution
computed tomography) (4), and more
direct evaluations using invasive measurements (bronchoscopy and
induced sputum) (5,6). Indirect and direct evaluation methods
have adverse effects through exposure to noxious agents and the
invasiveness of the sampling procedures, respectively (7). Therefore, the identification of
biomarkers for COPD has received increasing attention, aiming to
reflect the degree of disease severity with non-invasive
measurements, and to assist in monitoring pharmacological therapy
(8). Exhaled air and blood
analysis may be used to develop biomarkers of lung diseases.
Exhaled breath condensate (EBC), obtained through
cooling exhaled air during spontaneous breathing, offers promising
real-time measurement of pulmonary pathobiology. The advantages of
EBC include easy and non-invasive collection from patients using a
portable device, and its suitability for sequential and
longitudinal sampling of the lower respiratory tract (9,10).
Pulmonary surfactant proteins, defined as SP-A,
SP-B, SP-C and SP-D, are synthesized by type II pneumocytes, and
SP-A and SP-D have been associated with the host defense of the
lung (11–13). SP-A and SP-D lectins have been
reported to contribute to the pathogenesis of COPD (14,15),
and abnormal inflammatory responses and the involvement of
inflammatory cells and cytokines may affect the composition and
function of surfactant proteins (16). The present study focused on SP-A,
which functions in protecting the lungs from oxidants and
inflammatory and infectious stress (17,18).
The immunomodulatory protein, SP-A, in bronchoalveolar lavage (BAL)
fluid has also been reported to be associated with impaired
functions in chronic smokers (19,20).
The preoperative detection methods of SP-A are limited, and
predominantly include blood analysis and BAL fluid analysis
(18–21). However, blood analysis only
indirectly reflects the state of the lung, and BAL fluid analysis
cannot be used as a routine procedure due to the invasiveness of
the sampling procedure (22).
Therefore, a novel non-invasive method, which is easy to perform,
is required to measure the expression levels of SP-A and to
routinely monitor the severity of COPD.
The present study aimed to measure levels of SP-A in
the EBC of patients with COPD, to evaluate whether the expression
levels of SP-A in EBC are associated with those in the lung tissue,
and to assess whether SP-A in EBC is associated with lung
function.
Materials and methods
Subjects
The population recruited for the present study
comprised 60 subjects who underwent lobectomy for a solitary
peripheral lung nodule between September 2012 to January 2013. All
patients were recruited from the Tianjin Chest Hospital (Tianjin,
China). Patients who had lung infection or acute COPD exacerbation
within the month prior to commencement of the present study were
excluded. Patients suffering from severe diseases of the heart,
brain, liver or kidney were also excluded.
The diagnosis of patients with COPD was performed,
based on the following clinical symptoms: History of exposure to
risk factors and laboratory examination. The severity of COPD was
assessed according to the American Thoracic Society and European
Respiratory Society Consensus Statement (23), where post-bronchodilator forced
expiratory volume (FEV) in 1 sec/forced vital capacity ≤70%, and an
FEV of 1–80% confirmed the presence of airflow limitation that was
not fully reversible. The 60 subjects were divided into two groups,
according to pirometric evaluation: A COPD group (28 cases) and a
non-COPD group (32 cases) (Table
I). Written informed consent was obtained from all subjects,
and the protocol was approved by the Medical Ethics Committee of
Tianjin Chest Hospital.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | COPD | Non-COPD |
|---|
| Total cases (n) | 28 | 32 |
| Male (n) | 15 | 18 |
| Age (mean,
years) | 58.71±11.22 | 61.81±8.33 |
EBC
EBC (2 ml) was collected using a condenser
(Respiratory Research, Inc., Austin, TX, USA), as previously
described (24). Patients were
asked to rinse their mouth thoroughly, and were asked not to eat
food or take medicine containing nitrite or nitrate. This technique
allows the non-invasive collection of the on-gaseous components of
the expiratory air.
Tissue samples
Tissue samples of ~1 cm3 were obtained
during lobectomy at the Tianjin Chest Hospital (Tianjin, China)
through resection of adjacent lung tissues located >5 cm from
the nodule. The tissue samples destined for western blotting were
immediately snap-frozen in liquid nitrogen and stored at −80°C. The
tissues samples for immunohistochemistry were fixed using 10%
formalin buffer for <24 h. Then, each tissue block was embedded
in paraffin. Sections of 5 cm were cut from the tissue samples, and
then tissue sections underwent routine immunohistochemistry
procedures.
Enzyme-linked immunosorbent assay
(ELISA)
ELISA was used to measure the expression levels of
SP-A in the EBC of all subjects, using a Surfactant Associated
Protein A kit (Cloud-Clone Corp, Houston, TX, USA). Briefly,
dilutions (100 μl each) of standard, blank or EBC samples
were added to the appropriate well and incubated for 2 h at 37°C.
Subsequently, liquid from each well was removed, detection reagent
A (100 μl) was added to the wells of a micro-titer plate and
incubated at 37°C in the dark for 60 min. Following washing with
350 μl of 1X wash solution, detection reagent B (100
μl) was added to the wells, and the plate was incubated for
a further 30 min at 37°C in the dark. Subsequently, 90 μl
substrate solution was added to the wells and incubated for 15–25
min at 37°C in the dark. The reaction was terminated following the
addition of 50 μl stop solution, and the absorbance was
measured at 450 nm using a microplate reader 550 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
The expression levels of SP-A were detected using
western blot analysis, following standard procedures. Briefly,
fresh lung tissues were homogenized in radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Shanghai, China)
and protein concentrations were determined using the Bradford assay
(Bio-Rad Laboratories, Inc.). Equal quantities of protein (40
μg) were separated by 12% SDS-PAGE (SDS-PAGE Gel Preparation
kit; Beyotime Institute of Biotechnology) and transferred to
nitrocellulose blotting membranes (Schleicher & Schuell
Bioscience GmbH, Dassel, German). The membranes were then blocked
by incubating with 10% nonfat dried milk overnight at 4°C.
Subsequently, the membranes were incubated with mouse anti-human
SP-A monoclonal antibody (1:500; cat. no. PE-10; Fuzhou Maixin
Biotech Co., Ltd.) or mouse anti-actin antibody (1:1,000; cat. no.
MAB1501; EMD Millipore, Billerica, MA, USA) at room temperature for
1 h, followed by incubation with horseradish peroxidase-conjugated
rabbit anti-mouse secondary antibody (1:1,000; cat. no. sc-358914;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA). All experiments
were repeated in triplicate. The protein expression levels were
quantified using Photoshop Image Analysis software (Image-Pro Plus
6.0; Media Cybernetics Inc., Rockville, MD, USA).
Immunohistochemistry
For immunohistochemical analysis, sections were
deparaffinized and rehydrated, and antigen retrieval was achieved
by boiling (92–98°C) in citric acid buffer (pH 6.0) for 20 min.
Immunostaining of SP-A and thyroid transcription factor-1 (TTF-1)
were performed using mouse anti-human SP-A monoclonal antibody
(1:100; cat. no. PE-10; Fuzhou Maixin Biotech Co., Ltd.) and
anti-TTF-1 monoclonal antibody (1:100; cat. no. MX011; Fuzhou
Maixin Biotech Co., Ltd.), according to manufacturer's
instructions. The sections were counterstained with Mayer's
hematoxylin (Fuzhou Maixin Biotech Co., Ltd.). TTF-1 was used to
count the total type II pneumocytes including the SP-A-positive
type II cells on the adjacent serial sections.
Evaluation of the SP-A-positive type II pneumocytes
was performed using a light microscope (BX50; Olympus Corporation,
Tokyo, Japan) at ×400 magnification (manual counting). A total of
20 microscopic fields on each slide were evaluated for all
subjects, with the results expressed in cells/ml2.
Statistical analysis
The results are expressed as the mean ± standard
deviation. Differences between the groups of subjects were
determined using Student's t-test. Pearson's correlation
coefficients were used to evaluate the associations between
variables. The statistical software package, SPSS v.17 (SPSS, Inc.
Chicago, IL, USA), was used for analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
SP-A in EBC and lung tissue samples
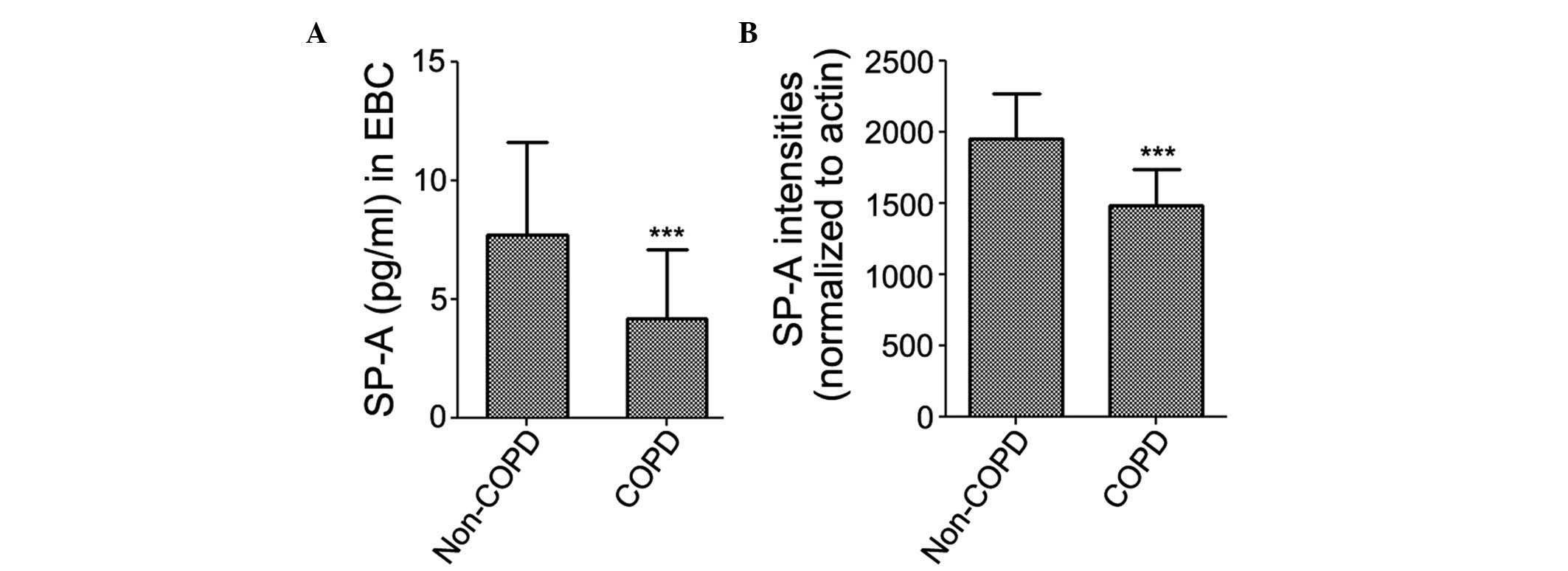
SP-A was detected in the EBC of all subjects. The
results of the ELISA revealed that the expression levels of SP-A in
the EBC were significantly decreased in the patients with COPD
(4.165±2.950 pg/ml), compared with the non-COPD subjects
(7.706±3.923 pg/ml; Fig. 1A). The
protein expression levels of SP-A were subsequently examined in the
lung tissue samples using western blot analysis. The protein
expression levels of SP-A were significantly decreased in the
patients with COPD (1,480.7±256.7), compared with the non-COPD
subjects (1,954.1±312.2; Fig.
1B).
SP-A-positive type II pneumocytes
The alveolar macrophages and certain type II
pneumocytes were SP-A-positive. The expression of TTF-1 was only
detected in the type II pneumocytes, with the alveolar macrophages
being TTF-1-negative. As type II pneumocytes and alveolar
macrophages have morphological differences, immunostaining with
TTF-1 and SP-A monoclonal antibodies was used to determine the
total number of type II pneumocytes and SP-A-positive type II
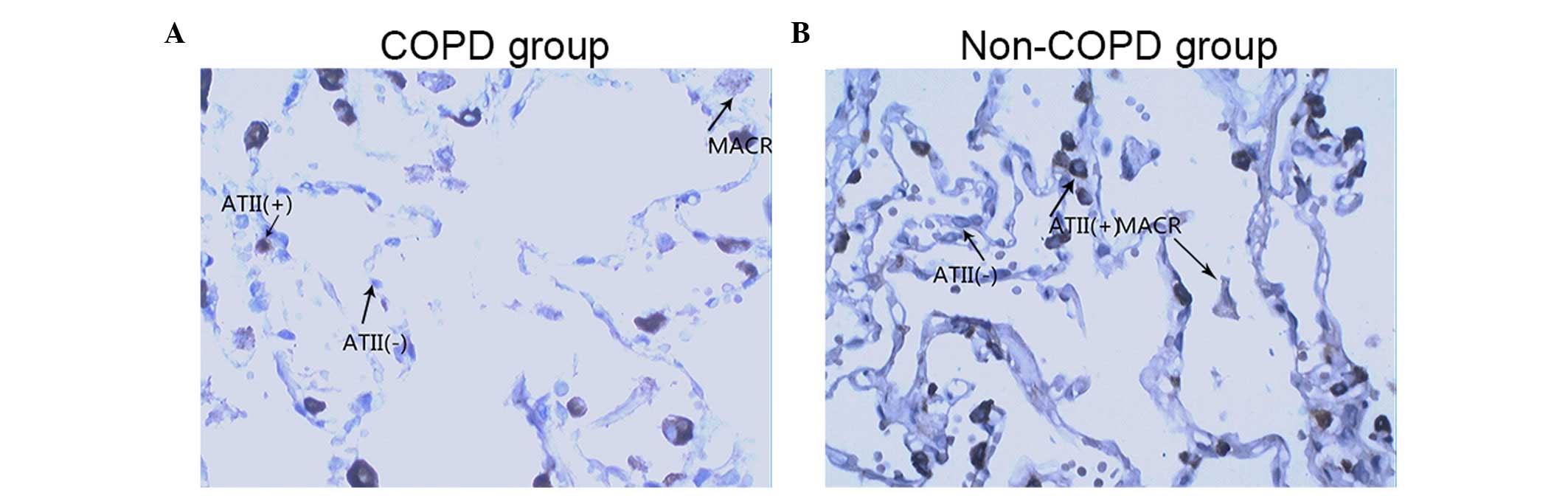
pneumocytes. Images of the SP-A immunostained tissues from a
patient with COPD and a patient without COPD are shown in Fig. 2A and B, respectively. The images in
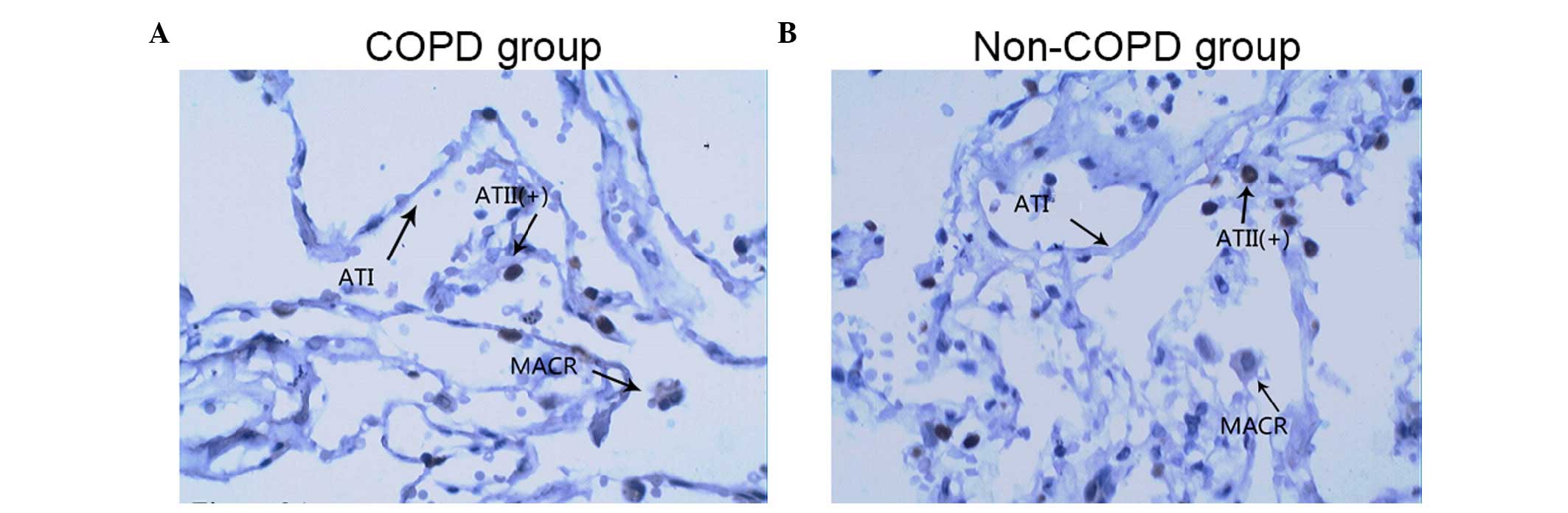
Fig. 3 show representative
TTF-1-immunostained images of lung tissue samples from a patient
with COPD (Fig. 3A) and a patient
without COPD (Fig. 3B). Although
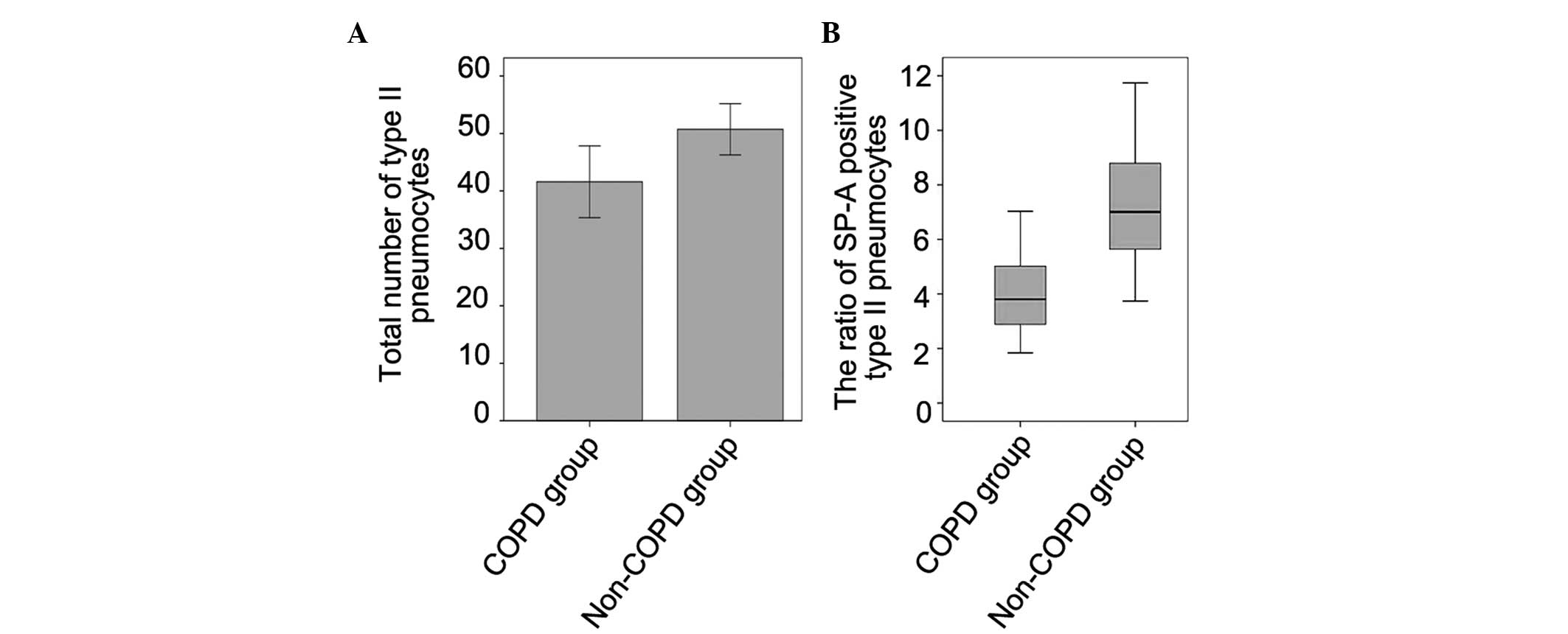
no significat difference was detected, the results demonstrated
that the total number of type II pneumocytes was lower in the COPD
group, compared with the non-COPD group, and the ratio of
SP-A-positive type II pneumocytes (SP-A-positive type II
pneumocytes/total type II pneumocytes, expressed as a percentage)
was decreased in the COPD group, compared with the non-COPD group
(Figs. 2Figure 3–4).
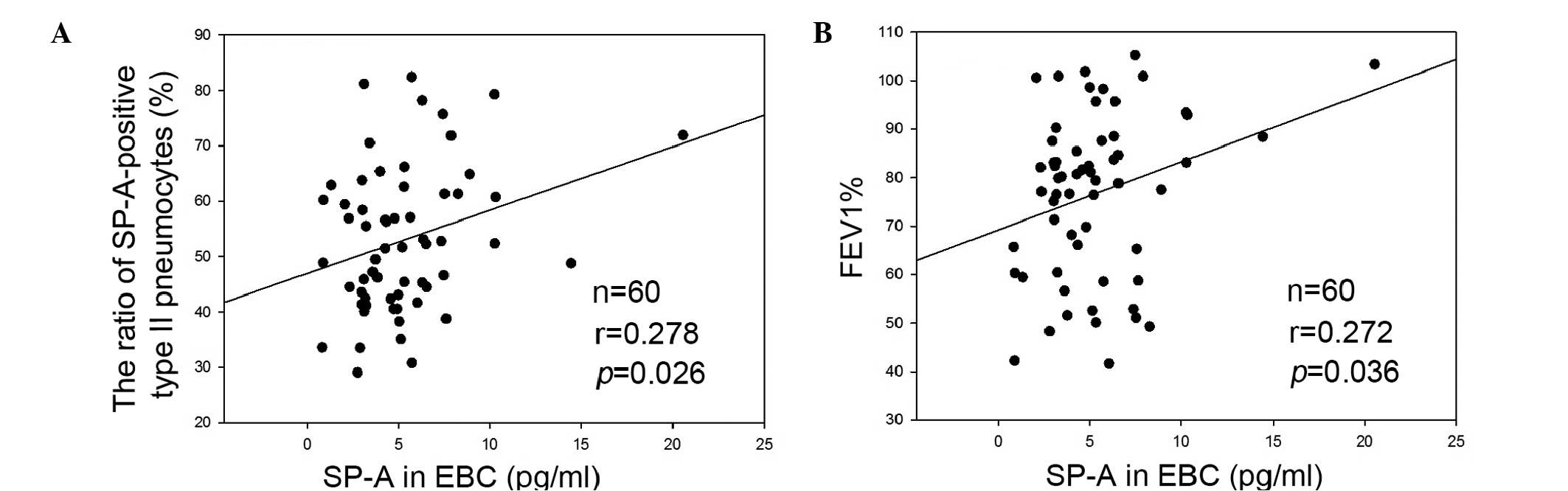
Correlation between the expression levels
of SP-A and EBC
As shown in Fig.
5A, the expression levels of SP-A in the EBC were correlated
with the ratio of SP-A-positive type II pneumocytes/total type II
pneumocytes (r=0.278; P=0.026). Decreased expression levels of SP-A
were associated with a decreased ratio of SP-A-positive type II
pneumocytes. In addition, the ratio of SP-A-positive type II
pneumocytes was correlated with the predicted FEV 1% (data not
shown), suggesting that the increased degree of airway obstruction
was associated with an increase in the SP-A ratio. The expression
levels of SP-A in the EBC were also correlated with the predicted
FEV 1% (r=0.272; P=0.036), suggesting that the decreased expression
levels of SP-A in EBC were associated with an increase in the
degree of airway obstruction (Fig.
5B).
Discussion
The non-invasive measurement of airway obstruction
in patients with COPD may prove useful for monitoring the disease.
EBC analysis is a non-invasive method for detecting airway lining
fluid composition, and the technique has numerous advantages, as it
is easy to perform and less expensive in terms of equipment and
personnel costs, compared with sampling BAL and induced sputum
(25,26).
Controversial results have been reported regarding
the expression levels of SP-A in the BAL or serum of patients with
COPD. Decreased expression levels of SP-A have been reported in
smokers who are otherwise healthy, elderly people and patients with
COPD (19,20,27).
Ohlmeier et al (28)
reported increased expression levels of SP-A in lung tissue samples
and the induced sputum of patients with COPD, compared with normal
or fibrotic lung tissue samples. Vlachaki et al (21) also reported that decreased
expression levels of SP-A in lung tissue samples of patients with
COPD are associated with the pathogenesis of COPD.
In the present study, investigation to identify a
non-invasive SP-A measurement technique and subsequent
investigation of the expression levels of SP-A in patients with
COPD were performed. The expression levels of SP-A were determined
in EBC collected, which was collected from patients with COPD and
non-COPD subjects. The results of the present study demonstrated
that the expression levels of SP-A were significantly decreased in
the patients with COPD, compared with the non-COPD subjects, and
the expression of SP-A in the EBC was correlated with that in the
lung tissue. Immunostaining of TTF-1 revealed that the total number
of type II pneumocytes in patients with COPD was increased,
compared with the number in the non-COPD subjects. The decrease in
the total number of type II pneumocytes may be due to the extensive
tissue damage in COPD (12), and
has been reported in previous studies following lung injury
(29,30). Type II pneumocytes proliferate
during lung injury and chronic inflammatory states, including COPD,
in order to produce type I neumocytes and specific products,
including SP-A), which are involved in lung defense (31).
The results of the present study demonstrated that
the ratio of SP-A-positive type II pneumocytes to the overall type
II pneumocytes was decreased in patients with COPD, compared with
the non-COPD subjects. One possible explanation is that
disease-induced epithelial destruction depletes the type II
pneumocytes, which are responsible for the production of SP-A.
Furthermore, the decreased expression levels of SP-A in EBC were
associated with a higher degree of airway obstruction, suggesting
that the measurement of SP-A in EBC may be a potential method for
monitoring airway obstruction in patients with COPD.
In conclusion, the present study performed
non-invasive SP-A measurement by detecting the expression levels of
SP-A in EBC collected from patients with COPD. Decreased expression
levels of SP-A in the EBC were observed in the patients with COPD,
compared with the non-COPD subjects, and these decreased expression
levels were also observed in the lung tissues. In addition, the
total number of type II pneumocytes in the patients with COPD were
decreased and the ratios of SP-A-positive type II pneumocytes were
reduced, compared with those in the non-COPD subjects. The
reduction in SP-A-positive type II pneumocytes was associated with
the expression levels of SP-A. Finally, the results of the present
study demonstrated that decreased expression levels of SP-A in the
EBC were associated with a higher degree of airway obstruction.
These results suggested that the measurement of SP-A in EBC may be
a potential method for monitoring airway obstruction in patients
with COPD. Further investigations are required in order to further
examine these observations and elucidate the underlying
mechanisms.
Acknowledgments
This present study was funded by grants from the
Tianjin Municipal Science and Technology Commission (grant no.
13ZCZDSY01800) and the Tianjin Health Bureau of Science and
Technology Funds (grant no. 2012KR16).
References
|
1
|
Vestbo J, Hurd SS, Agusti AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Mar tinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar
|
|
2
|
Barnes PJ: Chronic obstructive pulmonary
disease. N Engl J Med. 343:269–280. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pellegrino R and Brusasco V: On the causes
of lung hyperinflation during bronchoconstriction. Eur Respir J.
10:468–475. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baldi S, Miniati M, Bellina CR, Battolla
L, Catapano G, Begliomini E, Giustini D and Giuntini C:
Relationship between extent of pulmonary emphysema by
high-resolution computed tomography and lung elastic recoil in
patients with chronic obstructive pulmonary disease. Am J Respir
Crit Care Med. 164:585–589. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rutgers SR, Timens W, Kaufmann HF, van der
Mark TW, Koëter GH and Postma DS: Comparison of induced sputum with
bronchial wash, bronchoalveolar lavage and bronchial biopsies in
COPD. Eur Respir J. 15:109–115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhowmik A, Seemungal TA, Sapsford RJ,
Devalia JL and Wedzicha JA: Comparison of spontaneous and induced
sputum for investigation of airway inflammation in chronic
obstructive pulmonary disease. Thorax. 53:953–956. 1998. View Article : Google Scholar
|
|
7
|
Saetta M, Turato G, Maestrelli P, Mapp CE
and Fabbri LM: Cellular and structural bases of chronic obstructive
pulmonary disease. Am J Respir Crit Care Med. 163:1304–1309. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wielders PL and Dekhuijzen PN: Disease
monitoring in chronic obstructive pulmonary disease: Is there a
role for biomarkers? Eur Respir J. 10:2443–2445. 1997. View Article : Google Scholar
|
|
9
|
Hunt J: Exhaled breath condensate: An
evolving tool for noninvasive evaluation of lung disease. J Allergy
Clin Immunol. 110:28–34. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carter SR, Davis CS and Kovacs EJ: Exhaled
breath condensate collection in the mechanically ventilated
patient. Respir Med. 106:601–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anzueto A, Jubran A, Ohar JA, Piquette CA,
Rennard SI, Colice G, Pattishall EN, Barrett J, Engle M, Perret KA
and Rubin BK: Effects of aerosolized surfactant in patients with
stable chronic bronchitis: A prospective randomized controlled
trial. JAMA. 278:1426–1431. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bernhard W, Haslam PL and Floros J: From
birds to humans: New concepts on airways relative to alveolar
surfactant. Am J Respir Cell Mol Biol. 30:6–11. 2004. View Article : Google Scholar
|
|
13
|
Milic-Emili J: Does mechanical injury of
the peripheral airways play a role in the genesis of COPD in
smokers? COPD. 1:85–92. 2004. View Article : Google Scholar
|
|
14
|
Sin DD, Pahlavan PS and Man SF: Surfactant
protein D: A lung specific biomarker in COPD? Ther Adv Respir Dis.
2:65–74. 2008. View Article : Google Scholar
|
|
15
|
Guo X, Lin HM, Lin Z, Montaño M, Sansores
R, Wang G, DiAngelo S, Pardo A, Selman M and Floros J:
Polymorphisms of surfactant protein gene A, B, D, and of
SP-B-linked micro-satellite markers in COPD of a Mexican
population. Chest. 117:249S–250S. 2000. View Article : Google Scholar
|
|
16
|
Otto-Verberne CJ, Ten Have-Opbroek AA,
Franken C, Hermans J and Dijkman JH: Protective effect of pulmonary
surfactant on elastase-induced emphysema in mice. Eur Respir J.
5:1223–1230. 1992.PubMed/NCBI
|
|
17
|
Kishore U, Greenhough TJ, Waters P, Shrive
AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T and Chakraborty
T: Surfactant proteins SP-A and SP-D: Structure, function and
receptors. Mol Immunol. 43:1293–1315. 2006. View Article : Google Scholar
|
|
18
|
Pastva AM, Wright JR and Williams KL:
Immunomodulatory roles of surfactant proteins A and D: Implications
in lung disease. Proc Am Thorac Soc. 4:252–257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Honda Y, Takahashi H, Kuroki Y, Akino T
and Abe S: Decreased contents of surfactant proteins A and D in BAL
fluids of healthy smokers. Chest. 109:1006–1009. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Betsuyaku T, Kuroki Y, Nagai K, Nasuhara Y
and Nishimura M: Effects of ageing and smoking on SP-A and SP-D
levels in bronchoalveolar lavage fluid. Eur Respir J. 24:964–970.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vlachaki EM, Koutsopoulos AV, Tzanakis N,
Neofytou E, Siganaki M, Drositis I, Moniakis A, Schiza S, Siafakas
NM and Tzortzaki EG: Altered surfactant protein-A expression in
type II pneumocytes in COPD. Chest. 137:37–45. 2010. View Article : Google Scholar
|
|
22
|
Rahman I and Biswas SK: Non-invasive
biomarkers of oxidative stress: Reproducibility and methodological
issues. Redox Rep. 9:125–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Celli BR and MacNee W; ATS/ERS Task Force:
Standards for the diagnosis and treatment of patients with COPD: A
summary of the ATS/ERS position paper. Eur Respir J. 23:932–946.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mutti A, Corradi M, Goldoni M, Vettori MV,
Bernard A and Apostoli P: Exhaled metallic elements and serum
pneumoproteins in asymptomatic smokers and patients with COPD or
asthma. Chest. 129:1288–1297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kharitonov SA and Barnes PJ: Exhaled
markers of pulmonary disease. Am J Respir Crit Care Med.
163:1693–1722. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jöbsis Q, Raatgeep HC, Schellekens SL,
Kroesbergen A, Hop WC and de Jongste JC: Hydrogen peroxide and
nitric oxide in exhaled air of children with cystic fibrosis during
antibiotic treatment. Eur Respir J. 16:95–100. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo X, Lin HM, Lin Z, Montaño M, Sansores
R, Wang G, DiAngelo S, Pardo A, Selman M and Floros J: Surfactant
protein gene A, B and D marker alleles in chronic obstructive
pulmonary disease of a Mexican population. Eur Respir J.
18:482–490. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohlmeier S, Vuolanto M, Toljamo T, Vuopala
K, Salmenkivi K, Myllärniemi M and Kinnula VL: Proteomics of human
lung tissue identifies surfactant protein A as a marker of chronic
obstructive pulmonary disease. J Proteome Res. 7:5125–5132. 2008.
View Article : Google Scholar
|
|
29
|
Wirtz HR and Schmidt M: Acute influence of
cigarette smoke on secretion of pulmonary surfactant in rat
alveolar type II cells in culture. Eur Respir J. 9:24–32. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Subramaniam S, Whitsett JA, Hull W and
Gairola CG: Alteration of pulmonary surfactant proteins in rats
chronically exposed to cigarette smoke. Toxicol Appl Pharmacol.
140:274–280. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garcia O, Hiatt MJ, Lundin A, Lee J, Reddy
R, Navarro S, Kikuchi A and Driscoll B: Targeted type 2 alveolar
cell depletion provides a dynamic functional model for lung injury
repair. Am J Respir Cell Mol Biol. Jul 23–2015.Epub ahead of print.
View Article : Google Scholar
|