Introduction
Sumoylation, a post-translation modifier, is
involved in various biological processes and, as one of the most
intensively investigated fields in cancer pathogenesis, has been
suggested to modify proteins by attaching small ubiquitin-like
modifier (SUMO), which affects function of the target proteins in
organismal development (1,2). At present, three paralogs of SUMO
have been found, including SUMO-1, SUMO-2 and SUMO-3 (3,4). The
latest reports indicate a novel paralog, SUMO-4, however, no
evidence has been reported to demonstrate whether the SUMO-4
protein is an endogenously expressed protein in cells (5,6).
SUMO-specific protease 1 (SENP1), is one of the members of
desumoylation protease, which is curial in transcriptional
regulation and hypoxia signaling (7). Further investigations have suggested
that SENP1 is associated with the progression of prostate cancer
(8,9). In addition, SUMO-1 can alter the
activation of promyelocytic leukemia protein oncogenic domains
(10). Thus, sumoylation has been
regarded as an important pathway for disease pathogenesis, which
merits its further investigation.
Until now, substantial evidence indicates that SUMO
modification is involved in tumorigenesis (11,12).
The levels of ubiquitin-conjugating enzyme (UBC)9, one of
homologous of UBCs, have been found to be increased in several
types of cancer (13). In
addition, UBC9 promotes tumor cell growth (14). Protein inhibitor of activated STAT
(PIAs)3, one of the members of the SUMO E3 pathway, is also
increased in several types of cancer, including lung cancer,
pancreatic cancer, colorectal cancer and hepatocellular carcinoma
(15–17). Furthermore, sumoylation may
regulate tumor suppressor proteins, including p53, p63, p73 and
retinoblastoma protein (12). The
p53 protein is stimulated by a degradative pathway, which can be
modified by SUMO-1 sumoylation at the K386 site in the C-terminus
(18). Thus, SUMO-1 regulates the
crucial pathway of cancer progression in organisms or cells,
rendering it a potential target for therapeutic modulators.
PIAs contain PIAs1, PIAs3, PIAs1, PIAs3, PIAs3L and
PIAsy, as well as PIAsxα and β splice variants (19). These proteins are predominantly
expressed in the nucleus and are characterized by an N-terminal SAP
and C-terminal SP-RING domain (19). A previous study showed that PIAsxα
can stimulate E3 ligases in sumoylation (20). PIAsxα has also been shown to act as
a ligase of SUMO E3, and suppresses tumor development by promoting
the expression of phosphatase and tensin homologue deleted on
chromosome (PTEN) (21). These
findings suggest that PIAsxα is a novel SUMO E3 ligase in the
PTEN-associated cancer progression pathway.
In tumor cells, cell proliferation and division are
regulated by key regulators of the cell cycle, including cyclin D
kinase (CDK) and cyclin D proteins (22). If the expression of these genes are
disrupted, the cell cycle is arrested or delayed at checkpoints,
and results in the inhibition of cell proliferation. In addition,
CDK and cyclin D proteins stabilize the genome in the G1 phase,
which assists genome duplication in this process (23). Thus, the dysregulation of CDKs and
cyclins trigger disordered cell proliferation and cell division,
which are found in cancer cells.
In present study, an overexpression vector and
siRNAs were used to understand the effects of PIAsxα on U2-OS
osteosarcoma cells. In addition, the progression of the U2-OS cell
line was confirmed using flow cytometry. The expression levels of
CDKs and cyclin D were detected following transfection to induce
overexpression or with siRNAs, and treatment to rescue the effects
of overexpression and siRNA transfection, in U2-OS cells. The
results of these investigations may elucidate the effects of PIAsxα
and its mechanism on the progression of osteosarcoma.
Materials and methods
Patients
Tissue samples from patients with osteosarcoma were
collected from the Second Hospital of Xiangya, Central South
University (Changsha, China). The experiments were performed,
according to guidelines and following approval by Xiangya School of
Medicine Research Ethics Committee, Central South University.
Written informed consent was obtained from the patients, according
to the Declaration of Helsinki (24). The clinical parameters of the
patients, including age, gender and histological examination of the
state of invasiveness were also collected. The osteosarcoma and
adjacent tissues samples were obtained from 25 patients diagnosed
with TNM stage III osteosarcoma. These patients had not undergone
radical prostatectomy or any previous treatment.
Cell line and culture
The U2-OS osteosarcoma cell line was provided by the
Department of Orthopedic Surgery, Xiangya Hospital of Central South
University. The cells were seeded into 24-well plates at a density
of 1×106 cells per well. The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich, St. Louis,
MO, USA) with 10% bovine serum (Sigma-Aldrich) in a 37°C humidified
incubator. The concentration of CO2 in the humidified
incubator was 5%. Cell viability was confirmed using trypan blue
staining, and visualized using a Nikon Eclipse E400 microscope
(Nikon, Tokyo, Japan).
Transfection of PIAsxα vectors
To analysis the effect of PIAsxα on osteosarcoma
cell progression, a PIAsxα overexpression vector was constructed.
The overexpression vector was constructed using a pcDNA3.1(t)
plasmid (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The full-length coding sequence of PIAsxα was obtained from
GenBank (www.ncbi.nlm.nih.gov/genbank/), and the following
primers were designed to obtain the coding sequence with
XbaI and HindIII sites: Forward
5′-TTGTCTAGAATGGCGGATTTCGAAGAGTT-3′ and reverse
5′-AAGCTTTCACTGTTGCACAGTATCAGA-3′. The fragments were cloned using
a cDNA library from adjacent tissues. A T100™ thermal cycler was
used (Bio-Rad Laboratories, Inc., Richmond, CA, USA). The PCR
reaction comprised 30 cycles of 94°C for 30 sec, 52°C for 30 sec
and 72°C for 3 min. Subsequently, the PCR product was digested with
XbaI and HindIII (Shanghai Sangon Biotech Co., Ltd.,
Shanghai, China). The digested PCR fragments were inserted into the
pcDNA3.1(t) plasmid (Invitrogen; Thermo Fisher Scientific, Inc.)
using T4 DNA Ligase (Shanghai Sangon Biotech Co., Ltd.).
Subsequently, the vector was transfected into the U2-OS cells using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.).
Small interfering (si)RNAs of PIAsxα and the GAPDH
control were constructed using a Silencer siRNA Construction kit
(Ambion; Thermo Fisher Scientific, Inc.), according to a previous
report (21). The siRNA sequence
was 5′-GUGGAAGGCAACGAGUGGAUU-3, which was synthesize by Shanghai
Sangon Biotech Co., Ltd. The target sequence was 5′-AAT CCA CTC GTT
GCC TTC CAC-3′. The transfection protocol was performed, as
described previously.
The cells cultured in 12-well plates
(1×106) were randomly divided into five groups:
Untreated control group; PIAsxα interference group (20 µM
PIAsxα siRNA); PIAsxα overexpression group (1 µg PIAsxα
pcDNA3.1(t) vector transfection); PIAsxα interference rescue group
(20 µM siRNAs for 24 h following 1 µg pcDNA3.1(t)
PIAsxα vector transfection for 24 h); and PIAsxα overexpression
rescue group (1 µg pcDNA3.1(t) PIAsxα vector transfection
for 24 h following 20 µM siRNAs for 24 h). Each group was
treated in five independent parallel experiments. Following the
respective treatments, analyses of the cells were performed.
Flow cytometry
The cells were first fixed in 70% ethanol overnight
and resuspended in propidium iodide (50 µg/ml;
Sigma-Aldrich). The DNA content was measured using a flow cytometer
(FACScan; BD Biosciences, Franklin Lakes, NJ, USA). The cells were
treated with 0.5 µg/ml annexin V-phycoerythrin and 0.5
µg/ml 7-aminoactinomycin D (7-ADD; BD Biosciences) for 15
min at room temperature. Cell death was subsequently calculated
based on positive signals of annexin V, 7-AAD, or the two
combined.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The mRNA expression levels of the assessed genes,
including cyclin D and CDK, were detected using RT-qPCR. The total
RNAs of the cells were extracted using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacture's
protocol. Subsequently, 1 µg of the total RNAs was used to
synthesize the first chain of cDNA, which was constructed using a
Takara cDNA Library Construction kit (Takara Biotechnolohy Co.,
Ltd., Dalian, China). Subsequently, the expression levels of cyclin
D1, cyclin D3, CDK4, CDK6 and CDK8 were assayed on a 7500 Real-Time
PCR system Applied Biosystems; Thermo Fisher Scientific, Inc.). The
reaction mixture contained 1 µl cDNA, 10 µl 1X
reaction buffer. and 0.1 mM forward and reserve primers,
respectively. The primers for the cyclin D1, cyclin D3, CDK4, CDK6
and CDK8 genes were designed, as in a previous study (25), with the following sequences: cyclin
D1, forward 5′-TCG TTG CCC TCT GTG CCA CA-3′ and reverse: 5′-AGG
CAG TCC GGG TCA CAC TT-3′; cyclin D3, forward 5′-TGG TCC TAG GGA
AGC TCA AGT G-3′ and reverse 5′-CTG TAG CAC AGA GGG CCA AAA-3′;
CDK4, forward 5′-GCT ACC ACT CGA TAT GAA CCC GTG GCT GAA-3′ and
reverse 5′-GGT GCT TTG TCC AGG TAT GTC CGT AGG TCC-3′; CDK6,
forward 5′-ATC TAT AGT TTC CAG ATG GCT CTA ACC-3′ and reverse
5′-ACA AAC TTC TCA ATT GGT TGG GCA GAT TT-3′; CDK8, forward 5′-ATG
GAC TAT GAC TTT AAA GTG AAG CTG AGC AGC GA-3′ and reverse 5′-ATC
AGC ATG AGA CAG AAA CAC CTT TTG AAG-3′; and GAPDH, forward 5′-AAC
CCA GAA GAC TGT GGA TGG-3′ and reverse 5′-GGA GAC AAC CTG GTC CTC
AG-3′. The PCR conditions were as follows: 95°C for 10 min,
followed by 40 cycles at 95°C for 15 sec and 60°C for 45 sec. The
mRNA relative expression levels were analyzed using the
2−ΔΔCq method (26).
Western blot analysis
The protein expression levels of the assessed genes,
including cyclin D and CDK, were detected using western blot
analysis. The cells were first dissociated using
radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA), and centrifugation was performed at
20,000 × g for 15 min at 4°C. to isolate the protein in the cells.
The proteins were quantified using a Bradford kit (Beyotime
Institute of Biotechnology), according to the manufacturer's
protocol. Subsequently, 10 µg protein of each sample was
separated on 10% SDS-PAGE gels (Bio Rad Laboratories, Inc.,
Hercules, CA, USA) and then transferred onto polyvinylidene
fluoride membranes (Santa Cruz Biotechnology, Inc.). Following
blocking with 4% non-fat milk, the membranes were incubated at 4°C
overnight with following monoclonal rabbit primary antibodies:
anti-PIAsxα (cat. no. ab50226; Abcam, Cambridge, MA, USA),
anti-cyclin D1 (cat. no. ab7958; Abcam), anti-cyclin D3 (cat. no.
ab112034; Abcam), anti-CDK4 (cat. no. ab7955; Abcam), anti-CDK6
(cat. no. ab151247; Abcam), anti-CDK8 (cat. no. ab123940l Abcam)
and anti-GAPDH (cat. no. ab9485; Abcam). The membranes were then
washed three times using 1X phosphate-buffered saline with 0.05%
Tween-20, and incubated with secondary antibody (goat anti-rabbit
IgG; cat. no. ab6721; Abcam) at room temperature for 30 min. The
signals were detected using chemiluminescent substrate (ECL Plus;
GE Healthcare Life Sciences, Chalfont, UK). The primary antibodies
used in the present study were monoclonal antibodies of
recombination human proteins produced from rabbit: anti-PIAsxα
(cat. no. ab50226; Abcam), anti-cyclin D1 (cat. no. ab7958; Abcam),
anti-cyclin D3 (cat. no. ab112034; Abcam), anti-CDK4 (cat. no.
ab7955; Abcam), anti-CDK6 (cat. no. ab151247; Abcam), anti-CDK8
(cat. no. ab123940l Abcam) and anti-GAPDH (cat. no. ab9485; Abcam).
Quantification of protein expression levels was performed using
Gel-Pro Analyzer 4.0 (Media Cybernetics, Inc., Silver Spring, MD,
USA). The mean band intensity of three repeats was determined, and
the relative intensity of each blot was calculated as a percentage
of the blot.
Immunohistochemistry
The tissues were fixed in 10% formalin overnight and
were prepared as 10 µm thick tissue sections. Following
de-waxing, the samples were stained using primary antibodies at 4°C
overnight and secondary antibodies at room temperature for 30 min,
as described above. A horseradish peroxidase complex and
diaminobenzidine were used to visualize the signals (Beyotime
Institute of Biotechnology, Haimen, China). The slides were
mounted, according to the following criteria: 0, no staining; 1,
partial staining around cells; 2, strong staining of cells. For
each sample, 10 visual fields were randomly selected. Images were
obtained using an OLYMPUS CX31 microscope (Olympus Corporation,
Tokyo, Japan).
The treated U2-OS osteosarcoma cells were first
transferred onto sterile glass cover slips and then fixed with 4%
paraformaldehyde (Shanghai Sangon Biotech Co., Ltd.). Following
blocking with 5% fetal bovine serum, the cells were incubated with
primary antibodies, as described above, for 1 h at 37°C. Following
incubation with secondary antibodies with fluorescein
isothiocyanate-goat anti-rabbit IgG (Santa Cruz Biotechnology,
Inc.), the signals of the proteins were detected using a
fluorescence microscope (Nikon TMS; Nikon Corporation, Tokyo,
Japan).
Tumor formation in a nude mouse
model
To determine the function of PIAsxα in osteosarcoma
cells in vivo, 5-week-old nude mice were used as model of
tumor formation. The animal experiments performed were approved by
Xiangya School of Medicine Research Ethics Committee, Central South
University. The mice were maintained under specific pathogen-free
conditions in a 12 h light/dark cycle with a room temperature of
24±1°C. All mice were provided with rodent chow (Prolab RMH 3000;
PMI LabDiet, Richmond, IN, USA) and distilled deionized water.
Treatment groups were housed separately. A total of 20 female nude
mice were randomly divided into two groups (10 mice in each group).
The two groups were injected with U2-OS cells and cells
overexpressing PIAsxα 24 h following transfection, respectively.
For each individual, 20,000 cells in 1 ml DMEM were injected on the
dorsal side every 3 days for a total of 21 days. The tumor volume
was calculated as follows: Volume (mm3) = (length ×
width2) / 2. The length and width of the tumor was
measured using vernier calipers without anesthesia or surgery. The
gene expression levels were determined at the end of the 21 day
period. Following the experiments, the mice were anesthetized and
sacrificed under xylazine (10 mg/kg, intraperitoneally; Invitrogen;
Thermo Fisher Scientific, Inc.), and the tissues were collected for
examination.
Statistical analysis
The data are presented as the mean ± standard
deviation. All data were statistically analyzed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Significant differences in
expression were confirmed using one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
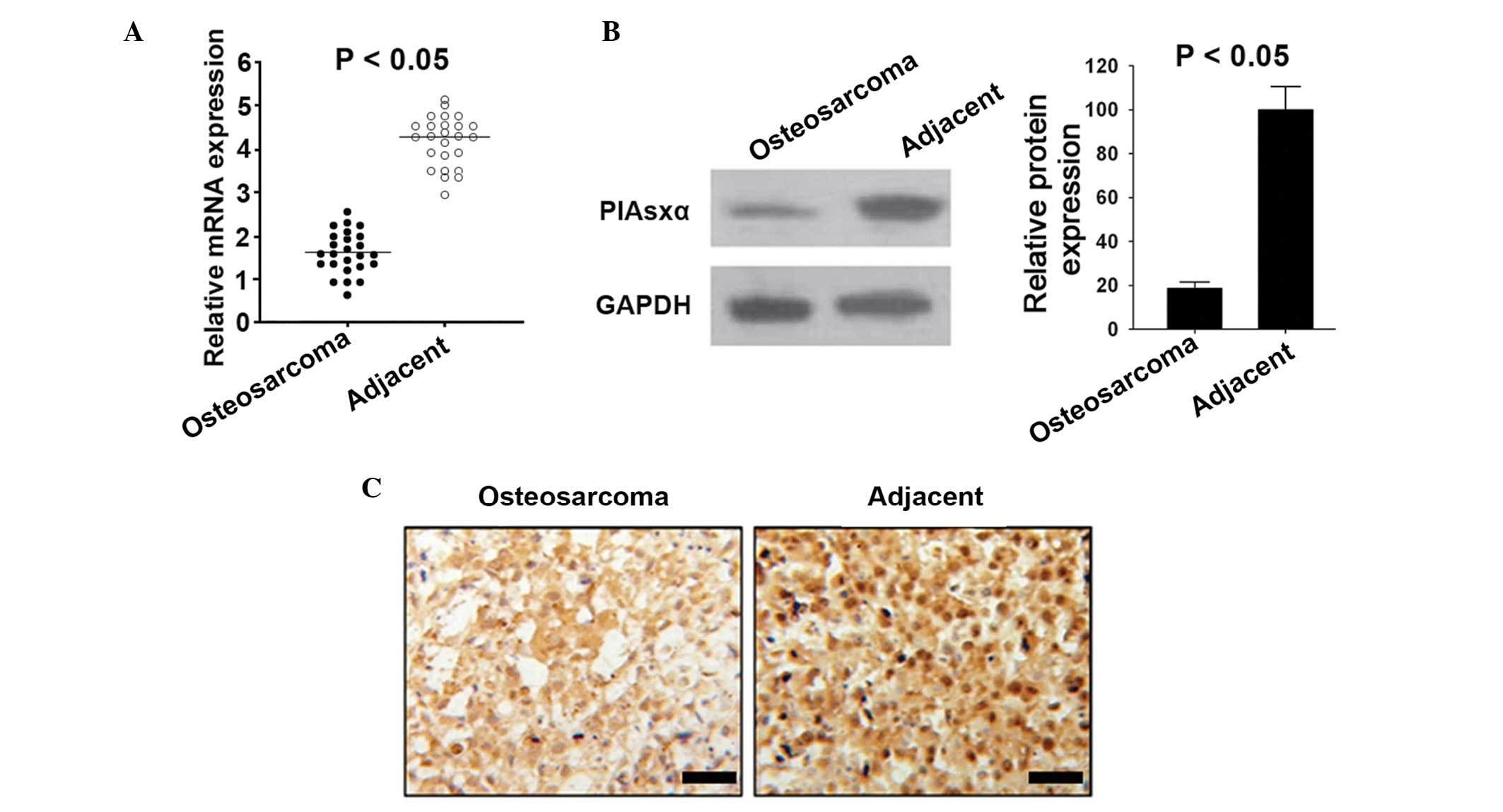
Expression of PIAsxα is reduced in
osteosarcoma
The mRNA expression levels of PIAsxα in osteosarcoma
were significantly lower in the osteosarcoma tissues, compared with
the adjacent normal tissues, which were determined using RT-qPCR
(Fig. 1A). Similarly, the protein
expression levels of PIAsxα were lower in the osteosarcoma tissues,
compared with the adjacent tissues (Fig. 1B and C).
PIAsxα induces apoptosis in the U2-OS
cell line
To understand the effects of PIAsxα on osteosarcoma
cell progression, the present study used overexpression and
interference techniques via an overexpression vector and siRNAs.
The results of the flow cytometric analysis revealed that the
levels of apoptosis were highest in the overexpression group
(Fig. 2A and B). In addition, the
rate of G2/M arrest was also highest in the overexpression group,
compared with the rates in the other groups assessed (Fig. 2C).
 | Figure 2Effects of PIAsxα overexpression and
interference on osteosarcoma cell progression. (A) Flow cytometric
analyses of the control, interference, overexpression,
overexpression rescue and interference rescue groups. (B) Apoptosis
indices in the control, interference, overexpression,
overexpression rescue and interference rescue groups. (C) Rates of
G2/M arrest in the control, interference, overexpression,
overexpression rescue and interference rescue groups. Data are
presented as the mean ± standard deviation. *P<0.05,
compared with the control. PIAsxα, protein inhibitor of activated
STAT xα; 7-ADD, 7-aminoactinomycin D; PE, phycoerythrin. |
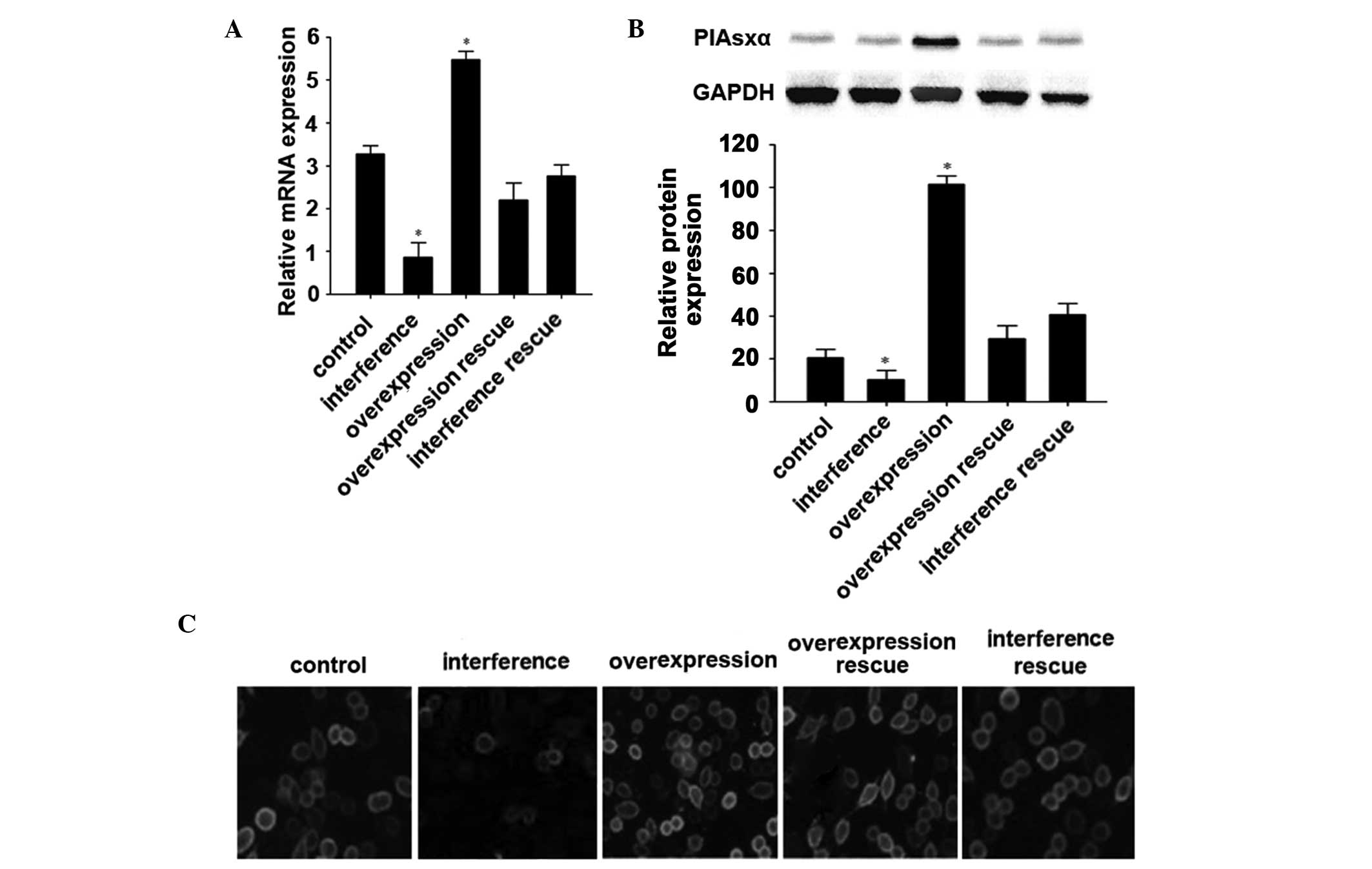
Overexpression and interference of PIAsxα
in the U2-OS cell line
The mRNA and protein expression levels in the U2-OS
cell line following overexpression and interference of PIAsxα were
detected using RT-qPCR, and western blot and immunofluorescence
analyses. The mRNA levels of PIAsxα in the U2-OS cells following
overexpression were the highest among all the groups, whereas the
PIAsxα interference group showed the lowest levels of expression.
The control, overexpression rescue and interference rescue groups
had the median expression levels among the groups (Fig. 3A), and the protein expression
levels were similar. The overexpression group and interference
group showed the highest and lowest protein expression levels among
all the groups in the western blot and immunofluorescence analyses,
respectively (Fig. 3B and C).
These results indicated that the induced overexpression and
interference of PIAsxα in the U2-OS cell line were successful.
PIAsxα downregulates the expression
levels of cyclin D and CDK in U2-OS cells
The present study aimed to further investigate the
inhibitory effects of PIAsxα on U2-OS cells by examine the
mechanism underlying these inhibitory effects. The results of the
present showed that, following overexpression of PIAsxα, the
expression levels of cyclin D1 and cyclin D3 were inhibited. By
contrast, the expression levels of cyclin D1 and cyclin D3
following PIAsxα interference were markedly increased, compared
with the control group. Similarly, the CDK genes including CDK4,
CDK6 and CDK8, increased markedly following PIAsxα interference. By
contrast, the expression levels of CDK4, CDK6 and CDK8 decreased
significantly in the overexpression group, compared with the other
groups (Fig. 4A and B). These
results demonstrated the inhibitory effects of PIAsxα on the cyclin
D and CDK pathways in the osteosarcoma cells.
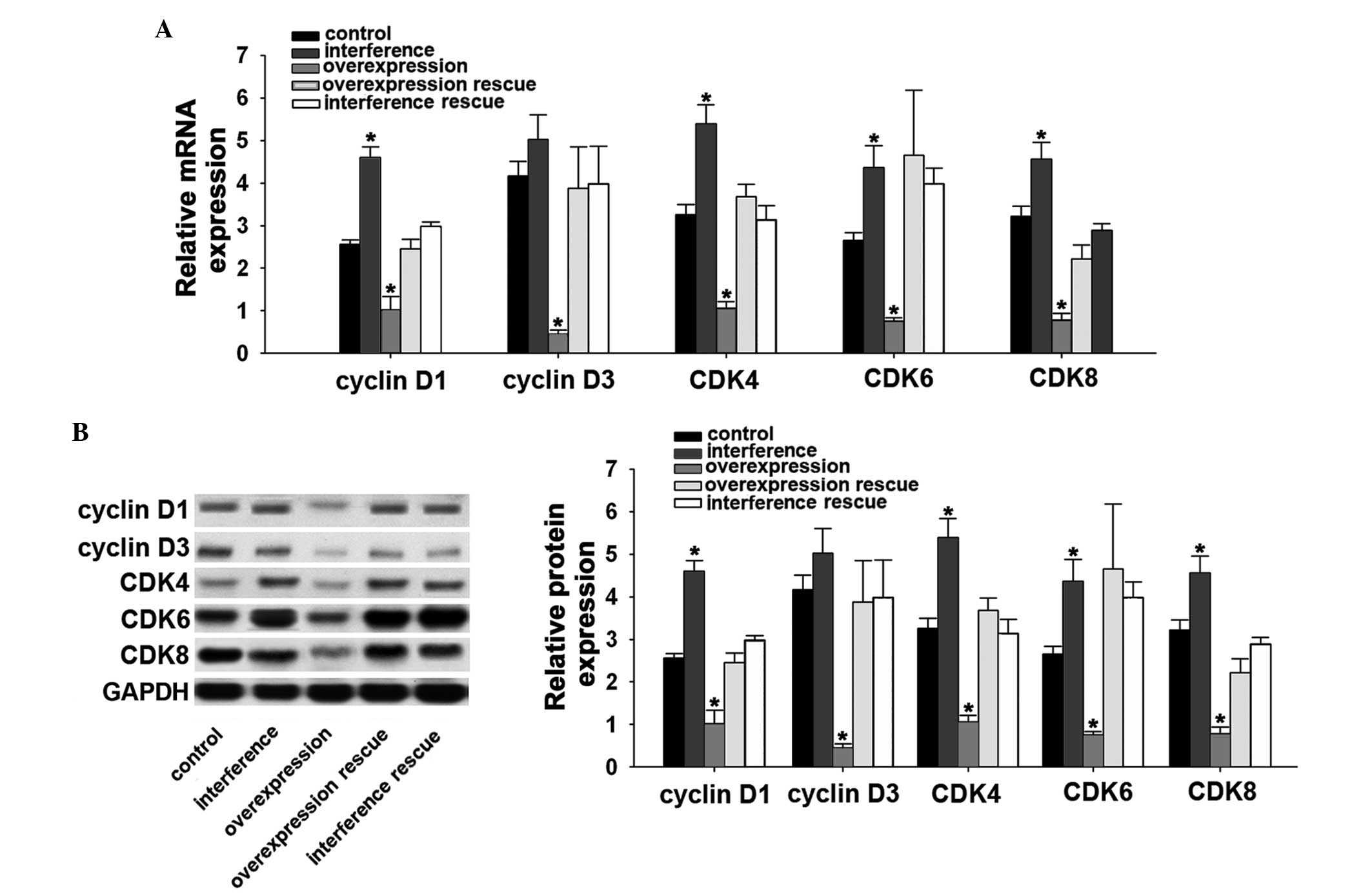 | Figure 4PIAsxα depresses cyclin D and CDK in
U2-OS cells. (A) Reverse transcription-quantitative polymerase
chain reaction analysis of cyclin D1, cyclin D3, CDK4, CDK6 and
CDK8 in the control, interference, overexpression, overexpression
rescue and interference rescue groups. (B) Western blot analysis of
cyclin D1, cyclin D3, CDK4, CDK6 and CDK8 in the control,
interference, overexpression, overexpression rescue and
interference rescue groups. Data are presented as the mean ±
standard deviation. *P<0.05, compared with the
control. PIAsxα, protein inhibitor of activated STAT xα; CDK,
cyclin D kinase. |
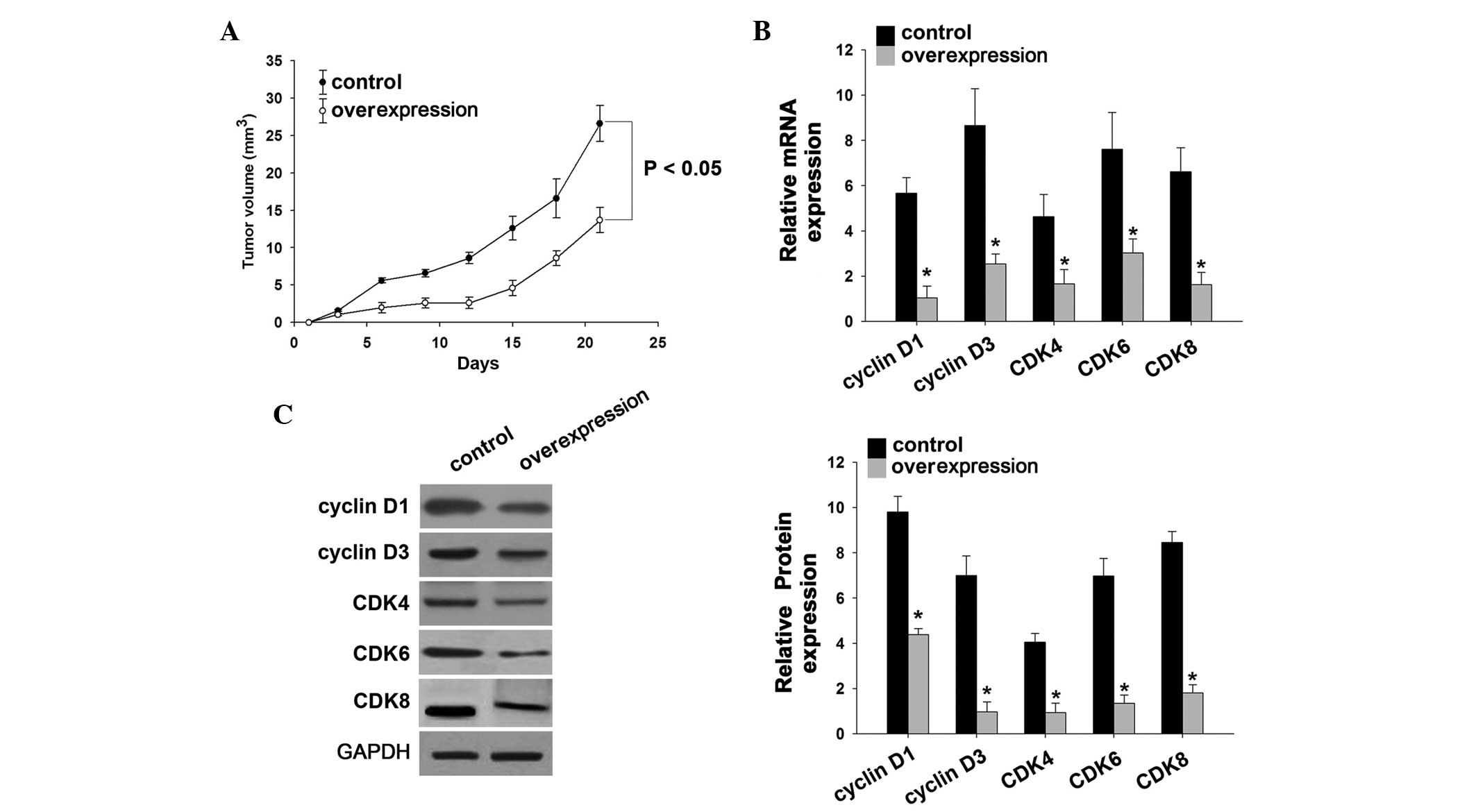
PIAsxα inhibits tumor formation in nude
mice
The xenograft inducing overexpression of PIAsxα in
the U2-OS cells depressed tumor formation, compared with the
untreated U2-OS cells (Fig. 5A).
In addition, the mRNA l and protein expression levels of cyclin D
and CDK were depressed by the increased expression of PIAsxα
(Fig. 5B and C), indicating that
the PIAsxα repressed these genes in the nude mouse tumor model.
Discussion
The results of the present study demonstrated that
the inhibitory effect of PIAsxα on the progression of osteosarcoma
cells occurred via the cyclin D and CDK pathway. The expression
levels of PIAsxα in osteosarcoma tissues were markedly lower,
compared with those in normal tissues, indicating impairment of the
mechanism mediating the formation of osteosarcoma. In addition, the
present study determined the expression levels of PIAsxα in
different groups, including control, overexpression, interference,
overexpression rescue and interference rescue groups. Of note, the
overexpression of PIAsxα induced the inhibition of osteosarcoma
cells, which is similar to other types of cancer cell, including
hepatic carcinoma cells and colon cancer cells (27–29).
In previous studies, it has been confirmed the downregulated
expression levels of PIAsxα may be associated with tumor
development and cell proliferation (29). Rodriguez et al reported that
PIAsxα affects the expression of sumoylate PML and PML-RARA,
affecting tumor development (30).
Shinbo et al also found that PIAsxα is essential for PML
degradation in lung carcinoma cells (31). These results are similar to those
of the present study and, together, these findings suggest that
PIAsxα is crucial in the regulation of oncogenic networks in tumor
cells.
Following elucidation of the inhibitory effect of
high expression levels of PIAsxα on osteosarcoma cells, the
metastatic and invasive activities of the cells following changes
in the expression of PIAsxα and changes in the cell cycle require
investigation. At present, reports that PIAsxα alters the cell
cycle in tumor cells directly are limited. Wang et al
reported that overexpression of PIAsxα arrests the cell cycle in
HeLa cells via a PTEN-dependent pathway (21). In the present study, the results
indicated that, following overexpression of PIAsxα, aptoptosis of
the osteosarcoma was induced. It appears that downregulated
expression levels of PIAsxα can control cell proliferation in
cancer cells. Notably, the present study observed no differences in
the overexpression rescue group, compared with the control group.
In addition, the PIAsxα overexpression group arrested the cells at
the G2/M stage. This is in accordance with the apoptotic effect of
PIAsxα on osteosarcoma cells, which suggested that increased
expression of PIAsxα may induce osteosarcoma cell apoptosis via
controlling the cell cycle. Furthermore, these effects indicated
that the progression between the S phase and G2 phase was inhibited
by PIAsxα during mitosis. Taken together, the increased expression
of PIAsxα triggered stagnancy of osteosarcoma cell proliferation at
the G2/M stage, and progression of the cell cycle into
mitosis resulted in the failure of cell division. This suggested
that PIAsxα inhibited the progression of osteosarcoma cells, and
has a regulatory role in cell cycle. However, the mechanisms
underlying the effects of PIAsxα on the cell cycle in osteosarcoma
cells require elucidation.
The expression levels of cyclin D1 and cyclin D3,
and CDK4, CDK6 and CDK8 factors in the control, overexpression,
interference, overexpression rescue and interference rescue groups
were determined to understand the mechanism underlying the effect
of PIAsxα on the inhibition of osteosarcoma cell progression. The
results indicated that all the assessed factors decreased
significantly in the overexpression group. It has been suggested
that cyclin D1 can control cancer cell division by regulating
transcription, mRNA amplification and stabilization (22). Similarly, cyclin D3 is also key in
stimulating cell progression, particularly in cancer cells
(22). The results of the present
study showed that upregulated PIAsxα suppressed the expression of
cyclin D1 and cyclin D3, at the mRNA and protein levels. The CDK
factors, including CDK4, CDK6 and CDK8, were also depressed by
upregulated PIAsxα at the mRNA and protein levels. CDK4, CDK6 and
CDK8 have been confirmed as cell cycle modulators via the
regulation of DNA synthesis and the progression between the G1 and
S stages (22,25). These factors also have been
confirmed to be involved in several types of cancer, which
indicates a potential therapeutic strategy. In the present study,
PIAsxα depressed the expression levels of CDK4, CDK6 and CDK8 in
vivo and in vitro. This suggested that the
overexpression of PIAsxα in osteosarcoma cells suppresses the
expression of cyclin D and CDK, offering a potential novel clinical
therapy for osteosarcoma. In addition, the present study
demonstrated that the upregulated expression of PIAsxα depressed
tumor formation in a nude mouse model, also indicating PIAsxα as a
potential therapeutic target in the treatment of osteosarcoma.
In conclusion, the present results demonstrated
that, by upregulating the expression of PIAsxα, osteosarcoma cells
were suppressed and arrested at the G2/M stage. The molecular
mechanism underlying these effects include the high expression
levels of PIAsxα depressing cyclin D and CDK factors. Therefore,
the expression of PIAsxα controls the expression levels of cyclin D
and CDKs in cell proliferation. These findings offer novel insight
for sumoylation and cell cycle in osteosarcoma, which may
contribute to the treatment of patients with osteosarcoma.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant. no. 81272947).
References
|
1
|
Geiss-Friedlander R and Melchior F:
Concepts in sumoylation: A decade on. Nat Rev Mol Cell Biol.
8:947–956. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeh ET: SUMOylation and De-SUMOylation:
Wrestling with life's processes. J Biol Chem. 284:8223–8227. 2009.
View Article : Google Scholar :
|
|
3
|
Saitoh H and Hinchey J: Functional
heterogeneity of small ubiquitin-related protein modifiers SUMO-1
versus SUMO-2/3. J Biol Chem. 275:6252–6258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson ES: Protein modification by SUMO.
Annu Rev Biochem. 73:355–382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Owerbach D, McKay EM, Yeh ET, Gabbay KH
and Bohren KM: A proline-90 residue unique to SUMO-4 prevents
maturation and sumoylation. Biochem Biophys Res Commun.
337:517–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mukhopadhyay D and Dasso M: Modification
in reverse: The SUMO proteases. Trends Biochem Sci. 32:286–295.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng J, Wang D, Wang Z and Yeh ET: SENP1
enhances androgen receptor-dependent transcription through
desumoylation of histone deacetylase 1. Mol Cell Biol.
24:6021–6028. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng J, Bawa T, Lee P, Gong L and Yeh ET:
Role of desumoylation in the development of prostate cancer.
Neoplasia. 8:667–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Xia N, Li T, Xu Y, Zou Y, Zuo Y,
Fan Q, Bawa-Khalfe T, Yeh ET and Cheng J: SUMO-specific protease 1
promotes prostate cancer progression and metastasis. Oncogene.
32:2493–2498. 2013. View Article : Google Scholar
|
|
10
|
Zhong S, Salomoni P and Pandolfi PP: The
transcriptional role of PML and the nuclear body. Nat Cell Biol.
2:E85–E90. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moschos SJ and Mo YY: Role of SUMO/Ubc9 in
DNA damage repair and tumorigenesis. J Mol Histol. 37:309–319.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alarcon-Vargas D and Ronai Z: SUMO in
cancer-wrestlers wanted. Cancer Biol Ther. 1:237–242. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mo YY, Yu Y, Theodosiou E, Ee PR and Beck
WT: A role for Ubc9 in tumorigenesis. Oncogene. 24:2677–2683. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu S, Sachdeva M, Wu F, Lu Z and Mo YY:
Ubc9 promotes breast cell invasion and metastasis in a
sumoylation-independent manner. Oncogene. 29:1763–1772. 2010.
View Article : Google Scholar :
|
|
15
|
Liu Lm, Yan Mg, Yang Dh, Sun WW and Zhang
JX: PIAS3 expression in human gastric carcinoma and its adjacent
non-tumor tissues. Clin Res Hepatol Gastroenterol. 35:393–398.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okumura F, Matsunaga Y, Katayama Y,
Nakayama KI and Hatakeyama S: TRIM8 modulates STAT3 activity
through negative regulation of PIAS3. J Cell Sci. 123:2238–2245.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Gao H, Bijukchhe SM, Wang Y and Li
T: PIAS3 may represent a potential biomarker for diagnosis and
therapeutic of human colorectal cancer. Med Hypotheses.
81:1151–1154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stehmeier P and Muller S: Regulation of
p53 family members by the ubiquitin-like SUMO system. DNA Repair
(Amst). 8:491–498. 2009. View Article : Google Scholar
|
|
19
|
Palvimo JJ: PIAS proteins as regulators of
small ubiquitin-related modifier (SUMO) modifications and
transcription. Biochem Soc Trans. 35:1405–1408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jackson PK: A new RING for SUMO: Wrestling
transcriptional responses into nuclear bodies with PIAS family E3
SUMO ligases. Genes Dev. 15:3053–3058. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Chen Y, Wang S, Hu N, Cao Z, Wang
W, Tong T and Zhang X: PIASxα ligase enhances SUMO1 modification of
PTEN protein as a SUMO E3 ligase. J Biol Chem. 289:3217–3230. 2014.
View Article : Google Scholar :
|
|
22
|
Hunter T and Pines J: Cyclins and cancer
II: Cyclin D and CDK inhibitors come of age. Cell. 79:573–582.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sherr CJ: G1 phase progression: Cycling on
cue. Cell. 79:551–555. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Association WM: World Medical Association
Declaration of Helsinki: Ethical principles for medical research
involving human subjects. Bulletin of the World Health
Organization. 79:3732001.
|
|
25
|
Neuveut C, Low KG, Maldarelli F, Schmitt
I, Majone F, Grassmann R and Jeang KT: Human T-cell leukemia virus
type 1 Tax and cell cycle progression: Role of cyclin D-cdk and
p110Rb. Mol Cell Biol. 18:3620–3632. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
27
|
Klampfer L: Signal transducers and
activators of transcription (STATs): Novel targets of
chemopreventive and chemotherapeutic drugs. Curr Cancer Drug
Targets. 6:107–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klampfer L: The role of signal transducers
and activators of transcription in colon cancer. Front Biosci.
13:2888–2899. 2008. View
Article : Google Scholar
|
|
29
|
Valentino L and Pierre J: JAK/STAT signal
transduction: Regulators and implication in hematological
malignancies. Biochem Pharmacol. 71:713–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rodriguez MS, Desterro JM, Lain S, Midgley
CA, Lane DP and Hay RT: SUMO-1 modification activates the
transcriptional response of p53. EMBO J. 18:6455–6461. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shinbo Y, Niki T, Taira T, Ooe H,
Takahashi-Niki K, Maita C, Seino C, Iguchi-Ariga SM and Ariga H:
Proper SUMO-1 conjugation is essential to DJ-1 to exert its full
activities. Cell Death Differ. 13:96–108. 2006. View Article : Google Scholar
|