Introduction
Medullary thyroid cancer (MTC) originates from the
thyroid parafollicular cells (also termed C cells), accounting for
5–10% of all thyroid malignancies (1). MTC is a type of malignant tumor, the
prevalence of which is marginally higher among the female
population compared with the male population. At present, surgical
removal of the tumor remains the preferred method for the treatment
of MTC. Unlike differentiated thyroid cancer, treatment with
radioiodine has no therapeutic effect on MTC, and the effects of
radiotherapy, radionuclide therapy and chemotherapy remain poor.
Biological immune treatments, including molecular targeted drugs,
cancer vaccines, monoclonal antibodies, and suicide or immune genes
have demonstrated therapeutic effects in clinical or pre-clinical
trials (2,3). In recent years, natural medicine has
had an important role in cancer treatment, and numerous natural
monomer compounds, such as paclitaxel and matrine, have been shown
to exhibit antitumor effects (4,5),
Therefore, investigating novel therapeutic targets for the
treatment of MTC is important for the identification of effective
drugs.
Lithospermum erythrorhizon is the dry root of
the perennial herb Comfrey Arnebia, and has a long medicinal
history in China. Studies have demonstrated that shikonin (SHI),
the main effective ingredient of L. erythrorhizon exhibits
antitumor effects towards HL60 human promyelocytic leukemia cell
lines (5), liver cancer (6), prostate cancer (7), colorectal cancer (8), oral squamous cell carcinoma (9), basal cell carcinoma (10) and osteosarcoma (11). SHI effects the metabolism,
proliferation, differentiation, signal transduction and gene
expression of tumor cells, and inhibits the activity of DNA
topoisomerase, oxidative stress, and proteasomes, thereby
inhibiting the growth of tumor cells (12,13).
SHI has also been demonstrated to increase the sensitivity of tumor
cells towards chemotherapeutic drugs, and may therefore serve as an
effective chemical sensitizer (12,13).
The death of tumor cells is predominantly induced via three
mechanisms: Necrosis, apoptosis and autophagy. Previous studies
reported that SHI induced tumor cell death through the apoptotic
signaling pathway, and may act by inhibiting the activation of
nuclear factor (NF)-κB (14);
upregulating caspase proteases (15); inhibiting the expression of
survivin (16); regulating the
mRNA and protein expression of B cell lymphoma 2 (Bcl-2)
family-associated genes (8), p53
(8), c-myc (7) and Fas (9); and altering the mitochondrial
membrane potential (17). Previous
studies have also reported that SHI induced cell death through the
non-apoptotic pathway (autophagy and necrosis-like programmed cell
death) (14,15).
Studies have yet to report SHI-induced cell death of
human TT medullary thyroid carcinoma cells or its underlying
mechanism. Thus, the present study investigated SHI-induced cell
death of TT cells.
Materials and methods
Materials and apparatus
SHI, bovine serum albumin and lead citrate were
obtained from Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 was
obtained from Gibco Life Technologies, (Carlsbad, CA, USA). MTT was
obtained from Fuzhou Maixin Biotechnology Development Co., Ltd.
(Fuzhou, China). Annexin V-phycoerythrin (PE)/7-aminoactinomycin D
(7-AAD) apoptosis detection kit, cell cycle kit and MitoScreen
(JC-1) were obtained from BD Biosciences (San Diego, CA, USA). The
following primary antibodies: Rabbit monoclonal anti-Bcl-2 (cat.
no. 2870), rabbit monoclonal anti-myeloid cell leukemia 1 (Mcl-1)
(cat. no. 5453), rabbit monoclonal anti-Bcl-extra large (xL) (cat.
no. 2764), rabbit monoclonal anti-Bcl-2-associated X protein (Bax)
(cat. no. 5023), rabbit polyclonal anti-Bcl-2 interacting protein
(Bid) (human-specific; cat. no. 2002), mouse monoclonal
anti-β-actin (cat. no. 3700), rabbit polyclonal anti-caspase 3
(cat. no. 9662), rabbit polyclonal anti-cleaved caspase 3 (Asp175)
(cat. no. 9661), rabbit polyclonal anti-caspase 9 (cat. no. 9502)
and rabbit polyclonal anti-cleaved caspase 9 (cat. no. 9501) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Polyvinylidene difluoride membrane was obtained from EMD Millipore
(Billerica, MA, USA). BD FACSCanto™ flow cytometer was obtained
from BD Biosciences Inc.
Cell culture
Human TT medullary thyroid carcinoma cells
(Sigma-Aldrich) were cultured in F-12K medium supplemented with 10%
fetal bovine serum (Gibco Life Technologies) in a humidified
incubator at 37°C in an atmosphere containing 5%
CO2.
MTT assay
The TT cells were seeded into a 96-well culture
plate at a density of 8–10×103 cells/well, and the
supernatant was discarded when the cells became adherent to the
wall. A total of 200 µl SHI at various final concentrations
(0.5, 1, 1.5, 2, 3, 4, 6 and 8 µg/ml) was subsequently
added, with five repeated wells per treatment group. A negative
control group, consisting only of RPMI-1640 solution, was also
established. Each treatment group was set up in three parallel
wells, and in the control group an equal volume of complete media
was added. Following drug-cultivation for 24, 48 and 72 h, an MTT
assay was performed to analyze the impact of SHI on TT cell growth.
The absorbance of each well at 570 nm was measured by iMark
microplate absorbance reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA), and the inhibition rates were calculated according to the
following equation: Inhibition rate = 1 − (ODdosing −
ODblank)/(ODnegative − ODblank) ×
100. Where OD is the optical density. The experiment was repeated
three times.
Flow cytometry
The cells were inoculated in 6-well culture plates
at 20–40×104 cells/well. When the cells became wall
adherent, 2 ml culture medium containing 2 or 4 µg/ml drug
was added to each well, and for the control group an equal volume
of complete medium was added. The cells were collected after 24 h
culture, and washed twice with phosphate-buffered saline (PBS),
then resuspended in 500 µl of 1X binding buffer. A total of
100 µl cell suspension was then added into 5 µl
Annexin V-PE/7-AAD apoptosis detection solution, prior to being
mixed and incubated in the dark at room temperature for 15 min.
Following the addition of 400 µl of 1X binding buffer, the
apoptosis rate was detected using a Guava® EasyCyte™
Plus flow cytometer (EMD Millipore).
Preparation of transmission electron
microscopy (TEM) samples and observation of apoptotic
morphology
The cells in the logarithmic growth phase were
seeded into the 6-well plates at a density of 1×106
cells/well, and the treatment drug was added 24 h after
inoculation. The blank control group (RPMI-1640-treated cells) and
the 24 h treatment groups (2 and 4 µg/ml SHI-treated TT
cells) samples were prepared and were sectioned using a Reichert
ultramicrotome (Reichert-Jung Inc., Vienna, Austria), in order to
obtain 70–90 nm sections, which were stained with lead citrate
solution and uranyl acetate (Sigma-Aldrich) 50%-saturated ethanol
solution for 15 min, respectively. Samples were then observed using
a TEM (JEM-1400 Plus; JEOL USA, Inc., Peabody, MA, USA).
Impact of SHI on mitochondrial membrane
potential (ΔΨm)
Cell culture was conducted as previously described
in the flow cytometry method, and once the cells became wall
adherent, they were washed twice with PBS, prior to treatment with
2 and 4 µg/ml SHI-containing medium, and equal volume of
complete medium for 16 h culture. The cells were collected, and a
JC-1 probe was added prior to 15 min incubation at room temperature
in the dark. The cells were then washed twice with MitoScreen
(JC-1) kit, and the ΔΨm change was detected with a flow cytometer
at 490 nm excitation wavelength, and mitochondrial morphology was
observed using TEM.
Western blot analysis
The cell cultures and drug treatments were conducted
as previously described in the flow cytometry methods section. The
total protein of the 2 and 4 µg/ml SHI-treated groups was
extracted, and protein concentration was measured using the DC
Protein Assay kit (Bio-Rad Laboratories, Inc.), according to the
manufacturer's protocol. The protein samples were subsequently
separated by 10% SDS-PAGE (Bio-Rad Laboratories, Inc.), then
transferred onto polyvinylidene fluoride membranes using the wet
method. The membranes were then blocked with 3% bovine serum
albumin for 1 h, prior to being incubated with primary antibodies
(1:1,000; Cell Signaling Technology, Inc.) at room temperature for
3 h. The membranes were then washed three times with Tris-buffered
saline with Tween® 20 (TBST), and incubated with the
horseradish peroxidase-labeled secondary antibody at room
temperature for 2 h, and washed three times with TBST. The
membranes were treated with ECL reagents (GE Healthcare, Milwaukee,
WI, USA) prior to analysis. Images of the target protein were
captured and the expression levels were quantified using ChemiDoc™
XRS+ system and Image Lab™ software (Bio-Rad
Laboratories, Inc.). The following protein expression levels were
detected in treated cells: Bcl-2, Bax, Bid, Bcl-xL, Mcl-1, cleaved
caspase 9, cleaved caspase 3, and cleaved poly (ADP-ribose)
polymerase, with β-actin serving as an internal control.
Statistical analysis
Comparisons between treatment groups were conducted
by one-way analysis of variance, Student's t-test and Pearson
correlation analysis. When the intergroup analysis showed no
homogeneity, a rank sum test was performed. The SPSS 13.0
statistical package (SPSS, Inc., Chicago, IL, USA) was used to
analyze the data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Impact of SHI on the proliferation of TT
cells
The effect of treatment with SHI on the
proliferation of TT cells as determined by the MTT assay
demonstrated that various concentrations of SHI affected the
proliferation of TT cells after 24–72 h of culture. TT cell
proliferation was shown to increase following treatment with SHI in
a dose-dependent manner (Table
I).
 | Table IHuman TT medullary thyroid carcinoma
cell growth inhibition rate following treatment with various
concentrations of SHI (%). |
Table I
Human TT medullary thyroid carcinoma
cell growth inhibition rate following treatment with various
concentrations of SHI (%).
| Time (h) | Blank control
group | Growth inhibition
rate following SHI treatment (µg/mg)
|
|---|
| 0.5 | 1 | 2 | 4 |
|---|
| 24 | – | 11.3±5.2 | 18.9±5.5 | 29.3±4.3a | 51.4±4.1a |
| 48 | – | 15.8±4.7 | 30.2±3.1a | 47.2±5.1a | 62.7±3.5a |
| 72 | – | 28.4±4.8a | 40.4±3.6a | 66.0±2.5a | 84.3±2.7a |
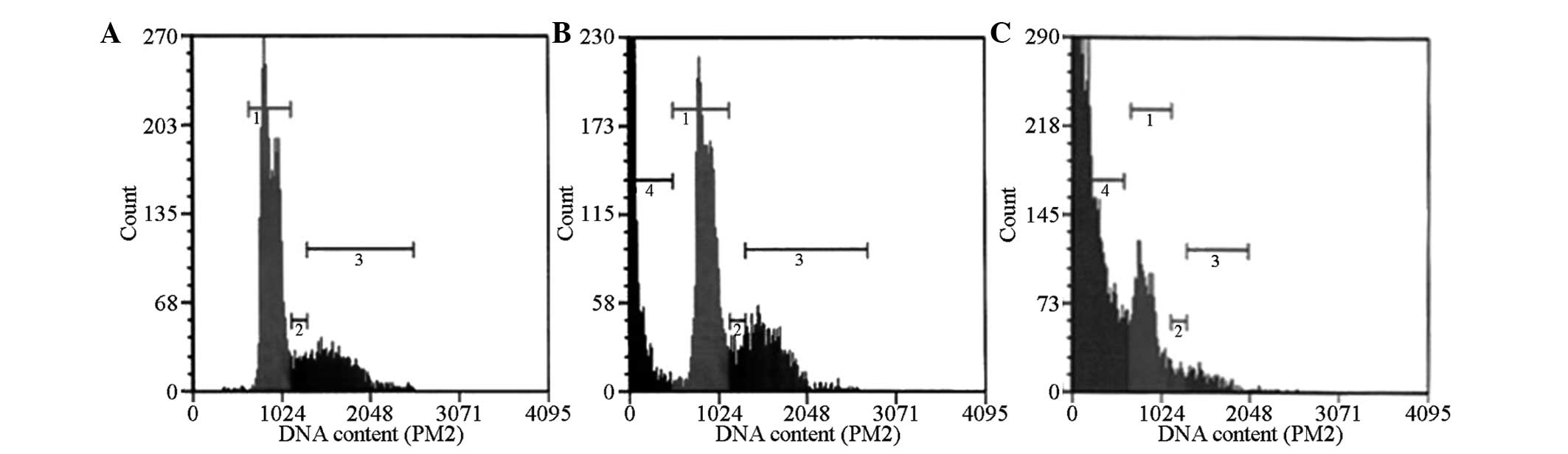
TT cell cycle changes
In the negative control group, flow cytometry
demonstrated the absence of a sub-diploid peak (apoptosis peak),
which appeared prior to the G0/G1 phase,
whereas in the 2 µg/ml SHI-treated cells, the sub-diploid
peak appeared after 24 h of culture. The 4 µg/ml SHI-treated
cells exhibited a marked pre-G0/G1 phase
sub-diploid peak (Fig. 1). The
following results in the cell cycle were observed following 24 h of
treatment with various concentrations of SHI. The percentages of
cells in the M1 (G0/G1), M2 (S), and M3 phase
(G2/M) in the 24 h treatment groups (2 and 4
µg/ml SHI-treated TT cells) showed no significant increase
when compared with the negative control group (P>0.05; Table II), thus the effects of SHI on the
percentages of cells in the various stages of the cell cycle were
not significant.
 | Table IIChanges in the 24 h TT human medullary
thyroid carcinoma cell cycle following treatment with various
concentrations of SHI. |
Table II
Changes in the 24 h TT human medullary
thyroid carcinoma cell cycle following treatment with various
concentrations of SHI.
| Group | M1
(G0/G1 phase) | M2 (S phase) | M3 (G2/M
phase) | M4 (sub
G0 phase) |
|---|
| Negative group | 68.8 | 5.7 | 25.0 | 0.2 |
| 2 µg/ml | 22.2 | 2.2 | 10.3 | 21.0 |
| 4 µg/ml | 13.3 | 1.3 | 3.0 | 52.3 |
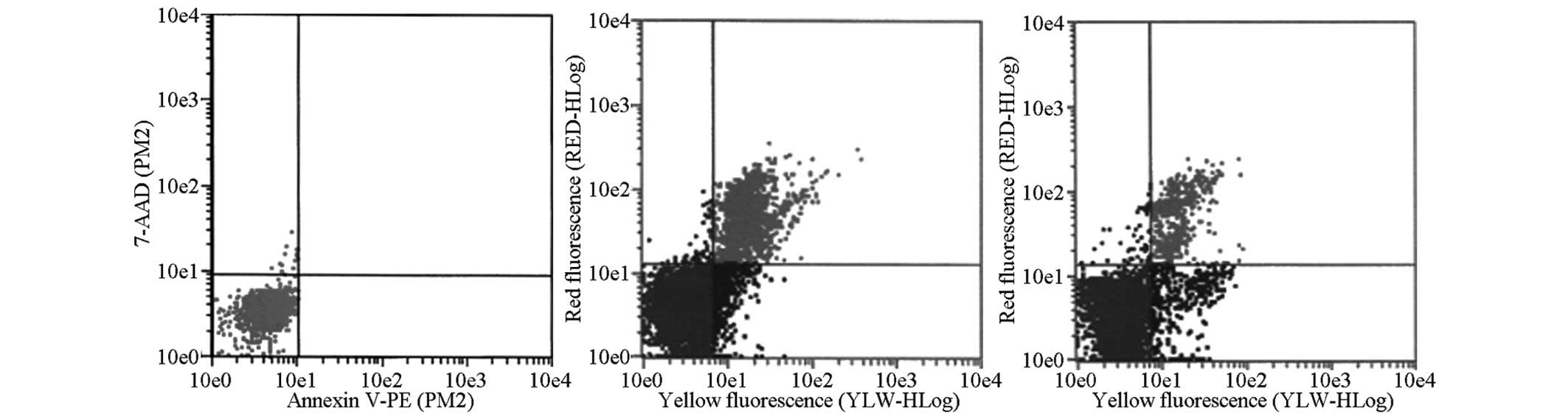
TT cell apoptosis
7-AAD and Annexin V-PE dual-labeling staining was
performed to measure apoptosis levels. Compared with the negative
control group, the 2 µg/ml SHI-treated group exhibited a
higher percentage (8.03%) of single-positive cells in early
apoptosis (Annexin V−PE+/7-AAD−)
after 24 h of treatment, compared with the control group (0.1%).
The percentage of double positive cells in late apoptosis, namely
the secondary necrosis phase (Annexin
V−PE+/7-AAD+), increased to
11.33%, compared with the control group (0.1%). After 48 h of
treatment, the percentage of single-positive cells in early
apoptosis (Annexin V−PE+/7-AAD−)
was increased to 24.99%, and that of the cells in late apoptosis
(Annexin V−PE+/7-AAD+) was
increased to 14.19%, compared with the control. After 72 h
treatment, the double positive cells (late apoptosis) were the
predominant cells, and the ratio markedly increased to 68.73%,
whereas single-positive cells accounted for only 6.1%. Intergroup
comparison revealed that these results were statistically
significant (P<0.01) (Fig.
2).
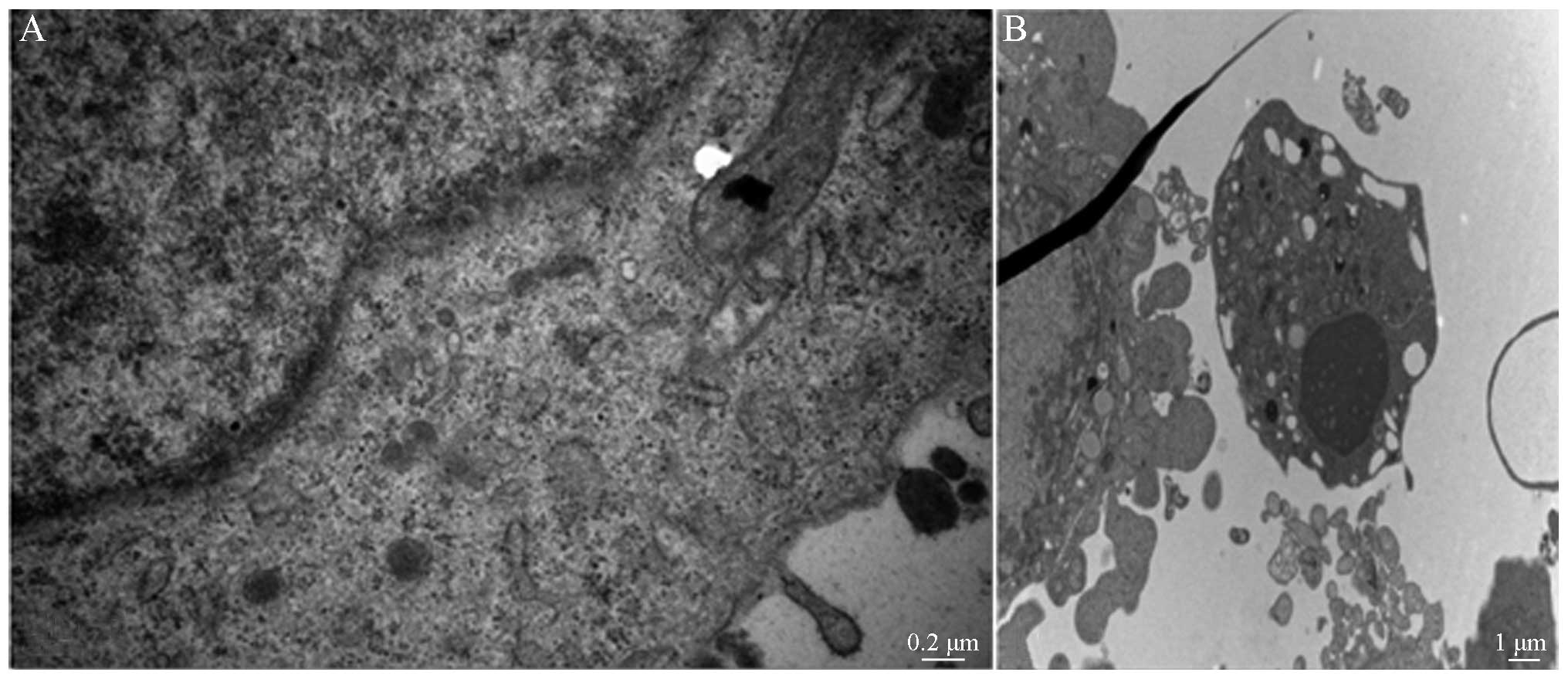
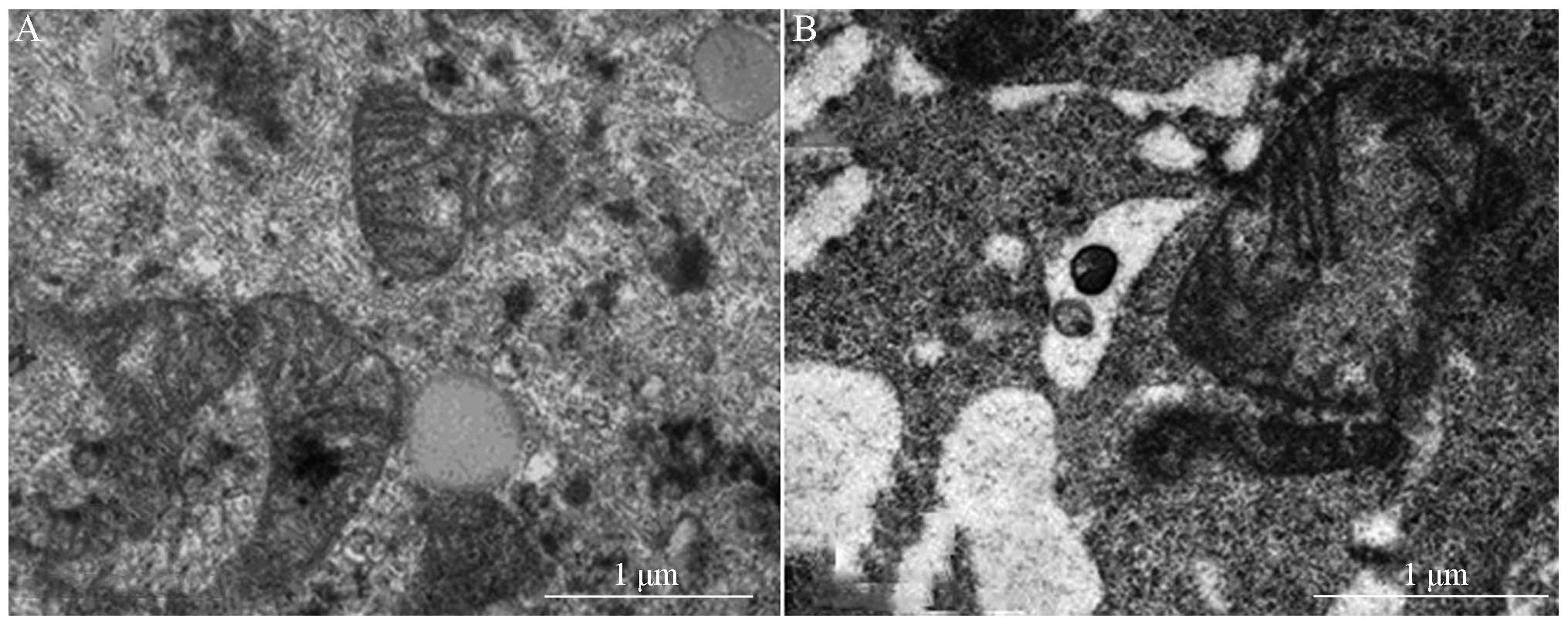
Apoptotic morphology observation
TEM was used to observe the cell morphology of the
control group after 24 h treatment (Fig. 3A). TEM observation revealed that
the apoptotic morphology of the 2 µg/ml SHI-treated group
exhibited morphological changes typical of apoptosis after 24 h
treatment (Fig. 3B).
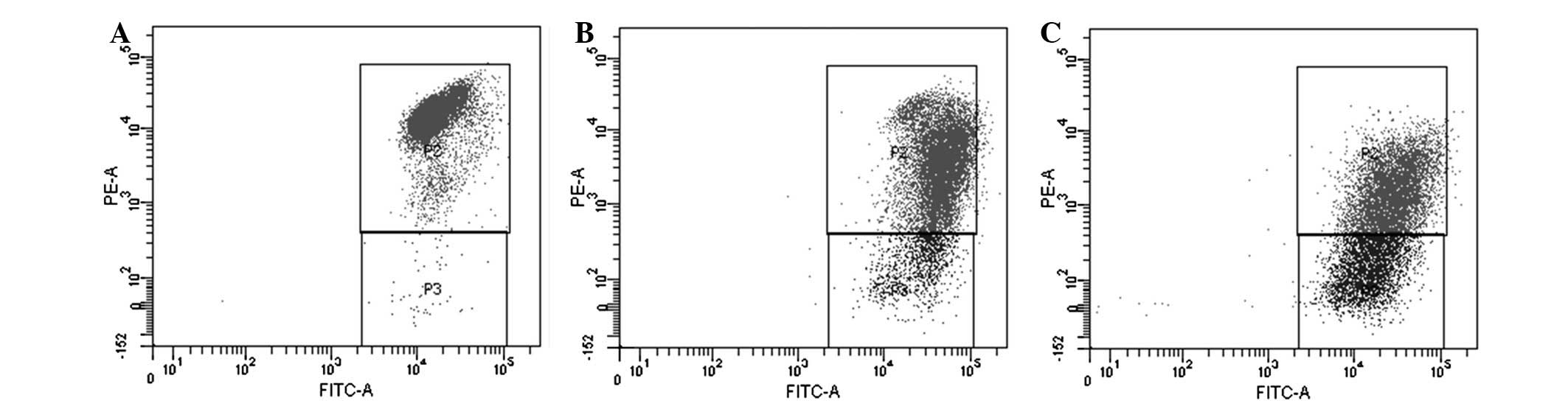
Detection of ΔΨm
Detection of the ΔΨm of the TT cells by flow
cytometry revealed that after 4 h of treatment, the percentage of 2
µg/ml SHI-treated cells with impaired mitochondria increased
from 10.1 to 23.4% (P<0.05), and that of the 4 µg/ml
SHI-treated cells increased to 42.1% (P<0.05), compared with the
control group. These results suggest that SHI may decrease the ΔΨm
in the TT cells, thereby damaging the mitochondrial membrane
(Fig. 4).
TEM was used to observe mitochondrial morphology
(Fig. 5), and demonstrated that
the mitochondria of the negative control group were stained, with a
clearly defined mitochondrial ridge structure (Fig. 5A). Conversely, after 24 h
treatment, the 2 µg/ml SHI-treated group exhibited
abnormally enlarged mitochondria, and the mitochondrial ridge and
membrane were both ruptured (Fig.
5B).
Western blotting determined that SHI
alters the expression levels of the Bcl-2 family proteins
Following SHI treatment, western blotting
demonstrated that the expression levels of anti-apoptotic proteins
Bax and Bid were markedly increased, whereas those of Bcl-2, Bcl-xL
and Mcl-1 were markedly decreased. This was shown to occur in a
dose-dependent manner (Fig. 6A).
In addition, following TT cell treatment with 2 and 4 µg/ml
SHI, the levels of caspase-9 and 3 increased in a dose-dependent
manner (Fig. 6B).
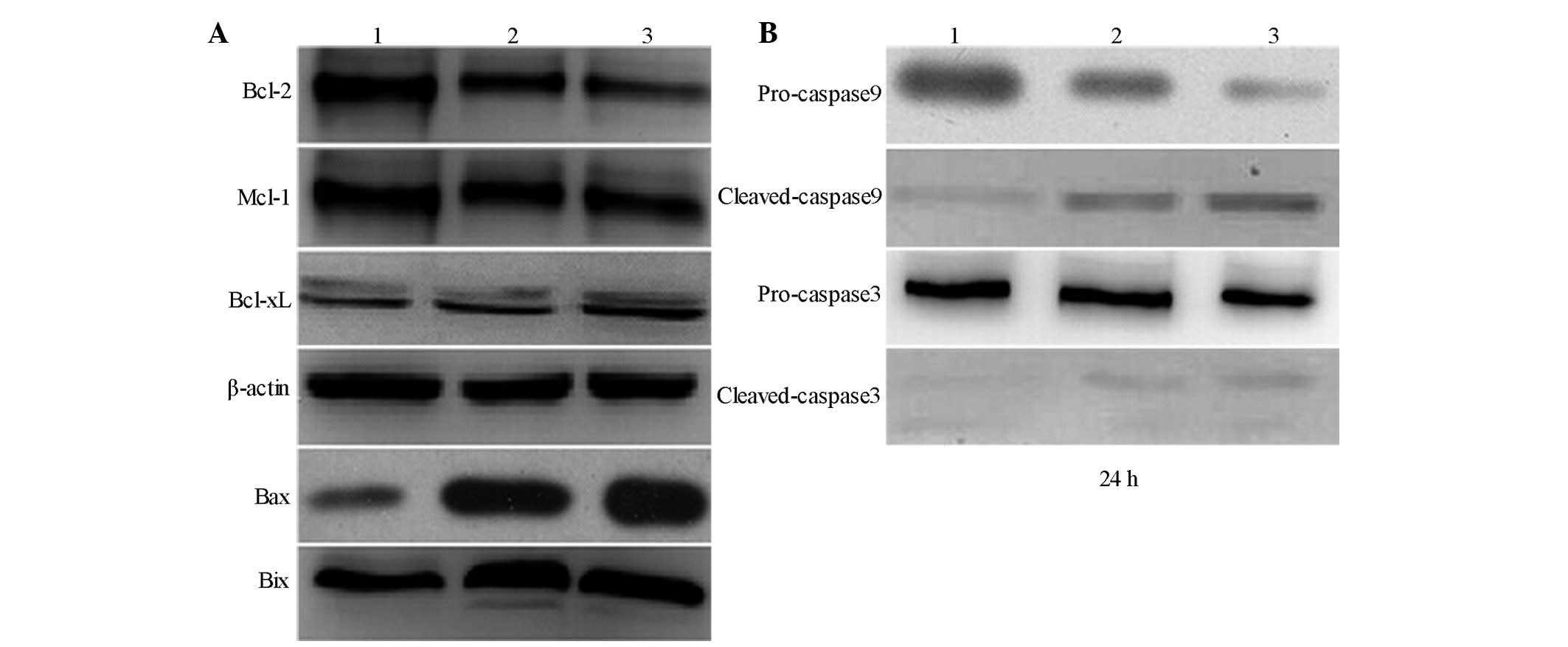 | Figure 6Effect of treatment with various
concentrations of SHI on the expression levels of Bax, Bcl-xl,
Bcl-2, Bik and caspases. (A) Effect of SHI on the expression of
Bax, Bcl-xl, Bcl-2 and Bik. (B) Effect of SHI on the expression of
caspase-9 and 3. Lane 1, control; lane 2, 2 µg/ml
SHI-treated cells; lane 3, 4 µg/ml SHI-treated cells. SHI,
shikonin; Bcl-2, B cell lymphoma 2; Bcl-xL, B cell lymphoma extra
large; Bax, Bcl-2-associated protein X; Bid, Bcl-2 interacting
protein; Mcl-1, myeloid cell leukemia 1. |
Discussion
SHI is the predominant active ingredient of the
traditional Chinese medicine Lithospermum erythrorhizon. Its
antitumor effects were first reported in 1977 by Sankawa et
al (18), who demonstrated
that treatment with 5–10 mg/kg/day SHI was able to effectively
inhibit the proliferation of mouse S180 ascites sarcoma cells.
Since then, further research has determined that SHI may be used as
an anticancer drug with multiple targets, exhibiting its antitumor
effects by inducing tumor cell apoptosis, inhibiting cell
proliferation, inducing cell differentiation and inhibiting tumor
cell metastasis (8,9).
A previous study demonstrated that mitochondria had
an important role in the apoptosis of malignant tumor cells
(19). Decreased ΔΨm is an early
manifestation of apoptosis, and is followed by mitochondrial
structural damage, during which small molecules are released,
including cytochrome c and apoptosis-inducing factors, and
the caspase-9 and 3 enzyme-linked reactions are activated (8). An association between SHI-induced TT
cell apoptosis and mitochondrial signaling has yet to be
reported.
The present study used TEM to observe mitochondria,
and demonstrated that following treatment with 2 µg/ml SHI
for 24 h, the mitochondria of the TT cells were abnormally enlarged
and swollen, and the mitochondrial ridge and membrane were
ruptured. Following 4 h of treatment with SHI, the ΔΨm of the TT
cells decreased, resulting in damage to the mitochondrial membrane.
These results suggested that SHI-induced apoptosis was associated
with a decrease in ΔΨm and changes in mitochondrial morphology.
The induction and regulation of mitochondrial outer
membrane permeabilization involved numerous proteins, specifically
those belonging to the Bcl-2 family. The pro-apoptotic Bcl-2 family
members, Bax and Bak, contributed to the mitochondrial outer
membrane permeabilization, thereby causing the activation of
caspase-9, whereas the anti-apoptotic Bcl-2 and Bcl-xL proteins
exhibited inhibitory effects. During the process of SHI-induced
apoptosis, the mitochondrial changes increased in a dose-dependent
manner, upregulating the expression of Bax and downregulating the
expression of Bcl-2, thereby increasing the Bax/Bcl-2 ratio. A
previous study also revealed that SHI was able to induce the
upregulation of Bax and the downregulation of Bcl-2 in other tumor
cells (20).
The increased Bax/Bcl-2 ratio indicated that the TT
cells underwent apoptosis via the mitochondrial signaling pathway,
and as the ΔΨm was decreased, the apoptotic cascade was activated.
These results demonstrated that SHI was able to increase the
Bax/Bcl-2 expression ratio. Therefore, the SHI-induced decrease in
ΔΨm may be viewed as the cause of the change in expression levels
of the Bcl-2 family proteins.
Previous studies demonstrated that in numerous cell
types, such as human bladder cancer cells and oral squamous cell
carcinoma cells, SHI may activate caspase-9 and caspase-3 via the
mitochondria-dependent signaling pathway (21–23),
thereby demonstrating their roles in the promotion of tumor cell
apoptosis. In the present study, western blot analysis demonstrated
that the expression levels of caspase-9 and caspase-3 in the
SHI-treated cells were downregulated. These results demonstrated
the important role of caspases in the SHI-induced TT apoptosis
process. The SHI-induced regulation of TT cell apoptosis was
mediated by the mitochondrial signaling pathway.
The results of the western blot analysis
demonstrated that the expression levels of caspase-9 and caspase-3
in the SHI-treated cells were downregulated, and RT-qPCR analysis
also revealed similar results. These results suggested that the
caspases had important roles in SHI-induced apoptosis of TT
cells.
In conclusion, SHI induced apoptosis of TT cells
through the mitochondrial signaling pathway. This was shown by an
increase in the expression levels of Bcl-2 apoptotic precursor
proteins Bax and Bid and decreased expression levels of
anti-apoptotic proteins Bcl-2, Bcl-xL and Mcl-1, thereby increasing
the Bax/Bcl-2 ratio. This resulted in a decrease in the ΔΨm,
activation of caspase-9 and caspase-3, and thus apoptosis.
Acknowledgments
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant no.
LY13H130003).
References
|
1
|
Cohen MS and Moley JF: Surgical treatment
of medullary thyroid carcinoma. J Intern Med. 253:616–626. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Minemura K, Takeda T, Nagasawa T, Zhang R,
Leopardi R and DeGroot LJ: Cell-specific induction of sensitivity
to ganciclovir in medullary thyroid carcinoma cells by
adenovirus-mediated gene transfer of herpes simplex virus thymidine
kinase. Endocrinology. 141:1814–1822. 2000.PubMed/NCBI
|
|
3
|
Soler MN, Milhaud G, Lekmine F,
Treilhou-Lahille F, Klatzmann and Lausson S: Treatment of medullary
thyroid carcinoma by combined expression of suicide and
interleukin-2 genes. Cancer Immunol Immunother. 48:91–99. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi Z, Chen J, Li CY, An N, Wang ZJ, Yang
SL, Huang KF and Bao JK: Antitumor effects of concanavalin A and
Sophora flavescens Iectin in vitro and in vivo. Acta Pharmcol Sin.
35:248–256. 2014. View Article : Google Scholar
|
|
5
|
Zhang LH, Wang H, Ma GL, Zhang C, Sun HX,
Song CX and Kong DS: Study on antitumor activity of
paclitaxel-loaded polymeric micelles. Int J Biomed Eng. 37:12–17.
2014.
|
|
6
|
Hashimoto S, Xu M, Masuda Y, Aiuchi T,
Nakajo S, Cao J, Miyakoshi M, Ida Y and Nakaya K:
Beta-hydroxyisovalerylshikonin inhibits the cell growth of various
cancer cell lines and induces apoptosis in leukemia HL-60 cells
through a mechanism different from those of Fas and etoposide. J
Biochem. 125:17–23. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang H, Zhou P, Huang H, Chen D, Ma N, Cui
QC, Shen S, Dong W, Zhang X, Lian W, et al: Shikonin exerts
antitumor activity via proteasome inhibition and cell death
induction in vitro and in vivo. Int J Cancer. 124:2450–2459. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu PC, Huang YT, Tsai ML, Wang YJ, Lin JK
and Pan MH: Induction of apoptosis by shikonin through coordinative
modulation of the Bcl-2 family, p27 and p53, release of cytochrome
c and sequential activation of caspases in human colorectal
carcinoma cells. J Agric Food Chem. 52:6330–6337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nam KN, Son MS, Park JH and Lee EH:
Shikonins attenuate microglial inflammatory responses by inhibition
of ERK, Akt and NF-kappaB: Neuroprotective implications.
Neuropharmacology. 55:819–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Min R, Tong J, Wenjun Y, Wenhu D, Xiaojian
Z, Jiacai H, Jian Z, Wantao C and Chenping Z: Growth inhibition and
induction of apoptosis in human oral squamous cell carcinoma
Tca-8113 cell lines by Shikonin was partly through the inactivation
of NF-kappaB pathway. Phytother Res. 22:407–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang IC, Huang YJ, Chiang TI, Yeh CW and
Hsu LS: Shikonin induces apoptosis through reactive oxygen
species/extracellular signal-regulated kinase pathway in
osteosarcoma cells. Biol Pharm Bull. 33:816–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu L, Qin A, Huang H, Zhou P, Zhang C, Liu
N, Li S, Wen G, Zhang C, Dong W, et al: Shikonin extracted from
medicinal Chinese herbs exerts anti-inflammatory effect via
proteasome inhibition. Eur J Pharmacol. 658:242–247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wiench B, Eichhorn T, Paulsen M and
Efferth T: Shikonin directly targets mitochondfia and causes
mitochondrial dysfunction in cancer cells. Evid Based Complement
Alteruat Med. 2012:7260252012.
|
|
14
|
Staniforth V, Wang SY, Shyur LF and Yang
NS: Shikonins, phytocompounds from Lithospermum erythrorhizon,
inhibit the transcriptional activation of human tumor necrosis
factor alpha promoter in vivo. J Biol Chem. 279:5877–5885. 2004.
View Article : Google Scholar
|
|
15
|
Hu X and Xuan Y: Bypassing cancer drug
resistance by activating multiple death pathways-a proposal from
the study of circumventing cancer drug resistance by induction of
necroptosis. Cancer Lett. 259:127–137. 2008. View Article : Google Scholar
|
|
16
|
Xuan Y and Hu X: Naturally-occurring
shikonin analogues-a class of necroptotic inducers that circumvent
cancer drug resistance. Cancer Lett. 274:233–242. 2009. View Article : Google Scholar
|
|
17
|
Villena J, Madrid A, Montenegro I, Werner
E, Cuellar M and Espinoza L: Diterpenylhydroquinones from natural
ent-labdanes induce apoptosis through decreased mitochondrial
membrane potential. Molecules. 18:5348–5359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sankawa U, Ebizuka Y, Miyazaki T, Isomura
Y and Otsuka H: Antitumor activity of shikonin and its derivatives.
Chem Pharm Bull (Tokyo). 25:2392–2395. 1977. View Article : Google Scholar
|
|
19
|
Kleibl Z, Raisová M, Novotný J, Pohlreich
P and Matous B: Apoptosis and its importance in the development and
therapy of tumors (review). Sb Lek. 103:1–13. 2002.In Czech.
|
|
20
|
Yingkun N, Lvsong Z and Huimin Y: Shikonin
inhibits the proliferation and induces the apoptosis of human HepG2
cells. Can J Physiol Pharmacol. 88:1138–1146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Z, Wu L, Li L, Tashiro S, Onodera S and
Ikejima T: P53-mediated cell cycle arrest and apoptosis induced by
shikonin via a caspase-9-dependent mechanism in human malignant
melanoma A375-S2 cells. J Pharmacol Sci. 94:166–176. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thangapazham RL, Singh AK, Seth P, Misra
N, Mathad VT, Raj K and Maheshwari RK: Shikonin analogue (SA)
93/637 induces apoptosis by activation of caspase-3 in U937 cells.
Front Biosci. 13:561–568. 2008. View
Article : Google Scholar
|
|
23
|
Yeh CC, Kuo HM, Li TM, Lin JP, Yu FS, Lu
HF, Chung JG and Yang JS: Shikonin-induced apoptosis involves
caspase-3 activity in a human bladder cancer cell line (T24). In
Vivo. 21:1011–1019. 2007.
|