Introduction
The hippocampus is a section of the forebrain, which
is important in regulating emotionality and cognitive processes,
including memory and learning (1,2).
Among the hippocampal subregions, the dentate gyrus (DG) grey
matter is a well-known neurogenic region, and neural progenitor
cells in the subgranular zone (SGZ) of the DG migrate into the
granule cell layer and differentiate into mature granule cells
(3–6). Newly formed granule cells in the DG
are closely associated with memory and learning (2,7). In
addition, it has been reported that neurogenesis in the hippocampus
is influenced by numerous factors, including age, pathological
conditions and pharmacological drugs (8–11).
Furthermore, numerous studies have focused on neurogenesis in
neurodegenerative diseases, and the stimulation of neurogenesis in
neurogenic regions may be a potential therapeutic strategy for
neurodegenerative diseases (12–15).
4-Hydroxy-3-methoxybenzaldehyde (vanillin) and
4-hydroxybenzyl alcohol (4-HBA) are phenolic constituents found in
various types of plants, including Gastrodia elata Blume
(Orchidaceae) (16,17). Previous studies have suggested that
vanillin and 4-HBA have several therapeutic properties, including
antioxidant, anti-inflammatory and anticancer properties (18–21).
It has also been reported that vanillin and 4-HBA have a variety of
beneficial effects against brain injury (22–24);
however, few studies, to the best of our knowledge, regarding the
effects of vanillin and 4-HBA on neurogenesis in the brain have
been reported.
The present study first investigated the effects of
vanillin and 4-HBA on cell proliferation and neuroblast
differentiation in the DG using 5-bromo-2′-deoxyuridine (BrdU; an
indicator for cell proliferation) labeling, Ki-67 (an endogenous
marker for cell proliferation) and doublecortin (DCX; a marker for
neuroblasts). In addition, the effects of the treatments on the
expression of brain-derived neurotrophic factor (BDNF) and
tropomyosin-related kinase B (TrkB, a BDNF receptor) in the DG of
adolescent mice, since BDNF is known to be implicated in adult
hippocampal neurogenesis through its primary receptor, TrkB
(25,26). The results of the present study may
provide further information on the enhancement of neurogenesis,
which is important as various neurological diseases are
characterised by impaired neurogenesis.
Materials and methods
Experimental animals
A total of 42 male adolescent ICR mice, aged 8
weeks, were obtained from Orientbio, Inc. (Seongnam, South Korea)
and used following 7 days of acclimation. The mice were housed in
an atmosphere of 23°C and 60% humidity with a 12 h light/dark cycle
and free access to food and water. The handling and caring of
animals conformed to the National Institute of Health Guide for the
Care and Use of Laboratory Animals (NIH Publication No. 85–23,
1985, revised 1996). The present study was approved by the
Institutional Animal Care and Use Committee of Kangwon National
University (KIACUC-12-0018). The utmost effort was made to minimize
the number of animals used in the present study, as well as the
suffering caused to them by the experiments performed.
Treatment with vanillin, 4-HBA and
BrdU
The animals were divided into three groups
(n=14/group): i) The vehicle-treated group (vehicle group); ii) the
40 mg/kg vanillin-treated group (vanillin group); iii) the 40 mg/kg
4-HBA-treated group (4-HBA group). Vanillin and 4-HBA were
purchased from Sigma-Aldrich (St. Louis, MO, USA) and were prepared
in 1 ml 10% Tween-80 solution dissolved in normal saline. The
experimental dosages of vanillin and 4-HBA were selected based on
our previous study (22), and
vehicle, vanillin and 4-HBA were administered orally using a
feeding needle once daily for 28 days, due to the fact that DCX is
exclusively expressed in immature neurons only between days 1–28 of
cell age (27,28). A 10% Tween-80 solution dissolved in
normal saline was injected into the mice of the vehicle group. The
animals were weighed twice weekly during drug treatment. No
significant differences were observed in the body weight of mice in
the experimental groups (data not shown). In order to label the
dividing cells in the DG, all animals received an intraperitoneal
injection of 50 mg/kg BrdU (Sigma-Aldrich) on days 8, 15, 22 and 27
of the experiment, as described in our previous study (29,30).
Tissue processing for histology
For histological analysis, the animals (n=7/group)
were anesthetized with 30 mg/kg Zoletil 50 (Virbac, Carros, France)
and perfused transcardially with 0.1 M phosphate-buffered saline
(PBS; pH 7.4), followed by 4% para-formaldehyde in 0.1 M PBS. The
brains were removed and post-fixed in the same fixative for 4 h at
room temperature. The brain tissues were subsequently cryoprotected
by infiltration with 30% sucrose overnight. The frozen tissues were
serially sectioned on a cryostat (Leica, Wetzlar, Germany) into 30
µm coronal sections and were subsequently collected into
6-well plates containing PBS for further analyses.
Immunohistochemistry
To obtain accurate data for immuno-histochemistry,
the tissue sections were carefully processed under identical
conditions. The tissue sections were selected between -1.46 and
-2.46 mm posterior to the bregma in reference to the mouse atlas
(31). The sections were
sequentially treated with 0.3% hydrogen peroxide in PBS for 30 min
at room temperature and 10% normal goat serum in 0.05 M PBS for 30
min at room temperature. They were subsequently incubated with
diluted polyclonal rabbit anti-Ki-67 (dilution, 1:100; cat. no.
ab15580; Abcam, Cambridge, UK) or polyclonal goat anti-DCX
(dilution, 1:100; cat. no. sc-8066; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) overnight at 4°C. The sections were exposed to
biotinylated goat anti-rabbit or rabbit anti-goat immunoglobulin G
(IgG; dilution, 1:200; cat. no. BA-1000; Vector Laboratories Inc.,
Burlingame, CA, USA) and streptavidin peroxidase complex (dilution,
1:200; cat. no. SA-5004; Vector Laboratories Inc.). The abtibodies
were visualized with 3,3′-diaminobenzidine tetrahydrochloride in
0.1 M Tris-hydrochloride buffer and mounted on gelatin-coated
slides. Following dehydration the sections were mounted in Canada
balsam (Kanto Chemical Co., Inc., Tokyo, Japan).
Images of Ki-67 and DCX-immunoreactive structures
were captured using an AxioM1 light microscope (BX53; Olympus,
Tokyo, Japan) equipped with a digital camera (DP72; Olympus)
connected to a personal computer monitor. The total number of Ki-67
or DCX positive cells in all groups were counted in six
sections/animal using an Image Analysis System equipped with a
computer-based CCD camera (Optimas 6.5; CyberMetrics, Scottsdale,
AZ, USA). The cell counts were obtained by averaging the counts
from the tissue sections obtained from each animal.
Double immunofluorescence
Double immunofluorescence staining for BrdU and
feminizing Locus on X 3 (NeuN) was performed in order to confirm
the differentiation from newly generated cells to mature neurons.
DNA denaturation was performed as follows: For BrdU immunostaining
to visualize BrdU-labeled nuclei, the cells were incubated for 2 h
in 50% formamide/2X SSC (0.3 M NaCl and 0.03 M sodium citrate) at
65°C and 30 min in 2 N HCl at 37°C, followed by rinsing for 10 min
in 0.1 M boric acid (pH 8.5). Following these steps, the tissue
sections were incubated in the mixture of monoclonal rat anti-BrdU
(dilution, 1:100; cat. no. MBS212468; BioSource International,
Camarillo, CA, USA) and polyclonal rabbit anti-NeuN (dilution,
1:500; cat. no. ABN78; Chemicon International, Temecula, CA, USA)
overnight at 4°C. They were subsequently incubated in a mixture of
fluorescein isothiocyanate-conjugated anti-rat IgG (dilution,
1:200; cat. no. 712-095-153; Jackson ImmunoResearch Labs, Inc.,
West Grove, PA, USA) and Cy3-conjugated anti-rabbit IgG (dilution,
1:500; cat. no. 711-165-152; Jackson ImmunoResearch Labs, Inc.) for
2 h at room temperature. The immunoreactions were observed under a
confocal microscope (LSM 510 META NLO; Carl Zeiss, Jena, Germany).
Cell counts were performed, as described above.
Western blot analysis
In order to examine the changes in the protein
expression levels of DCX, BDNF and TrkB in the DG following
vanillin or 4-HBA treatment for 28 days, 7 animals from each group
were anesthetized with 30 mg/kg Zoletil 50 (Virbac, Carros,
France), sacrificed by cervical dislocation, and used for western
blot analysis, as described in our previous study (30). Briefly, following sacrifice by
cervical dislocation, the mice were decapitated and the brains were
removed. The brains were then serially and transversely cut into
400 µm thick tissue sections using a vibratome (Leica Camera
AG, Wetzlar, Germany). Subsequently, the DG was dissected using a
surgical blade. The tissues were homogenized in 50 mM PBS (pH 7.4)
containing ethylene glycol tetraacetic acid (pH 8.0), 0.2% NP-40,
10 mM ethylenediaminetetraacetic acid (pH 8.0), 15 mM sodium
pyrophosphate, 100 mM β-glycerophosphate, 50 mM sodium fluoride,
150 mM NaCl, 2 mM sodium orthvanadate, 1 mM phenylmethylsulfonyl
fluoride and 1 mM dithiothreitol (DTT).
Following centrifugation at 16,000 × g for 20 min at
4°C, a Micro bicinchoninic acid Protein Assay kit with bovine serum
albumin as a standard (Pierce Chemical, Rockford, IL,. USA) was
used to determine the protein level in the supernatants. Aliquots
containing 50 µg total protein were boiled in loading
buffer, which contained 250 mM Tris (pH 6.8), 10 mM DTT, 10% sodium
dodecyl sulfate, 0.5% bromophenol blue and 50% glycerol. The
aliquots were subsequently loaded onto a 10% polyacrylamide gel
(Sigma-Aldrich).
Following electrophoresis, the gels were transferred
onto nitrocellulose membranes (Pall Corp., Pittsburgh, PA, USA).
The same stripped nitrocellulose membranes were used to incubate
all antibodies. In order to reduce background staining, the
membranes were incubated with 5% non-fat dry milk in Tris buffered
saline containing 0.1% Tween 20 for 45 min. The membranes were
subsequently incubated overnight at 4°C with polyclonal goat
anti-DCX (dilution, 1:100; cat. no. sc-8066; Santa Cruz
Biotechnology, Inc.), which produced a band at ~40 kDa, polyclonal
rabbit anti-BDNF (dilution, 1:500; cat. no. ab6200; Abcam), which
produced a band at ~28 kDa, and polyclonal rabbit anti-TrkB
(dilution, 1:500; cat. no. sc-8316; Santa Cruz Biotechnology,
Inc.), which produced two bands [truncated TrkB (95 kDa) and
full-length TrkB (145 kDa)]. The membranes were subsequently
exposed to peroxidase-conjugated rabbit anti-goat (cat. no.
sc-2768; dilution 1:5,000; Santa Cruz Biotechnology, Inc.) and goat
anti-rabbit IgG (cat. no. sc-2004; dilution 1:5,000; Santa Cruz
Biotechnology, Inc.) and an enhanced chemiluminescence kit (GE
Healthcare Life Sciences, Chalfont, UK).
The result of the western blot analysis was scanned
and densitometric analysis was performed for the quantification of
the bands. Scion Image 4.0.2 software (Scion Corp., Frederick, MD,
USA) was used to calculate the relative optical density (ROD): A
ratio of the ROD was calibrated as %, with the vehicle group
designated as 100%.
Statistical analysis
The data are presented as the mean ± standard error.
Statistical analysis of the differences between the groups was
performed using one-way analysis of variance with Duncan's post-hoc
test with SPPS software version 17.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Changes in cell proliferation
Ki-67 positive (Ki-67+) cells were
predominantly detected in the SGZ of the DG in all experimental
groups (Fig. 1). In the vehicle
group, numerous Ki-67+ cells were observed in the SGZ
(Fig. 1A). In both the vanillin
and 4-HBA groups, the number of Ki-67+ cells was
significantly increased compared with the vehicle group; however,
no significant differences were identified in the distribution and
number of Ki-67+ cells between the vanillin and 4-HBA
groups (Fig. 1B–D).
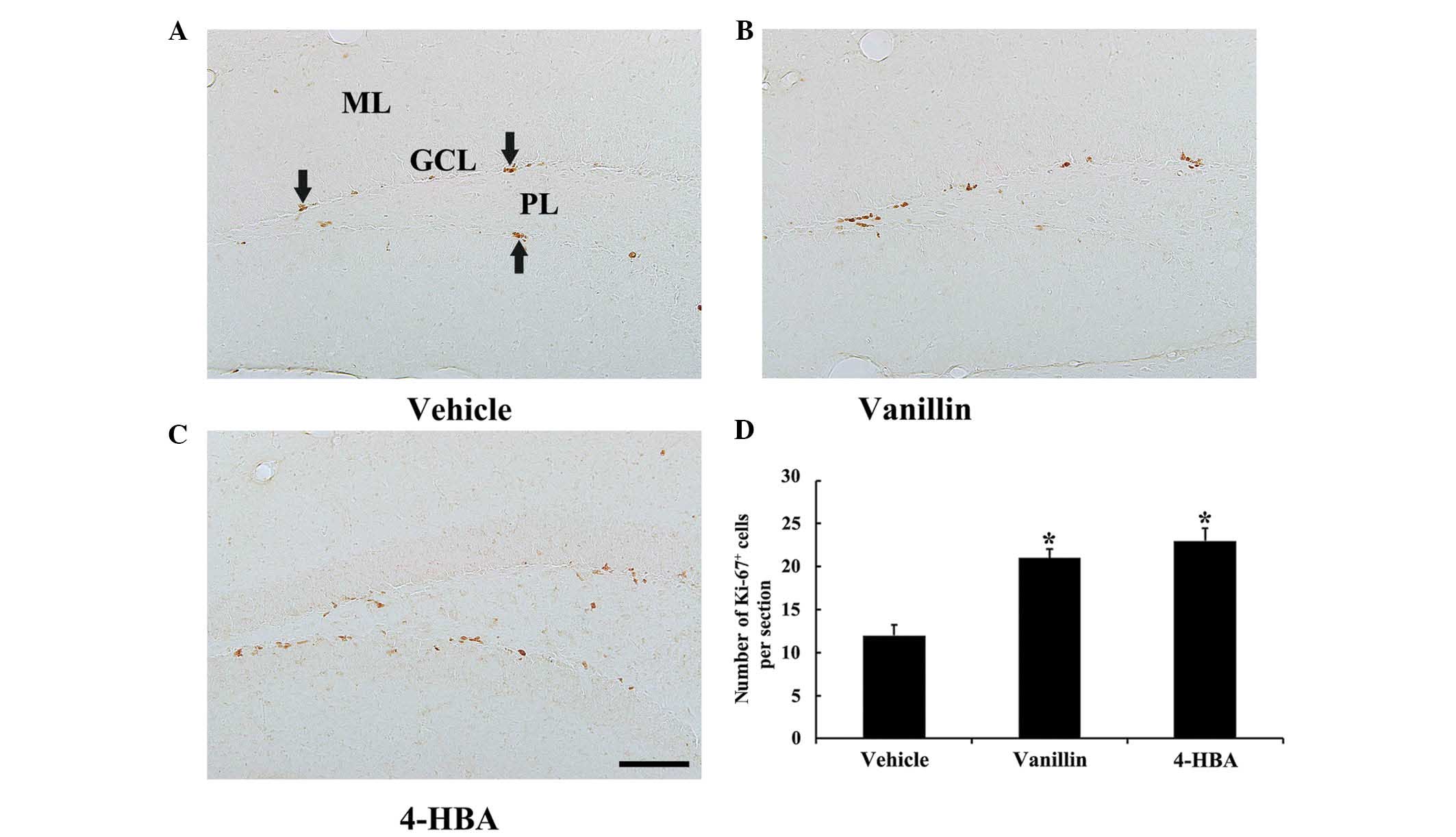 | Figure 1Immunohistochemistry for Ki-67 in the
DG of the (A) vehicle, (B) vanillin and (C) 4-HBA groups.
Ki-67+ cells (arrows) were easily observed in the
vehicle group. Ki-67+ cells in the vanillin and 4-HBA
groups were more abundant compared with the vehicle group. (Scale
bar, 100 µm). (D) The mean number of Ki-67+ cells
per section in the DG in the vehicle, vanillin and 4-HBA groups
were calculated. The data are presented as the mean ± standard
error (n=7/group; *P<0.05, vs. the vehicle group).
ML, molecular layer; GCL, granule cell layer; PL, polymorphic
layer; DCX, doublecortin; DG, dentate gyrus; HBA, hydroxybenzyl
alcohol; vanillin, 4-hydroxy-3-methoxybenzaldehyde. |
Changes in neuroblast
differentiation
In all experimental groups, DCX+
neuroblasts were predominantly detected in the SGZ of the DG
(Fig. 2). In the vehicle group,
numerous DCX+ neuroblasts were observed in the SGZ, some
with poorly-developed and others with well-developed dendrites with
tertiary branches, which extended into the molecular layer of the
DG (Fig. 2A and D). The number of
DCX+ neuroblasts was significantly increased in both the
vanillin and 4-HBA groups, as compared with the vehicle group,
although no significant differences were observed in the number of
DCX+ neuroblasts between the vanillin and 4-HBA groups
(Fig. 2B–D). In addition, the
dendrites of DCX+ neuroblasts in the vanillin and 4-HBA
groups were considerably long and thick compared with the ones in
the vehicle group (Fig. 2B and
C).
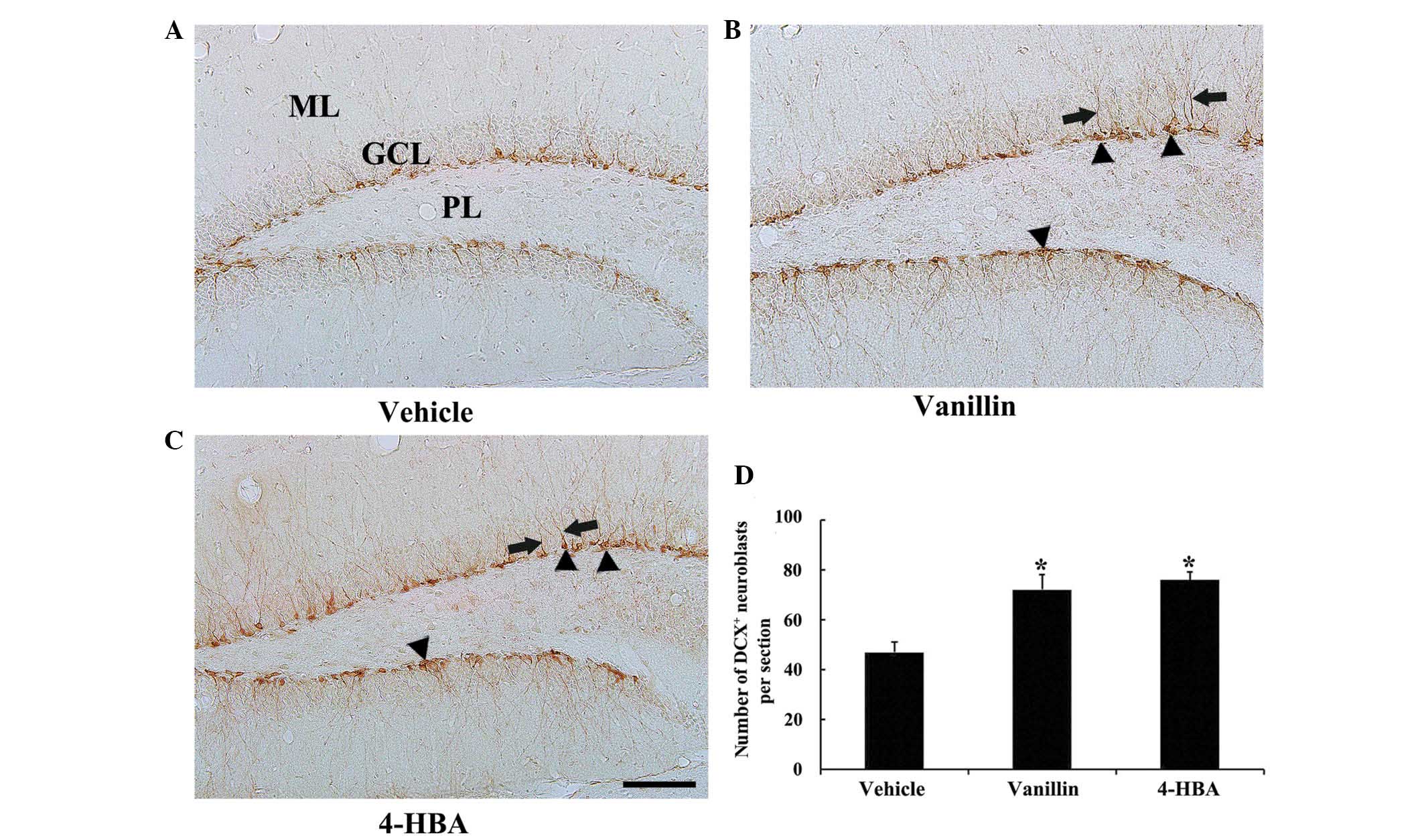 | Figure 2Immunohistochemistry for DCX in the
DG of the (A) vehicle, (B) vanillin and (C) 4-HBA groups. In the
vanillin and 4-HBA groups, the number of DCX+
neuroblasts (arrowheads) was significantly increased, and their
dendrites (arrows) were considerably longer and thicker compared
with the vehicle control group. (Scale bar, 100 µm). (D) The
mean number of DCX+ neuroblasts per section in the DG of
the vehicle, vanillin and 4-HBA groups were calculated. The data
are expressed as the mean ± standard error (n=7/group;
*P<0.05, vs. the vehicle group). ML, molecular layer;
GCL, granule cell layer; PL, polymorphic layer; DCX, doublecortin;
DG, dentate gyrus; HBA, hydroxybenzyl alcohol; vanillin,
4-hydroxy-3-methoxybenzaldehyde. |
BrdU+/NeuN+
neurons
In all experimental groups, newly generated
BrdU+ neurons with NeuN immunoreactivity were detected
in the SGZ and granular cell layer of the DG (Fig. 3). In the vanillin and 4-HBA groups,
the number of BrdU+/NeuN+ neurons was
revealed to be significantly increased (~2-fold) compared with that
in the vehicle group (Fig. 3).
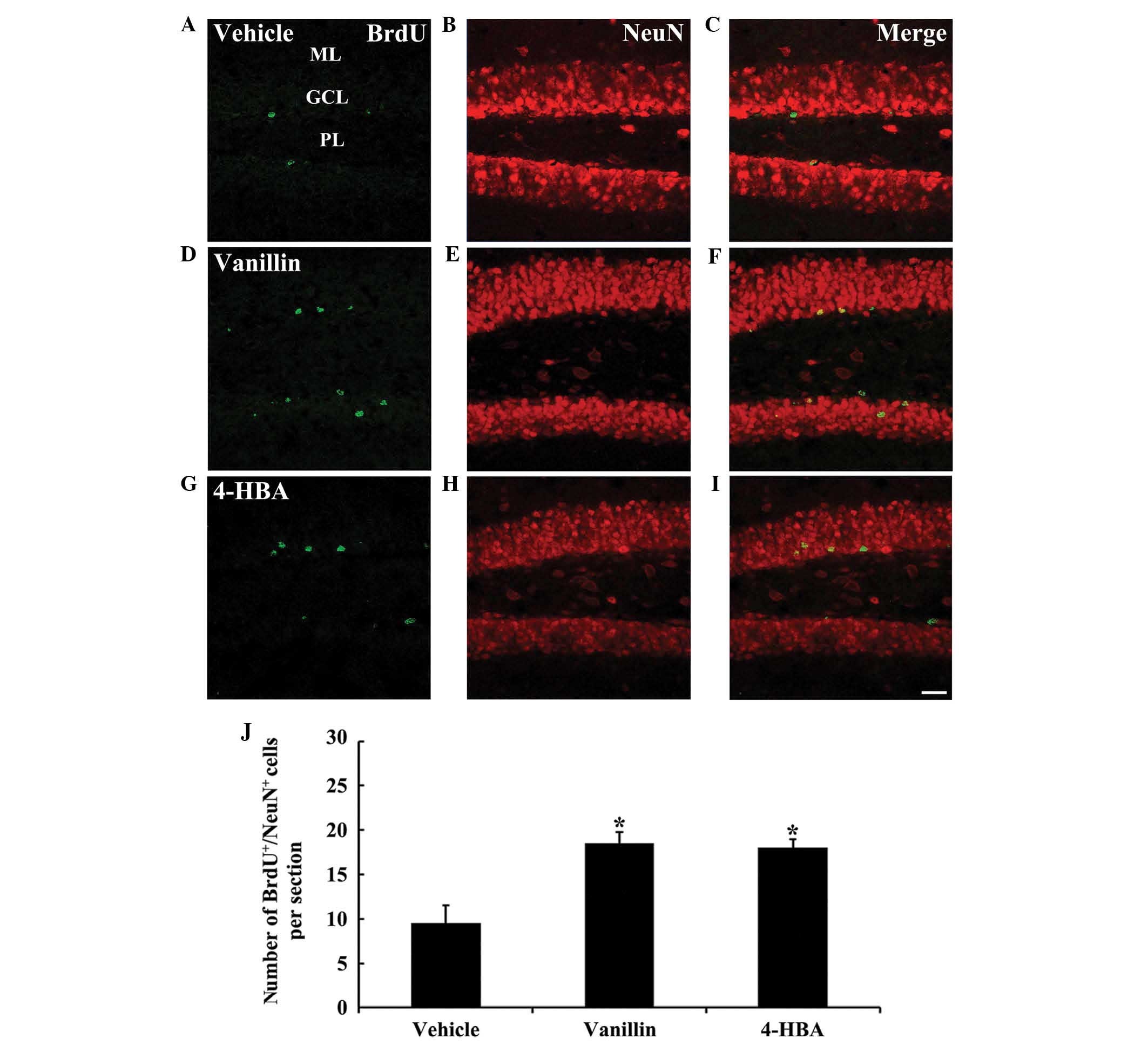 | Figure 3(A-I) Confocal images of cells
double-labeled with BrdU (green; A, D and G), NeuN (red; B, E and
H) and merged images (C, F and I) in the DG of the (A-C) vehicle,
(D-F) vanillin and (G-I) 4-HBA groups. In the vanillin and 4-HBA
groups, the number of BrdU+/NeuN+ neurons
were significantly increased compared with the vehicle group.
(Scale bar, 40 µm). (J) The mean number of
BrdU+/NeuN+ neurons per section in the DG of
the vehicle, vanillin and 4-HBA groups. The data are presented as
the mean ± standard error (n=7/group; *P<0.05, vs.
the vehicle group). ML, molecular layer; GCL, granule cell layer;
PL, polymorphic layer; DG, dentate gyrus; HBA, hydroxybenzyl
alcohol; vanillin, 4-hydroxy-3-methoxybenzaldehyde; BrdU,
5-bromo-2′-deoxyuridine; NeuN, feminizing Locus on X 3. |
Changes in the protein expression levels
of DCX, BDNF and TrkB
In the present study, changes in the protein
expression levels of DCX, BDNF and TrkB (full-length and truncated
forms) were examined in the DG by western blot analysis (Fig. 4). In both the vanillin and 4-HBA
groups, the protein levels of DCX (~1.7-fold in the vanillin and
~2-fold in 4-HBA group), BDNF (~1.5-fold in each group) and
full-length TrkB (~1.5-fold in each group) were significantly
increased compared with those in the vehicle group; however, no
significant differences were observed in the protein expression of
truncated TrkB, a dominant negative inhibitor of BDNF signaling via
full-length TrkB (32), between
the vanillin or 4-HBA, and the vehicle groups.
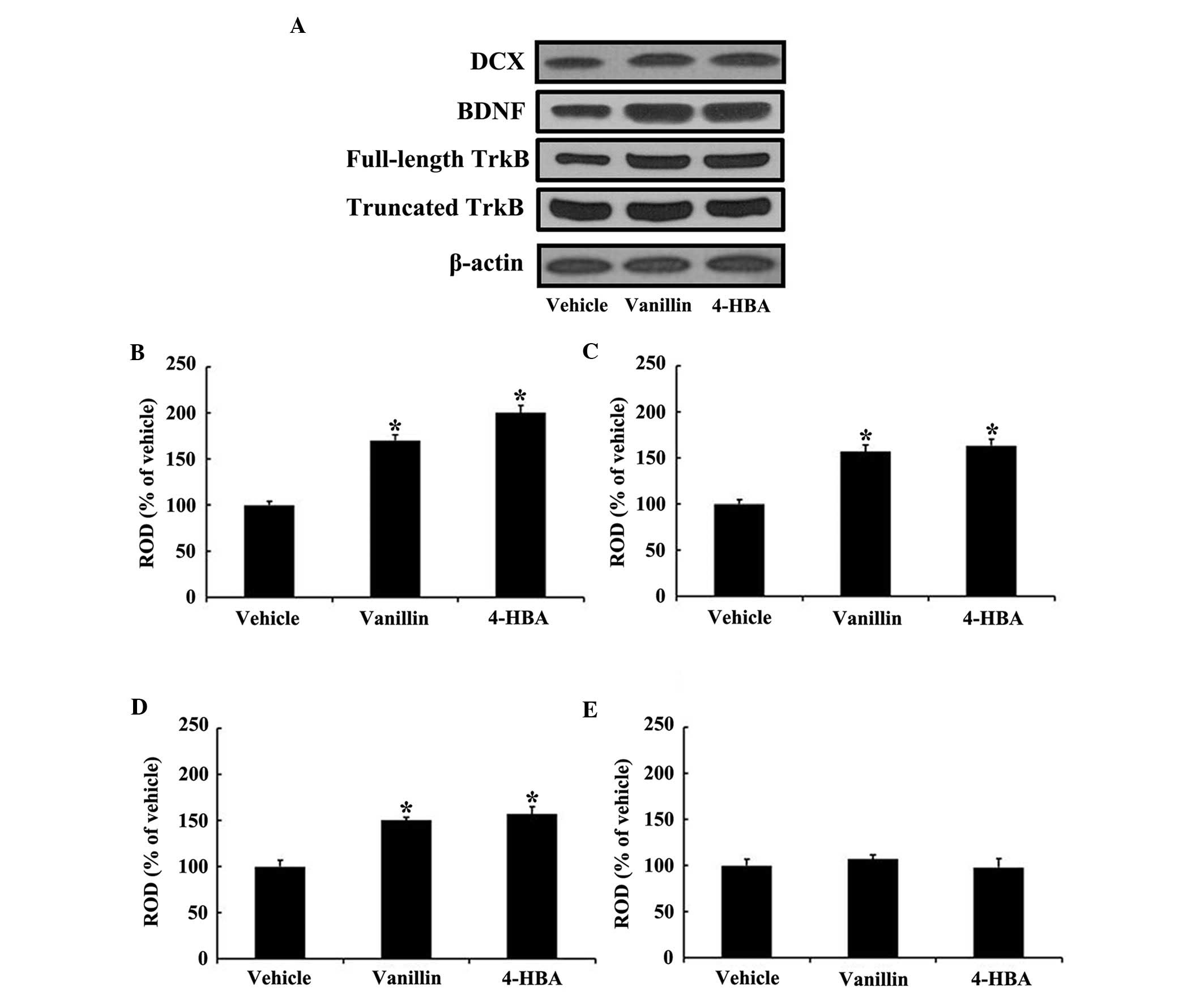 | Figure 4Western blot analysis of (A) the
expression levels of DCX, BDNF and TrkB (full-length and truncated
forms) in the DG of the vehicle, vanillin and 4-HBA groups. ROD, as
percentage of the immunoblot band is shown for (B) DCX, (C) BDNF,
(D) full-length TrkB, and (E) truncated TrkB. The data are
presented as the mean ± standard error (n=7 per group;
*P<0.05, vs. the vehicle group). ROD, relative
optical density; DCX, doublecortin; BDNF, brain-derived
neurotrophic factor; TrkB, tropomyosin-related kinase B; HBA,
hydroxybenzyl alcohol; vanillin,
4-hydroxy-3-methoxybenzaldehyde. |
Discussion
Adult neurogenesis in the DG is considered to have
an important role in hippocampal functions associated with learning
and memory (7). It is well-known
that the suppression of neurogenesis in the DG by aging or
treatments with certain pharmacological drugs leads to an
impairment of the hippo-campus-dependent memory (33,34).
By contrast, numerous previous studies have reported that
neurogenesis in the DG is increased in response to environmental
conditions, including exercise, dietary energy restrictions and
environmental enrichment, and that enhanced neurogenesis may
improve learning and memory (1,27,35,36).
In the present study, the effects of vanillin and
4-HBA treatments on cell proliferation and neuroblast
differentiation in the SGZ of the DG in adolescent mice were first
examined. The results revealed that the number of Ki-67+
cells and DCX+ neuroblasts were significantly increased
in both the vanillin and 4-HBA groups compared with the vehicle
group. In addition, the number of BrdU+/NeuN+
double-labeled granule cells was significantly increased in both
the vanillin and 4-HBA groups. This finding was consistent with the
findings of previous studies showing that treatments with phenolic
compounds found in plants, including curcumin and
(-)-epigallocatechin-3-gallate, increased neurogenesis in the
hippocampus of adult mice (37,38).
It has been previously reported that adult
hippocampal neurogenesis is regulated by various growth factors,
including BDNF (39,40). BDNF is a member of the neurotrophin
family, which is involved in neuronal survival and plasticity and
exerts its effects by binding to the TrkB, which regulates the
survival and differentiation of neurons and synaptic plasticity of
the central nervous system (41–43).
In order to explain the increased neurogenesis following vanillin
and 4-HBA treatments, the present study investigated the
alterations in the protein expression levels of BDNF and TrkB in
the DG. It was revealed that, in both the vanillin and 4-HBA
groups, the expression levels of BDNF and TrkB were markedly
increased in the DG, as compared with the vehicle group. BDNF is
known to influence the developmental processes of the brain
(44,45). Scharfman et al (40) reported that administration of BDNF
significantly increased neurogenesis in the DG of rats, whereas
other previous studies reported that the knockdown of BDNF reduced
neurogenesis in the DG of both adult rats and mice (35,46).
In addition, it was previously shown that BDNF-TrkB signaling is
closely associated with hippocampal neurogenesis (25,26).
Sairanen et al (47)
reported that a decrease in the protein expression of BDNF or TrkB
activity causes reductions in neurogenesis in the mouse DG.
Furthermore, it was previously shown that exercise-induced
increases in the expression of BDNF and TrkB in the hippo-campus
were associated with the increase in cell proliferation in the
hippocampal DG (48).
The results of the present study revealed that cell
proliferation, as well as neuroblast differentiation and
integration into granule cells, were markedly increased in the DG
of adolescent mice treated with vanillin or 4-HBA. In addition, the
expression levels of BDNF and TrkB were found to be significantly
increased by vanillin or 4-HBA treatment, indicating that vanillin
and 4-HBA enhanced cell proliferation, neuroblast differentiation
and integration of granule cells in the DG of adolescent mice.
These neurogenic effects of vanillin and 4-HBA may be closely
associated with increases in BDNF and TrkB. Based on these
findings, it was hypothesized that vanillin and 4-HBA have high
therapeutic potential for the prevention and treatment of
neurological disorders that involve impaired neurogenesis,
including depression (49) and
Alzheimer's disease (50).
Acknowledgments
The authors would like to thank Mr. Seung Uk Lee
(Department of Neurobiology, School of Medicine, Kangwon National
University, Chuncheon, South Korea) for his technical assistance in
the present study. The present study was supported by the Basic
Science Research Program of the National Research Foundation of
Korea funded by the Ministry of Science, ICT and future Planning
(grant no. NRF-2013R1A2A2A01068190), and by the National Research
Foundation of Korea (grant no. NRF-2013M3A9B6046563), which was
funded by the Ministry of Science, ICT, and Future Planning.
References
|
1
|
Sahay A, Scobie KN, Hill AS, O'Carroll CM,
Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A and Hen R:
Increasing adult hippocampal neurogenesis is sufficient to improve
pattern separation. Nature. 472:466–470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shimazu K, Zhao M, Sakata K, Akbarian S,
Bates B, Jaenisch R and Lu B: NT-3 facilitates hippocampal
plasticity and learning and memory by regulating neurogenesis.
Learn Mem. 13:307–315. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kempermann G: The neurogenic reserve
hypothesis: What is adult hippocampal neurogenesis good for? Trends
Neurosci. 31:163–169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li H, Lee CH, Yoo KY, Choi JH, Park OK,
Yan BC, Byun K, Lee B, Hwang IK and Won MH: Chronic treatment of
exendin-4 affects cell proliferation and neuroblast differentiation
in the adult mouse hippocampal dentate gyrus. Neurosci Lett.
486:38–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong J, Wu G, Zou Y, Tao J and Chen L:
Electroacupuncture promotes neurological functional recovery via
the retinoic acid signaling pathway in rats following cerebral
ischemia-reperfusion injury. Int J Mol Med. 31:225–231. 2013.
|
|
6
|
Zhang L, Yan R, Zhang Q, Wang H, Kang X,
Li J, Yang S, Zhang J, Liu Z and Yang X: Survivin, a key component
of the Wnt/β-catenin signaling pathway, contributes to traumatic
brain injury-induced adult neurogenesis in the mouse dentate gyrus.
Int J Mol Med. 32:867–875. 2013.PubMed/NCBI
|
|
7
|
Bruel-Jungerman E, Rampon C and Laroche S:
Adult hippo-campal neurogenesis, synaptic plasticity and memory:
Facts and hypotheses. Rev Neurosci. 18:93–114. 2007. View Article : Google Scholar
|
|
8
|
Hwang IK, Yi SS, Song W, Won MH, Yoon YS
and Seong JK: Effects of age and treadmill exercise in chronic
diabetic stages on neuroblast differentiation in a rat model of
type 2 diabetes. Brain Res. 1341:63–71. 2010. View Article : Google Scholar
|
|
9
|
Feng X, Xing J, Feng G, Sang A, Shen B, Xu
Y, Jiang J, Liu S, Tan W, Gu Z and Li L: Age-dependent impaired
neurogenic differentiation capacity of dental stem cell is
associated with Wnt/β-catenin signaling. Cell Mol Neurobiol.
33:1023–1031. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niu Y, Li Y, Zang J, Huang H and Deng J,
Cui Z, Yu D and Deng J: Death receptor 5 and neuroproliferation.
Cell Mol Neurobiol. 32:255–265. 2012. View Article : Google Scholar
|
|
11
|
Zhang XY, Yang YJ, Xu PR, Zheng XR, Wang
QH, Chen CF and Yao Y: The role of β-catenin signaling pathway on
proliferation of rats neural stem cells after hyperbaric oxygen
therapy in vitro. Cell Mol Neurobiol. 31:101–109. 2011. View Article : Google Scholar
|
|
12
|
Fuster-Matanzo A, Llorens-Martin M,
Hernández F and Avila J: Role of neuroinflammation in adult
neurogenesis and Alzheimer disease: Therapeutic approaches.
Mediators Inflamm. 2013:2609252013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Sullivan SS, Johnson M, Williams DR,
Revesz T, Holton JL, Lees AJ and Perry EK: The effect of drug
treatment on neurogenesis in parkinson's disease. Mov Disord.
26:45–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garcez RC, Teixeira BL, Schmitt Sdos S,
Alvarez-Silva M and Trentin AG: Epidermal growth factor (EGF)
promotes the in vitro differentiation of neural crest cells to
neurons and melanocytes. Cell Mol Neurobiol. 29:1087–1091. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He N, Wang Z, Wang Y, Shen H and Yin M:
ZY-1, a novel nicotinic analog, promotes proliferation and
migration of adult hippocampal neural stem/progenitor cells. Cell
Mol Neurobiol. 33:1149–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung JW, Yoon BH, Oh HR, Ahn JH, Kim SY,
Park SY and Ryu JH: Anxiolytic-like effects of Gastrodia elata and
its phenolic constituents in mice. Biol Pharm Bull. 29:261–265.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee YS, Ha JH, Yong CS, Lee DU, Huh K,
Kang YS, Lee SH, Jung MW and Kim JA: Inhibitory effects of
constituents of Gastrodia elata Bl. on glutamate-induced apoptosis
in IMR-32 human neuroblastoma cells. Arch Pharm Res. 22:404–409.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim EJ, Kang HJ, Jung HJ and Park EH:
Anti-angiogenic, anti-inflammatory and anti-nociceptive activity of
4-hydroxy-benzyl alcohol. J Pharm Pharmacol. 59:1235–1240. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lirdprapamongkol K, Sakurai H, Kawasaki N,
Choo MK, Saitoh Y, Aozuka Y, Singhirunnusorn P, Ruchirawat S,
Svasti J and Saiki I: Vanillin suppresses in vitro invasion and in
vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci.
25:57–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J and Mori A: Antioxidant and
pro-oxidant activities of p-hydroxybenzyl alcohol and vanillin:
Effects on free radicals, brain peroxidation and degradation of
benzoate, deoxyribose, amino acids and DNA. Neuropharmacology.
32:659–669. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murakami Y, Hirata A, Ito S, Shoji M,
Tanaka S, Yasui T, Machino M and Fujisawa S: Re-evaluation of
cyclooxygenase-2-inhibiting activity of vanillin and guaiacol in
macrophages stimulated with lipopolysaccharide. Anticancer Res.
27:801–807. 2007.PubMed/NCBI
|
|
22
|
Kim HJ, Hwang IK and Won MH: Vanillin,
4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent
hippocampal CA1 cell death following global ischemia. Brain Res.
1181:130–141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Makni M, Chtourou Y, Barkallah M and
Fetoui H: Protective effect of vanillin against carbon
tetrachloride (CCl4)-induced oxidative brain injury in
rats. Toxicol Ind Health. 28:655–662. 2012. View Article : Google Scholar
|
|
24
|
Yu SS, Zhao J, Lei SP, Lin XM, Wang LL and
Zhao Y: 4-hydroxy-benzyl alcohol ameliorates cerebral injury in
rats by antioxidant action. Neurochem Res. 36:339–346. 2011.
View Article : Google Scholar
|
|
25
|
Donovan MH, Yamaguchi M and Eisch AJ:
Dynamic expression of TrkB receptor protein on proliferating and
maturing cells in the adult mouse dentate gyrus. Hippocampus.
18:435–439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu
SY, Lo CP and Kuo YM: Exercise enhances the proliferation of neural
stem cells and neurite growth and survival of neuronal progenitor
cells in dentate gyrus of middle-aged mice. J Appl Physiol (1985).
105:1585–1594. 2008. View Article : Google Scholar
|
|
27
|
Brown J, Cooper-Kuhn CM, Kempermann G, Van
Praag H, Winkler J, Gage FH and Kuhn HG: Enriched environment and
physical activity stimulate hippocampal but not olfactory bulb
neurogenesis. Eur J Neurosci. 17:2042–2046. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Couillard-Despres S, Winner B, Schaubeck
S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG
and Aigner L: Doublecortin expression levels in adult brain reflect
neurogenesis. Eur J Neurosci. 21:1–14. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen BH, Yan BC, Park JH, Ahn JH, Lee DH,
Kim IH, Cho JH, Lee JC, Kim SK, Lee B, et al: Aripiprazole, an
atypical antipsychotic drug, improves maturation and complexity of
neuroblast dendrites in the mouse dentate gyrus via increasing
superoxide dismutases. Neurochem Res. 38:1980–1988. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee TH, Lee CH, Kim IH, Yan BC, Park JH,
Kwon SH, Park OK, Ahn JH, Cho JH, Won MH and Kim SK: Effects of
ADHD therapeutic agents, methylphenidate and atomoxetine, on
hippocampal neurogenesis in the adolescent mouse dentate gyrus.
Neurosci Lett. 524:84–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franklin KBJ and Paxinos G: The mouse
brain in stereotaxic coordinates. Academic Press; San Diego:
1997
|
|
32
|
Eide FF, Vining ER, Eide BL, Zang K, Wang
XY and Reichardt LF: Naturally occurring truncated trkB receptors
have dominant inhibitory effects on brain-derived neurotrophic
factor signaling. J Neurosci. 16:3123–3129. 1996.PubMed/NCBI
|
|
33
|
Kuhn HG, Dickinson-Anson H and Gage FH:
Neurogenesis in the dentate gyrus of the adult rat: Age-related
decrease of neuronal progenitor proliferation. J Neurosci.
16:2027–2033. 1996.PubMed/NCBI
|
|
34
|
Shors TJ, Miesegaes G, Beylin A, Zhao M,
Rydel T and Gould E: Neurogenesis in the adult is involved in the
formation of trace memories. Nature. 410:372–376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee J, Duan W and Mattson MP: Evidence
that brain-derived neurotrophic factor is required for basal
neurogenesis and mediates, in part, the enhancement of neurogenesis
by dietary restriction in the hippocampus of adult mice. J
Neurochem. 82:1367–1375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nilsson M, Perfilieva E, Johansson U,
Orwar O and Eriksson PS: Enriched environment increases
neurogenesis in the adult rat dentate gyrus and improves spatial
memory. J Neurobiol. 39:569–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim SJ, Son TG, Park HR, Park M, Kim MS,
Kim HS, Chung HY, Mattson MP and Lee J: Curcumin stimulates
proliferation of embryonic neural progenitor cells and neurogenesis
in the adult hippocampus. J Biol Chem. 283:14497–14505. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoo KY, Choi JH, Hwang IK, Lee CH, Lee SO,
Han SM, Shin HC, Kang IJ and Won MH: (−)-Epigallocatechin-3-gallate
increases cell proliferation and neuroblasts in the subgranular
zone of the dentate gyrus in adult mice. Phytother Res.
24:1065–1070. 2010.
|
|
39
|
Aberg MA, Aberg ND, Hedbäcker H, Oscarsson
J and Eriksson PS: Peripheral infusion of IGF-I selectively induces
neurogenesis in the adult rat hippocampus. J Neurosci.
20:2896–2903. 2000.
|
|
40
|
Scharfman H, Goodman J, Macleod A, Phani
S, Antonelli C and Croll S: Increased neurogenesis and the ectopic
granule cells after intrahippocampal BDNF infusion in adult rats.
Exp Neurol. 192:348–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang EJ and Reichardt LF: Neurotrophins:
Roles in neuronal development and function. Ann Rev Neurosci.
24:677–736. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim
CJ, Kim SH, Baek SS, Lee EK and Jee YS: Treadmill exercise prevents
aging-induced failure of memory through an increase in neurogenesis
and suppression of apoptosis in rat hippocampus. Exp Gerontol.
45:357–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu Y, Christian K and Lu B: BDNF: A key
regulator for protein synthesis-dependent LTP and long-term memory?
Neurobiol Learn Mem. 89:312–323. 2008. View Article : Google Scholar
|
|
44
|
Bramham CR and Messaoudi E: BDNF function
in adult synaptic plasticity: The synaptic consolidation
hypothesis. Prog Neurobiol. 76:99–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yoo DY, Nam SM, Kim W, Lee CH, Won MH,
Hwang IK and Yoon YS: N-acetylserotonin increases cell
proliferation and differentiating neuroblasts with tertiary
dendrites through upregulation of brain-derived neurotrophic factor
in the mouse dentate gyrus. J Vet Med Sci. 73:1411–1416. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Taliaz D, Stall N, Dar DE and Zangen A:
Knockdown of brain-derived neurotrophic factor in specific brain
sites precipitates behaviors associated with depression and reduces
neurogenesis. Mol Psychiatry. 15:80–92. 2010. View Article : Google Scholar :
|
|
47
|
Sairanen M, Lucas G, Ernfors P, Castrén M
and Castrén E: Brain-derived neurotrophic factor and antidepressant
drugs have different but coordinated effects on neuronal turnover,
proliferation and survival in the adult dentate gyrus. J Neurosci.
25:1089–1094. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heo YM, Shin MS, Kim SH, Kim TW, Baek SB
and Baek SS: Treadmill exercise ameliorates disturbance of spatial
learning ability in scopolamine-induced amnesia rats. J Exerc
Rehabil. 10:155–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schmidt HD and Duman RS: The role of
neurotrophic factors in adult hippocampal neurogenesis,
antidepressant treatments and animal models of depressive-like
behavior. Behav Pharmacol. 18:391–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Haughey NJ, Nath A, Chan SL, Borchard AC,
Rao MS and Mattson MP: Disruption of neurogenesis by amyloid
beta-peptide and perturbed neural progenitor cell homeostasis, in
models of Alzheimer's disease. J Neurochem. 83:1509–1524. 2002.
View Article : Google Scholar : PubMed/NCBI
|


















