Introduction
Cartilage lesions in the joints remain a major
clinical challenge due to their poor healing ability following
injury. At present, the most commonly used treatments comprise
microfracture, osteochondral autologous transplantation and
autologous chondrocyte implantation, which can provide short-term
success (1,2). Previously, mesenchymal stem cells
(MSCs) have been suggested as an alternative cell-source for
cartilage regeneration, due to their ease of isolation and
amenability to ex vivo expansion (3–5).
Therefore, MSCs may provide a cell source for novel treatment
strategies in cartilage regeneration (4). Histologically, it has been observed
that autologous matrix-assisted MSC implantation generates
significantly more cartilage matrix into cartilage defects in
animal experiments (6,7). However, histological evaluation of
cartilage repair tissue is invasive, and a non-invasive method is
required to systematically evaluate the integrity of the repair
tissue.
Magnetic resonance imaging (MRI), as a non-invasive
approach, has been widely adapted to examine cartilage and repair
tissue (8–10). In addition, advanced high-field MRI
techniques can generate images with high spatial resolution, which
enables visualization of the regenerated repair tissue in more
detail (11,12). Qualitative T2 mapping is
able to differentiate hyaline cartilage from repair tissues, and
assess collagen fibril network organization of the native hyaline
cartilage following cartilage repair procedures (13–15).
Furthermore, diffusion-weighted imaging (DWI) is able to detect
changes in water mobility within the cartilage repair tissue, and
may provide a method of quantification of cartilage maturation and
tissue quality (16,17). Therefore, additional information
regarding cartilage repair tissue can be obtained with
T2-mapping and DWI, in addition to standard
morphological MRI (18).
In the present study, the quality of cartilage
repair tissue following SMSC treatment was investigated in a rabbit
large osteochondral defect model using routine morphological MRI,
T2 mapping and a DWI MRI technique with a 9.4T
high-field. It was hypothesized that osteochondral repair using
SMSCs facilitates the repair of appropriate tissue structure.
Materials and methods
Harvesting synovial cells from
rabbits
In the present study, three New Zealand white
rabbits (male, 5 months old, weighing ~3 kg) were used as the
source of synovial cells. Rabbits were maintained at 20–25°C in a
12/12 h light/dark cycle. Synovium-derived MSCs (SMSCs) were
isolated and expanded, as previously described (19). The rabbits were anesthetized with
pentobarbital (Sigma-Aldrich, St. Louis, MO, USA) and the synovial
tissue (1×1×1 cm) was harvested from the knee joint. Harvested
synovial tissue samples were finely sectioned with scissors and
digested with 0.02% collagenase (Sigma-Aldrich) in high-glucose
Dulbecco's modified Eagle's medium (DMEM, GE Healthcare Life
Sciences, Chalfont, UK) supplemented with 10% fetal bovine serum
(FBS, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
antibiotics (100 U/ml penicillin and 100 U/ml streptomycin; GE
Healthcare Life Sciences). Following overnight incubation at 37°C,
the cells were collected by centrifugation (1,000 × g for 5 min at
room temperature), washed twice with phosphate-buffered saline
(PBS), and resuspended in high-glucose DMEM supplemented with 10%
FBS and antibiotics. The SMSCs were then seeded into culture flasks
(1×105 cells/ml) and incubated in complete medium
(low-glucose DMEM supplemented with 10% FBS and antibiotics) at
37°C in a humidified atmosphere containing 5% CO2 for
proliferation. The complete medium was replaced once every 3 days.
When the attached cells reached 90% confluence, following 9–12 days
of primary culture, the cells were washed twice with sterilized PBS
solution, collected by treatment with trypsin-EDTA (0.25% trypsin
and 1 mM EDTA; Cell Applications, Inc., San Diego, CA USA), and
seeded in new culture flasks at a dilution of 1:4 for the first
sub-culture. The medium was replaced after 2 days to allow cell
adhesion and to remove the adherent cells.
Animal experiments
All animal experiments were approved by the internal
Animal Care and Use Committee at Shanghai Jiaotong University
(Shanghai, China). A total of 15 white New Zealand rabbits (male, 5
months old, weighing 2.7±0.5 kg) were used for the present study.
In the normal group (n=5), a surgical incision was introduced in
the right knee joint without osteochondral defect model
establishment. The remaining 10 rabbits underwent a surgical
procedure to establish an osteochondral defect in the right knee.
Among these, five rabbits subsequently received SMSC therapy (SMSC
group), whereas the remaining five rabbits received no further
treatment (control group). All animals underwent anesthesia by
intravenous administration of 3% pentobarbital (30 mg/kg). Briefly,
the animal was placed in a supine position on the operative table.
Following skin preparation and disinfection, a midline incision was
made, and a lateral parapatellar arthrotomy was performed to expose
the femoral trochlea. A round full-thickness cartilage defect, 6 mm
in diameter and 3 mm in depth, was created in the central portion
of the femoral trochlea groove using a ring-drill (Osteochondral
Autograft Transfer System; Arthrex, Inc., Naples, FL, USA). In the
SMSC group, the defect was injected with the 1% high availability
solution (Shanghai YuanYe Biological Technology Co., Ltd.,
Shanghai, China) containing SMSCs (2×105 cells/ml, 0.5
ml/defect). Following treatment, the patella was reduced, the joint
capsule was closed with interrupted sutures and the wound was
closed in layers. Postoperatively, the animals were returned to
their cages and allowed free cage activity without
immobilization.
All rabbits were sacrificed 12 weeks following the
surgical procedures for examination, using an overdose of
pentobarbital. The macroscopic appearance of the defects was scored
using a newly developed semi-quantitative macroscopic scoring
system, developed by Goebel et al (8). This reverse scale consists of five
major parameters, and a total of 20 points indicates the worst
possible result.
Evaluation by 9.4 T high-field MRI
Immediately following sacrifice, knee tissue samples
were harvested for MRI examination. MRI scans were performed on a
9.4T/400 mm wide bore MRI scanner (Agilent DD2 NMR console; Agilent
Technologies, Inc., Santa Clara, CA, USA) using a volume RF coil
(inner diameter, 40 mm; Bruker Corporation, Billerica, MA, USA). A
3D spoiled gradient echo (GRE) sequence was selected to perform
isovolumetric scans of the osteochondral samples. Minimum voxel
size was set to 125×86×86 µm, and optimized imaging
parameters were evaluated as: Repetition time (TR), 5.4 ms; time
echo (TE), 3 ms; flip angle (FA), 7°; number of excitations (NEX),
9 and bandwidth (BW), 50 kHz. The cartilage defects were evaluated
using a 2D magnetic resonance observation of cartilage repair
tissue (MOCART) score (20).
Qualitative T2 maps were collected using a multi-echo
multi-slice sequence (TR=2,500 ms, TE=10 ms, NEX = 1, FOV = 25 mm
*25 mm, matrix = 256×256, slice thickness = 1 mm).
Diffusion-weighted imaging was selected using a spin echo sequence
(TR/TE = 2,300/36.5 ms, NEX = 1, matrix = 128×128, FOV = 25 mm
*25 mm, section thickness = 1 mm). Pixel-wise
calculation of the apparent diffusion coefficient maps was
performed numerically.
Histology evaluation
Following MRI scans, all samples were fixed in 10%
neutral-buffered formalin solution (Wuhan Goodbio Technology Co.,
Ltd., Wuhan, China) for 2 days, and the samples were then
decalcified in 10% EDTA solution for ~4 weeks at room temperature.
The samples were sectioned (5 µm) through the center of the
defect using a microtome (SM2500; Leica Microsystems GmbH,
Mannheim, Germany), and these sections were stained with
hematoxylin and eosin (Wuhan Goodbio Technology Co., Ltd.) and
Safranin-O (Sigma-Aldrich). The slides were visualized using
inverted light microscopy (IX71SBF-2; Olympus Corporation, Tokyo,
Japan). Digital images were captured using a DP Manager camera
(Olympus Corporation). Two investigators performed histological
analysis in a blinded-manner, in which five histological sections
in each group were scored according to the modified O'Driscoll
scale (21), with a lower score
indicating lower histological quality of the cartilage repair
tissue.
Data analysis
Statistical analysis was performed using Stata 10.0
software (Stata Corp, College Station, TX, USA), and data are
presented as the mean ± standard deviation. Statistical analysis of
the quantitative results was performed using one-way analysis of
variance or a Kruskal-Wallis test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Macroscopic results
No complications were observed in the rabbits
throughout the course of the present study. At 12 weeks following
surgery, the macroscopic appearance of the control group showed
complete defect filling. The surface of the repair tissue was not
flat, compared with that of the normal group. In four samples of
the SMSC group, virtually complete defect filling was observed,
whilst one sample of the SMSCs group exhibited incomplete defect
filling with a severe fissure. For all samples of the SMSC group,
the appearance of the repair tissue was similar to that of the
adjacent cartilage tissue.
MRI results
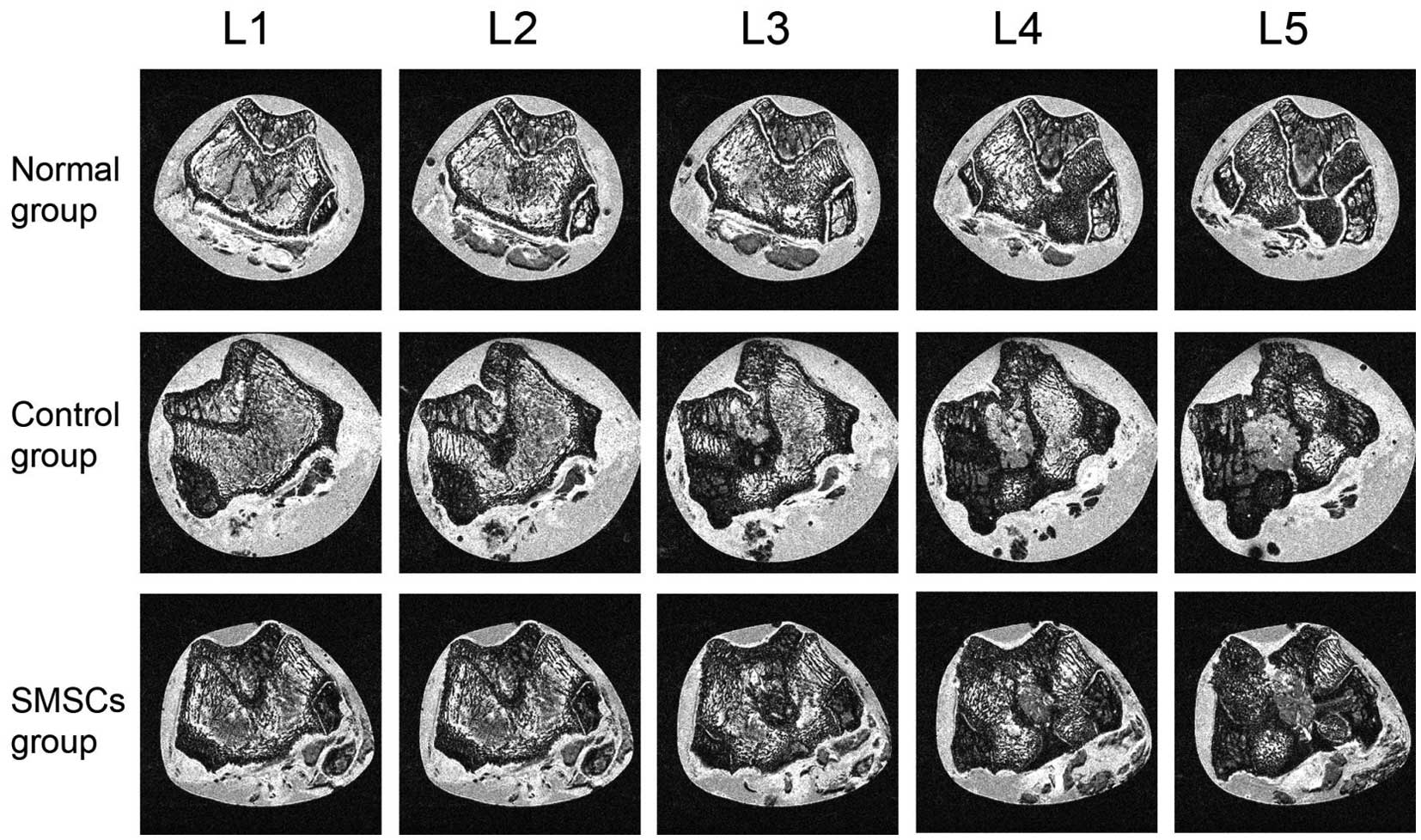
The GRE images captured of the continuous cross
section of articular cartilage and cartilage-bone interface are
shown in Fig. 1. In the normal
group, the surface appearance and signal intensity throughout the
cartilage were flat, and the tide mark was clearly visible. In the
control group, defect filling was complete and the defect remained
visible. The signal intensity of the repair tissue was also
abnormal, and the subchondral lamina was not intact. In addition,
the signal intensity of the osteochondral defect site was higher,
compared with that of the adjacent cartilage. In the single sample
with severe fissure in the SMSC group, the osteochondral site
revealed a similar pattern as the control group sample, with high
signal intensity at the site of repair (data not shown). For the
remaining samples of the SMSC group, the defect filling was almost
complete, and a demarcation border was visible. The signal
intensity of the repair tissue was almost normal, and the
subchondral lamina was intact. Granulation tissue was also observed
in the subchondral bone in the SMSC group, and the signal intensity
of the granulation tissue was continuous, compared with that of the
adjacent cartilage.
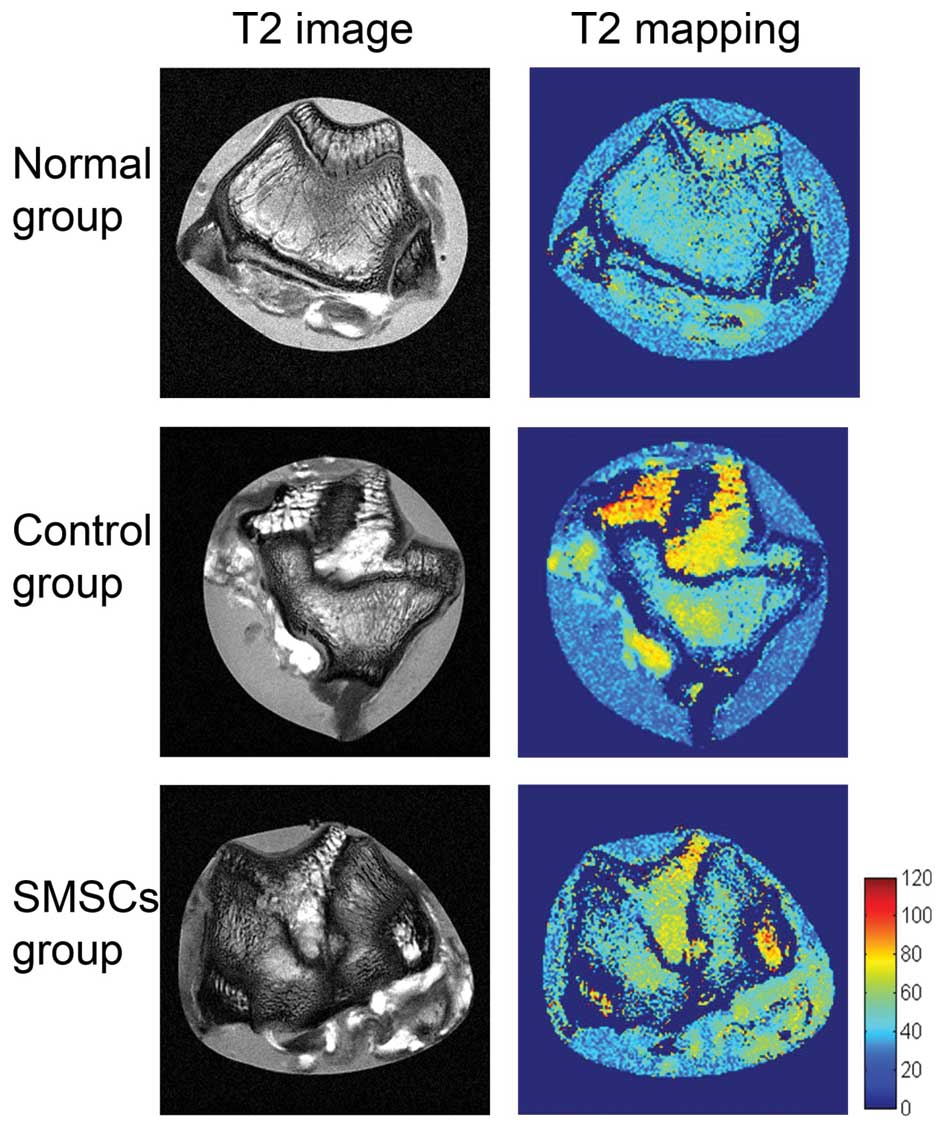
The results of the T2 mapping for each
group are shown in Fig. 2. In the
normal group, T2 mapping of the cartilage revealed a
homogenous T2 pattern, with no extensive signal
variation between the deep and the superficial cartilage. In the
control group, the T2 values in the repair tissue were
significantly higher, compared with those in the normal group
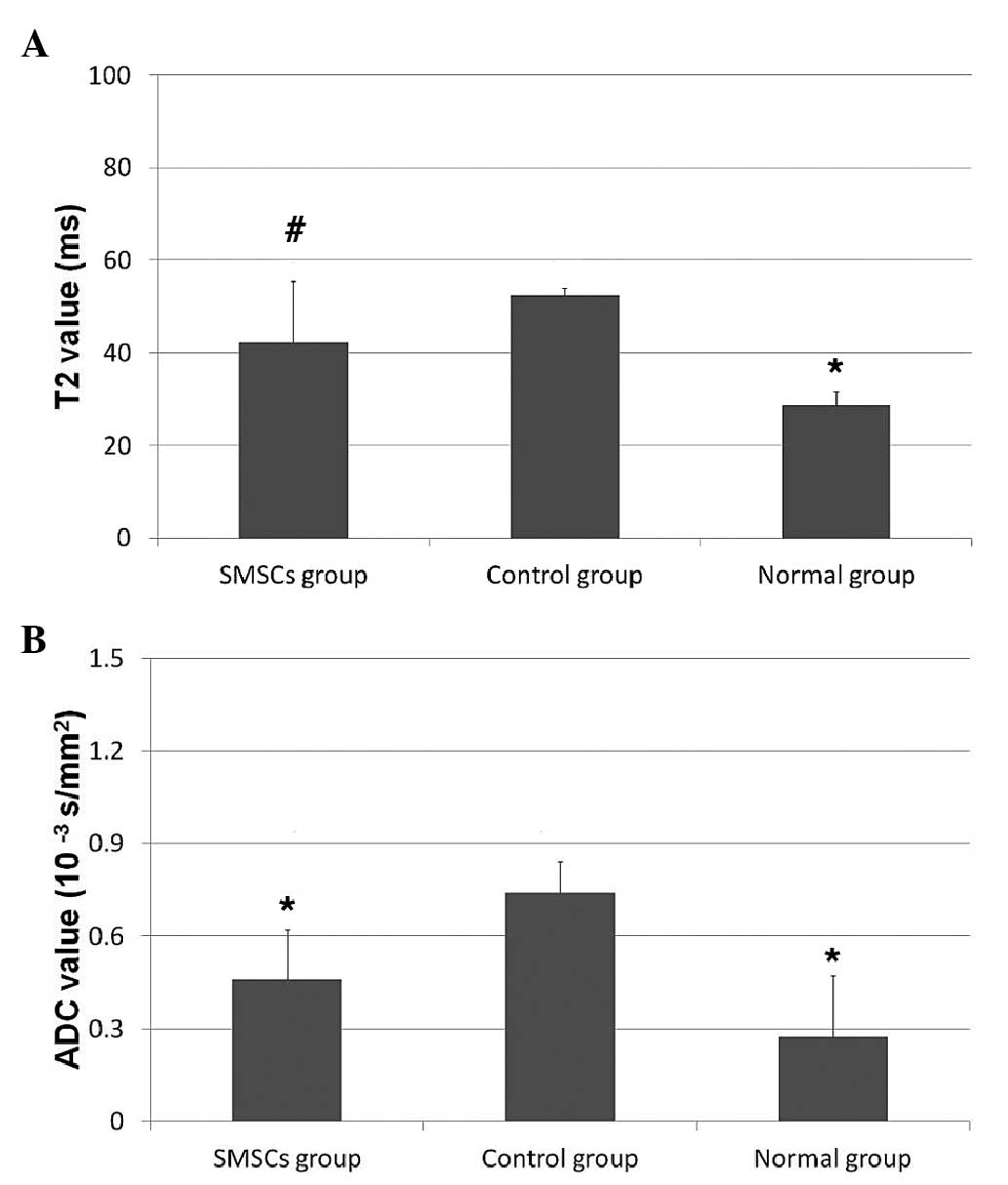
(52.48±1.60, vs. 28.60±3.20ms; P= 0.009; Fig. 3A). The T2 mapping showed
areas of long T2 components at the cartilage lesion,
particularly in the subchondral region. At 12 weeks following SMSC
transplantation, the repair tissue of the SMSC group also showed a
significantly higher T2 value, compared with the normal
cartilage group (42.26±13.09, vs. 28.60±3.20ms; P=0.0283; Fig. 3A). The repair tissue in the SMSC
group had a shorter T2, compared with that of the
control group, although no significant difference was detected
(P>0.05; Fig. 3A). No
significant variation in T2 values were observed from
the deep to superficial cartilage between the control and SMSC
groups. All three groups exhibited areas of short T2
components in the superficial cartilage.
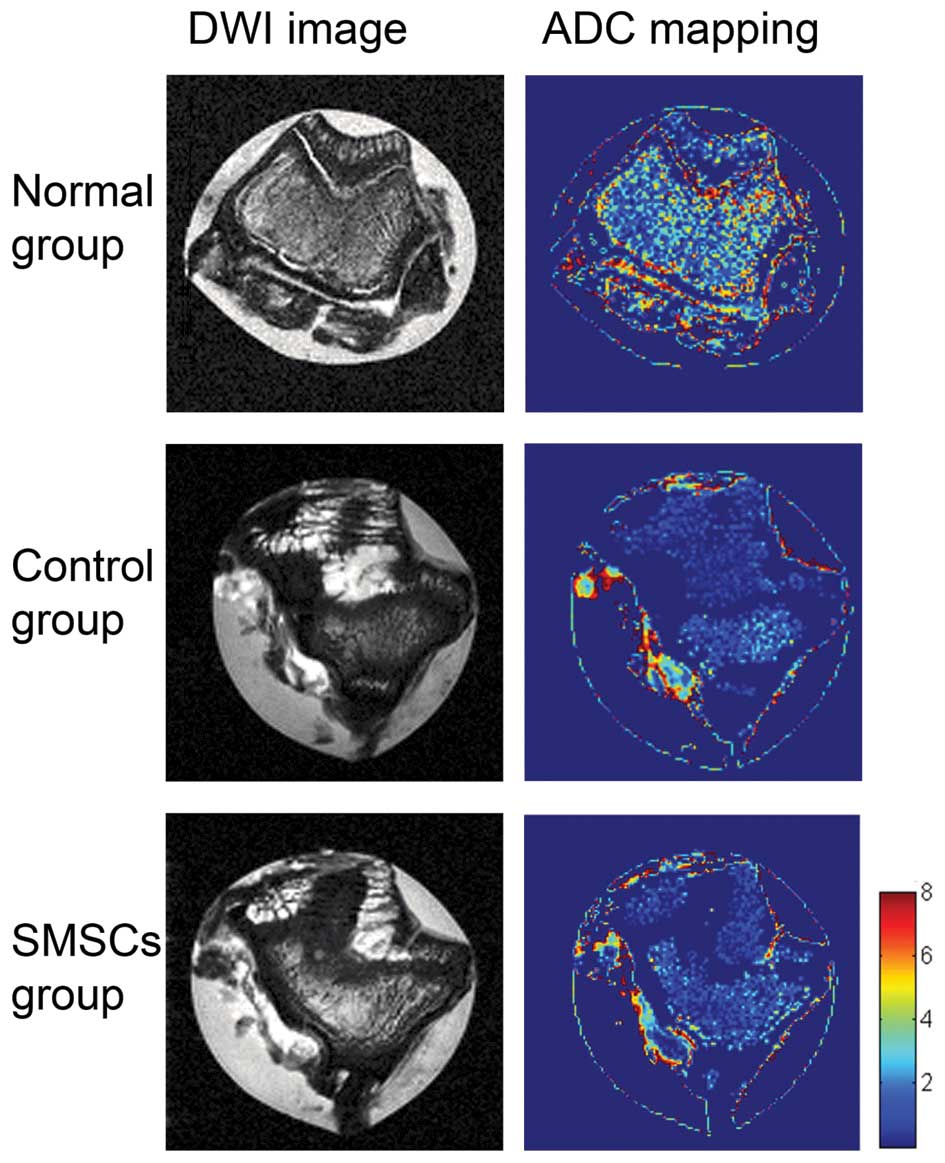
In the DWI imaging, the repair tissue in the SMSC
group exhibited a significantly lower ADC value, compared with the
control group (0.46±0.16×10−3, vs.
0.74±0.10×10−3 s/mm2; P=0.016; Fig. 3B). No significant differences were
observed between the ADC values of the SMSC group and the normal
group (0.46±0.16×10−3, vs. 0.27±0.20×10−3
s/mm2; P=0.17; Fig.
3B).DWI imaging of the normal group showed clear signal
variation in the normal cartilage, with a signal transition between
low and high ADC values observed between the deep and superficial
cartilage (Fig. 4). However,
cartilage repair tissue in the control group showed no signal
variation in the ADC values, suggesting a lack of correct collagen
arrangement. The repair tissue area of the SMSC group showed no
transition between low and high ADC values between the deep and
superficial articular layers of the repair site.
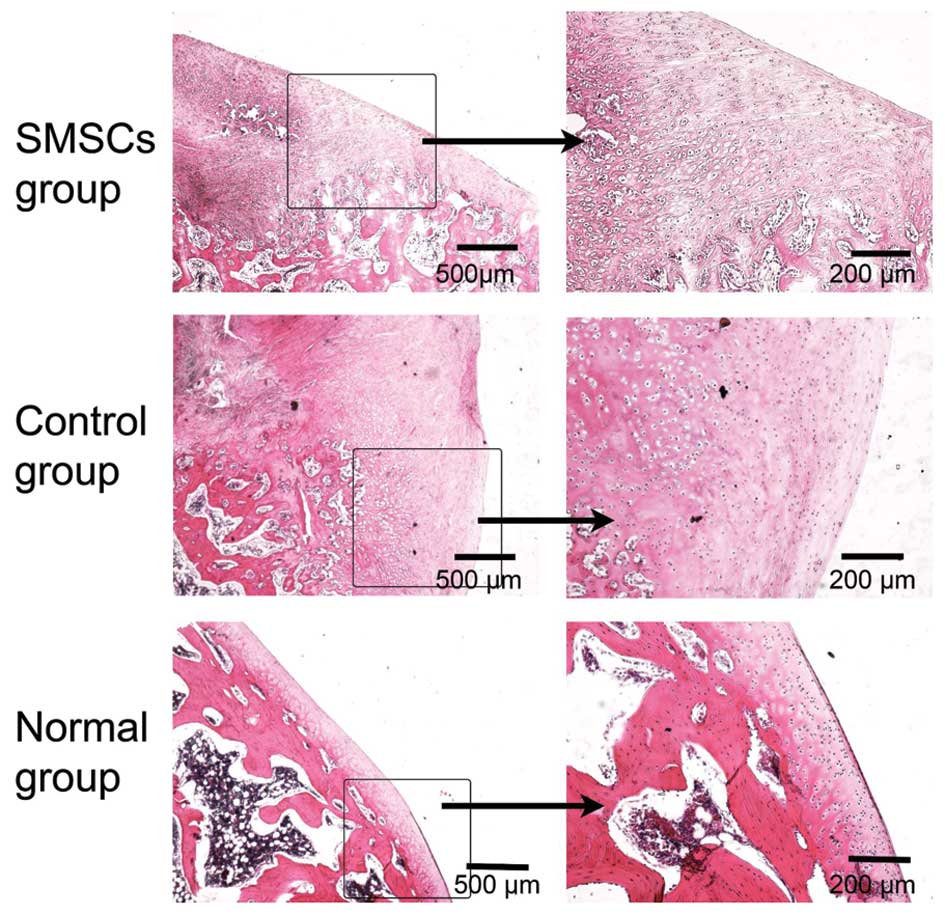
Histological results
As determined by the histological analysis, the
cartilage tissues in the normal group were compact and exhibited a
structural arrangement (Fig. 5).
At 12 weeks following the SMSC transplantation procedure, the
defects in the SMSC group and the control group had been filled
with repair tissues. In the control group, fibrous repair tissue
penetrated deeply into the subchondral bone, however, there was no
evidence of hyaline-like cartilage or restored cartilage surface,
and a severe fissure was observed at the cartilage interface. The
defects were predominantly filled with granulation and fibrous
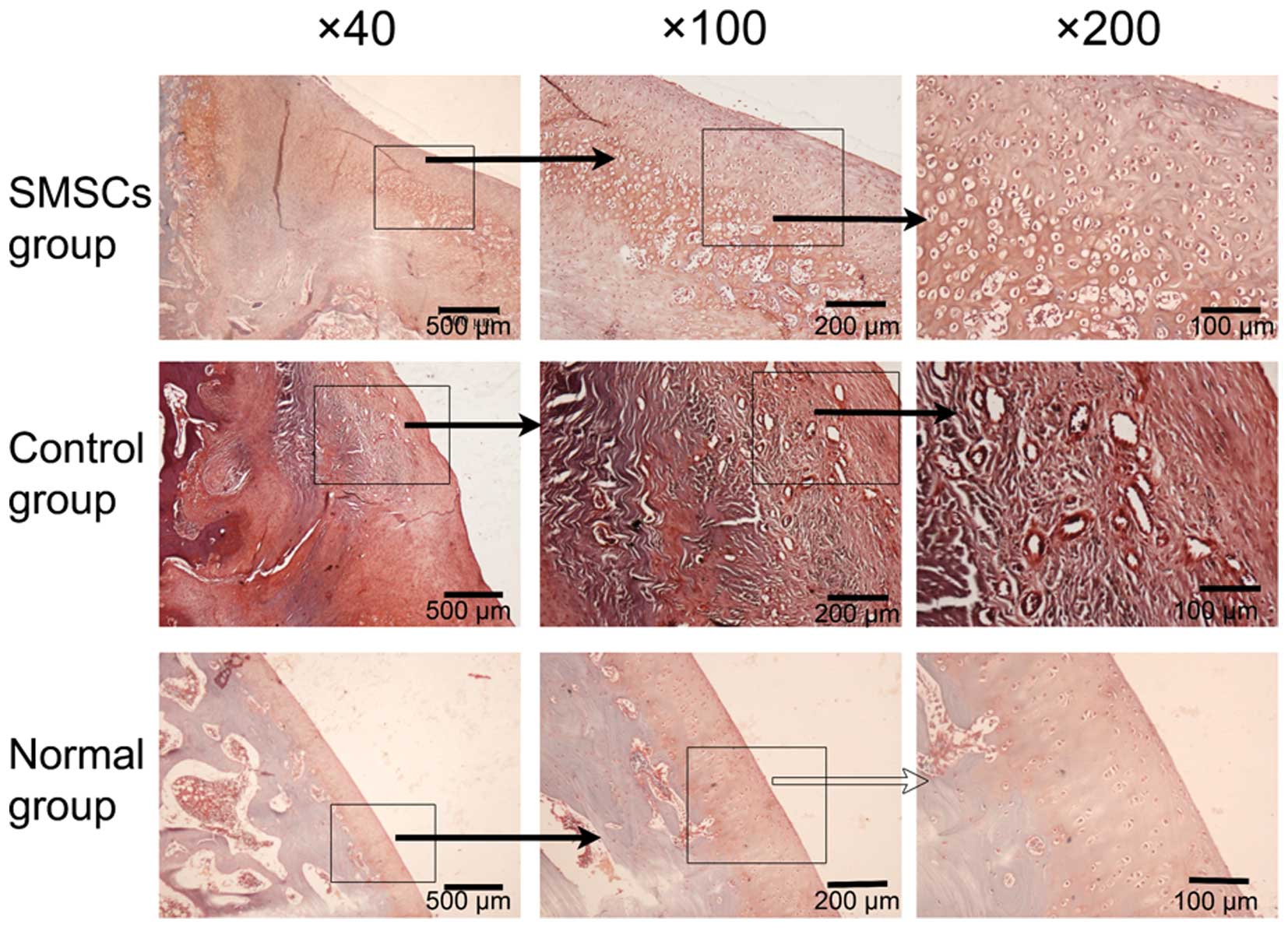
tissue, which exhibited poor Safranin-O staining (Fig. 6). In the SMSC group, repair tissue
covered the lesion site of the osteochondral samples. Fibrous
tissue was also observed in the superficial layer of the SMSC
group, and subchondral bone formation was present. Small, flat
cells were observed in the surface layer, and large, round cells
were located in the deep layer (Fig.
5). Safranin-O staining revealed repair tissues, which were
stained red and regularly distributed in the SMSC group, which was
indicated by the columnar alignment of the cells in the deep layer
and the small tangentially organized cells in the superficial area
(Fig. 6).
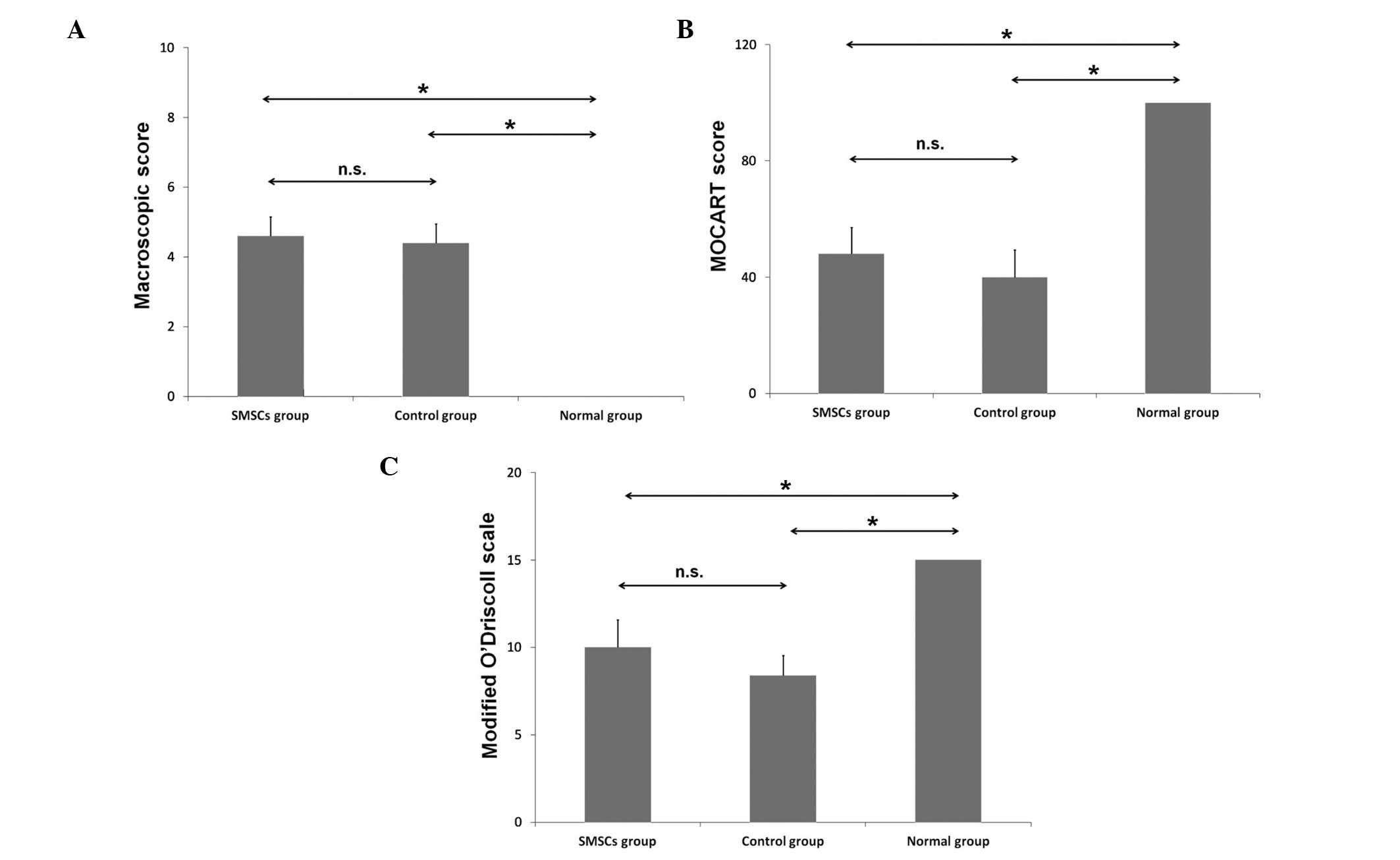
The macroscopic score and 2D MOCART score, and the
modified O'Driscoll scale are shown in Fig. 7. No significant differences were
observed between the control group and the SMSC group in the
macroscopic score (P>0.05), the 2D MOCART score (P>0.05) or
the modified O'Driscoll scale (P>0.05).
Discussion
In the present study, tissue repair following SMSC
transplantation in a rabbit osteochondral defect model was analyzed
using 9.4T high-field MRI. The DWI technique revealed unique SMSC
healing outcomes, demonstrating regional variation in cartilage
organization. The SMSC group exhibited increased cartilage healing,
compared with the control group, resulting from the production of
the regularly distributed organization of repair tissue at the
repair site.
Previous investigations have demonstrated that MSC
grafts improve the early healing response, increasing collagen type
II-positive cross-sectional areas of the regenerated tissue and
increasing aggrecan content (6,22).
It has also been demonstrated that intra-articular bone
marrow-derived MSCs enhance cartilage repair quality, with
increased aggrecan content and tissue firmness (23). In the present study, it was
observed that SMSC implantation increased repair tissue quality,
with columnar alignment of cells in the deep layer and small
tangentially organized cells in the superficial layer.
In addition, MRI at 9.4T revealed that the signal
intensity of the osteochondral defect site was higher than that of
the adjacent cartilage. However, graft signal intensity was not
directly associated with graft histological appearance (24). Therefore, T2 mapping and
the DWI technique were subsequently used to differentiate the
repair tissue. T2 mapping uses the water content of
cartilage and its transverse relaxation time as biochemical markers
for changes in cartilage collagen structure (25,26).
A significant trend of increasing T2 values, from the
deep to the superficial layer, is found in hyaline cartilage,
whereas fibrous tissue sites exhibit no significant change in
T2 value with depth (13,27).
In the present study, the cartilage of the normal tissue specimens
revealed a homogenous T2 pattern, with no significant
regional variation between the deep and superficial cartilage. All
three groups exhibited areas of short T2 in the
superficial cartilage, possibly owing to the effect of
neutral-buffered formalin on the cartilage.
DWI exploits the mobility of water protons in
biological tissues, and thus can reveal the structure of biological
tissue at a microscopic level (16,18,28).
Whereas T2 mapping is specific for collagen network and
water-content, DWI is specific for collagen and proteoglycan
content (29). Mean diffusivity,
evidence by the ADC, is linearly correlated to progressive
proteoglycan extraction in articular cartilage (30). The ADC value of normal cartilage
increases from the bone-cartilage interface to the articular
surface (12). In the present
study, DWI imaging of the normal group showed marked regional
variation, with a transition between low and high ADC values from
the deep to superficial regions. It was hypothesized that the DWI
reflects the biochemical constitution of cartilage as a combination
of collagen content/orientation, hydration and glycosaminoglycan
content (18). Furthermore, in the
repair tissue area of the SMSC group, no transition between low and
high ADC values were observed between the deep and superficial
areas. These results suggested that SMSC implantation alone was not
able to restore the native order of cartilage structure, however,
it increased the columnar alignment of cells in deep layer and
small tangentially organized cells in the superficial layer.
Although the repair tissue area of the SMSC group had a
significantly lower ADC value, compared with the control group, no
significant difference in ADC values were observed between the SMSC
and the normal group. Therefore, the ADC maps provided a more
sensitive technique for the detection of improved repair tissue
quality in the SMSC group.
The present study presented with a number of
limitations. Firstly, the cartilage defect samples were examined
ex vivo, therefore, the results cannot be generalized to
in vivo conditions. Secondly, the present study evaluated
only a single time point, with a small number of samples. Future
in-depth investigations with a larger sample size is required to
provide valuable information regarding the validity of these
results. Finally, there was the lack of direct histological
correlation for the T2 and ADC measurements.
In conclusion, the present study demonstrated that
osteochondral repair using SMSCs facilitated the repair of
appropriate tissue structure. In addition, clinical scoring,
morphological MRI, T2 mapping and DWI at 9.4T high field
provided additional information in the evaluation of cartilage
repair tissue quality. The DWI technique may be applied to evaluate
the treatment progress of osteochondral lesion.
Acknowledgments
This study was supported by the Nano Project of
Shanghai Municipal Science and Technology Commission (grant nos.
1052nm03701 and 11nm0504900), the Project of Shanghai Municipal
Science and Technology Commission (grant no. 11JC1401700) and the
National Natural Science Foundation of China (grant nos. 81201068,
81472142, 81401812 and U1232212).
References
|
1
|
Harris JD, Siston RA, Pan X and Flanigan
DC: Autologous chondrocyte implantation: A systematic review. J
Bone Joint Surg Am. 92:2220–2233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bekkers JE, Tsuchida AI, van Rijen MH,
Vonk LA, Dhert WJ, Creemers LB and Saris DB: Single-stage
cell-based cartilage regeneration using a combination of chondrons
and mesenchymal stromal cells: Comparison with microfracture. Am J
Sports Med. 41:2158–2166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roelofs AJ, Rocke JP and De Bari C:
Cell-based approaches to joint surface repair: A research
perspective. Osteoarthritis Cartilage. 21:892–900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee KB, Hui JH, Song IC, Ardany L and Lee
EH: Injectable mesenchymal stem cell therapy for large cartilage
defects-a porcine model. Stem Cells. 25:2964–2971. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sampat SR, O'Connell GD, Fong JV,
Alegre-Aguarón E, Ateshian GA and Hung CT: Growth factor priming of
synovium-derived stem cells for cartilage tissue engineering.
Tissue Eng Part A. 17:2259–2265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung M, Kaszap B, Redöhl A, Steck E,
Breusch S, Richter W and Gotterbarm T: Enhanced early tissue
regeneration after matrix-assisted autologous mesenchymal stem cell
transplantation in full thickness chondral defects in a minipig
model. Cell Transplant. 18:923–932. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koga H, Muneta T, Nagase T, Nimura A, Ju
YJ, Mochizuki T and Sekiya I: Comparison of mesenchymal
tissues-derived stem cells for in vivo chondrogenesis: Suitable
conditions for cell therapy of cartilage defects in rabbit. Cell
Tissue Res. 333:207–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goebel L, Orth P, Müller A, Zurakowski D,
Bücker A, Cucchiarini M, Pape D and Madry H: Experimental scoring
systems for macroscopic articular cartilage repair correlate with
the MOCART score assessed by a high-field MRI at 9.4 T-comparative
evaluation of five macroscopic scoring systems in a large animal
cartilage defect model. Osteoarthritis Cartilage. 20:1046–1055.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rautiainen J, Lehto LJ, Tiitu V, Kiekara
O, Pulkkinen H, Brünott A, van Weeren R, Brommer H, Brama PA,
Ellermann J, et al: Osteochondral repair: Evaluation with sweep
imaging with fourier transform in an equine model. Radiology.
269:113–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Madelin G, Babb J, Xia D, Chang G,
Krasnokutsky S, Abramson SB, Jerschow A and Regatte RR: Articular
cartilage: Evaluation with fluid-suppressed 7.0-T sodium MR imaging
in subjects with and subjects without osteoarthritis. Radiology.
268:481–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glaser C: New techniques for cartilage
imaging: T2 relaxation time and diffusion-weighted MR imaging.
Radiol Clin North Am. 43:641–653. vii2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raya JG, Horng A, Dietrich O, Krasnokutsky
S, Beltran LS, Storey P, Reiser MF, Recht MP, Sodickson DK and
Glaser C: Articular cartilage: In vivo diffusion-tensor imaging.
Radiology. 262:550–559. 2012. View Article : Google Scholar
|
|
13
|
Welsch GH, Mamisch TC, Domayer SE, Dorotka
R, Kutscha-Lissberg F, Marlovits S, White LM and Trattnig S:
Cartilage T2 assessment at 3-T MR imaging: In vivo differentiation
of normal hyaline cartilage from reparative tissue after two
cartilage repair procedures-initial experience. Radiology.
247:154–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eshed I, Trattnig S, Sharon M, Arbel R,
Nierenberg G, Konen E and Yayon A: Assessment of cartilage repair
after chondrocyte transplantation with a fibrin-hyaluronan
matrix-correlation of morphological MRI, biochemical T2 mapping and
clinical outcome. Eur J Radiol. 81:1216–1223. 2012. View Article : Google Scholar
|
|
15
|
Domayer SE, Apprich S, Stelzeneder D,
Hirschfeld C, Sokolowski M, Kronnerwetter C, Chiari C, Windhager R
and Trattnig S: Cartilage repair of the ankle: First results of T2
mapping at 7.0 T after micro-fracture and matrix associated
autologous cartilage transplantation. Osteoarthritis Cartilage.
20:829–836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Friedrich KM, Mamisch TC, Plank C, Langs
G, Marlovits S, Salomonowitz E, Trattnig S and Welsch G:
Diffusion-weighted imaging for the follow-up of patients after
matrix-associated autologous chondrocyte transplantation. Eur J
Radiol. 73:622–628. 2010. View Article : Google Scholar
|
|
17
|
Apprich S, Trattnig S, Welsch GH,
Noebauer-Huhmann IM, Sokolowski M, Hirschfeld C, Stelzeneder D and
Domayer S: Assessment of articular cartilage repair tissue after
matrix-associated autologous chondrocyte transplantation or the
microfracture technique in the ankle joint using diffusion-weighted
imaging at 3 Tesla. Osteoarthritis Cartilage. 20:703–711. 2010.
View Article : Google Scholar
|
|
18
|
Welsch GH, Trattnig S, Domayer S,
Marlovits S, White LM and Mamisch TC: Multimodal approach in the
use of clinical scoring, morphological MRI and biochemical
T2-mapping and diffusion-weighted imaging in their ability to
assess differences between cartilage repair tissue after
microfracture therapy and matrix-associated autologous chondrocyte
transplantation: A pilot study. Osteoarthritis Cartilage.
17:1219–1227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Bari C, Dell'Accio F, Tylzanowski P and
Luyten FP: Multipotent mesenchymal stem cells from adult human
synovial membrane. Arthritis Rheum. 44:1928–1942. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marlovits S, Singer P, Zeller P, Mandl I,
Haller J and Trattnig S: Magnetic resonance observation of
cartilage repair tissue (MOCART) for the evaluation of autologous
chondrocyte transplantation: Determination of interobserver
variability and correlation to clinical outcome after 2 years. Eur
J Radiol. 57:16–23. 2006. View Article : Google Scholar
|
|
21
|
Zalewski T, Lubiatowski P, Jaroszewski J,
Szcześniak E, Kuśmia S, Kruczyński J and Jurga S: Scaffold-aided
repair of articular cartilage studied by MRI. MAGMA. 21:177–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilke MM, Nydam DV and Nixon AJ: Enhanced
early chondrogenesis in articular defects following arthroscopic
mesenchymal stem cell implantation in an equine model. J Orthop
Res. 25:913–925. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McIlwraith CW, Frisbie DD, Rodkey WG,
Kisiday JD, Werpy NM, Kawcak CE and Steadman JR: Evaluation of
intra-articular mesenchymal stem cells to augment healing of
microfractured chondral defects. Arthroscopy. 27:1552–1561. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tins BJ, McCall IW, Takahashi T,
Cassar-Pullicino V, Roberts S, Ashton B and Richardson J:
Autologous chondrocyte implantation in knee joint: MR imaging and
histologic features at 1-year follow-up. Radiology. 234:501–508.
2005. View Article : Google Scholar
|
|
25
|
Domayer SE, Kutscha-Lissberg F, Welsch G,
Dorotka R, Nehrer S, Gäbler C, Mamisch TC and Trattnig S: T2
mapping in the knee after microfracture at 3.0 T: Correlation of
global T2 values and clinical outcome-preliminary results.
Osteoarthritis Cartilage. 16:903–908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kijowski R, Blankenbaker DG, Munoz Del Rio
A, Baer GS and Graf BK: Evaluation of the articular cartilage of
the knee joint: Value of adding a T2 mapping sequence to a routine
MR imaging protocol. Radiology. 267:503–513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
White LM, Sussman MS, Hurtig M, Probyn L,
Tomlinson G and Kandel R: Cartilage T2 assessment: Differentiation
of normal hyaline cartilage and reparative tissue after
arthroscopic cartilage repair in equine subjects. Radiology.
241:407–414. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Apprich S, Trattnig S, Welsch GH,
Noebauer-Huhmann IM, Sokolowski M, Hirschfeld C, Stelzeneder D and
Domayer S: Assessment of articular cartilage repair tissue after
matrix-associated autologous chondrocyte transplantation or the
microfracture technique in the ankle joint using diffusion-weighted
imaging at 3 Tesla. Osteoarthritis Cartilage. 20:703–711. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roemer FW, Crema MD, Trattnig S and
Guermazi A: Advances in imaging of osteoarthritis and cartilage.
Radiology. 260:332–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raya JG, Melkus G, Adam-Neumair S,
Dietrich O, Mützel E, Kahr B, Reiser MF, Jakob PM, Putz R and
Glaser C: Change of diffusion tensor imaging parameters in
articular cartilage with progressive proteoglycan extraction.
Invest Radiol. 46:401–409. 2011. View Article : Google Scholar : PubMed/NCBI
|