Introduction
Stroke continues to be a leading worldwide cause of
human mortality and long-term disability, with ~70% of stroke
survivors experiencing reduced work capacity and ≤30% requiring
self-care assistance (1). A
reduction or complete blockage of blood flow to regions of the
brain results in oxygen and glucose deficiencies, which may lead to
ischemic stroke (2,3). The weight of the brain is 2% of the
total body weight; however, it uses nearly 20% of the body's
cardiac output to achieve its supply of essential nutrients,
including oxygen and glucose (4).
Glucose is the primary source of energy that sustains cellular
activity and homeostasis in the brain. Under the conditions of a
stroke, more glucose is required due to the rapid depletion of
oxygen from compensatory metabolic alterations (5). Severe reduction in the supply of
glucose and oxygen to the brain, even for a short period of time,
often initiates brain ischemia-reperfusion, which leads to a
complex cascade of cellular events, resulting in neuronal death
and, consequently, loss of brain function (6).
Glucose transporters (GLUTs) are present in all
types of cells and are responsible for the entry of glucose into
cells without consuming energy throughout the periphery and the
brain. Therefore, the expression, regulation and activity of GLUTs
are important for neural homeostasis (7). Additionally, the enhanced activity of
GLUTs protects cells during energy depletion under hypoxic
conditions (8). Hypoxia-inducible
factor 1α (HIF-1α), an important regulator of the cellular response
to oxygen deprivation (9), is a
key transcription factor for various genes involved in glucose
uptake, angiogenesis, glycolysis, pH balance and metastasis
(10). In a hypoxic environment,
the HIF-1α signaling pathway is activated and, in turn, GLUTs and
glycolytic enzymes are activated, promoting glucose uptake in cells
through the transcription of GLUTs (11) to maintain adenosine triphosphate
(ATP) levels essential for cell survival (12,13).
Astrocytes are star-shaped glial cells of the
central nervous system, and provide an important link between
endothelial cells and neurons (14). The regulation of glucose uptake in
astrocytes is important for normal brain function because glucose
taken up by astrocytes is also used to supply neurons with
metabolic substrates required to sustain neuronal functions, such
as synaptic transmission (10,15).
Although numerous isoforms of GLUTs have been identified in the
brain, GLUT-1 and GLUT-3 are the most abundant (16,17).
Under physiological conditions, GLUT-1 is primarily expressed in
neurons and at the plasma membranes in astrocytes; however,
controversy remains as to whether GLUT-3 is also expressed in
astrocytes (16,18). A previous study indicated that
GLUT-3 was not expressed in astrocytes but in neurons (19); however, other studies have detected
GLUT-3 at extremely low levels in cultured astrocytes under
physiological conditions (10,18).
Numerous physiological pathways, each with a
distinctive time frame, are spontaneously activated following the
onset of stroke (20). It is
unclear whether the regulation of GLUT-1, GLUT-3 and glucose
homeostasis behave in a time-dependent manner under hypoxic
conditions. CoCl2 is a hypoxia mimetic agent that may
produce a hypoxic-like environment and activate HIF-1α under
normoxic conditions in vitro and in vivo (13,21).
CoCl2 exposure also leads to mitochondrial damage and
increases the generation of reactive oxygen species (20,22).
These observations suggest that a model of hypoxic damage induced
by CoCl2 is a suitable tool to investigate the
mechanisms of cell injury (23).
Furthermore, hypoxia and CoCl2 exposure were associated
with compromised ATP production, possibly associated with metabolic
alterations, which resulted in death of the astrocytes (24). Therefore, the current study was
undertaken to investigate the regulation of GLUTs and glucose
homeostasis including glucose uptake and consumption during
CoCl2 treatment, which may suggest an optimal time frame
following stroke to commence with therapy. Further understanding of
hypoxia-induced alterations in the homeostasis of glucose uptake
and consumption would facilitate the design of effective
rehabilitative strategies.
Materials and methods
Materials
All cell culture reagents, including media,
antibiotics, fetal bovine serum (FBS) and phosphate-buffered saline
(PBS), were purchased from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). Mouse monoclonal anti-GLUT-1, rabbit monoclonal
anti-GLUT-3, mouse monoclonal anti-HIF-1α and mouse monoclonal
anti-β-actin were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Cell culture plastics were purchased from
Corning Incorporated (Corning, NY, USA). TRIzol reagent,
SuperScript III reverse transcriptase and Platinum SYBR-Green qPCR
SuperMix were purchased from Invitrogen (Thermo Fisher Scientific,
Inc.). A cytotoxicity detection kit was purchased from Roche
Diagnostics (Indianapolis, IN, USA) and an Amplex Red Glucose Assay
kit was purchased from Life Technologies (Thermo Fisher Scientific,
Inc). An ATP assay kit was purchased from Merck Millipore
(Darmstadt, Germany). The pyruvate assay kit and the glycogen assay
kit were purchased from BioVision, Inc. (Milpitas, CA, USA). The
remaining chemicals used were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Animals
A total of 22, 1-day-old BALB/c mice, weighing 2±0.3
g were obtained from the Experimental Animal Centre of Zhengzhou
University (Zhengzhou, China), housed in a pathogen-free
environment, maintained on a 12-h light-dark cycle and fed with
commercial pellets. All experiments were approved by the Ethics
Committee of Life Sciences, Zhengzhou University (Zhengzhou,
China).
Primary astrocyte culture
The primary astrocyte culture was prepared as
described previously (22).
Briefly, the neonatal mice were sacrificed by decapitation
following anesthesia with 30 mg/kg Zoletil 100 (Virbac
Laboratories, Carros, France), cerebral cortices were removed, and
tissue was transferred to complete Dulbecco's modified Eagle's
medium (DMEM; supplemented with 10% FBS, 25 mM glucose, 50 U/ml
penicillin and 50 mg/ml streptomycin-sulfate) and dissociated with
0.0025% trypsin/ethylenediaminetetraacetic acid. Cells were seeded
at a density of 3×104 cells/cm2 in complete
DMEM at 37°C in a humidified atmosphere with 5% CO2. The
medium was renewed on day in vitro (DIV) 1, DIV 5 and DIV 7.
On DIV 9, microglia were discarded using the shake-off method
(23). Astrocytes were harvested
with trypsin and reseeded at a density of 3×104
cells/cm2 in complete DMEM/F12 medium. The homogeneity
of astrocytes was 90–95% by detection of glial fibrillary acidic
protein with 5–10% of cells being B4 isolectin+
microglia. Astrocytes were reseeded at a density of
5×104 cells/cm2 and incubated at 37°C with 5%
CO2. Experiments were performed on cells 10–18 days
after plating at which point they formed an incomplete monolayer of
stellate- and flat-shaped astrocytes. cells were exposed to 100
µM CoCl2 and tested at distinct time points.
Reverse transcription-quantitative
polymerase chain reac- tion (RT-qPCR)
To examine GLUT-1 and GLUT-3 gene expression induced
by CoCl2 treatment according to the time courses,
astrocytes were harvested following incubation with 100 µM
CoCl2 at different time points (0, 2, 4, 6, 8, 10, 12,
18 and 24 h). Total RNA was extracted by using TRIzol reagent,
according to the manufacturer's protocol, and subjected to DNase
treatment using a FastQuant RT kit (Tiangen Biotech Co., Ltd.,
Beijing, China). RNA quantity and quality was determined
spectrophotometrically at 260 and 280 nm using an EU-2200R
Ultraviolet spectrophotometer (Shanghai Onlab Instruments Co.,
Ltd., Shanghai, China). Reverse transcription of 20 ng RNA was
performed using a SuperScript III reverse transcriptase kit
(Invitrogen; Thermo Fisher Scientific, Inc.) in preparation for
qPCR.
GLUT-1, GLUT-3, HIF-1α and β-actin expression levels
were evaluated by qPCR using Platinum SYBR-Green qPCR SuperMix. The
running conditions were 50°C for 2 min and 95°C for 10 min,
followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. qPCR
was performed using the ABI 7500 sequence detector (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The design of the
specific primers is presented in Table
I.
 | Table ISpecific primers for the expression
of GLUT-1, GLUT-3, HIF-1α and β-actin. |
Table I
Specific primers for the expression
of GLUT-1, GLUT-3, HIF-1α and β-actin.
| Gene name | Primer sequence
(5′-3′) | Length, bp | Amplicon, nt |
|---|
| GLUT-1 | F:
GACCCTGCACCTCATTGG | 18 | 106 |
| R:
GATGCTCAGATAGGACATCCAAG | 23 | |
| GLUT-3 | F:
GGAGGAAGACCAAGCTACAGAG | 22 | 130 |
| R:
GAGCTCCAGCACAGTCACCT | 20 | |
| HIF-1α |
F:AACAGAATGGAACGGAGCAA | 20 | 119 |
| R:
TTCACAATCGTAACTGGTCAGC | 22 | |
| β-actin | F:
CTAAGGCCAACCGTGAAAAG | 20 | 104 |
| R:
ACCAGAGGCATACAGGGACA | 20 | |
The quantities of gene-specific mRNA expression were
determined by the quantification cycle (Cq) method and the Cq value
for β-actin was used as an internal calibrator. The comparative Cq
method, or 2−ΔΔCq, was used for relative quantization
(25) using the following
equation: ΔΔCq = (Cqtarget −
Cqcalibrator)experimental −
(Cttarget − Cqcalibrator)control.
Data are presented as ratios over control (time point, 0 h).
Western blot analysis
The astrocytes were exposed to 100 µM
CoCl2 for the aforementioned periods of time, then the
cells were collected and solubilized in lysis buffer (60 mM HEPES,
pH 7.4, 150 mM NaCl, 3 mM KCl, 5 mM Na3 EDTA, 3 mM EGTA,
and 1% Triton X-100) with protease inhibitors. Following
centrifugation at 14,000 × g for 15 min at 4°C, the supernatant was
collected and solubilized in loading buffer. Samples were
normalized to protein concentration and proteins were separated
using 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis with a running voltage of 200 v for 30 min and
electrophoretically transferred to polyvinylidene difluoride
membranes at 100 V for 1 h. The membranes were blocked with 5.0%
fat-free milk in PBS with 0.05% Tween-20 (PBST) for 1 h at room
temperature, and incubated separately with mouse monoclonal
anti-GLUT-1 (cat. no. sc-377228; dilution 1:1,000), rabbit GLUT-3
(cat. no. sc-74399; dilution 1:1,000), mouse monoclonal anti-HIF-1α
(cat. no. sc-71247; dilution 1:1,000) or mouse β-actin (cat. no.
sc-47778; dilution 1:2,000; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. Subsequently, the membranes were washed with PBST
three times for 10 min each. Following incubation with Alexa
Fluor® 488 donkey anti-rabbit IgG (cat. no. R37118) and
Alexa Fluor® 680 rabbit anti-mouse IgG (cat. no.
A-21065; Thermo Fisher Scientific, Inc.; dilution 1:10,000) for 60
min at room temperature in the dark, blots were detected on an
Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE,
USA) and relative density units were estimated from the mean pixel
density using Image Studio Lite version 4.0 (LI-COR Biosciences)
and normalized to β-actin. Data are presented as ratios over
control (time point: 0 h).
Measuring intracellular glucose
concentration
Astrocytes were seeded at a density of
5×104 cells/cm2 in a 12 well plate and
exposed to 100 µM CoCl2 for the aforementioned
time periods when cells were confluent. In the final 2 h of each
time course, astrocytes were incubated in glucose-free medium (25
mM mannose) for 1 h, and then in 25 mM glucose medium for 1 h.
During the incubation in medium containing 25 mM mannose and 25 mM
glucose, astrocytes of the CoCl2 treatment group were
still treated with 100 µM CoCl2. Subsequently,
cells were rapidly chilled on ice and washed four times with
ice-cold PBS, then they were collected and lysed in 500 µl
of ice-cold lysis buffer (10 mM HEPES, 50 mM NaCl, 5 mM EDTA, 1 mM
benzamidine, 0.5% Triton X-100) with protease inhibitors
(Sigma-Aldrich). The intracellular glucose concentration in cell
lysates was determined using the Amplex Red Glucose Assay kit
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Subsequently, 50 µl cell lysate was mixed with 50
µl reaction mixture and incubated for 30 min at room
temperature in the dark. The absorbance was then measured at 560 nm
using an SP-Max 2300A2 microplate reader (Shanghai Flash Spectrum
Biological Technology Co., Ltd., Shanghai, China). Intracellular
glucose concentration was determined from a standard curve
generated using the various concentrations normalized to the
protein concentration. Data are presented as the percentage of
glucose concentration compared with control (time point of
CoCl2 treatment, 0 h), which is expressed as 100%.
Quantification of intracellular
glycogen
Total glycogen concentration was determined using a
colorimetric glycogen assay kit (BioVision, Inc.), according to the
manufacturer's protocol. The astrocytes were homogenized with
dH2O on ice and the homogenates were boiled for 5 min to
inactivate the enzymes present. The boiled samples were spun at
15,000 × g at 4°C for 5 min to remove insoluble material, then the
supernatant was collected for assaying optical density, which was
measured at 570 nm using a microplate reader (SP-Max 2300A2;
Shanghai Flash Spectrum Biological Technology Co., Ltd.). Glycogen
concentration was determined from a standard curve generated using
various concentrations of glycogen and normalized to protein
concentration. Data are presented as the percentage glycogen
concentration compared with control (time point, 0 h), which is
expressed as 100%.
Measuring intracellular pyruvate
Pyruvate was extracted from the astrocytes using 4
volumes of the pyruvate assay buffer. The cells were centrifuged
(10,000 × g; 10 min; 4°C) to remove insoluble material and collect
the supernatant. A total of 2–50 µl supernatant was added
into a 96-well plate and the volume adjusted to 50 µl/well
with pyruvate assay buffer. Next, the 50 µl reaction mixture
(containing 46 µl pyruvate assay buffer, 2 µl
pyruvate probe and 2 µl enzyme mix) was added. Following
incubation for 30 min at room temperature in the dark,
intracellular pyruvate was measured by colorimetric assay using a
pyruvate assay kit (BioVision, Inc.). Optical density was measured
at 570 nm using a microplate reader (SP-Max 2300A2; Shanghai Flash
Spectrum Biological Technology Co., Ltd.). Intracellular pyruvate
was determined from a standard curve generated using various
concentrations of pyruvate and normalized to protein concentration.
Data are presented as the percentage pyruvate concentration
compared with the control (time point of CoCl2
treatment=0 h), which is expressed as 100%.
Measuring intracellular ATP content
Astrocytes were seeded at a density of
5×104 cells/cm2 in a 12-well plate and were
exposed to 100 µM CoCl2 for testing at distinct
time points. Cells of the control group were generated at the same
time points without CoCl2 treatment. Following the
aforementioned cell preparations, intracellular ATP content was
determined by bioluminescence assay using a commercial ATP assay
kit (Merck Millipore), according to the manufacturer's protocol.
The astrocytes were treated with 100 µl nuclear releasing
reagent for 5 min at room temperature while gently shaking, then 1
µl ATP monitoring enzyme was added to the cell lysate.
Bioluminescence of intracellular ATP content was determined from a
standard curve generated using various concentrations of ATP and
normalized to protein concentration.
Measuring cell viability
Astrocyte cell viability was demonstrated at
distinct time points following CoCl2 treatment using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay, according to the manufacturer's protocol. Astrocytes of the
control group were cultured without CoCl2 treatment and
generated at the same time points. MTT solution (5.0 mg/ml) was
added to the cells and incubated at 37°C for 4 h. The culture
medium was aspirated, and 1 ml dimethyl sulfoxide was added and
thoroughly mixed for 10 min. MTT absorbance was measured at 570 and
630 nm using a microplate reader (SP-Max 2300A2; Shanghai Flash
Spectrum Biological Technology Co., Ltd.).
Measuring lactate dehydrogenase
release
To measure lactate dehydrogenase (LDH) release in
the astrocyte supernatant, the supernatant was collected by
centrifugation at 250 × g at 4°C for 10 min and transferred into a
96-well plate following exposure to CoCl2 treatment for
the indicated time points. Astrocytes of the control group were
cultured without CoCl2 treatment and the supernatant was
generated at the same time points. LDH was determined using a
cytotoxicity detection kit (Roche Diagnostics) according to the
manufacturer's protocol, with Triton X-100 (2.0%) serving as a
positive control. Absorbance was measured using a microplate reader
(SP-Max 2300A2; Shanghai Flash Spectrum Biological Technology Co.,
Ltd.) at a test wavelength of 490 nm and a reference wavelength of
630 nm.
Statistical analysis
Statistical analyses was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Data from multiple
experiments were processed and are expressed as the mean ± standard
deviation. Statistical differences between the different time
points were determined using one-way analysis of variance followed
by a Student-Newman-Keuls test. Statistical analyses of
intracellular ATP content, cell viability and LDH release at all
time points between two groups were performed using the
independent-samples t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of CoCl2 treatment on
mRNA expression levels of GLUT-1, GLUT-3 and HIF-1α
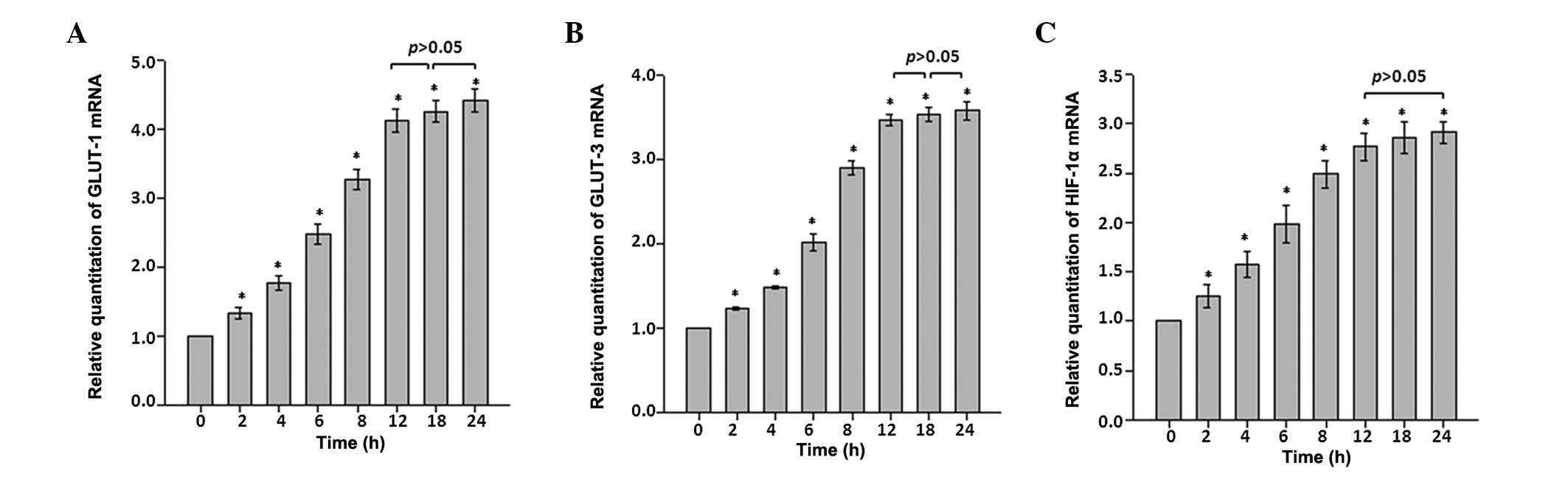
The time course analysis using RT-qPCR indicated
that GLUT-1 and GLUT-3 mRNA expression levels in astrocytes changed
in a time-dependent manner following CoCl2
administration. Expression levels of GLUT-1 and GLUT-3 mRNA
increased immediately and significantly in each time interval
during the first 12 h time period. Throughout the rest of the
recording period, the expression levels for GLUT-1 and GLUT-3 mRNAs
remained at ~4.5- and 3.5-fold higher than that of the control
(P<0.05 vs. control; Fig. 1A and
B).
HIF-1α mRNA expression in astrocytes was also
analyzed as an indication of hypoxic stress. HIF-1α mRNA expression
exhibited time-dependent alterations similar to those observed with
GLUT-1 and GLUT-3. Following exposure to CoCl2
treatment, the astrocytes exhibited a ~2.9-fold increase in HIF-1α
mRNA expression during the first 12 h time course, and the increase
remained constant throughout the remaining recording period
(Fig. 1C).
Effects of CoCl2 treatment on
protein levels of GLUT-1, GLUT-3 and HIF-1α
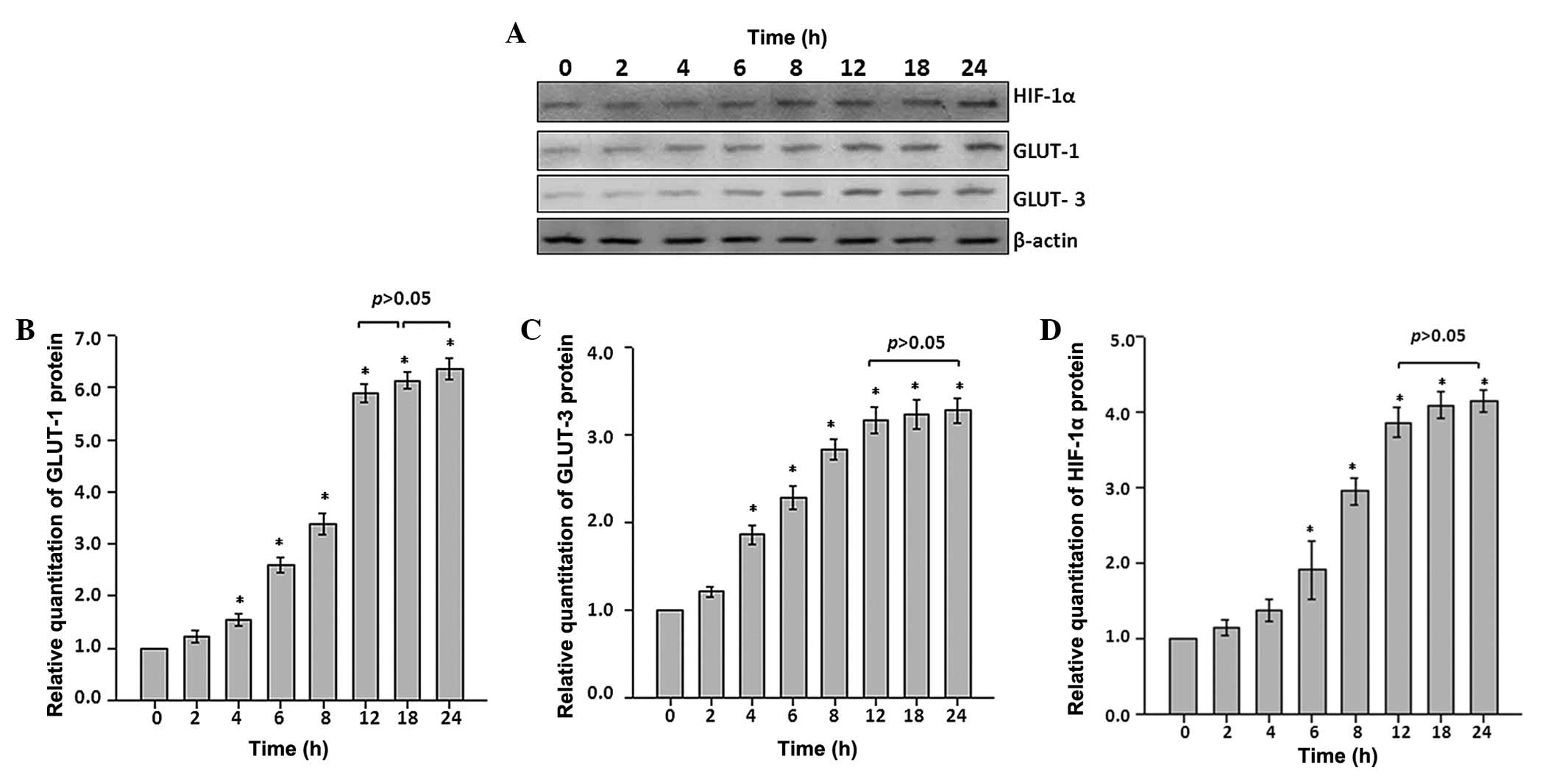
Protein expression levels of GLUT-1, GLUT-3 and
HIF-1α were determined by western blot analysis (Fig. 2A). Quantitative analysis
demonstrated that treatment withCoCl2 in the first 2 h
resulted in no significant differences in GLUT-1 and GLUT-3 protein
expression levels in astrocytes (P>0.05 vs. control; Fig. 2B and C); however, a significant
increase in the protein expression of GLUT-1 and GLUT-3 occurred
from the 4 h time point throughout the experiments (P<0.05 vs.
control; Fig. 2B and C). In
particular, 6.3- and 3.2-fold increases for GLUT-1 and GLUT-3,
respectively, were observed from the 12 h time point throughout the
remaining recording period (Fig. 2B
and C).
Additionally, to investigate whether
CoCl2 treatment induced GLUT-1 and GLUT-3 activity, and
whether HIF-1α behaved in an independent manner, protein expression
levels for HIF-1α were determined. As a reflection of hypoxic
stress, HIF-1α protein expression behaved similarly to that of
GLUT-1 and GLUT-3. There was no statistically significant
alteration within the initial 4 h, however a significant increase
was identified at the 6 h time point. The increase reached
~4.0-fold at the 12 h time point and remained at that level
throughout remaining recording period (P<0.05 vs. control;
Fig. 2D).
Effects of CoCl2 treatment on
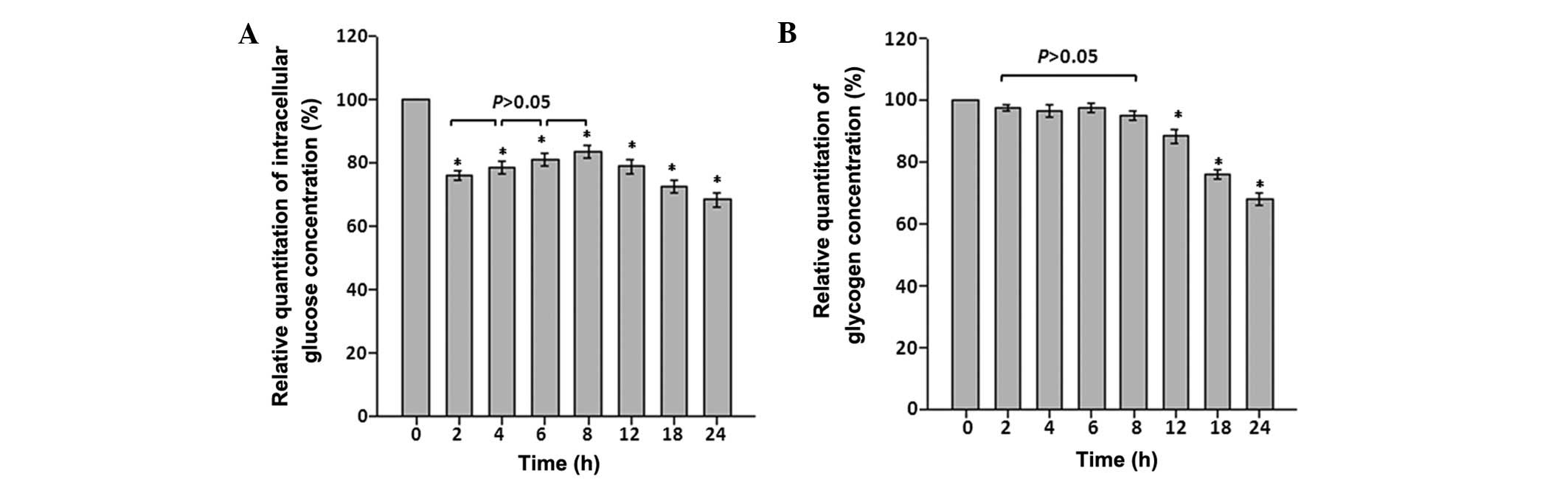
intracellular glucose concentrations and glycogen storage
To determine the effects of CoCl2
treatment on intracellular glucose concentrations and glycogen
storage at different time points, intracellular glucose and
glycogen concentrations were determined. When astrocytes were
exposed to CoCl2 treatment, intracellular glucose
concentration immediately fell by >25% between 0 and 2 h
(P<0.05). Although an increase occurred between the 4–8 h time
points, intracellular glucose and glycogen concentrations were
significantly reduced compared with the control by the 24 h time
point (P<0.05; Fig. 3A and
B).
Effects of CoCl2 treatment on
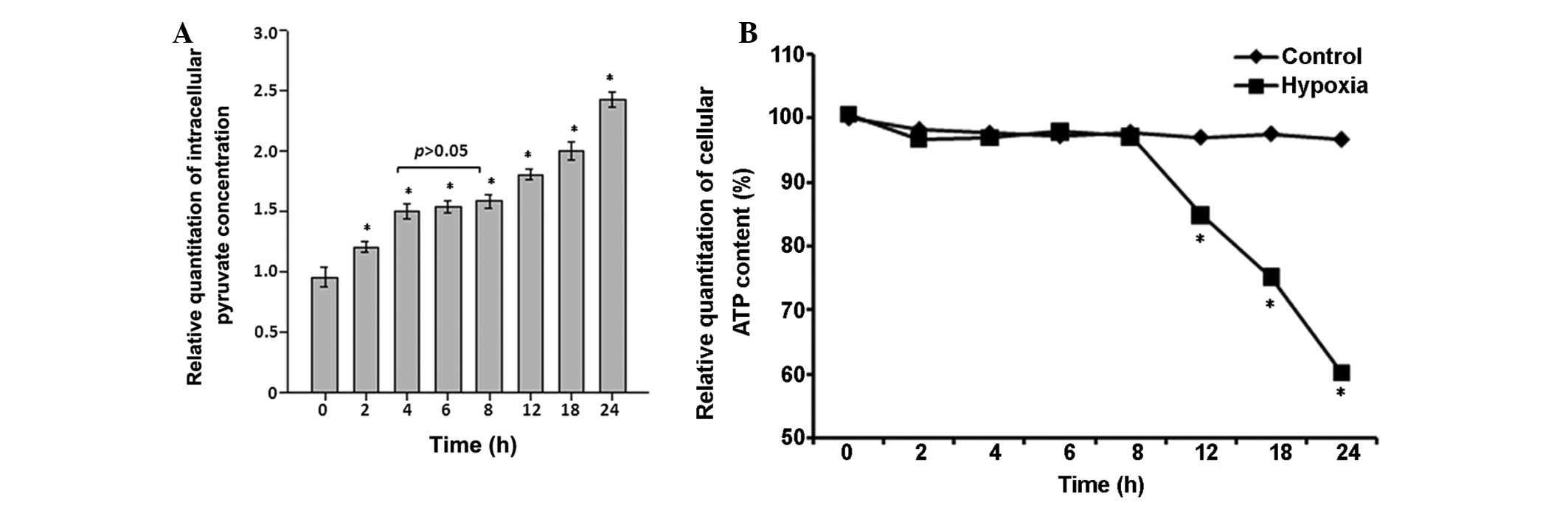
intracellular pyruvate concentration and ATP content
Intracellular pyruvate in astrocytes (Fig. 4A) immediately increased by
~1.2-fold within the first 2 h (P<0.05 vs. control), but
remained steady at a level of ~1.6-fold increase relative to the
control between 4 and 8 h (P<0.05 vs. control). Subsequently,
the intracellular pyruvate concentration further increased from the
12 h time point to ~2.5-fold at the 24 h time point (P<0.05;
Fig. 4A).
By contrast, there were no statistically significant
differences between the two groups in terms of intracellular ATP
content in the first 8 h subsequent to exposure of astrocytes to
100 µM of CoCl2 (P>0.05; Fig. 4B); however, intracellular ATP
content significantly reduced in the hypoxia group each subsequent
time interval (Fig. 4B).
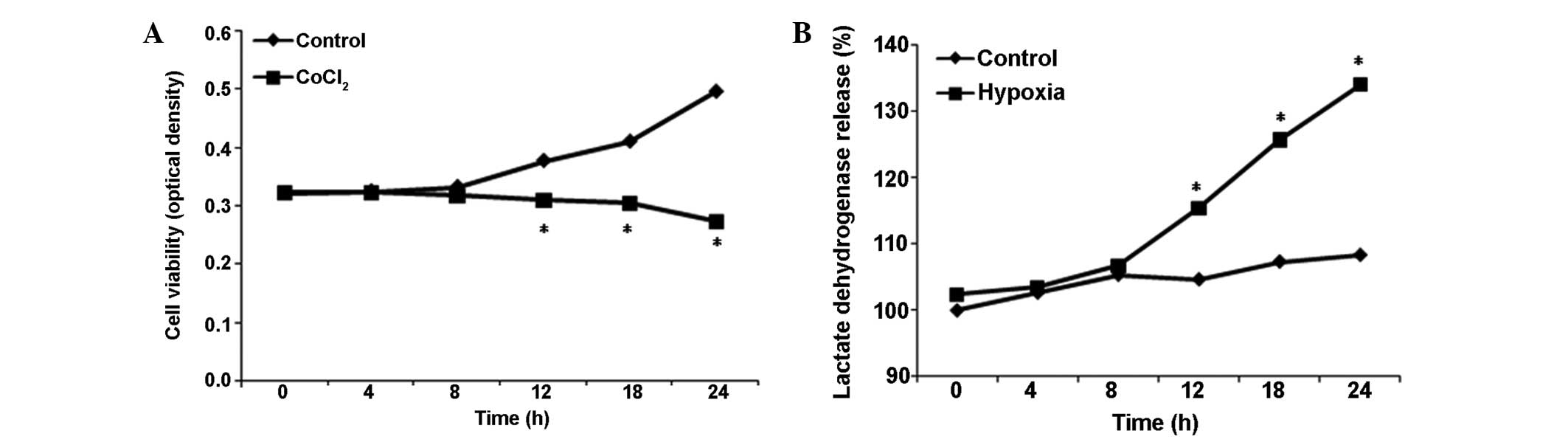
Effects of CoCl2 treatment on
cell viability and cytotoxicity in astrocytes
To further investigate the effects of
CoCl2 treatment on cell viability and cytotoxicity in
astrocytes, cell viability and LDH release activity were
determined. Compared with the control group, cell viability and LDH
release activity in the hypoxia group remained at the baseline
level for the first 8 h of CoCl2 treatment (P>0.05
vs. control; Fig. 5A and B);
however, cell viability reduced significantly following exposure to
100 µM CoCl2 for >12 h, while LDH release
activity continued to increase in each subsequent time
interval.
Discussion
To investigate whether astrocytic expression of
GLUT-1 and GLUT-3 is crucial for regulation of glucose uptake under
CoCl2 treatment, mRNA and protein expression levels of
GLUT-1 and GLUT-3 were determined along with HIF-1α, an indicator
of hypoxic stress and a key regulator of hypoxia (9). It has been reported that levels of
GLUT-1 and GLUT-3 are controlled at the transcriptional level by
HIF-1 (13,26,27).
However, under hypoxic conditions, the flux of glucose through the
glycolytic pathway may be increased by HIF-1α in order to maintain
the requisite ATP levels necessary to sustain life (12). The current study determined that
protein and mRNA levels of GLUT-1 and GLUT-3 were elevated in a
time-dependent manner under CoCl2 treatment, followed by
HIF-1α accumulation resulting from the increased expression.
Expression levels of GLUT-1, GLUT-3 and HIF-1α mRNA increased
immediately and significantly at each time interval during the
first 12 h and remained at the higher levels. Additionally, it was
determined that the mRNA expression levels of GLUT-1, GLUT-3 and
HIF-1α were higher following treatment with CoCl2, and
the regulation occurred at the transcriptional level prior to
protein alteration. Under physiological conditions (0 h time
point), protein expression of GLUT-1 and GLUT-3 were detected at
low levels, and in the first 2 h of CoCl2 treatment,
protein expression levels of GLUT-1 and GLUT-3 did not change.
However, protein expression levels of GLUT-1 and GLUT-3 increased
significantly during the 4–12 h time points and remained at higher
levels throughout the remaining recording period (P<0.05 vs.
control). By contrast, protein expression of HIF-1α remained at
baseline levels in the first 4 h of CoCl2 treatment, but
significantly increased from the 6–12 h time points and increased
further to ~4-fold relative to the control. Therefore, it is
possible that GLUT-1 and GLUT-3 may be regulated by HIF-1α;
however, they have also been reported to be affected by other
regulators, such as connexin 43, c-Src and the Akt/protein kinase A
signaling pathway (10,28,29).
As hypoxia has been indicated to have significant effects on pH
homeostasis due to lactate production and upregulation of
glycolysis to maintain ATP production. Na+/H+
exchanger isoform 1 regulation and pattern of intracellular pH
changes were investigated (data not shown). It had been observed
that during the early period (in the first 2 h of CoCl2
treatment), both NHE1 activity and pHi dropped immediately with
reducing NHE1 mRNA expression, whereas expression levels of NHE1
protein were not altered. In the later period of CoCl2
treatment, activity of NHE1 and value of pHi markedly increased,
and was associated with increased mRNA and protein expression
levels of NHE1 (data not shown).
GLUTs are critical for cell survival under hypoxic
conditions. It is controversial whether GLUT-3 is expressed in
astrocytes (16,18). The present study indicates that
GLUT-3 was detected at extremely low levels in cultured astrocytes
under physiological conditions, however whether GLUT-3 may be
induced in adult brain in vivo still remains unclear. GLUT-3
transports extracellular glucose ~7 times faster than GLUT-1
(19), as GLUT-3 only takes up
extracellular glucose and does not release intracellular glucose to
the extracellular space, even if the intracellular glucose
concentration is higher than the extracellular concentration
(8). Additionally, compared with
GLUT-3 mRNA and protein levels, CoCl2 treatment induced
a higher increase in GLUT-1 mRNA and protein levels (P<0.05). It
is likely that GLUT-1 is important for the increased glucose influx
into astrocytes (30); therefore,
these results indicate that inducing GLUT-3 may in turn support
GLUT-1 activities, and promote the enhancement of glucose uptake
and transport into the astrocytes to meet cellular energy
requirements for maintaining cell viability during hypoxia
(10,31).
By contrast, glucose was quickly consumed to recover
or sustain the homeostasis of cells; therefore, intracellular
glucose and glycogen levels were then investigated. Intracellular
glucose concentration fell immediately by ~25% and increased only
marginally between the 2–8 h time points. Intracellular glucose
concentration then continued to reduce throughout the remaining
recording period. Similarly, intracellular glycogen was reduced in
the first 8 h and then significantly reduced in the remaining
hours, although protein expression levels of GLUT-1 and GLUT-3 were
increased. To explain this phenomenon, increased glycolysis, which
serves to ameliorate metabolic disorders following hypoxia, may be
responsible for breaking down glucose into pyruvate as one of the
means by which ATP is generated (32).
To assess this hypothesis, the time-dependent manner
by which glucose was consumed following astrocyte exposure to 100
µM CoCl2 was determined for distinct time
periods. It was determined that the intracellular pyruvate of
astrocytes increased immediately within the first 2 h following
CoCl2 treatment, and then remained at a steady level of
~1.6-fold relative to the control during the 4–8 h time points, and
continued to significantly increase throughout the remaining
recording period, reaching ~2.5-fold at the 24-h time point
relative to the control. By contrast, intracellular ATP content did
not change within the first 8 h but began to significantly reduce
during the remaining hours. It has been reported that
CoCl2 exposure and oxygen deprivation lead to ATP
depletion in astrocyte cultures; however, ATP is reduced to a lower
extent by hypoxia, suggesting that CoCl2 has additional
cellular targets (26).
Furthermore, CoCl2 is an established blocker of
voltage-gated calcium channels, which may explain its inhibition of
morphological differentiation induced by cyclic adenosine
monophosphate in astrocytes (26,33).
The observations of the present study suggest that, under
CoCl2 treatment, increased glucose uptake allows
astrocytes to utilize more glucose for increased glycolysis, which
may also be increased by HIF-1α to maintain the requisite ATP
levels essential for cell survival and necessary to sustain life,
resulting in an increase in pyruvate and lactate production.
Glycolysis generates a net gain of only two
molecules of ATP per glucose molecule, a markedly smaller quantity
of energy compared with the net gain of 38 molecules of ATP that is
produced by respiration. Thus, normal cells gain only 10% of their
energy through glycolysis (11,34)
and require more glucose uptake to obtain sufficient energy. During
the first 8 h of CoCl2 treatment, increased glucose
uptake compensated for glucose consumption in the cells by
maximizing their energy production. Thus, there was no significant
change in cell viability and LDH release compared with minor
changes in intracellular glycogen storage and ATP content; however,
due to the increase in glycolysis, the intracellular pyruvate
concentration increased and the glucose concentration was reduced.
Intracellular glucose concentration recovered only marginally
during the 4–8 h time points as a result of increased expression
levels of GLUT-1 and GLUT-3; however, it remained at a lower level
compared with the control. However, after 8 h, the increase in
glucose uptake did not meet the demand of enhanced glycolysis,
leading to a breakdown of glucose homeostasis, depletion of
glycogen, deficit of ATP and the accumulation of pyruvate. As a
regular supply of energy is essential to maintain normal cell
function, the loss of the energy supply, even for a short period of
time, results in cell death (7).
In the current study, reduced cell viability and increased LDH
release activity were observed 8 h subsequent to CoCl2
treatment.
In conclusion, the current study indicated that
glucose homeostasis in astrocytes under CoCl2 treatment
is time-dependent. CoCl2 treatment exerted early effects
(in the first 8 h) on glucose homeostasis, leading to minor
alterations in LDH release activity, intracellular glycogen storage
and ATP content; however a significant increase in pyruvate
concentration and a reduction in glucose concentration in
astrocytes was observed. This may be a mechanism for the
maintenance of cell viability as a result of increased glycolysis.
However, 8 h of treatment led to increased LDH release activity,
accumulating pyruvate concentration and reduced intracellular
glucose concentration, a shortage of ATP content, and a deficit in
glycogen storage associated with reduced cell viability. It was
also determined that the expression levels of GLUT-1 and GLUT-3
were regulated by CoCl2 treatment in a time-dependent
manner, and were associated with the regulation of HIF-1α.
Therefore, the present study provides novel evidence that the
regulation of GLUT-1 and GLUT-3 may be responsible for alterations
in cell viability and energy production under CoCl2
treatment, as a mimic of hypoxia in astrocytes, and these
observations emphasize the relevance of hypoxia in GLUT function
regulation and glucose homeostasis in astrocytes. Further
investigation is required to elucidate the mechanisms by which
injury occurs following human stroke, to develop neuroprotective
strategies and to determine an optimal time frame to commence
therapeutic procedures following a stroke.
Acknowledgments
The current study was supported by grants from the
China Scholarship Council (grant no. 201207045015), the '5451'
Project of Henan Health Department (grant no. 201201065), the
Foundation of the Henan Educational Committee (grant nos. 12A310010
and 16A310003), the Foundation for University Key Teacher of Henan
Educational Committee (grant no. 2011GGJS-013), the Science and
Technology Planning Project of Henan (grant nos. 122300410338,
122300410082, 132300410029, 132102310138 and 142102310140), the
Science and Technology Planning Project of Zhengzhou (grant nos.
10PTGS484 7, 121PPTGG507-22, 2PPTSF302 and N201250105) and the
National Natural Science Foundation of China (grant no.
81500433).
References
|
1
|
Cunningham LA, Candelario K and Li L:
Roles for HIF-1α in neural stem cell function and the regenerative
response to stroke. Behav Brain Res. 227:410–417. 2012. View Article : Google Scholar
|
|
2
|
Wang C, Wang Z, Zhang X, Zhang X, Dong L,
Xing Y, Li Y, Liu Z, Chen L, Qiao H, et al: Protection by silibinin
against experimental ischemic stroke: up-regulated pAkt, pmTOR,
HIF-1α and Bcl-2, down-regulated Bax, NF-κB expression. Neurosci
Lett. 529:45–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zemke D, Smith JL, Reeves MJ and Majid A:
Ischemia and ischemic tolerance in the brain: An overview.
Neurotoxicology. 25:895–904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ratan RR, Siddiq A, Smirnova N, Karpisheva
K, Haskew-Layton R, McConoughey S, Langley B, Estevez A, Huerta PT,
Volpe B, et al: Harnessing hypoxic adaptation to prevent, treat,
and repair stroke. J Mol Med Berl. 85:1331–1338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vemula S, Roder KE, Yang T, Bhat GJ,
Thekkumkara TJ and Abbruscato TJ: A functional role for
sodium-dependent glucose transport across the blood-brain barrier
during oxygen glucose deprivation. J Pharmacol Exp Ther.
328:487–495. 2009. View Article : Google Scholar :
|
|
6
|
Agrawal M, Kumar V, Kashyap MP, Khanna VK,
Randhawa GS and Pant AB: Ischemic insult induced apoptotic changes
in PC12 cells: protection by trans resveratrol. Eur J Pharmacol.
666:5–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reagan LP, Magariños AM, Lucas LR, van
Bueren A, McCall AL and McEwen BS: Regulation of GLUT-3 glucose
transporter in the hippocampus of diabetic rats subjected to
stress. Am J Physiol. 276:E879–E886. 1999.PubMed/NCBI
|
|
8
|
Iwabuchi S and Kawahara K: Inducible
astrocytic glucose transporter-3 contributes to the enhanced
storage of intracellular glycogen during reperfusion after
ischemia. Neurochem Int. 59:319–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Badawi Y, Ramamoorthy P and Shi H:
Hypoxia-inducible factor 1 protects hypoxic astrocytes against
glutamate toxicity. ASN Neuro. 4:231–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valle-Casuso JC, González-Sánchez A,
Medina JM and Tabernero A: HIF-1 and c-Src mediate increased
glucose uptake induced by endothelin-1 and connexin43 in
astrocytes. PLoS One. 7:e324482012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pereira KM, Chaves FN, Viana TS, Carvalho
FS, Costa FW, Alves AP and Sousa FB: Oxygen metabolism in oral
cancer: HIF and GLUT-s. Oncol Lett. 6:311–316. 2013.Review.
PubMed/NCBI
|
|
12
|
Seagroves TN, Ryan HE, Lu H, Wouters BG,
Knapp M, Thibault P, Laderoute K and Johnson RS: Transcription
factor HIF-1 is a necessary mediator of the pasteur effect in
mammalian cells. Mol Cell Biol. 21:3436–3444. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinni H, Guo M, Ding JY, Konakondla S,
Dornbos D III, Tran R, Guthikonda M and Ding Y: Cerebral metabolism
after forced or voluntary physical exercise. Brain Res. 1388:48–55.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brix B, Mesters JR, Pellerin L and Jöhren
O: Endothelial cell-derived nitric oxide enhances aerobic
glycolysis in astrocytes via HIF-1α-mediated target gene
activation. J Neurosci. 32:9727–9735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rouach N, Koulakoff A, Abudara V, Willecke
K and Giaume C: Astroglial metabolic networks sustain hippocampal
synaptic transmission. Science. 322:1551–1555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duelli R and Kuschinsky W: Brain glucose
transporters: Relationship to local energy demand. News Physiol
Sci. 16:71–76. 2001.PubMed/NCBI
|
|
17
|
Nijland PG, Michailidou I, Witte ME, Mizee
MR, van der Pol SM, van Het Hof B, Reijerkerk A, Pellerin L, van
der Valk P, de Vries HE and van Horssen J: Cellular distribution of
glucose and monocarboxylate transporters in human brain white
matter and multiple sclerosis lesions. Glia. 62:1125–1141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cidad P, Garcia-Nogales P, Almeida A and
Bolaños JP: Expression of glucose transporter GLUT-3 by endotoxin
in cultured rat astrocytes: the role of nitric oxide. J Neurochem.
79:17–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maher F, Davies-Hill TM and Simpson IA:
Substrate specificity and kinetic parameters of GLUT-3 in rat
cerebellar granule neurons. Biochem J. 315:827–831. 1996.
View Article : Google Scholar
|
|
20
|
Efrati S, Fishlev G, Bechor Y, Volkov O,
Bergan J, Kliakhandler K, Kamiager I, Gal N, Friedman M, Ben-Jacob
E and Golan H: Hyperbaric oxygen induces late neuroplasticity in
post stroke patients - randomized, prospective trial. PLoS One.
8:e537162013. View Article : Google Scholar :
|
|
21
|
Saxena S, Shukla D and Bansal A:
Augmentation of aerobic respiration and mitochondrial biogenesis in
skeletal muscle by hypoxia preconditioning with cobalt chloride.
Toxicol Appl Pharmacol. 264:324–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Björklund O, Shang M, Tonazzini I, Daré E
and Fredholm BB: Adenosine A1 and A3 receptors protect astrocytes
from hypoxic damage. Eur J Pharmacol. 596:6–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McCarthy KD and de Vellis J: Preparation
of separate astroglial and oligodendroglial cell cultures from rat
cerebral tissue. J Cell Biol. 85:890–902. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karovic O, Tonazzini I, Rebola N, Edström
E, Lövdahl C, Fredholm BB and Daré E: Toxic effects of cobalt in
primary cultures of mouse Astrocytes Similarities with hypoxia and
role of HIF-1a. Biochem Pharmacol. 73:694–708. 2007. View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Schubert D: Glucose metabolism and
Alzheimer's disease. Ageing Res Rev. 4:240–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones NM and Bergeron M: Hypoxic
preconditioning induces changes in HIF-1 target genes in neonatal
rat brain. J Cereb Blood Flow Metab. 21:1105–1114. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang KR, Jiang T, Wu TT, Zhou SH, Yao HT,
Wang QY and Lu ZJ: Expression of hypoxia-related markers in
inflammatory myofibroblastic tumors of the head and neck. World J
Surg Oncol. 11:2942013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Papa Pde C, Sousa LM, Silva Rdos S, de
Fátima LA, da Fonseca VU, do Amaral VC, Hoffmann B, Alves-Wagner
AB, Machado UF and Kowalewski MP: Glucose transporter 1 expression
accompanies hypoxia sensing in the cyclic canine corpus luteum.
Reproduction. 147:81–89. 2014. View Article : Google Scholar
|
|
30
|
Yamada T, Uchida M, Kwang-Lee K, Kitamura
N, Yoshimura T, Sasabe E and Yamamoto T: Correlation of
metabolism/hypoxia markers and fluorodeoxyglucose uptake in oral
squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral
Radiol. 113:464–471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu O, Li X, Qu Y, Liu S, An J, Wang M, Sun
Q, Zhang W, Lu X, Pi L, et al: Regulation of glucose transporter
protein-1 and vascular endothelial growth factor by hypoxia
inducible factor 1α under hypoxic conditions in Hep-2 human cells.
Mol Med Rep. 6:1418–1422. 2012.PubMed/NCBI
|
|
32
|
Dornbos D III, Zwagerman N, Guo M, Ding
JY, Peng C, Esmail F, Sikharam C, Geng X, Guthikonda M and Ding Y:
Preischemic exercise reduces brain damage by ameliorating metabolic
disorder in ischemia/reperfusion injury. J Neurosci Res.
91:818–827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
MacVicar BA: Morphological differentiation
of cultured astrocytes is blocked by cadmium or cobalt. Brain Res.
420:175–177. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Eckert AW, Lautner MH, Schütze A, Taubert
H, Schubert J and Bilkenroth U: Coexpression of hypoxia-inducible
factor-1α and glucose transporter-1 is associated with poor
prognosis in oral squamous cell carcinoma patients. Histopathology.
58:1136–1147. 2011. View Article : Google Scholar : PubMed/NCBI
|