Introduction
Atrial fibrillation (AF) is a common type of
tachyarrhythmia (1), and has drawn
the attention of researchers due to its high incidence and serious
complications (2,3). However, the optimal method of
treating AF has remained controversial (4,5).
Therefore, it is crucial to identify more effective and safer
treatments, particularly those using atrial-selective blockers.
The current of Kv1.5 channels
(Ikur) is the main outward ion flow during atrial
action potential re-polarization. The KCNA5 gene encodes the
Kv1.5 sub-unit, which is the main molecular component of
Ikur. Ikur is present in the
atrium (6), but not in ventricles
(7); therefore,
Ikur is a potential target for atrium-specific
arrhythmia therapy.
Myricetin (Myr) is a flavonoid compound present in
numerous plants, including Myrica rubra (Lour.) Zucc. Its
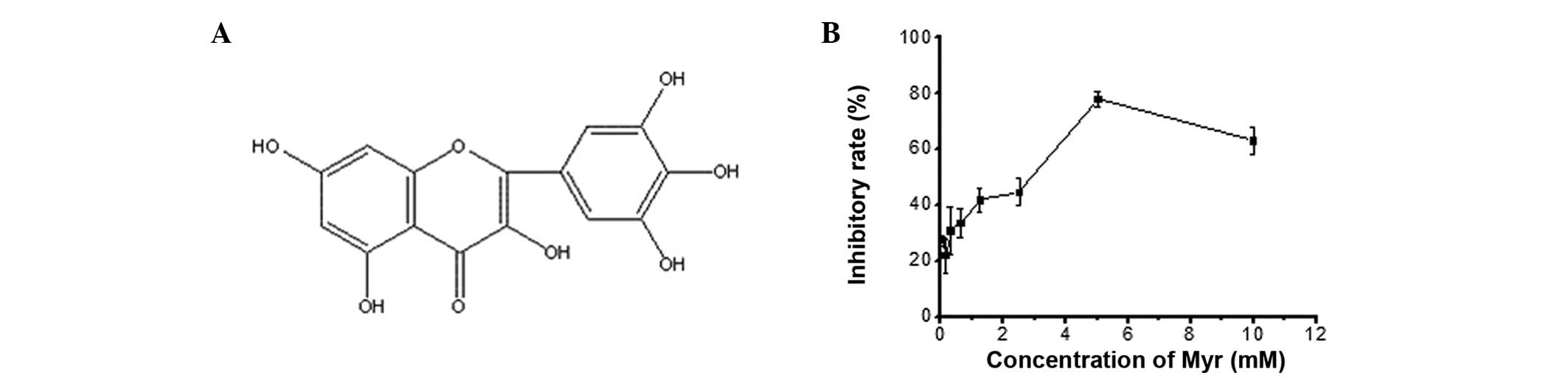
chemical structure is shown in Fig.
1A. Myr has a variety of pharmacological effects, including
anti-bacterial (8), analgesic
(8), anti-tumor (9,10),
anti-allergic (11-13), anti-oxidant (14,15),
blood sugar-lowering (16) and
hepatoprotective actions (17). It
also exerts protective effects against cardiovascular diseases,
including atherosclerosis (18),
ischemia-reperfusion injury (19),
myocardial infarction (20) and
hypertension (21,22). However, the mechanisms by which Myr
exerts anti-arrhythmic effects have remained elusive. It has been
indicated that a Myr derivative, hexamethyl Myr, is able to inhibit
Ikur (23);
therefore, the present study examined whether Myr also inhibits
Ikur and investigated the underlying
mechanisms.
Materials and methods
Reagents
Myr (98% purity) was provided by Dr Yong Ye (College
of Pharmacy, Guangxi Medical University, Nanning, China). It was
prepared as a 10-mM stock solution in dimethyl sulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) and stored in aliquots at 4°C
prior to use. For use in the subsequent experiments, it was diluted
to 0.5, 3 or 10 µM with extracellular fluid, as described
below.
Solutions
The Ikur recording solution
(extracellular solution) consisted of 130 mM NaCl and 0.33 mM
Na2HPO4 x12H2O (both from KeLong
Chemical, Chengdu, China) as well as 5.4 mM KCl, 1 mM
CaCl2, 1 mM MgCl2 and 10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (all
from Sangon Biotech Co. Ltd., Shanghai, China), 5.5 mM glucose (Bio
Basic Inc, Markham, OT, Canada) and NaOH (Jinshan Chemical Reagent
Co. Ltd, Chengdu, China) to adjust the pH to 7.4. The electrode
solution contained 110 mM l-aspartate (Sigma-Aldrich), 110
mM KOH (Sangon Biotech Co. Ltd.), 20 mM KCl, 8 mM NaCl, 1 mM
MgCl2, 1 mM CaCl2, 10 mM ethylene glycol
tetraacetic acid (all from Sigma-Aldrich), 10 mM HEPES (Sangon
Biotech Co. Ltd.) and KOH to adjust the pH to 7.2; 5 mM
Mg-adenosine triphosphate (Sigma-Aldrich) was added immediately
prior to use, and the solution was filtered through 0.22-µm
microporous membranes (Merck Millipore, Carrigtwohill, Republic of
Ireland). The lysis buffer consisted of 250 mM glucose, 20 mM
3-(N-morpholino)propanesulfonic acid and l mM dithiothreitol
(Sigma-Aldrich); 1% (v/v) protease inhibitor (Calbiochem; EMD
Millipore, Billerica, MA, USA) was added prior to use.
Cell culture and transfection
HEK293 cells (American Type Tissue Collection,
Manassas, VA, USA) were cultured in RPMI-1640 medium (Thermo Fisher
Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine
serum (Thermo Fisher Scientific) and penicillin/streptomycin
(Sigma-Aldrich). Cells were transiently transfected with
hKv1.5-overexpression vector in six-well plates
(Corning-Costar, Corning, NY, USA) using 3 µl Entranster™-H
transfection reagent (Engreen Biosystem Co. Ltd, Beijing, China)
and 3 µg of hKv1.5 cDNA in pEGFP-N1 vector
(Sangon Biotech Co. Ltd.). A pEGFP-N1 was used as a negative
control. Subsequent assays were performed at 24 h subsequent to
transfection. Cells used for electrophysiology experiments were
seeded at a density of 1×104 cells/35-mm dish with glass
cover slips.
MTT assay
MTT assays were used to assess the toxicity of Myr.
Logarithmically growing cells were seeded at a density of
1–5×104 cells/well in 96-well plates (Corning-Costar)
and were allowed to adhere for 24 h at 37°C. The medium was then
replaced with fresh medium containing various concentrations of
Myr. The cells were maintained for two days and the toxicity of Myr
was determined using MTT (Amresco LLC, Solon, OH, USA). Ten
microliters of MTT (5 mg/ml stock) was added to each well followed
by incubation for 4 h. The seeding medium was then discarded and
0.15 ml DMSO was added to each well. The absorbance (A) was read
using a multimode microplate reader (Infinite M200; Tecan Group,
Ltd., Männedorf, Switzerland) at 570 nm and the cell viability was
determined as follows: (1−Atreated group/Acontrol
group ×100.
Western blot analysis
Cells (4×105/ml) were incubated with or
without Myr (0–100 µM) for 24 h in six-well plates. Cells
were collected and lysed in lysis buffer. After sonication in ice
water, crude lysates were cleared by centrifugation at 12,000 ×g
for 5 min at 4°C. The total protein concentration of the lysates
was measured using a bicinchoninic acid protein assay kit
(Solarbio, Beijing, China). Lysates were diluted at a 4:1 ratio
with 5X loading buffer (Beyotime Institute of Biotechnology,
Haimen, China) and boiled for 5 min. Equal amounts of protein (10
µg) were loaded onto 10% SDS-polyacrylamide gels and
separated by electrophoresis using an electrophoresis apparatus
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The separated
proteins were then electrotransferred onto polyvinylidene fluoride
(PVDF) membranes (Immun-Blot® PVDF membrane; Bio-Rad
Laboratories, Inc.). Following electrotransfer, the membranes were
blocked with 5% non-fat milk (Yili Industrial Group Co. Ltd, Inner
Mongolia, China) in Tris-buffered saline with Tween 20 for 60 min
on an orbital shaker (Qilinbeier Instrument and Manufacture Co.
Ltd, Haimen, China) and then incubated with rabbit polyclonal
primary antibodies against Kv1.5 (A03K0017; 1:200
dilution; BlueGene Biotech Co., Ltd., Shanghai, China) overnight at
4°C followed by biotin (AP132B; 1:10,000; EMD Millipore) for 1 h
and goat anti-rabbit horseradish peroxidase-conjugated antibodies
(sc-2004; 1:50,000; Santa Cruz Biotechnology, Dallas, TX, USA) for
30 min at room temperature. Rabbit polyclonal antibody of the
endogenous protein GAPDH (Santa Cruz Biotechnology) was used as a
loading control. Protein bands were detected using enhanced
chemiluminescence western blotting substrate (Pierce Biotechnology
Inc, Rockford, IL, USA), imaged using the Universal Hood II system
(Bio-Rad Laboratories, Inc.) and quantified using Quantity one
software v4.6.2 (Bio-Rad Laboratories, Inc.).
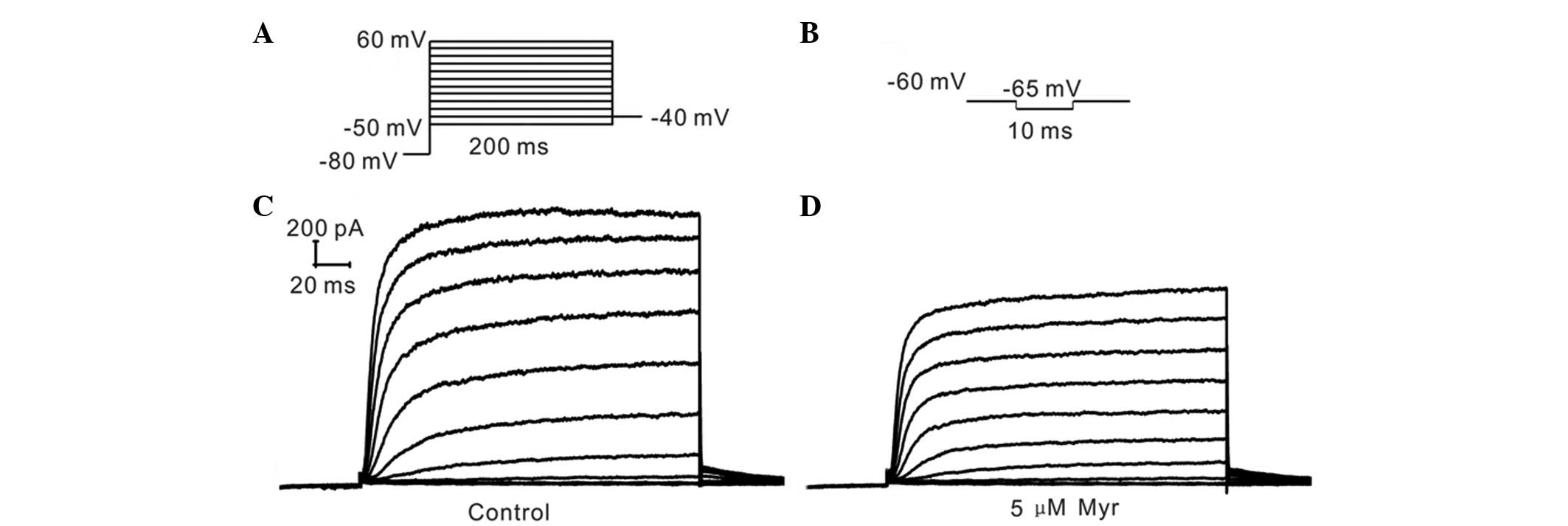
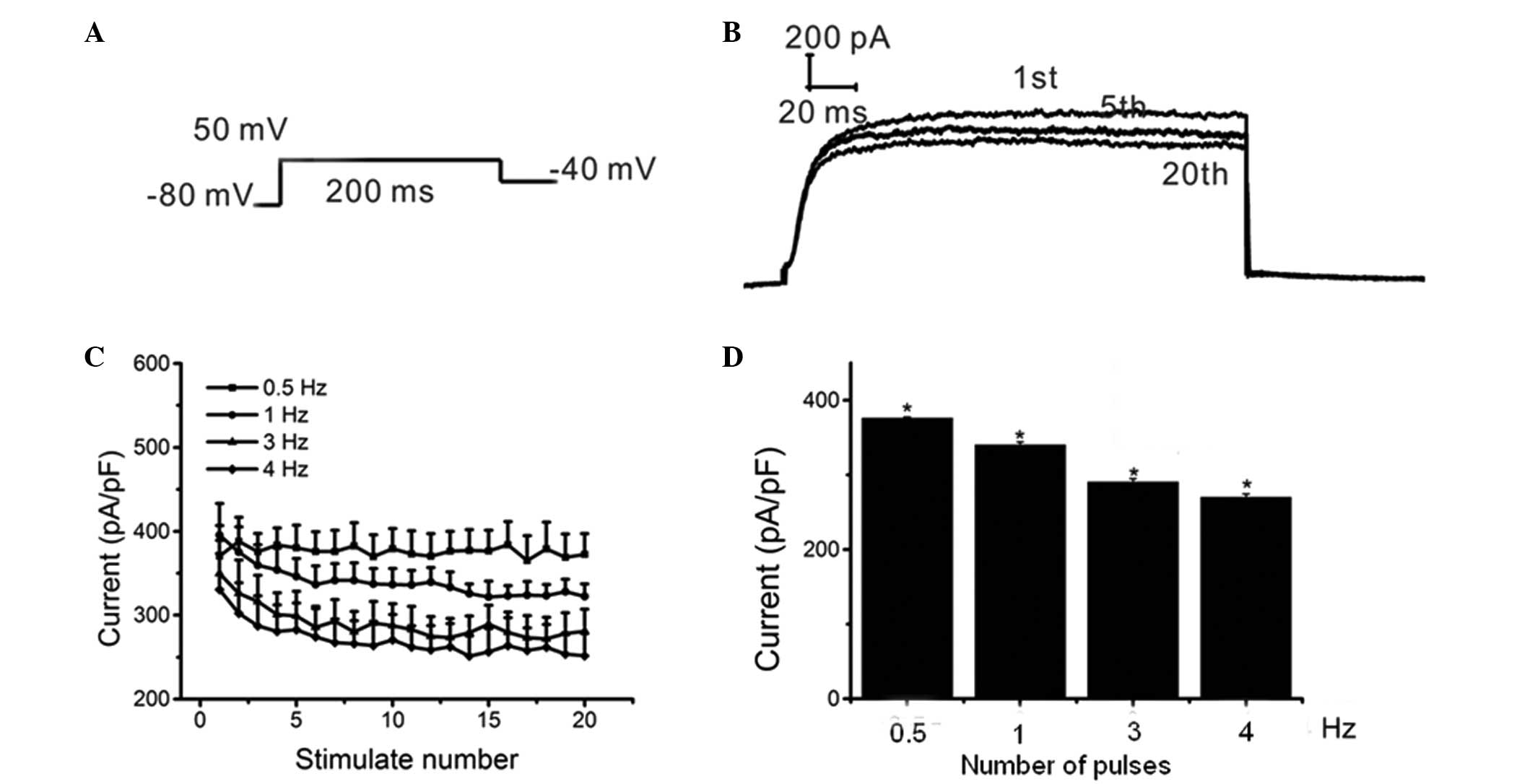
Patch-clamp recordings
Currents were recorded using the whole-cell
patch-clamp technique at room temperature (~22°C). The protocol
used 200-msec voltage steps between −50 mV and +60 mV from a
holding potential of −80 mV, followed by a return to −40 mV
(Fig. 2A). The current amplitude
was measured from 150 msec to the end of the depolarization step.
Borosilicate glass electrodes (1.2 mm optical density) were pulled
using a Brown-Flaming puller (model P-97; Sutter Instrument Co,
Novato, CA, USA); they had a tip resistance of 5–7 MΩ when filled
with the electrode solution. The membrane current was recorded in
voltage-clamp mode using an EPC-10 amplifier and Pulse software
(Heka Elektronik, Lambrecht, Germany). A 3 M NaCl-agar salt bridge
was used as the reference electrode. The tip potential was set to
zero before the patch pipette touched the cell. After a GΩ seal was
obtained, the cell membrane was ruptured using gentle suction to
establish the whole-cell configuration. The series resistance (Rs)
and membrane capacitance were compensated prior to the onset of
recordings. The membrane capacity (Cm) was recorded using 10-msec
voltage steps to −65 mV from a holding potential of −60 mV,
followed by a return to −60 mV (Fig.
2B). The formula used to calculate the membrane capacity was as
follows: Cm=0.98× A × τ/5, with amp as the current amplitude and τ
as the time constant. Ikur was recorded at
various time-points in the presence of various concentrations of
Myr, and Myr-untreated cells were used as a control. Prior to
measurements, cells were perfused with extracellular solution
containing various concentrations of Myr at a rate of 3 ml/min. The
perfusion volume was ~15 ml per experiment so that the original
solution was replaced completely. The Myr incubation
time-dependency was analyzed using the mean ± standard error of the
current suppression ratio
(IC−IA)/IC,
where IC represents the Ikur in
the control group and IA stands for
Ikur in the presence of Myr.
Statistical analysis
Electrophysiological data were analyzed using
Patchmaster (Heka Elektronik), Clampfit 10.0 (Axon Instruments,
Molecular Devices, Sunnyvale, CA, USA), and Origin 9 software
(OriginLab, Northampton, MA, USA). The results were analyzed using
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA), and values are expressed
as the mean ± standard error. Statistical comparisons were made
using Student's t-tests for two groups of data, or analysis
of variance for multiple groups of data. P<0.05 was considered
to indicate a statistically significant difference.
Results
Toxicity of Myr
To determine an effective, non-toxic concentration
of Myr for use in the patch-clamp assay, MTT assays were performed
following treatment of HEK293 cells with Myr at a range of doses.
Myr caused a dose-dependent decrease in cell growth between 2 and 6
mM with an IC50 of 4.66 mM (Fig. 1B). However, no cell growth
inhibition was observed at 0–100 µM Myr, which was therefore
used in the subsequent experiments.
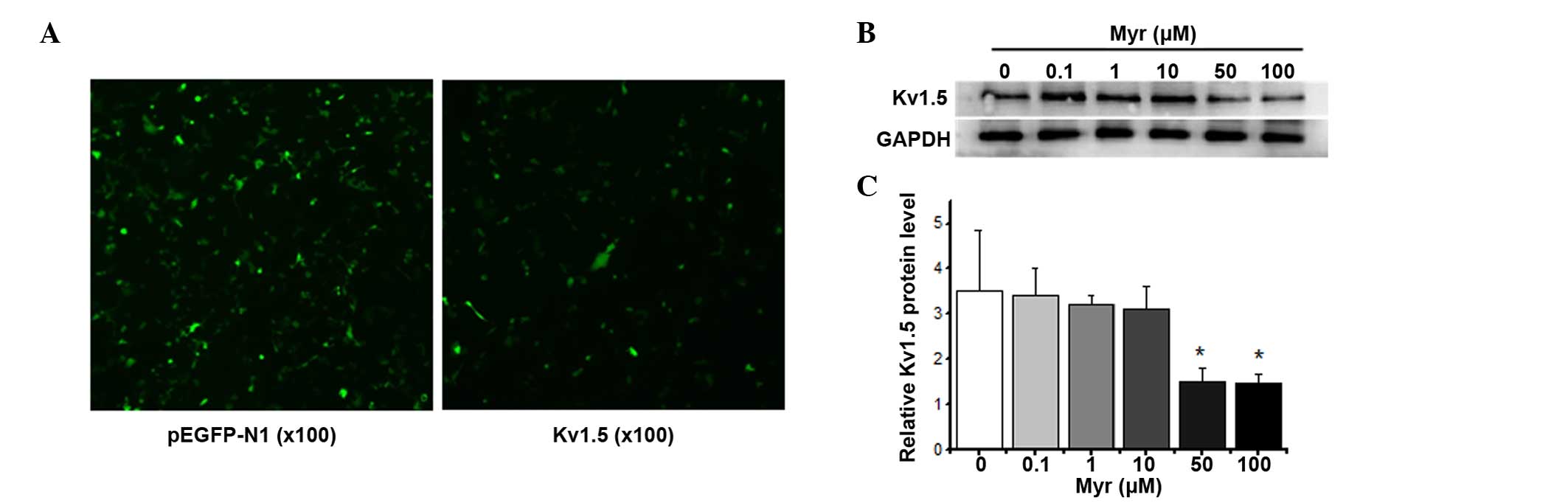
Myr inhibits hKv1.5 protein
expression in a dose-dependent manner
A Kv1.5 wild-type overexpression plasmid
was transiently transfected into HEK293 cells using Entranster™-H
transfection reagent (Engreen Biosystem Co., Ltd., Beijing, China)
for 24 h (Fig. 2A), and the levels
of Kv1.5 protein were assessed using western blot
analysis after treatment with Myr (0–100 µM) for 24 h
(Fig. 2B). Myr induced a
dose-dependent decrease in the expression of Kv1.5 after
24 h.
Myr inhibits Ikur
Patch clamp experiments on cells perfused with
extracellular solution containing various concentrations of Myr
showed that 5 µM Myr reduced the current amplitude from
215.01±40.59 to 77.72±17.94 pA/pF, suggesting that
Ikur was significantly inhibited by Myr (P=0.011
vs. control; n=5) (Fig. 3).
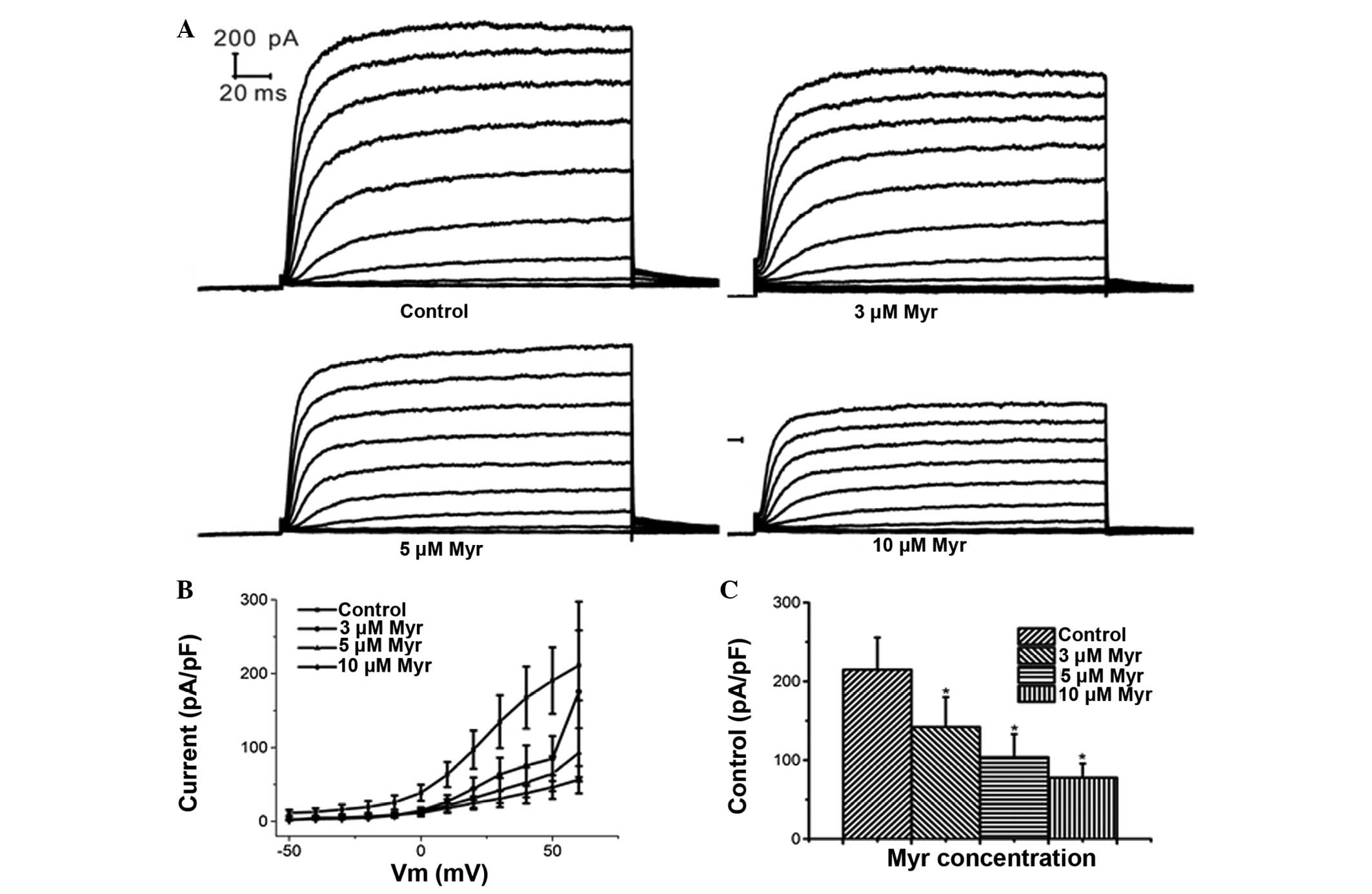
Myr inhibits Ikur in a
dose-dependent manner
At a clamping voltage of 60 mV, the current density
(Im/Cm, pA/pF) was 142.15±37.80, 103.75±29.32 and 77.72±17.94 in
the presence of 3, 5 and 10 µM Myr, respectively (Fig. 4A–C), which was significantly
different from that in the control group (215.01±40.59 pA/pF; all
P<0.05; n=5). This suggested that Myr inhibited
Ikur at concentrations of 3–10 µM in a
concentration-dependent manner.
Myr inhibits Ikur in a
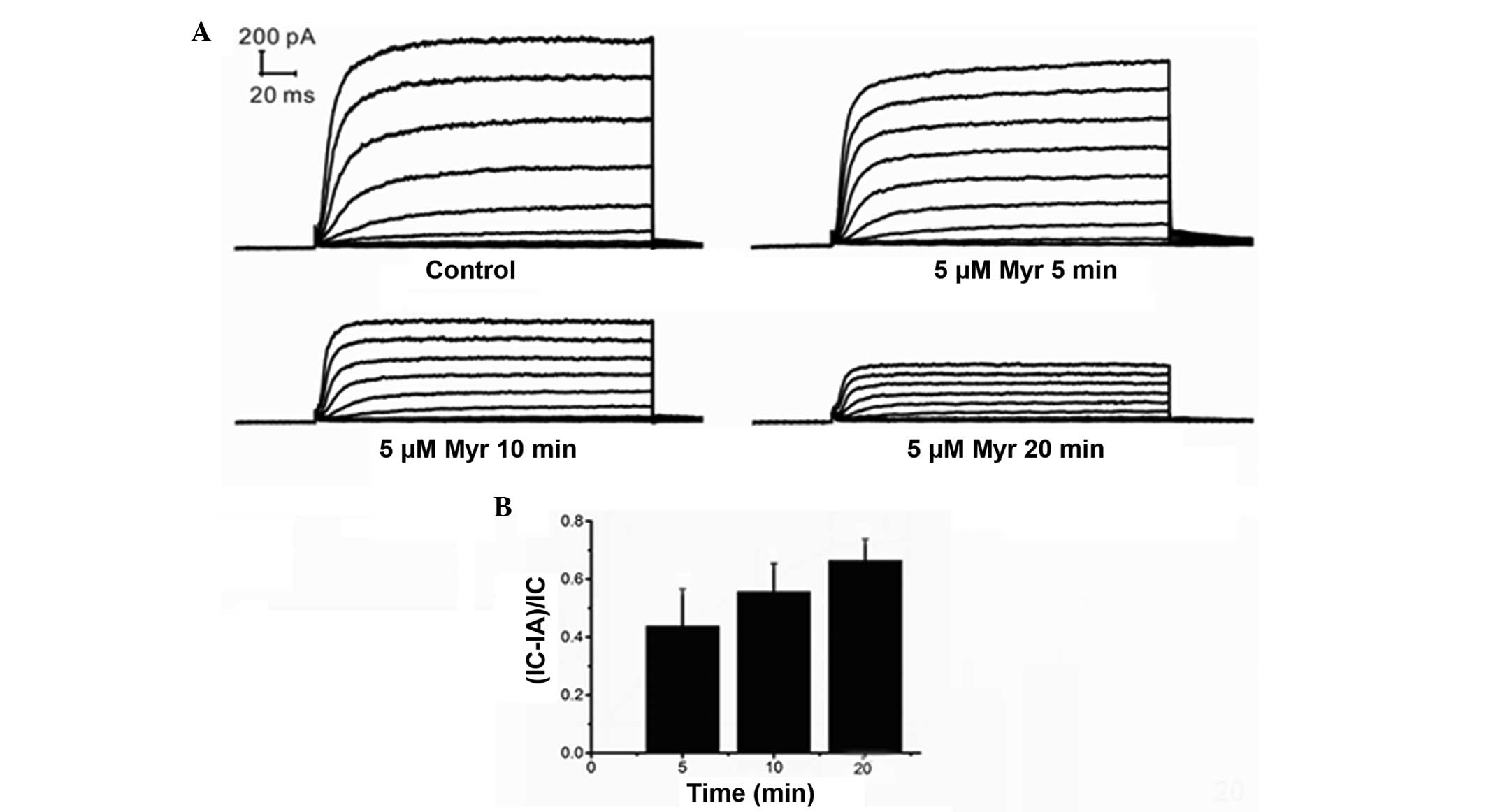
time-dependent manner
Ikur was recorded from 5 to 30 min
after the addition of Myr to the chamber to evaluate the
time-dependent effects of Myr on Ikur. The
results indicated that the effects of Myr were time-dependent
(Fig. 5A and B). The effects of
time were analyzed using the mean ± standard error of the current
suppression ratio (IC-IA)/IC, where IC represents the
Ikur in the control group and IA stands for
Ikur in the presence of Myr. The (IC-IA)/IC was
0.3101±0.1234 at 5 min (n=7; P=0.046 vs. control), 0.4091±0.1180 at
10 min (n=7; P=0.066 vs. 5 min; P=0.013 vs. control),
0.05352±0.0978 at 15 min (n=7, P=0.004 vs. 10 min; P=0.009 vs. 5
min; P=0.002 vs. control), and 0.5497±0.1060 at 20 min (n=7;
P=0.453 vs. 15 min; P=0.023 vs. 10 min; P=0.004 vs. control). These
results demonstrated that the Ikur amplitude
decreased with time and that the inhibitory effects of Myr
increased over time.
Myr inhibits Ikur in a
frequency-dependent manner
A train of 20 pulses, each with a 200-msec single
voltage step, were used from a holding potential of −80 mV to +50
mV at frequencies of 0.5, 1, 3 and 4 Hz (Fig. 6A). The inhibitory effects of Myr on
Ikur increased with the continuous depolarization
voltage pulses. In addition, the inhibition of
Ikur by Myr was dependent on the number of pulses
(Fig. 6B and C), which suggested
that Myr inhibited Ikur in a rate- or
frequency-dependent manner. Specifically, Ikur
was 376.23±1.30, 340.01±4.25, 290.59±4.44 and 270.193±4.28 pA/pF at
0.5, 1, 3 and 4 Hz, respectively, and changes between each set of
two groups were significant (n=5, P<0.001). Furthermore, the
inhibition of Ikur significantly increased when
the frequency was increased from 0.5–4 Hz (P<0.05) (Fig. 6D). These results suggested that Myr
inhibited Ikur in HEK293 cells in a
frequency-dependent manner.
Discussion
Ikur participates in phases I and
II of atrial re-polarization, and affects atrial rhythm and
frequency by changing the action potential duration and effective
refractory period. Therefore, abnormal electrical activity is
closely associated with the occurrence and maintenance of AF.
Furthermore, Ikur is present in the atria
(6), but not in the ventricles of
the human heart (11). Human
atrial Ikur is encoded by the
hKv1.5 (or hKCNA5) gene (7,11,24).
Due to its atrial specificity, drugs that affect
Ikur have atrium-specific effects without
affecting ventricular function. Certain studies suggested that
Kv1.5 channels may represent novel targets for the
treatment of AF (25,26). The present study demonstrated that
Myr was able to block Ikur in a dose-, time- and
frequency-dependent manner.
The treatment of AF using drug- or
non-drug-associated methods, including catheter-based or surgical
interventions, has received significant attention in recent years
(27). Catheter ablation has been
proposed as an effective nonpharmacological alternative for AF that
is often, however not always, the second-line treatment (28). In addition, surgical ablation of AF
is commonly performed in cases with other indications for cardiac
surgery and it is limited to cases with low surgical risk (29). However, non-drug treatments are
limited by factors including their indications, medical conditions
and costs. Therefore, drug-based therapies remain the primary
method used to treat AF (30).
Western medicine is widely applied for anti-arrhythmic treatment;
however, its effectiveness is only 30–60%, and varying degrees of
arrhythmogenic effects have been reported (31). By contrast, Traditional Chinese
Medicine comprises effective anti-arrhythmic treatments, which may
provide novel anti-arrhythmia therapies for clinical use.
Flavonoids are a class of compounds with the structure of 2-phenyl
chromone, observed to be ubiquitously observed in food, including
fruits, vegetables, nuts and plant-derived beverages (tea and wine)
(32,33). Flavonoids possess a variety of
pharmacological activities, including against infection (bacterial
and viral diseases) and degenerative diseases such as
cardiovascular diseases, cancer and additional age-associated
diseases (34). Epidemiological
studies suggest that the beneficial cardiovascular health effects
of diets high in fruit and vegetables are associated with their
flavonoid content (35).
Flavonoids such as apigenin have been reported to reduce the
occurrence of various cardiovascular diseases including coronary
disease, arrhythmia, atherosclerosis, hypertension, ischemic stroke
and peripheral arteriopathy, congestive heart failure (36). In addition, certain flavonoids such
as luteolin-7-O-β-D-glucopyranoside (36), catechin (37), quercetin (38), hesperidin/hesperetin (39), silymarin (40) and genistein (41) exhibited cardioprotective effects.
Acacetin, a natural flavone, has been reported to selectively
inhibits human atrial repolarization potassium currents and
prevents atrial fibrillation in dogs (42). Ampelopsin exerts anti-arrhythmic
effects in an aconitine-induced rat arrhythmic model, and the
underlying electrophysiological mechanism was demonstrated to be
partly associated with the inhibition of INa and
enhancement of IK1, and prolongation of action
potential duration (43). Myr is a
natural flavonol identified to be present in onions, tea and other
natural plants, is advantageous due to its cardioactive components
(44). Its chemical structure is
similar to that of ampelopsin, which exerts anti-arrhythmic effects
(43). Previous studies have
suggested that Myr possesses cardio-protective effects (19,20).
The present study was designed to identify the possible
anti-arrhythmic mechanism of action of Myr in order to provide a
theoretical basis for anti-arrhythmic treatments using Myr and
other Traditional Chinese Medicinal compounds.
The results of the present study revealed that Myr
inhibited Ikur in vitro, which provided
the basis for further in vivo experiments. However, the
conclusions of the present study do not warrant effects on the
human body due to the lack of knowledge of the complex regulatory
mechanisms in vivo. Questions regarding the mechanisms of
action of Myr, for example whether it functions by binding to
binding sites, whether these include Kv1.5 channels and
whether it affects other ion channels, remain to be investigated in
further studies.
Acknowledgments
The present study was funded by the China
Postdoctoral Science Foundation. The authors would like to thank
LetPub (www.letpub.com) for its linguistic
assistance during the preparation of the manuscript.
References
|
1
|
Jones C, Pollit V, Fitzmaurice D and Cowan
C; Guideline Development Group: The management of atrial
fibrillation: Summary of updated NICE guidance. BMJ. 348:g36552014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Bruijn RF, Heeringa J, Wolters FJ,
Franco OH, Stricker BH, Hofman A, Koudstaal PJ and Ikram MA:
Association between atrial fibrillation and dementia in the general
population. JAMA Neurol. Sep 21–2015.Epub ahead of print.
View Article : Google Scholar
|
|
3
|
Schotten U, Hatem S, Ravens U, Jaïs P,
Müller FU, Goette A, Rohr S, Antoons G, Pieske B, Scherr D, et al
EUTRAF investigators: The European network for translational
research in atrial fibrillation (EUTRAF): Objectives and initial
results. Europace. Sep 12–2015.Epub ahead of print. View Article : Google Scholar
|
|
4
|
Patel NJ, Patel A, Agnihotri K, Pau D,
Patel S, Thakkar B, Nalluri N, Asti D, Kanotra R, Kadavath S, et
al: Prognostic impact of atrial fibrillation on clinical outcomes
of acute coronary syndromes, heart failure and chronic kidney
disease. World J Cardiol. 7:397–403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Desai NR and Giugliano RP: Can we predict
outcomes in atrial fibrillation? Clin Cardiol. 35(Suppl 1): 10–14.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fedida D, Wible B, Wang Z, Fermini B,
Faust F, Nattel S and Brown AM: Identity of a novel delayed
rectifier current from human heart with a cloned K+ channel
current. Circ Res. 73:210–216. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li GR, Feng J, Yue L, Carrier M and Nattel
S: Evidence for two components of delayed rectifier K+ current in
human ventricular myocytes. Circ Res. 78:689–696. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naz S, Siddiqi R, Ahmad S, Rasool SA and
Sayeed SA: Antibacterial activity directed isolation of compounds
from Punica granatum. J Food Sci. 72:M341–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phillips PA, Sangwan V, Borja-Cacho D,
Dudeja V, Vickers SM and Saluja AK: Myricetin induces pancreatic
cancer cell death via the induction of apoptosis and inhibition of
the phosphati-dylinositol 3-kinase (PI3K) signaling pathway. Cancer
Lett. 308:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Siegelin MD, Gaiser T, Habel A and
Siegelin Y: Myricetin sensitizes malignant glioma cells to
TRAIL-mediated apoptosis by down-regulation of the short isoform of
FLIP and bcl-2. Cancer Lett. 283:230–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park HH, Lee S, Son HY, Park SB, Kim MS,
Choi EJ, Singh TS, Ha JH, Lee MG, Kim JE, et al: Flavonoids inhibit
histamine release and expression of proinflammatory cytokines in
mast cells. Arch Pharm Res. 31:1303–1311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geraets L, Moonen HJ, Brauers K, Wouters
EF, Bast A and Hageman GJ: Dietary flavones and flavonoles are
inhibitors of poly (ADP-ribose) polymerase-1 in pulmonary
epithelial cells. J Nutr. 137:2190–2195. 2007.PubMed/NCBI
|
|
13
|
Medeiros KC, Figueiredo CA, Figueredo TB,
Freire KR, Santos FA, Alcantara-Neves NM, Silva TM and Piuvezam MR:
Anti-allergic effect of bee pollen phenolic extract and myricetin
in ovalbumin-sensitized mice. J Ethnopharmacol. 119:41–46. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang KA, Wang ZH, Zhang R, Piao MJ, Kim
KC, Kang SS, Kim YW, Lee J, Park D and Hyun JW: Myricetin protects
cells against oxidative stress-induced apoptosis via regulation of
PI3K/Akt and MAPK signaling pathways. Int J Mol Sci. 11:4348–4360.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sim GS, Lee BC, Cho HS, Lee JW, Kim JH,
Lee DH, Kim JH, Pyo HB, Moon DC, Oh KW, et al: Structure activity
relationship of antioxidative property of flavonoids and inhibitory
effect on matrix metalloproteinase activity in UVA-irradiated human
dermal fibroblast. Arch Pharm Res. 30:290–298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghaffari MA and Mojab S: Influence of
flavonols as in vitro on low density lipoprotein glycation. Iran
Biomed J. 11:185–191. 2007.PubMed/NCBI
|
|
17
|
Matić S, Stanić S, Bogojević D, Vidaković
M, Grdović N, Dinić S, Solujić S, Mladenović M, Stanković N and
Mihailović M: Methanol extract from the stem of Cotinus coggygria
Scop., and its major bioactive phytochemical constituent myricetin
modulate pyrogallol-induced DNA damage and liver injury. Mutat Res.
755:81–89. 2013. View Article : Google Scholar
|
|
18
|
Ong KC and Khoo HE: Biological effects of
myricetin. Gen Pharmacol. 29:121–126. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scarabelli TM, Mariotto S, Abdel-Azeim S,
Shoji K, Darra E, Stephanou A, Chen-Scarabelli C, Marechal JD,
Knight R, Ciampa A, et al: Targeting STAT1 by myricetin and
delphinidin provides efficient protection of the heart from
ischemia/reperfusion-induced injury. FEBS Lett. 583:531–541. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tiwari R, Mohan M, Kasture S, Maxia A and
Ballero M: Cardioprotective potential of myricetin in
isoproterenol-induced myocardial infarction in Wistar rats.
Phytother Res. 23:1361–1366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borde P, Mohan M and Kasture S: Effect of
myricetin on deoxycorticosterone acetate (DOCA)-salt-hypertensive
rats. Nat Prod Res. 25:1549–1559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Godse S, Mohan M, Kasture V and Kasture S:
Effect of myricetin on blood pressure and metabolic alterations in
fructose hypertensive rats. Pharm Biol. 48:494–498. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu JX: Effects of HMM on the hKv1.5
channel in HEK293 cells (unpublished PhD thesis). Huazhong
University of Science and Technology; 2010
|
|
24
|
Feng J, Wible B, Li GR, Wang Z and Nattel
S: Antisense oligodeoxynucleotides directed against Kv1.5 mRNA
specifically inhibit ultrarapid delayed rectifier K+ current in
cultured adult human atrial myocytes. Circ Res. 80:572–579. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ou XH, Li ML, Liu R, Fan XR, Mao L, Fan
XH, Yang Y and Zeng XR: Remodeling of Kv1.5 channel in right atria
from Han Chinese patients with atrial fibrillation. Med Sci Monit.
21:1207–1213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baczko I, Liknes D, Yang W, Hamming KC,
Searle G, Jaeger K, Husti Z, Juhasz V, Klausz G, Pap R, et al:
Characterization of a novel multifunctional resveratrol derivative
for the treatment of atrial fibrillation. Br J Pharmacol.
171:92–106. 2014. View Article : Google Scholar :
|
|
27
|
Lawrance CP, Henn MC and Damiano RJ Jr:
Surgery for atrial fibrillation. Cardiol Clin. 32:563–571. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Prystowsky EN, Padanilam BJ and Fogel RI:
Treatment of atrial fibrillation. JAMA. 314:278–288. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abo-Salem E, Lockwood D, Boersma L, Deneke
T, Pison L, Paone RF and Nugent KM: Surgical Treatment of Atrial
Fibrillation. J Cardiovasc Electrophysiol. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Reiffel JA, Camm AJ, Belardinelli L, Zeng
D, Karwatowska-Prokopczuk E, Olmsted A, Zareba W, Rosero S and
Kowey P; HARMONY Investigators: The HARMONY trial: Combined
ranolazine and dronedarone in the management of paroxysmal atrial
fibrillation: Mechanistic and therapeutic synergism. Circ Arrhythm
Electrophysiol. 8:1048–1056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Christ T and Ravens U: Do we need new
antiarrhythmic compounds in the era of implantable cardiac devices
and percutaneous ablation? Cardiovasc Res. 68:341–343. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu J, Jia YY, Chen SR, Ye JT, Bu XZ, Hu Y,
Ma YZ, Guo JL and Liu PQ:
(E)-1-(4-ethoxyphenyl)-3-(4-nitrophenyl)-prop-2-en-1-one suppresses
LPS-induced inflammatory response through inhibition of NF-κB
signaling pathway. Int Immunopharmacol. 15:743–751. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Glossman-Mitnik D: A comparison of the
chemical reactivity of naringenin calculated with the M06 family of
density functionals. Chem Cent J. 7:1552013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar S and Pandey AK: Chemistry and
biological activities of flavonoids: An overview. Scientific World
Journal. 2013:1627502013. View Article : Google Scholar
|
|
35
|
Dayoub O, Andriantsitohaina R and Clere N:
Pleiotropic beneficial effects of epigallocatechin gallate,
quercetin and delphinidin on cardiovascular diseases associated
with endothelial dysfunction. Cardiovasc Hematol Agents Med Chem.
11:249–264. 2013. View Article : Google Scholar
|
|
36
|
Singh M, Kaur M and Silakari O: Flavones:
An important scaffold for medicinal chemistry. Eur J Med Chem.
84:206–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Islam MA: Cardiovascular effects of green
tea catechins: Progress and promise. Recent Pat Cardiovasc Drug
Discov. 7:88–99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Zhang J, Dong X, Deng H and Yang F:
Quercetin protects against lipopolysaccharide-induced cardiac
injury in mice. Nan Fang Yi Ke Da Xue Xue Bao. 35:1068–1072.
2015.In Chinese. PubMed/NCBI
|
|
39
|
Roohbakhsh A, Parhiz H, Soltani F, Rezaee
R and Iranshahi M: Molecular mechanisms behind the biological
effects of hesperidin and hesperetin for the prevention of cancer
and cardiovascular diseases. Life Sci. 124:64–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zholobenko A and Modriansky M: Silymarin
and its constituents in cardiac preconditioning. Fitoterapia.
97:122–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liew R, Stagg MA, Chan J, Collins P and
MacLeod KT: Gender determines the acute actions of genistein on
intracellular calcium regulation in the guinea-pig heart.
Cardiovasc Res. 61:66–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li GR, Wang HB, Qin GW, Jin MW, Tang Q,
Sun HY, Du XL, Deng XL, Zhang XH, Chen JB, et al: Acacetin, a
natural flavone, selectively inhibits human atrial repolarization
potassium currents and prevents atrial fibrillation in dogs.
Circulation. 117:2449–2457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang Y, Fu L, Wang L, Xu L and Yang B:
Electrophysiological study on the antiarrhythmic mechanism of
ampelopsin in rats. Zhonghua Xin Xue Guan Bing Za Zhi. 42:675–679.
2014.In Chinese. PubMed/NCBI
|
|
44
|
Huang H, Chen AY, Ye X, Li B, Rojanasakul
Y, Rankin GO and Chen YC: Myricetin inhibits proliferation of
cisplatin-resistant cancer cells through a p53-dependent apoptotic
pathway. Int J Oncol. 47:1494–1502. 2015.PubMed/NCBI
|