Introduction
Combined radiation and wound injury (CRWI) is
characterized as radiation injury coupled with wounds, and is
expected to occur following nuclear explosions and accidents,
radiological or nuclear terrorism, and radiation therapy in
combination with surgery or other modalities (1). Wound healing is a complicated
process, and exposure to radiation may significantly aggravate the
degree of damage and prolong healing time (2,3).
Currently, there is no effective medical countermeasure for the
management of CRWI. Emerging stem cell-based therapy is considered
to be promising for the treatment of CRWI; however, the cell source
remains a challenging issue (4).
Among various stem cell populations, bone marrow-derived
mesenchymal stromal cells (BMSCs) are commonly applied in
experimental models and clinical trials (5–8).
However, the lymphohematopoietic system is particularly sensitive
and vulnerable to radiation exposure (9), which indicates that bone marrow is
not a good source for autologous cell therapy in CRWI, and there is
an urgent requirement to develop alternative cell sources.
Skin is the largest organ in the body and during the
past decade the importance of the dermis as an easily accessible
source of stem cell populations, and their promising significance
in wound repair and other diseases has been established (10–12).
Recently, the granulation tissue-derived cells (GTCs) were further
characterized as an abundant cell source for their important
therapeutic efficacy in wound healing and tissue repair (13). As skin is relatively insensitive to
radiation, the present study hypothesized that the GTCs from CRWI
may represent an alternative source of adult stem cells for
transplantation. The aim of the present study was to investigate
the biological features of GTCs from the skin wounds (SWs) of CRWI
mice (C-GTCs). Multiple biological characteristics, including the
radiation sensitivity of C-GTCs, were investigated and compared
with BMSCs from CRWI mice, dermal stem cells (DSCs) from neonatal
C57BL/6 mice and GTCs from unirradiated SWs.
Materials and methods
Animals and wound model
A total of 14 female C57/BL mice (age, 6 weeks;
weight, 20–22 g) were purchased from the Center of Experimental
Animals at the Third Military Medical University (Chongqing,
China). The mice were randomly divided into two groups (7
mice/group): CRWI group and SW group. Neonatal mice (age, 1 day)
were raised and used for neonatal DSC isolation.
Total-body irradiation was delivered at a rate of
0.70 Gy/min from a 60Co gamma-ray source at the
Radiation Center of the Third Military Medical University. The mice
from the CRWI group were exposed to a total of 6 Gy in a single
dose, and an SW was created 30 min after irradiation. In each
group, the SW was implemented as described previously (14). The mice were anaesthetized by
intraperitoneal injection with 1% pentobarbital (30 mg/kg; Merck
Millipore, Darmstadt, Germany) and the back hair was shaved. A
circular, full-thickness SW (~1.5 cm in diameter) was made in the
center of the back using sterilized ophthalmic scissors and forceps
following disinfection of the mouse skin with iodophor (Jinshan
Co., Ltd., Chengdu, China). The mice were group-housed under
standard conditions throughout the study, under a 12-h light/dark
cycle with ad libitum access to food and water. All
procedures on the mice were approved by the ethics committee of the
Third Military Medical University.
Cell isolation and culture
To obtain GTCs from the CRWI and SW mice, the mice
were sacrificed by cervical dislocation 7 days after the SW was
created and the granulation tissues were acquired. The tissues were
washed twice with 75% ethanol and phosphate-buffered saline (PBS)
and sliced into small sections (~1 mm3). The sections
were digested with 0.25% collagenase I (Worthington Biochemical
Corp., Lakewood, NJ, USA) and the cells were agitated into cell
suspension for 2 h at 37°C, then cultured in Iscove's modified
Dulbecco's medium (IMDM; GE Healthcare Life Sciences, Logan, UT,
USA) supplemented with Gibco 10% fetal bovine serum (FBS; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 1%
penicillin/streptomycin (1 ml/100 ml; Beyotime Institute of
Biotechnology, Shanghai, China) at 37°C under an atmosphere of 5%
CO2.
BMSCs were obtained from the femurs and tibiae of
CRWI mice. The cells were flushed out using a 1 ml syringe and
filtered with a 200 Mesh CellCribble (Sangon Biotech Co., Ltd.,
Shanghai, China). A single-cell suspension was created as described
above.
To obtain DSCs, isolation was performed as described
previously (15). Full-thickness
skin tissue was obtained from four neonatal C57BL/6 mice (age, 1
day), according to a previous study (16). The tissue was washed in 75% ethanol
and PBS and the subcutaneous tissue was removed. The skin tissue
was sliced into small sections (~4 mm2), transferred to
0.25% trypsin (HyClone; GE Healthcare Life Sciences) and digested
overnight at 4°C. The epidermis was discarded and the dermal layer
was sliced into smaller sections (~0.5 mm2). The tissue
sections were flushed into a cell suspension and cultured as
described above. Passage 0–3 cells were used in the further
experiments.
Cell attachment and proliferation
To investigate cell adhesion, a cell attachment
assay was performed as described previously (17). Resuspended cells from the different
populations were seeded into 24-well plates at a density of
1.0×104 cells/well. At 0.5, 1, 2 and 4 h after
inoculation, the cells were washed twice with PBS, fixed with 4%
paraformaldehyde (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) and stained with DAPI (Beyotime Institute of Biotechnology)
for 10 min. The cell number in 10 randomly selected fields was
counted under a fluorescence microscope (BX51TRF; Olympus
Corporation, Tokyo, Japan) and cell adhesion was presented as the
mean cell number. A cell proliferation assay was performed as
described previously (18). Cells
were seeded in 96-well plates at a density of 3,000 cells/well and
cultured at 37°C. At 0, 1, 2, 3, 4 and 5 days after seeding, the
media was replaced with 100 µl PBS and 10 µl Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) for each well, and cells were incubated for
another 2 h at 37°C. The absorbance was measured at 450 nm using a
Model 680 Microplate Reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Colony formation assay
To detect the single-cell colony formation ability
of the different cell types, a colony formation assay was performed
as described previously (19).
Cells were plated into 6-well plates (3,000 cells/well) with IMDM
supplemented with 10% FBS and cultured at 37°C. After 12 days,
cells were fixed with 4% paraformaldehyde, stained using a
Wright-Giemsa staining kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) and washed twice with distilled water.
The number of colonies that contained >50 cells was counted and
images of the colonies were randomly captured using a light
microscope (CK40-F200; Olympus Corporation). The colony area was
measured using ImageJ software 1.48 (NIH, Bethesda, MA, USA) and
the mean area of the colonies was calculated.
Detection of senescence-associated
β-galactosidase (SA-β-gal) activity
To establish the senescence state of the cells,
SA-β-gal activity was determined as described previously (20). Briefly, passage 2 cells were
harvested and plated into 6-well plates (1.0×104
cells/well), and 24 h after incubation the SA-β-gal activity was
detected using an SA-β-gal staining kit (C0602; Beyotime Institute
of Biotechnology) according to the manufacturer's instruction. The
numbers of SA-β-gal-positive and total cells in 10 randomly
selected fields were counted under a light microscope (CK40-F200),
and the percentage of senescent cells was displayed as the ratio of
the number of SA-β-gal-positive cells to total cells.
Cell migration assay
To observe the migration ability of the cells, a
scratch-wound assay was performed as described previously (21). Confluent cells were continuously
scratched through the entire monolayer using a sterile P200 pipette
tip (Axygen Scientific Inc., Union City, CA, USA). After washing
with PBS, the wells were cultured with fresh medium at 37°C. Images
of the wounds were captured (magnification, ×400) 0, 12, 24 and 36
h after scratching. The rate of wound closure (%) at various points
was calculated as follows: [(Original wound area − residual wound
area) / original wound area] × 100.
Examination of cell differentiation
To investigate the differentiation ability of the
cells, the cells were incubated in osteogenic and adipogenic
differentiation media [Cyagen Biosciences (Guangzhou) Inc.,
Guangzhou, China]. After a 3-week incubation at 37°C, the cells
were stained with Alizarin Red (EMD Millipore, Billerica, MA, USA)
and quantitative analysis of osteogenic differentiation was
performed using an osteogenesis assay kit (MUBMX-90021; EMD
Millipore), according to the manufacturer's instruction. For
adipogenic differentiation, induced lipid droplets were visualized
with Oil Red O (Sigma-Aldrich, St. Louis, MO, USA). Quantitative
analyses of the lipids were performed by spectrophotometry (1510;
Thermo Fisher Scientific, Inc.) of isopropanol-extracted Oil Red O
staining.
Statistical analysis
All data were analyzed by SPSS 13.0 and expressed as
the mean ± standard error of the mean. Statistical significance was
examined with an independent-samples t test for comparison
of C-GTCs and BMSCs from mice with CRWI, and by one-way analysis of
variance for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
C-GTCs and BMSCs exhibit different
sensitivities to radiation
The proliferative capacity of BMSCs and C-GTCs
derived from mice with CRWI were compared. The primary passage (P0)
C-GTCs demonstrated comparable proliferation ability following
subculture (P1). However, the BMSCs presented markedly inhibited
growth following subculture (Fig.
1A). To further determine the difference, the proliferative
ability of the P1 of the two populations was examined; the result
indicated marked growth suppression in the BMSCs (Fig. 1B). Furthermore, the biomarker,
SA-β-gal was used to evaluate cellular senescence 24 h after
incubation. A higher percentage of blue-stained BMSCs was detected
(Fig. 2A and B), indicating a
greater quantity of aging cells in BMSCs. These experiments
indicate that the BMSCs exhibited a higher sensitivity to damage by
CRWI.
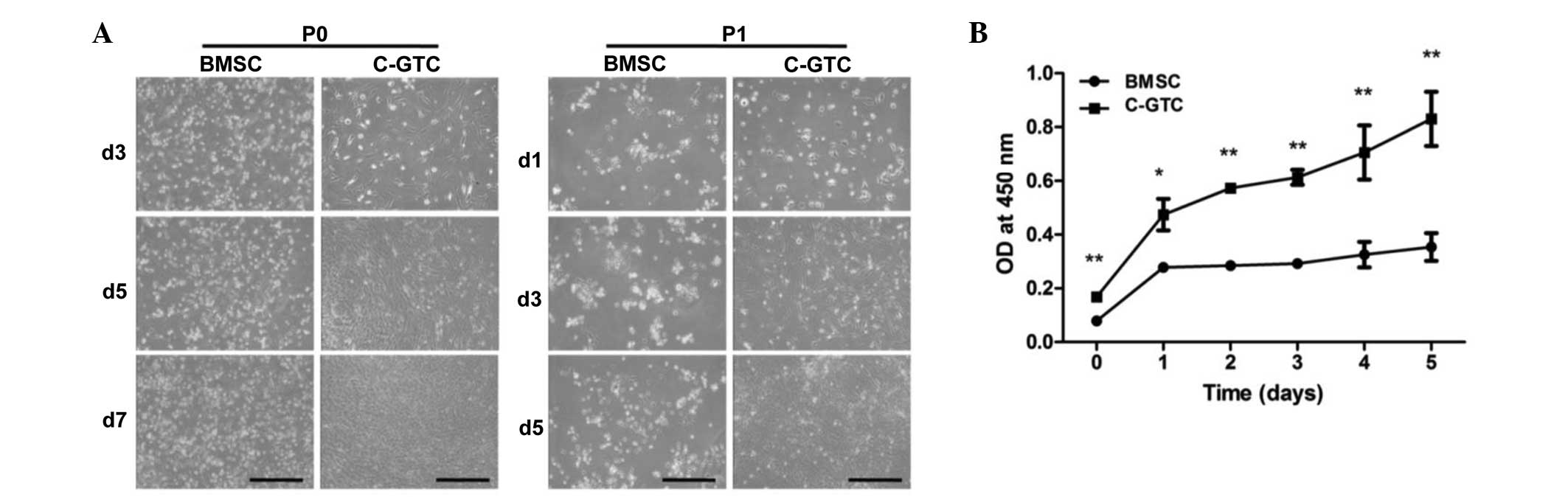 | Figure 1Comparison of proliferative capacity
between C-GTCs and BMSCs in the CRWI group. (A) The growth of cells
was observed at days 3, 5 and 7 for P0, and at days 1, 3 and 5 for
P1 (scale bar, 500 µm). (B) Quantitative analysis of C-GTCs
and BMSCs using the Cell Counting Kit-8. *P<0.05,
**P<0.01 vs. BMSC group. BMSC, bone marrow-derived
mesenchymal stromal cells; C-GTC, granulation tissue-derived cells
from the skin wounds of CRWI mice; CRWI, combined radiation and
wound injury; P0, primary passage; P1, subculture; d, day; OD,
optical density. |
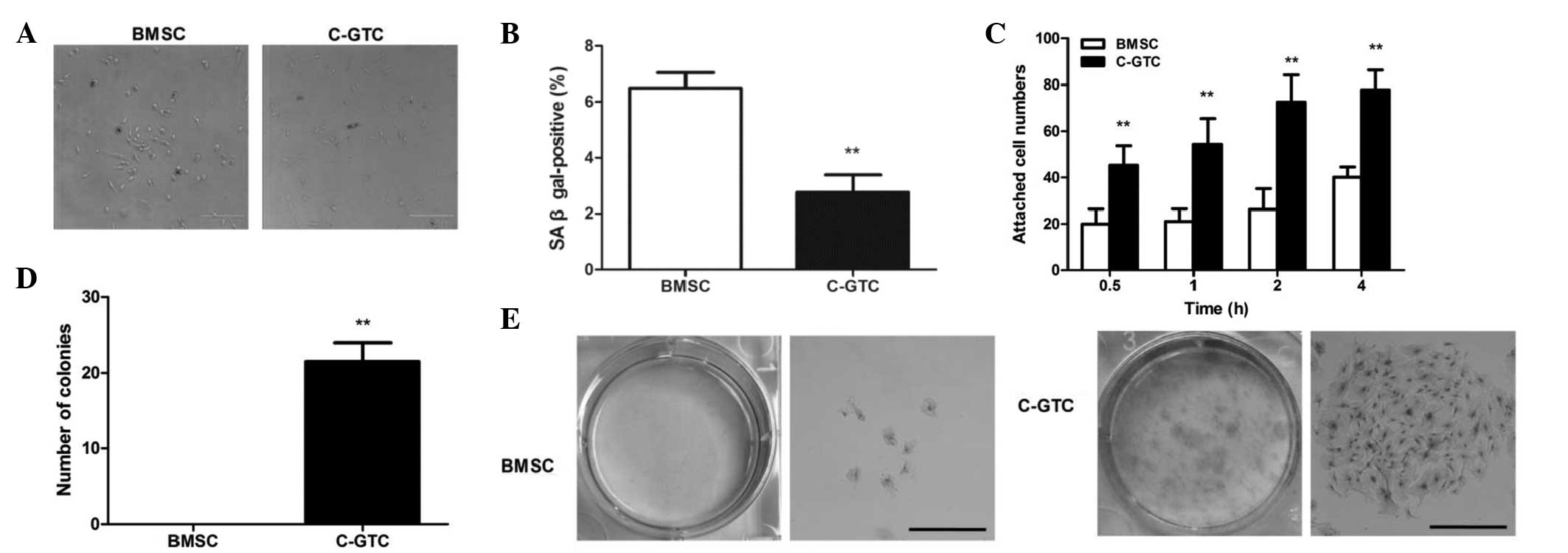 | Figure 2Senescence, adhesion and
colony-formation of C-GTCs and BMSCs. (A) SA-β-gal activity
analysis in which positive cells were stained blue (dark stain in
the figure). (B) Percentage of SA-β-gal-positive cells in a high
power field (×200) was calculated. (C) Cell adhesion assay. At the
time-point of 0.5, 1, 2 and 4 h after plating, the cells were
stained and cell attachment was measured by the number of cells per
field. (D) Quantitative analysis of colony formation ability. (E)
Colonies were cultured for 12 days and stained with Wright-Giemsa
[left: Visual observation; right: Microscopic image (magnification,
×200)]; scale bar, 500 µm. **P<0.01 vs. BMSC
group. BMSC, bone marrow-derived mesenchymal stromal cells; C-GTC,
granulation tissue-derived cells from the skin wounds of CRWI mice;
CRWI, combined radiation and wound injury; SA-β-gal,
senescence-associated β-galactosidase. |
To further elucidate the biological characteristics
of C-GTCs and BMSCs, the cell adherence and colony formation
abilities were evaluated. C-GTCs and BMSCs adhered to plastic
surfaces; however, C-GTCs demonstrated more rapid and greater
attachment than BMSCs at 30 mins after cell plating, and the
significant difference persisted throughout the 4 h of culture
(Fig. 2C). With regard to the
colony forming assay, no colonies were observed in the BMSC group,
while C-GTCs demonstrated a significantly enhanced colony forming
capability (P<0.01; Fig. 2D and
E).
Morphology, colony formation and
proliferation of C-GTCs
C-GTCs were demonstrated to be more radioresistant
and easily accessible when compared with BMSCs from mice with CRWI.
To further investigate the biological features of C-GTCs, neonatal
DSCs and GTCs from wounds without irradiation (GTCs) were used as
control cells.
The morphologies of cells from the three groups
(C-GTC, GTC and DSC) were analyzed under a light microscope using
unstained cells. GTCs and DSC had an elongated, fibroblast-like
morphology, whereas C-GTCs were larger, irregularly-shaped and
appeared flattened (Fig. 3A). The
CCK-8 assay result suggested that the patterns of cell
proliferation exhibited by C-GTCs, GTCs and DSCs were comparable
(Fig. 3B). Furthermore, the colony
forming capacity, which is a feature associated with stem cells,
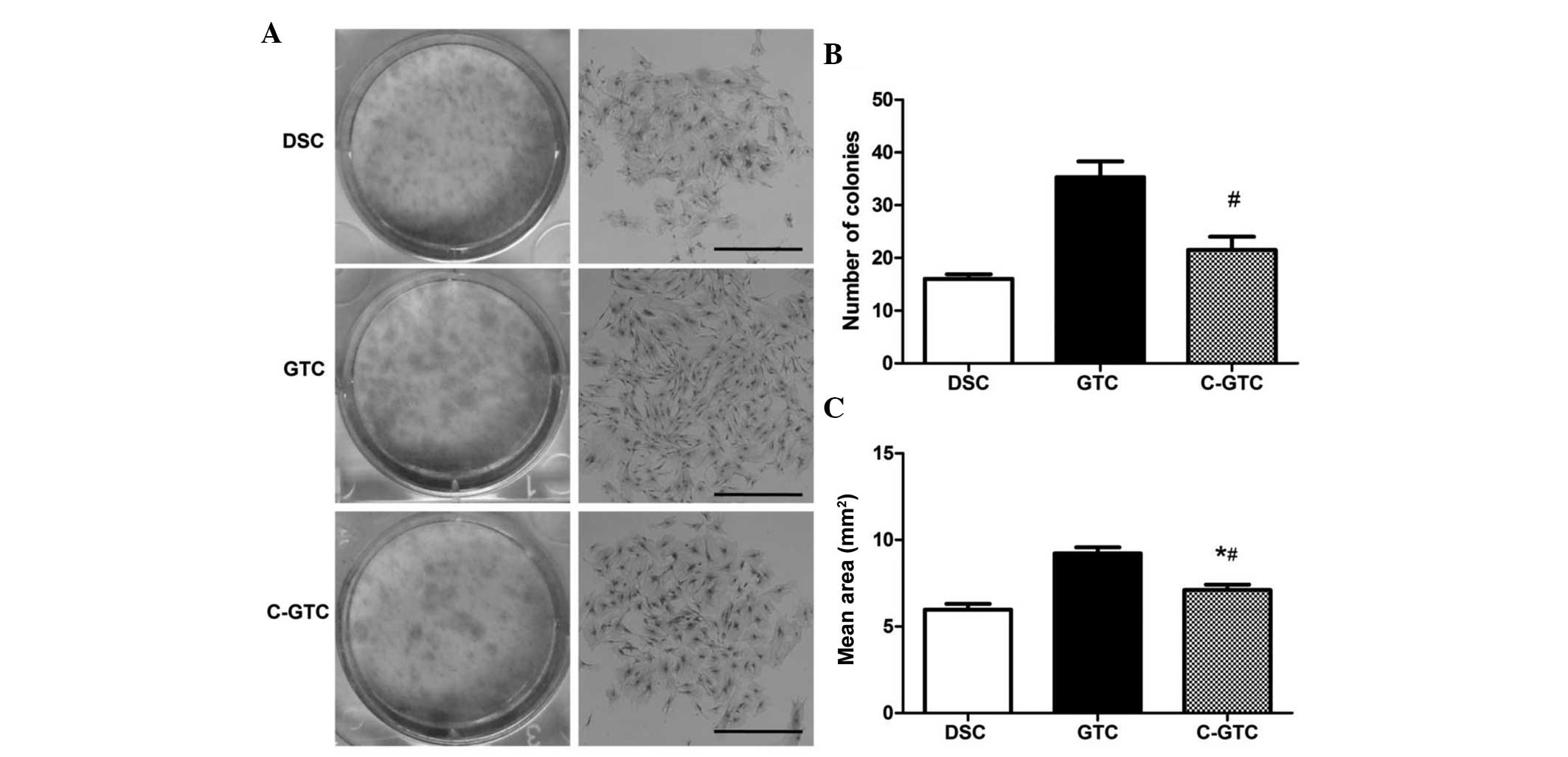
was determined. The colony forming experiments demonstrated that
GTCs formed the most colonies, followed by C-GTCs and finally DSCs
(Fig. 4). Accordingly, the mean
area of the colonies from large to small was also in the order
GTCs, C-GTCs and DSCs (Fig.
4).
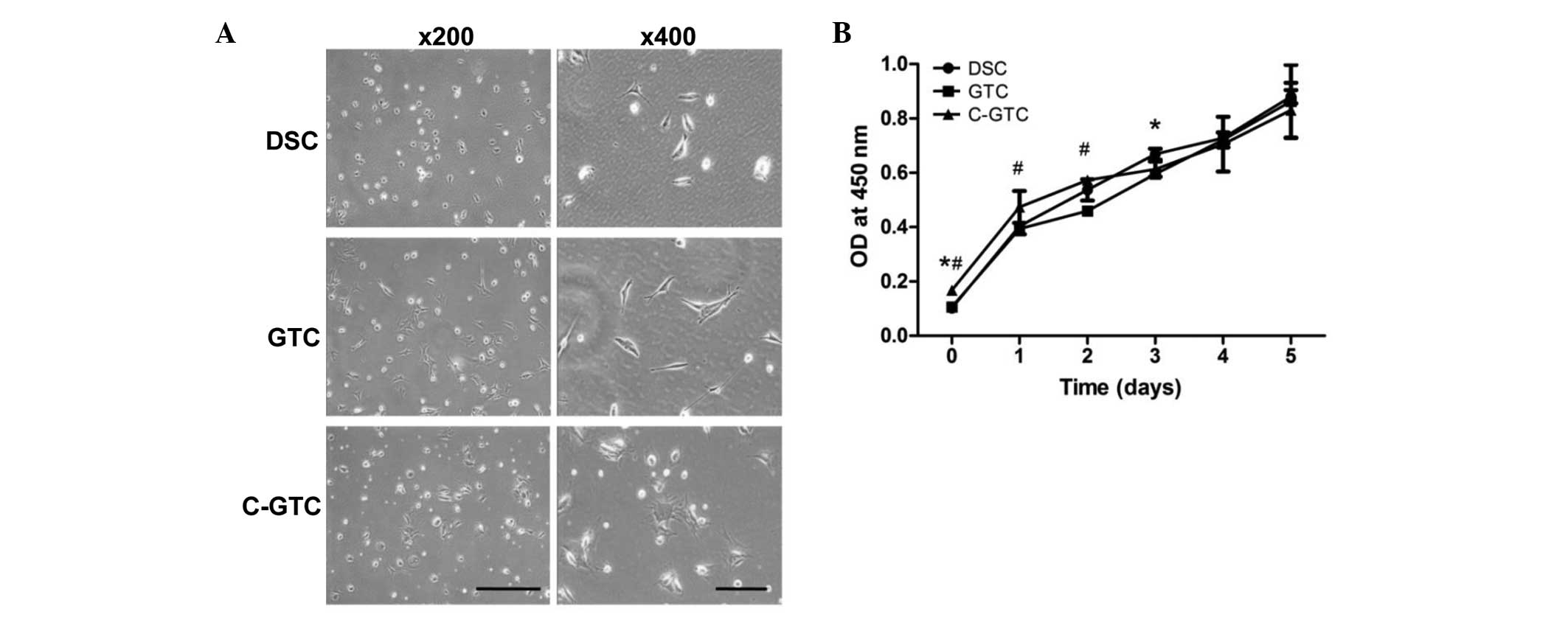 | Figure 3Morphological observation and
proliferation of DSCs, GTCs and C-GTCs. (A) Unirradiated DSCs and
GTCs demonstrated typical elongated, fibroblast-like morphology,
however the C-GTCs exhibited a flattened phenotype. Scale bar, 500
µm with magnification, ×200; scale bar, 200 µm with
magnification, ×400. (B) Quantitative analysis using the Cell
Counting Kit-8. *P<0.05 vs. DSCs and
#P<0.05 vs. GTCs. DSC, dermal stromal cells; GTC,
granulation tissue-derived cells; C-GTCs, GTCs from the skin wounds
of CRWI mice; CRWI, combined radiation and wound injury; OD,
optical density. |
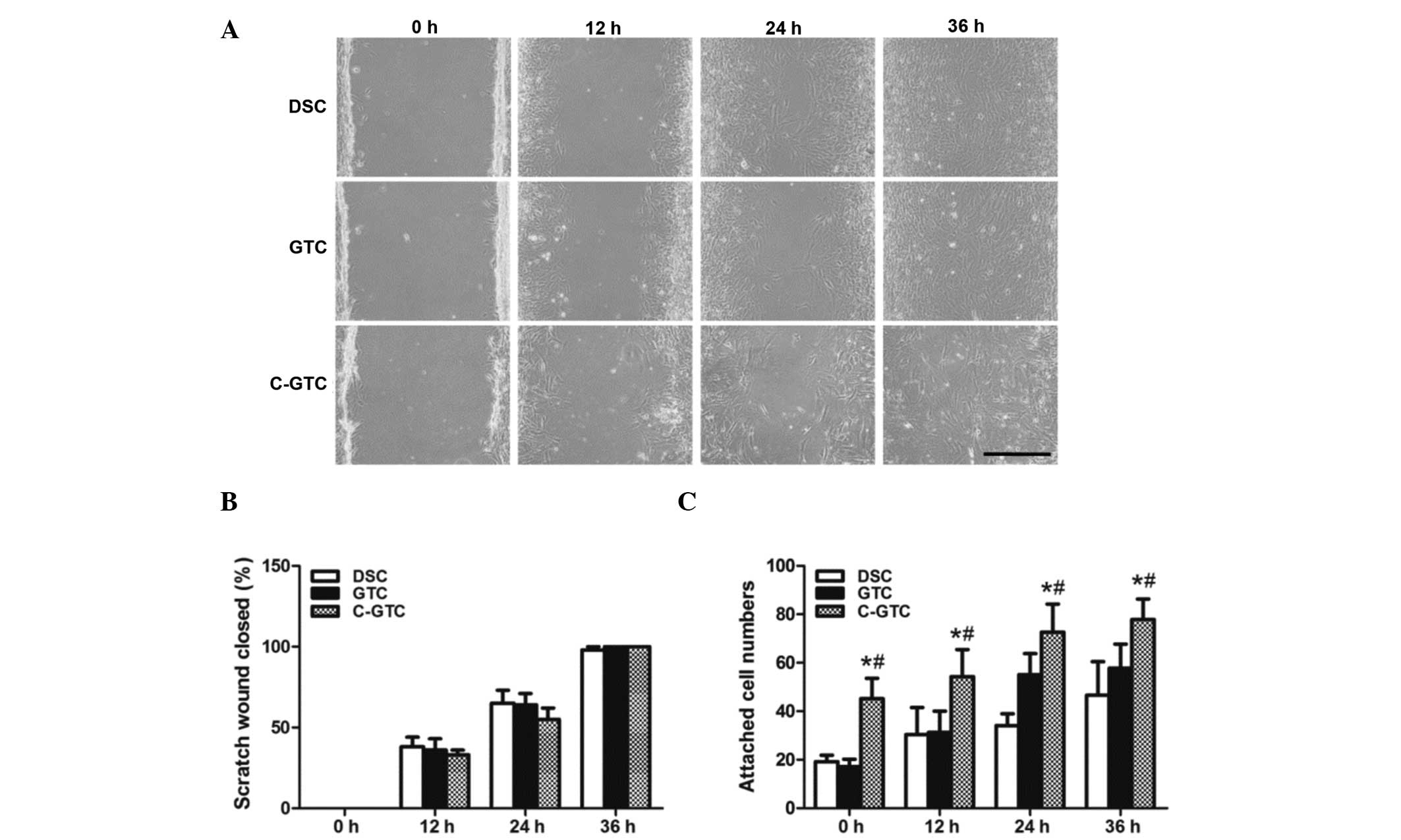
Migration and adhesion of C-GTCs
To examine whether CRWI affects cell migration, the
cell migration ability of C-GTCs were evaluated using a scratch
wound assay. Images of scratch wounds were captured at 0, 12, 24
and 36 h after treatment, and the wounds in C-GTCs, GTCs and DSCs
were completely closed by 36 h. The wound area was then quantified
using Image J software and the healing rate was calculated. The
data indicated that there was no significant difference in the rate
of C-GTCs when compared with that of GTCs and DSCs (P>0.05;
Fig. 5A and B).
To investigate the effect of radiation on cell
adhesion, equal numbers of C-GTCs, GTCs and DSCs were plated into
24-well plates and attachment was monitored over 4 h. The result
demonstrated that C-GTCs had a marked attachment ability and
exhibited processes and flattened shapes (Fig. 5C), indicating that irradiation may
improve the adhesive capacity of mesenchymal stem cells (MSCs).
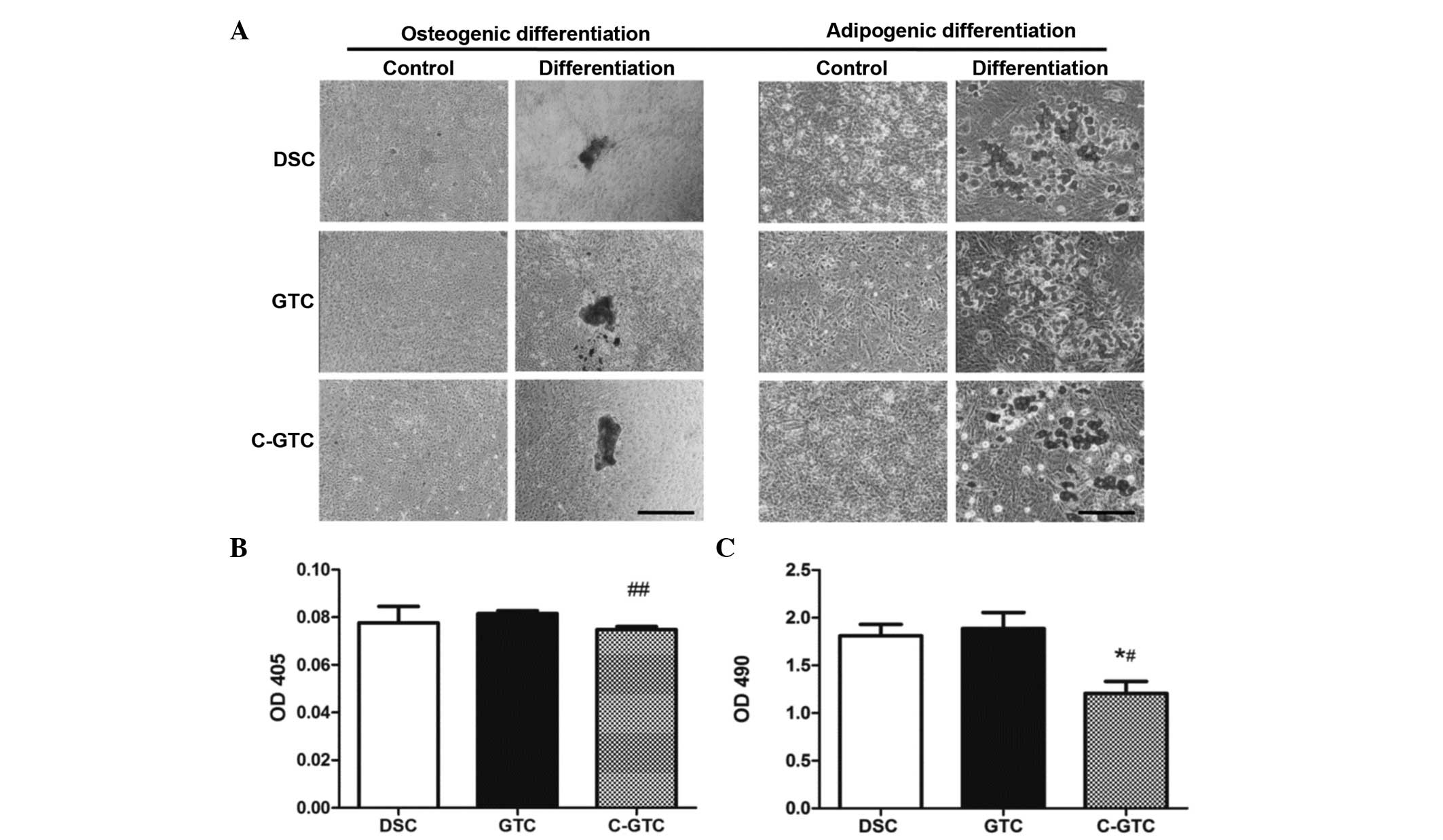
Differentiation potential of C-GTCs
The osteogenic and adipogenic differentiation
potential is a unique characteristic of MSCs. Therefore, the
differentiation capability of C-GTCs were evaluated, with DSCs and
GTCs serving as controls. Fig. 6A
demonstrates that C-GTCs were able to differentiate into osteocytes
(with the mineralized nodules highlighted by Alizarin Red
staining). However, quantitative analysis indicated that C-GTCs
formed fewer mineralized extracellular matrices than GTCs
(P<0.01; Fig. 6B). In addition,
when cultured in adipo-inductive media for 7 days, all three
populations were able to form lipid globules; the lipid droplets
were stained with Oil Red O (Fig.
6A). The results indicate that all three cell populations
displayed the potential to differentiate into adipocytes, including
C-GTCs, although absorbance of the Oil Red O extract was lower in
the C-GTC group than those of the control groups (GTCs and DSCs;
Fig. 6C). These results indicated
that the differentiation ability of GTCs was not abrogated by
CRWI.
Discussion
Adult stem cell-based therapy presents as a
promising treatment strategy for diseases and injuries, including
CRWI (22). Stem cells are
isolated from different types of tissue, including bone marrow,
adipose tissue, the skin and umbilical cords (23). However, the cell source is a
challenge for the management of wounds that are difficult to heal,
such as CRWI. In the current study, the effects of radiation on the
isolation and proliferation of C-GTCs and BMSCs in mice with CRWI
were investigated. Although it has been reported that BMSCs exhibit
a certain quantity of radioresistance in order to retain their stem
cell characteristics, including proliferation, adherence, colony
formation ability and differentiation potential (24), the present results demonstrated
that BMSCs were more sensitive to the damage caused by CRWI. In
addition, it was particularly difficult to harvest ideal cells from
the bone marrow of mice with CRWI. Notably, the isolated C-GTCs
demonstrated higher resistance to CRWI and exhibited a
significantly lower level of senescence when compared with BMSCs,
and preserved their self-renewal and multilineage differentiation
capacities as effectively as neonatal DSCs and GTCs from
unirradiated SWs.
Skin is the largest organ of the body and the dermis
has been shown to contain various stem cells populations (25). It has been established that stem
cells are important in wound healing and that, following wounding,
newly formed granulation tissue is enriched in cells that express
stem cell surface markers (26).
Previous studies have reported the therapeutic implications of GTCs
in different animal models (26,27).
The GTCs are able to secrete important factors, such as vascular
endothelial growth factor and maintain their trilineage
differentiation ability in vitro (28). Furthermore, GTCs may mitigate
damage and accelerate repair in liver and kidney injury (28,29).
Therefore, granulation tissue is emerging as a potential source of
multifunctional cells for transplantation therapy.
Previous studies have demonstrated that
transplantation of mesenchymal stem/stromal cells is a potent
therapeutic method for radiation-induced damage in various organs
and tissues, such as salivary glands (8), lungs (30), the liver (31,32),
skin (33), bone marrow (34) and intestines (35,36).
Studies have demonstrated that MSCs exert their therapeutic effects
via multiple mechanisms, including engraftment and differentiation
into target cell types, immunomodulation and anti-inflammation
activities [by decreasing the expression levels of inflammatory
cytokines, such as interleukin (IL)-1α, IL-β and tumor necrosis
factor-α], and promoting the paracrine action of growth factors
associated with neovascu-larization (33,36).
In addition, the transplantation of MSCs was reported to upregulate
the expression of cell cycle-associated genes, including
cyclin-dependent kinase inhibitor 1A (37). Furthermore, oxidative stress may be
reduced following MSC therapy (31). In our previous study, it was
reported for the first time, to the best of our knowledge, that
systemic transplantation of neonatal dermal multipotent cells
significantly promoted survival, and accelerated hematopoietic
recovery and wound healing in rats with CRWI. This indicated that
stem cell therapy achieves multiple therapeutic effects and
provides a potential novel strategy for the treatment of severe
traumatic injuries comprised of multiple tissue/organ damage, such
as radiation combined injuries (38). In the present study, it was
verified that the isolated C-GTCs possessed comparable stem
cell-associated properties with neonatal DSCs and GTCs from normal
wounds without irradiation. Considering the ease of accessibility,
granulation tissue is proposed to be an optimal, autologous source
of stem/progenitor cells for therapeutic applications in CRWI, for
the replacement of skin, as well as for tissue repair of other
organs.
The source of MSCs for transplantation therapy of
CRWI has been widely investigated. Previous research has
demonstrated that granulation tissue derived from the dermis is a
promising stem cell source and that the GTCs exhibit stem
cell-associated properties. In the current study, the
characteristics of C-GTCs (obtained from CRWI mice) were
investigated, and the C-GTCs displayed advantageous properties when
compared with BMSCs, including improved radiation resistance and
biological characteristics, such as proliferative, colony formation
and adhesion abilities. In addition, C-GTCs better retained their
stem cell characteristics when compared with DSCs and GTCs that
were obtained from normal granulation tissue. In conclusion, C-GTCs
have been demonstrated as a potential autologous source for the
treatment of CRWI.
Acknowledgments
The present study was supported by the State Key
Basic Research Development Program (grant no. 2012CB518103), the
Natural Science Foundation Programs (grant no. 81072523), the
Program of New Century Excellent Talents in University from the
Ministry of Education (grant no. NCET-11-0869), the Innovation Team
Building Program of Chongqing University (grant no. KJTD201338) and
Intramural Research Project grants from the Third Military Medical
University (grant nos. BWS13C016 and AWS14007-01).
References
|
1
|
Cheng T, Chen Z, Yan Y, Ran X, Su Y and Ai
G: Experimental studies on the treatment and pathological basis of
combined radiation and burn injury. Chin Med J (Engl).
115:1763–1766. 2002.
|
|
2
|
Vegesna V, Withers HR, Holly FE and
McBride WH: The effect of local and systemic irradiation on
impairment of wound healing in mice. Radiat Res. 135:431–433. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Boerma M, Fu Q and Hauer-Jensen M:
Radiation responses in skin and connective tissues: Effect on wound
healing and surgical outcome. Hernia. 10:502–506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Huang X, Wang H, Liu X, Zhang T,
Wang Y and Hu D: The challenges and promises of allogeneic
mesenchymal stem cells for use as a cell-based therapy. Stem Cell
Res Ther. 6:2342015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bey E, Prat M, Duhamel P, Benderitter M,
Brachet M, Trompier F, Battaglini P, Ernou I, Boutin L, Gourven M,
et al: Emerging therapy for improving wound repair of severe
radiation burns using local bone marrow-derived stem cell
administrations. Wound Repair Regen. 18:50–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Gong JF, Zhang W, Zhu WM and Li
JS: Effects of transplanted bone marrow mesenchymal stem cells on
the irradiated intestine of mice. J Biomed Sci. 15:585–594. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Linard C, Busson E, Holler V, Strup-Perrot
C, Lacave-Lapalun JV, Lhomme B, Prat M, Devauchelle P, Sabourin JC,
Simon JM, et al: Repeated autologous bone marrow-derived
mesenchymal stem cell injections improve radiation-induced
proctitis in pigs. Stem Cells Transl Med. 2:916–927. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim JY, Yi T, Choi JS, Jang YH, Lee S, Kim
HJ, Song SU and Kim YM: Intraglandular transplantation of bone
marrow-derived clonal mesenchymal stem cells for amelioration of
post-irradiation salivary gland damage. Oral Oncol. 49:136–143.
2013. View Article : Google Scholar
|
|
9
|
Karkanitsa LV: Radiation damage to
hematopoiesis: What do we know better? Stem Cells. 15(Suppl 2):
S71–S73. 1997. View Article : Google Scholar
|
|
10
|
Chen Z, Wang Y and Shi C: Therapeutic
implications of newly identified stem cell populations from the
skin dermis. Cell Transplant. 24:1405–1422. 2015. View Article : Google Scholar
|
|
11
|
Perng CK, Ku HH, Chiou SH, Chen IL, Tsai
FT, Yang YP, Chang KY and Kao CL: Evaluation of wound healing
effect on skin-defect nude mice by using human dermis-derived
mesenchymal stem cells. Transplant Proc. 38:3086–3087. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zabierowski SE, Fukunaga-Kalabis M, Li L
and Herlyn M: Dermis-derived stem cells: A source of epidermal
melanocytes and melanoma? Pigment Cell Melanoma Res. 24:422–429.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spyrou GE, Watt DA and Naylor IL: The
origin and mode of fibroblast migration and proliferation in
granulation tissue. Br J Plast Surg. 51:455–461. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang T, Feng Y, Sun H, Zhang L, Hao L, Shi
C, Wang J, Li R, Ran X, Su Y and Zou Z: miR-21 regulates skin wound
healing by targeting multiple aspects of the healing process. Am J
Pathol. 181:1911–1920. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao L, Liu F, Tan L, Liu T, Chen Z and Shi
C: The immunosuppressive properties of non-cultured dermal-derived
mesenchymal stromal cells and the control of graft-versus-host
disease. Biomaterials. 35:3582–3588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lichti U, Anders J and Yuspa SH: Isolation
and short-term culture of primary keratinocytes, hair follicle
populations and dermal cells from newborn mice and keratinocytes
from adult mice for in vitro analysis and for grafting to
immunodeficient mice. Nat Protoc. 3:799–810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong
TH, Zhou G, Baggett LS, Mikos AG and Cao Y: Donor age and cell
passage affects differentiation potential of murine bone
marrow-derived stem cells. BMC Cell Biol. 9:602008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng Y, Yang J, Zhang E, Sun H, Wang Q,
Wang T, Su Y and Shi C: Human positive coactivator 4 is a potential
novel therapeutic target in non-small cell lung cancer. Cancer Gene
Ther. 19:690–696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou
J, Jin Y and Chen FM: Comparison of mesenchymal stem cells derived
from gingival tissue and periodontal ligament in different
incubation conditions. Biomaterials. 34:7033–7047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su W, Chen Y, Zeng W, Liu W and Sun H:
Involvement of Wnt signaling in the injury of murine mesenchymal
stem cells exposed to X-radiation. Int J Radiat Biol. 88:635–641.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith AN, Willis E, Chan VT, Muffley LA,
Isik FF, Gibran NS and Hocking AM: Mesenchymal stem cells induce
dermal fibroblast responses to injury. Exp Cell Res. 316:48–54.
2010. View Article : Google Scholar
|
|
22
|
Coppes RP, van der Goot A and Lombaert IM:
Stem cell therapy to reduce radiation-induced normal tissue damage.
Semin Radiat Oncol. 19:112–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klopp AH, Gupta A, Spaeth E, Andreeff M
and Marini F III: Concise review: Dissecting a discrepancy in the
literature: Do mesenchymal stem cells support or suppress tumor
growth? Stem Cells. 29:11–19. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicolay NH, Sommer E, Lopez R, Wirkner U,
Trinh T, Sisombath S, Debus J, Ho AD, Saffrich R and Huber PE:
Mesenchymal stem cells retain their defining stem cell
characteristics after exposure to ionizing radiation. Int J Radiat
Oncol Biol Phys. 87:1171–1178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Toma JG, Akhavan M, Fernandes KJ,
Barnabé-Heider F, Sadikot A, Kaplan DR and Miller FD: Isolation of
multipotent adult stem cells from the dermis of mammalian skin. Nat
Cell Biol. 3:778–784. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diaz-Flores L Jr, Gutierrez R, Madrid JF,
Varela H, Valladares F and Diaz-Flores L: Adult stem cells and
repair through granulation tissue. Front Biosci (Landmark Ed).
14:1433–1470. 2009. View
Article : Google Scholar
|
|
27
|
Singh AK, Patel J, Litbarg NO, Gudehithlu
KP, Sethupathi P, Arruda JA and Dunea G: Stromal cells cultured
from omentum express pluripotent markers, produce high amounts of
VEGF and engraft to injured sites. Cell Tissue Res. 332:81–88.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patel J, Gudehithlu KP, Dunea G, Arruda JA
and Singh AK: Foreign body-induced granulation tissue is a source
of adult stem cells. Transl Res. 155:191–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patel J, Pancholi N, Gudehithlu KP,
Sethupathi P, Hart PD, Dunea G, Arruda JA and Singh AK: Stem cells
from foreign body granulation tissue accelerate recovery from acute
kidney injury. Nephrol Dial Transplant. 27:1780–1786. 2012.
View Article : Google Scholar
|
|
30
|
Wang H, Yang YF, Zhao L, Xiao FJ, Zhang
QW, Wen ML, Wu CT, Peng RY and Wang LS: Hepatocyte growth factor
gene-modified mesenchymal stem cells reduce radiation-induced lung
injury. Hum Gene Ther. 24:343–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Francois S, Mouiseddine M, Allenet-Lepage
B, Voswinkel J, Douay L, Benderitter M and Chapel A: Human
mesenchymal stem cells provide protection against radiation-induced
liver injury by antioxidative process, vasculature protection,
hepatocyte differentiation and trophic effects. Biomed Res Int.
2013:1516792013. View Article : Google Scholar
|
|
32
|
Mouiseddine M, François S, Souidi M and
Chapel A: Intravenous human mesenchymal stem cells transplantation
in NOD/SCID mice preserve liver integrity of irradiation damage.
Methods Mol Biol. 826:179–188. 2012. View Article : Google Scholar
|
|
33
|
Horton JA, Hudak KE, Chung EJ, White AO,
Scroggins BT, Burkeen JF and Citrin DE: Mesenchymal stem cells
inhibit cutaneous radiation-induced fibrosis by suppressing chronic
inflammation. Stem Cells. 31:2231–2241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang X, Balakrishnan I, Torok-Storb B and
Pillai MM: Marrow stromal cell infusion rescues hematopoiesis in
lethally irradiated mice despite rapid clearance after infusion.
Adv Hematol. 2012:1425302012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bessout R, Sémont A, Demarquay C,
Charcosset A, Benderitter M and Mathieu N: Mesenchymal stem cell
therapy induces glucocorticoid synthesis in colonic mucosa and
suppresses radiation-activated T cells: New insights into MSC
immuno-modulation. Mucosal Immunol. 7:656–669. 2014. View Article : Google Scholar
|
|
36
|
Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H
and Jin X: Multi-therapeutic effects of human adipose-derived
mesen-chymal stem cells on radiation-induced intestinal injury.
Cell Death Dis. 4:e6852013. View Article : Google Scholar
|
|
37
|
Lange C, Brunswig-Spickenheier B,
Cappallo-Obermann H, Eggert K, Gehling UM, Rudolph C,
Schlegelberger B, Cornils K, Zustin J, Spiess AN and Zander AR:
Radiation rescue: Mesenchymal stromal cells protect from lethal
irradiation. PLoS One. 6:e144862011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi C, Cheng T, Su Y, Mai Y, Qu J, Lou S,
Ran X, Xu H and Luo C: Transplantation of dermal multipotent cells
promotes survival and wound healing in rats with combined radiation
and wound injury. Radiat Res. 162:56–63. 2004. View Article : Google Scholar : PubMed/NCBI
|