Introduction
Gonadotropin-releasing hormone (GnRH) stimulates the
secretion of follicle-stimulating hormone (FSH) and luteinizing
hormone (LH) from the pituitary gland, which regulate the secretion
of steroid hormones, such as estrogen and progesterone (1). GnRH and LH receptors have been
identified in the human and rat gastrointestinal (GI) tract
(2–4), where GnRH inhibits cell proliferation
(5,6). LH stimulation fragments the migrating
myoelectric complex in the small intestine (7) and reduces survival of enteric rat
neurons in culture (8). A number
of patients suffer from GI side effects during treatment with GnRH
analogs, and a few patients develop chronic intestinal
pseudo-obstruction (CIPO) or enteric dysmotility (ED) with
degenerative and inflammatory changes in the GI tract (3,9).
Previous rat studies have shown that GnRH analogs
cause loss of submucosal and myenteric neurons in the fundus, ileum
and colon, without any effect on body weight, circulating
inflammatory biomarkers or morphometry of the bowel wall (4,10).
The numbers of mast cells and eosinophils were shown to be
unaltered; however, signs of ganglioneuritis with infiltration of
T-lymphocytes in the enteric ganglia were identified (4,11).
Elevated estradiol levels in the plasma and a thickened uterine
muscle layer were observed, along with reduced relative numbers of
neurons immunoreactive to LH receptors and increased
imunnoreactivity of activated caspase-3 (4,10).
Thus, buserelin treatment in rats constitutes a rat model of
enteric neuropathy, and the neuronal loss is anticipated to be
mediated through elevated LH release and hyperactivation of LH
receptor-bearing enteric neurons (4,10,11),
resulting in increased apoptosis (4,12).
Prior results are based on studies where the animals
were sacrificed shortly after the final buserelin treatment
(4,10,11).
The aim of the present study was to investigate the long-term
effect of buserelin-induced enteric neuropathy on body weight and
on the GI tract regarding neuronal cell loss, morphology, and
inflammatory changes.
Materials and methods
Animals
Female Sprague-Dawley rats (n=20; age, 7 weeks;
weight, 170–180 g), purchased from Charles River Laboratories
(Sulzfeld, Germany) were used. The rats were allowed to acclimatize
to the climate- and light-controlled animal facility for at least 5
days prior to experimentation. Standard rat chow (4% fat/g)
(Lactamin R36, Stockholm, Sweden) and water were supplied ad
libitum. The experimental design was approved by the Animal
Ethics Committee, Lund and Malmö, Sweden (M350–12, date of
approval: 14.11.12). Animals were used in accordance with the
European Communities Council Directive (2010/63/EU) and the Swedish
Animal Welfare Act (SFS 1988:534).
Study design
Rats (n=12) were administered 20 μg of the
GnRH analog buserelin (1 mg/ml) (Suprefact, Sanofi-Aventis, Bromma,
Sweden) (dissolved in 0.2 ml saline) subcutaneously, once daily for
5 days, followed by 3 weeks of recovery, representing one session
of treatment (4). The dosage and
administration of buserelin are based on previous studies which
have shown reliable physiological effects in terms of uterine
hypertrophy, without any adverse effects (4,10,13).
Control animals (n=8) received 0.2 ml saline injections. The
animals were weighed prior to inclusion in the study, and weekly in
the morning during the study. Thirty-two weeks after the start of
the study, and 4 months after the last treatment session, rats were
anesthetized using chloral hydrate (300 mg/kg body weight; C8383;
Sigma-Aldrich, St. Louis, MO, USA) and killed by aorta puncture.
Tissue samples from the fundus, distal ileum, proximal colon, and
distal part of the uterine horn were collected and processed for
histological evaluation.
Tissue preparation
The fundus/corpus region of the stomach, ileum
(starting 8 cm proximally to the cecum and continuing in the
proximal direction), and colon (starting 2 cm distally to the cecum
and continuing in the distal direction) were opened and flattened
on filter paper. One section of each gut region was fixed in
Stefanini's fixative (a mixture of 2% formaldehyde (Sigma-Aldrich)
and 0.2% picric acid (Sigma-Aldrich) in phosphate buffer, pH 7.2)
for 22 h at 4°C, and the other section and the uterine horns were
fixed in 4% paraformaldehyde (Sigma-Aldrich) in 0.1 M phosphate
buffer for 22 h at 4°C. Stefanini-fixed specimens were rinsed three
times in Tyrode's solution containing 10% sucrose, before being
orientated and mounted for cross- and longitudinal sectioning in
Tissue-Tek (Sakura, Histolab, Gothenburg, Sweden), frozen on dry
ice, and sectioned (10 μm) in a cryostat (HM500; Microm
GmbH, Walldorf, Germany). Paraformaldehyde-fixed specimens were
dehydrated in ethanol, cleared in xylene, orientated for cross- and
longitudinal sectioning, embedded in paraffin, and sectioned (5
μm). Sections were processed for immunocytochemistry and
histochemistry.
Histochemistry
Morphometry was performed on scanned,
deparaffinized, hydrated, and hematoxylin and eosin-stained
paraffin (all Histolab Products AB, Gothenburg, Sweden) sections
from cross-sectioned uterine horns and longitudinally cut GI
regions using a computerized, image-analyzing system (Imagescope,
Aperio ScanScope GL SS5082, Vista, CA, USA). The myometrium and the
intestinal layers of fundus, distal ileum, and proximal colon
(mucosa and circular and longitudinal muscle layers) were indicated
manually, and then measured using a computerized binary cursor.
Mean values of 6–10 representative measurements were calculated
from each rat in each region.
Immunocytochemistry
For studies on enteric neuronal survival, monoclonal
mouse antibodies against human neuronal protein (HuC/D) (dilution
1:100, cat. no. A-2127, Thermo Fisher Scientific, Inc., Stockholm,
Sweden) were used as general neuronal marker. Paraffin sections
from the fundus, ileum and colon were deparaffinized, hydrated and
subjected to antigen retrieval by boiling in citrate acid buffer
(0.01 M, pH 6; Sigma-Aldrich) in a microwave oven (650 W) for 7 min
twice. The sections were cooled and washed in distilled water
followed by phosphate-buffered saline (PBS)/Triton X-100
(Sigma-Aldrich). Sections were exposed to biotinylated, primary
antibodies against HuC/D at 4°C overnight. For visualization of
biotinylated HuC/D, a VECTASTAIN ABC kit containing horseradish
peroxidase (HRP) and 3,3′-diaminobenzidine tetrahydrochloride (DAB)
was used (Vector Laboratories, Inc., Burlingame, CA, USA).
HuC/D-immunoreactive neurons stained dark brown and were counted in
submucosal and myenteric ganglia in longitudinally cut whole-wall
sections using the computerized, image-analyzing system Imagescope
(Aperio ScanScope). Since antigens for testing the specificity of
antibodies against HuC/D are not commercially available, omission
of the primary antibodies served as a control. Numbers of HuC/D
neurons were counted in a total length of at least 20 mm, cut at
6–9 different depths in each region and rat. Results are expressed
as numbers of HuC/D-immunoreactive submucosal or myenteric neurons
per mm region length of GI tract.
Mast cells
Toluidine blue-staining (Histolab Products AB) was
performed on paraffin sections to identify mast cells in the GI
tract (14). Sections from the
fundus, ileum and colon were deparaffinized, hydrated, and stained
with 0.1% toluidine blue in 60% ethanol for 60 min in room
temperature. Cross- and longitudinally cut, whole-wall sections
were scanned and analyzed in Imagescope (Aperio ScanScope). Mucosa,
submucosa, and circular and longitudinal muscle layers were
evaluated separately and all mast cells were counted for at least
12 mm, cut at 6–9 different depths from each region and rat.
Results are expressed as numbers of mast cells per mm region length
of GI tract.
Eosinophils
A staining procedure based on cyanide-resistant
eosinophilic peroxidase (EPO) was used to identify eosinophils
(15). Cryo sections from the
fundus, ileum, and colon were washed in PBS buffer (pH 7.2), DAB
(75 mg/100 ml), H2O2 (100 μl/100 ml)
and NaCN (50 mg/100 ml) for 8 min and then rinsed in water for 10
min prior to being mounted in glycerine gelatine (Kaiser, Merck K,
Damstadt, Germany). Both cross- and longitudinally cut whole-wall
sections were analyzed using a light microscope (Olympus BX43, with
attached camera Nikon XC30, LRI instrument, Hamburg, Germany). The
number of cells were evaluated separately in the mucosa, submucosa,
and muscle layers of each region in a range from 0 to +++, where 0
indicates no cells, + indicates a few cells, ++ indicates a
moderate number, and +++ indicates a high number, as previously
described (4,15). In regions which showed a difference
between groups, eosinophils were counted for at least 30 mm, cut at
6–9 different depths from each region and rat. Results are
expressed as the number of eosinophils per mm or cm region length
of GI tract.
T-lymphocytes
The presence of CD3-immunoreactive T-lymphocytes was
analyzed on cross- and longitudinally cut whole-wall paraffin
sections from the fundus, ileum and colon. The sections were
incubated over night with primary polyclonal antibodies against CD3
raised in goat (dilution 1:1,000; cat. no. 1127, Santa Cruz
Biotechnology, Inc., CA, USA). The sections were then exposed to
DyLight-TM-594-conjugated donkey anti-rabbit IgG (dilution 1:1,000;
Jackson ImmunoResearch Europe, Ltd., Newmarket, UK) for 1 h and
mounted in phosphate buffer:glycerol 1:1. The fluorophore was
excited and T-lymphocytes were visualized at 488 nm using a Olympus
BX43 microscope.
T-lymphocytes were separately evaluated in
epithelia, mucosa, submucosa, circular and longitudinal muscle
layers, and serosa of the fundus, ileum and colon, in a range from
0 to +++, where 0 indicates no cells, + indicates few, ++ indicates
a moderate number, and +++ indicates a high number, as previously
described (4,15). Only those regions which showed a
difference between groups were counted, for at least 20 mm GI
length, cut at 6–9 different depths from each region and rat.
Results are expressed as numbers of T-lymphocytes per mm region
length of GI tract.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). Results are
presented as the medians and spreads, expressed as the 25th and
75th percentile or as the mean ± standard error of the mean.
Statistical analyses were performed using unpaired Student's t-test
regarding the normally distributed body weight and by Mann-Whitney
U-test for other data. P<0.05 was considered to indicate a
statistically significant difference.
Results
General observations
All rats looked healthy and exhibited normal healthy
behavior. One of the buserelin-treated rats presented with a
subcutaneous tumor during week 22 and was sacrificed (not included
in the study), leaving eight rats in the control group (C) and 11
rats in the buserelin-treated group (B). There was a significant
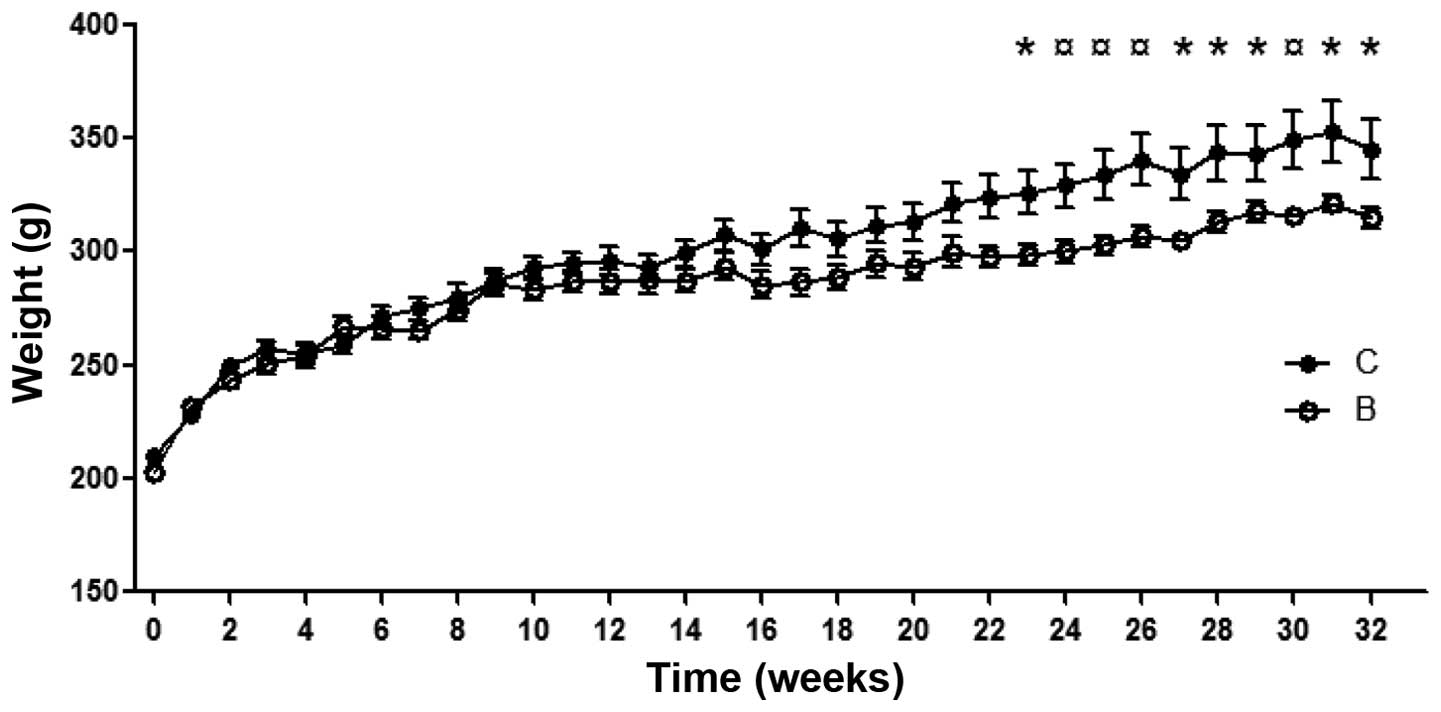
difference in body weight between controls and buserelin-treated
rats from week 23 to the end of the study (Fig. 1). At autopsy, the visceral organs
were inspected, and no lesions or abnormalities were
identified.
Morphology and morphometry
Hematoxylin and eosin staining of sections from the
uterus, fundus, ileum and colon revealed normal histology in the
controls and buserelin-treated rats. No differences in mucosal
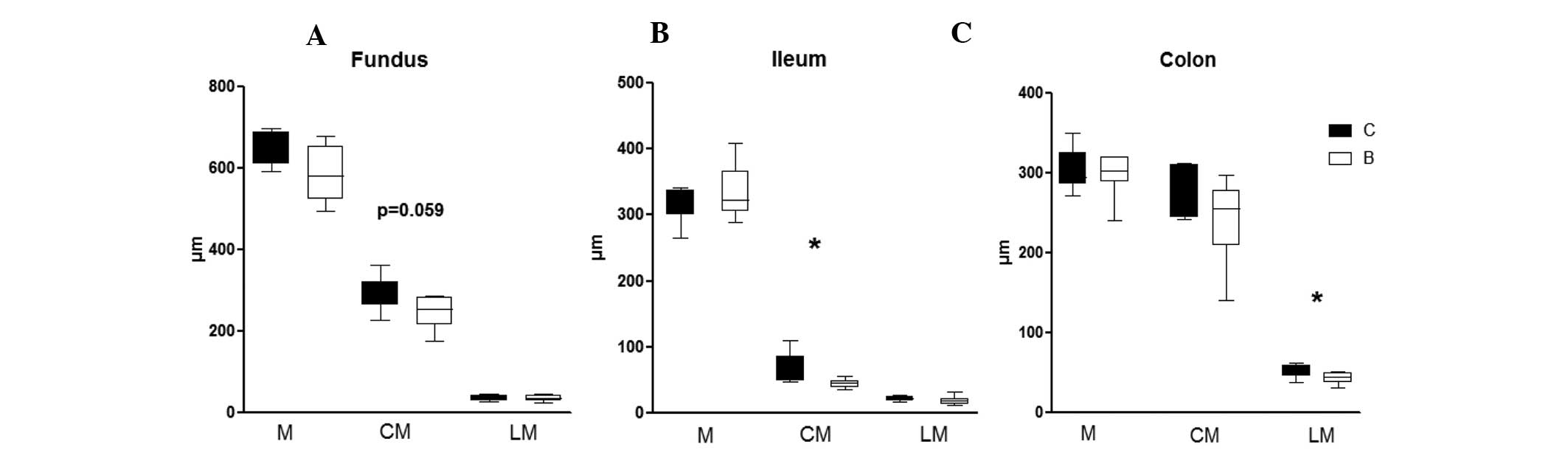
thickness were detected between the two groups. However, a thinner
circular muscle layer of the fundus was observed in
buserelin-treated rats, as compared with the control rats (P=0.059)
(Fig. 2A). A thinning of the
circular muscle layer in the ileum (P<0.05) (Fig. 2B) and the longitudinal muscle layer
in colon (P<0.05) (Fig. 2C)
were also noted in buserelin-treated rats, as compared with the
controls. No difference in the thickness of uterine muscle layer
was detected between the two groups (data not shown).
Neuronal survival
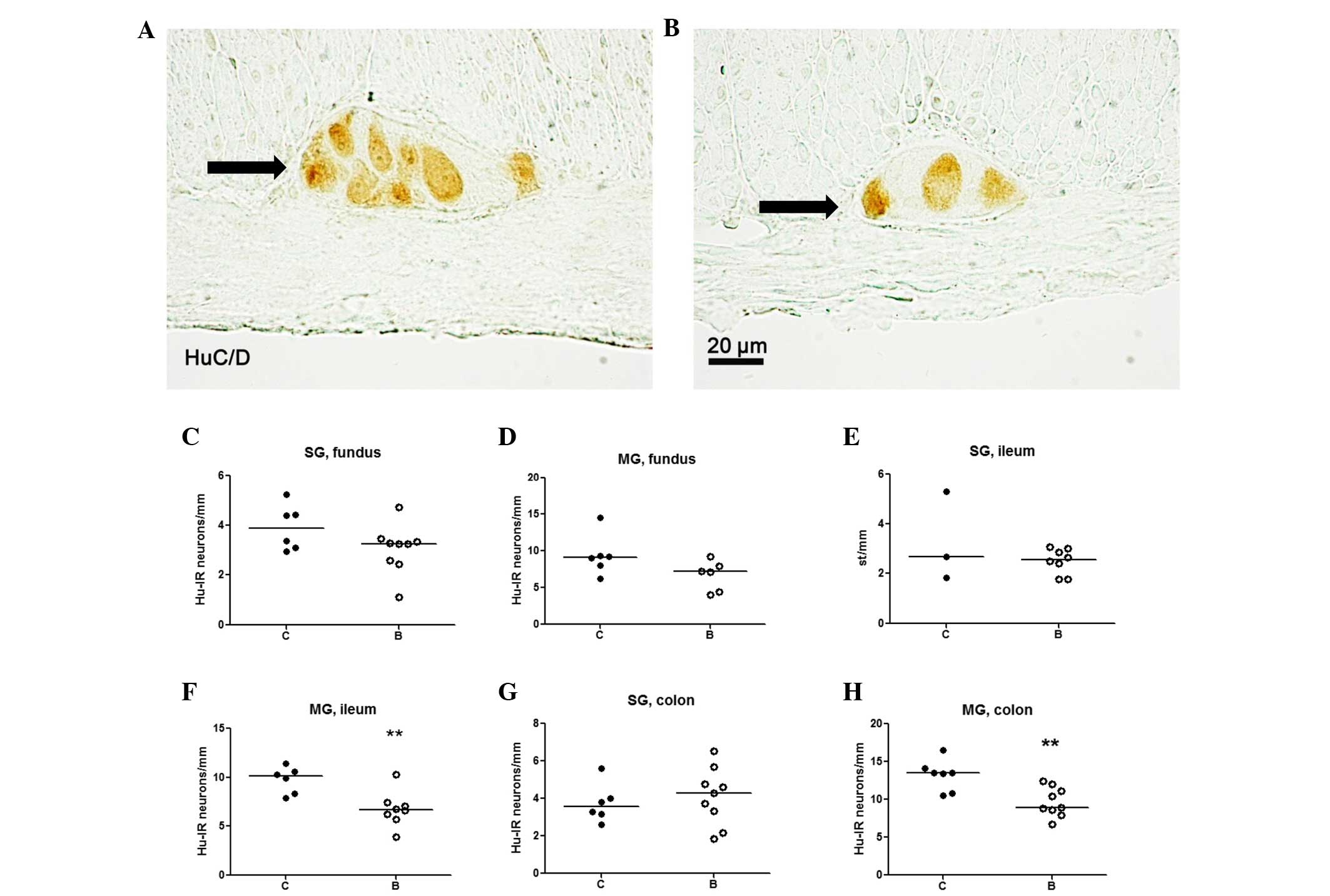
Neurons displaying HuC/D immunoreactivity were
regularly found all along the fundus, ileum, and colon (Fig. 3A and B). In addition, the number of
neurons was determined in the fundus, ileum and colon (Fig. 3C–H). No significant difference was
identified in the numbers of neurons in the fundus between the
controls and the buserelin-treated group (Fig. 3C and D).
In the ileum and colon, the numbers of submucosal
neurons did not differ between the two groups (Fig. 3E and G), whereas reduced numbers of
myenteric neurons were found in the buserelin-treated group
(P<0.01) (Fig. 3F and H).
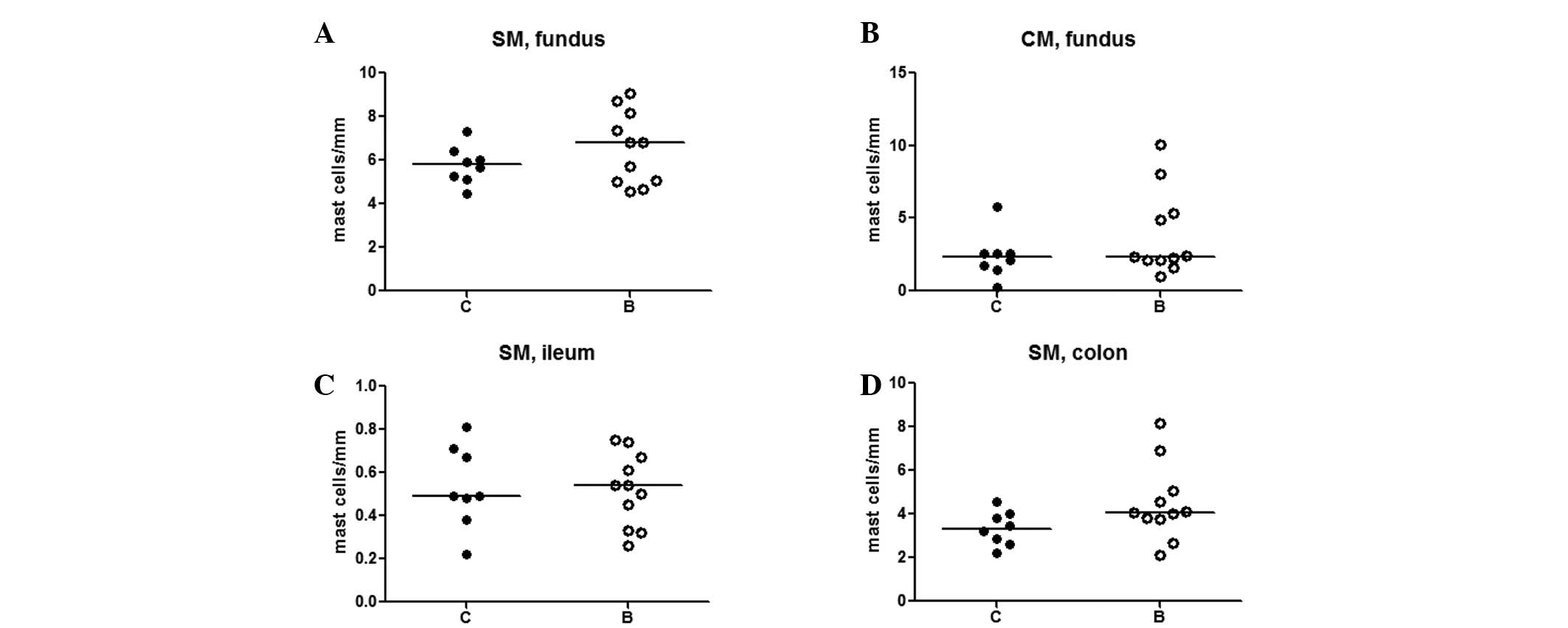
Mast cells
In the fundus, numerous mast cells were identified
in the submucosa and in the circular muscle layer, with a high
individual variation, independent of treatment (Fig. 4A and B). In the ileum and colon,
the mast cells were only found in the submucosa, without any
difference between the groups (Fig. 4C
and D).
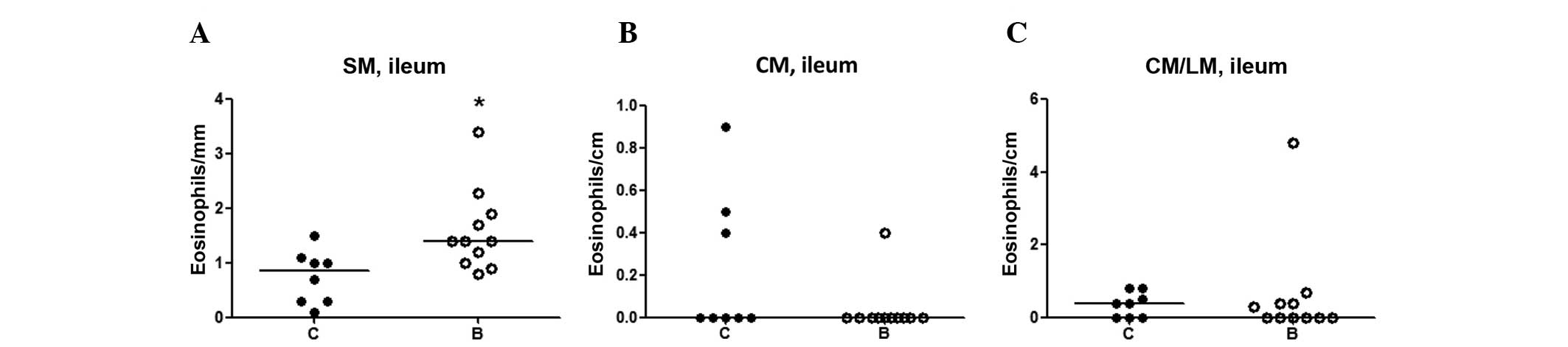
Eosinophils
Eosinophils were abundant in the lamina propria of
the entire GI mucosa, without any difference between the groups.
Eosinophils were sparsely found in the submucosa, smooth muscle
layers and serosa in the two groups. An increased number of
eosinophils was detected in the submucosa of the ileum in
buserelin-treated rats compared with controls (P<0.05) (Fig. 5A), whereas no changes were
identified in the circular muscle layer or in-between the circular
and longitudinal muscle layers (Fig.
5B and C).
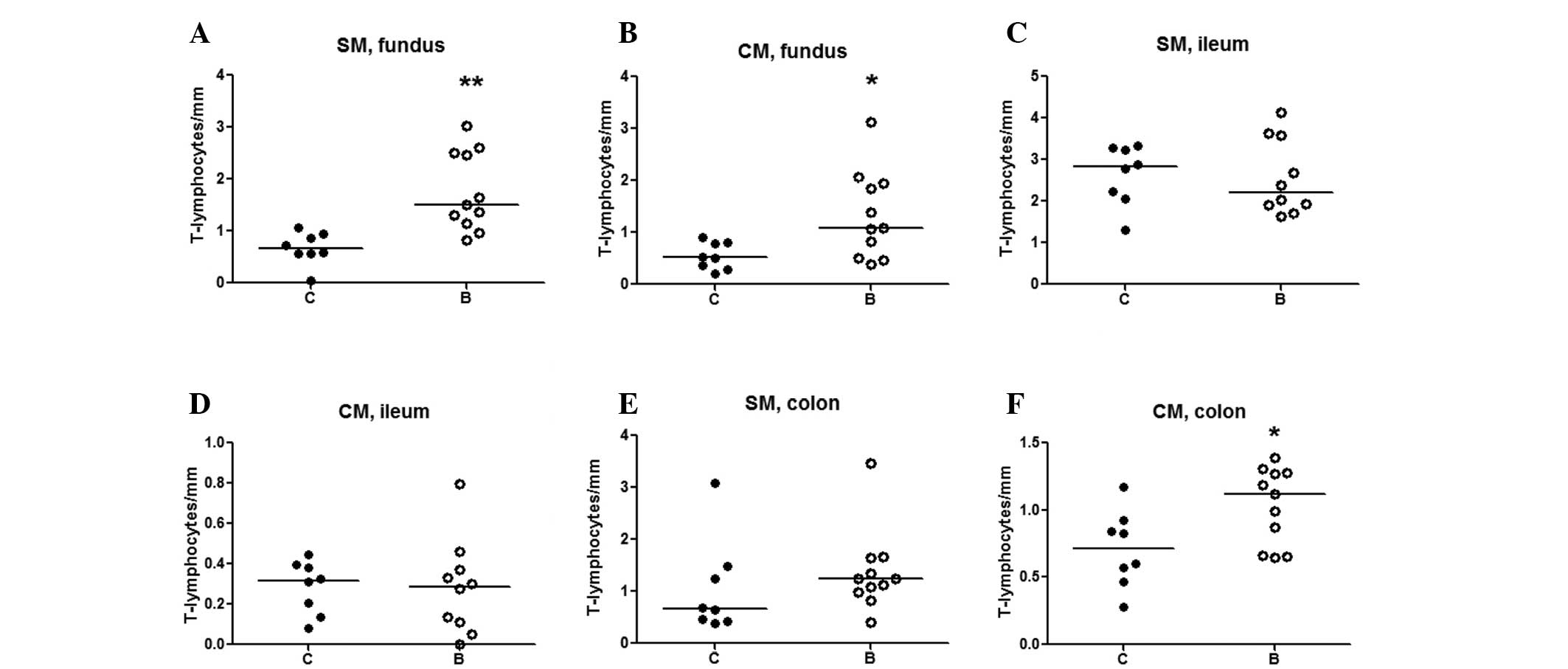
T-lymphocytes
In the two groups, the majority of T-lymphocytes
were identified in the mucosa, positioned in the lamina propria and
intraepithelially. T-lymphocytes were only rarely detected in the
longitudinal muscle layer.
The number of T-lymphocytes was significantly
increased in the submucosa (P<0.01) (Fig. 6A) and circular muscle layer of
fundus (P<0.05) (Fig. 6B) in
the buserelin-treated group, as compared with the controls.
In the ileum, no significant changes in the numbers
of T-lymphocytes in the submucosa or circular muscle layer were
detected between the two groups (Fig.
6C and D). In the colon, the number of T-lymphocytes in the
submucosa did not differ between the two groups, whereas in the
circular muscle layers, the numbers of T-lymphocytes were increased
in the buserelin-treated group, as compared with the controls
(P<0.05) (Fig. 6E and F).
Discussion
Reduced numbers of myenteric neurons in the ileum
and colon were detected in buserelin-treated rats compared with
controls in the present long-term study. Buserelin treatment
resulted in a lower body weight at the end of the study, a thinner
circular muscle layer in the ileum, a thinner longitudinal muscle
layer in the colon, an increased number of eosinophils in the
submucosa of the ileum, and a higher number of T-lymphocytes in the
submucosa of the fundus, and circular muscle layers of the fundus
and colon, compared with controls.
Sixteen weeks after the last treatment session, at
study week 32, the rats treated with buserelin showed a loss of
myenteric neurons in the ileum and colon, as described in previous
studies where the rats were sacrificed shortly after the end of the
treatment (4,10). In accordance with former studies,
the neuronal loss was more pronounced in the ileum and colon than
in the fundus, and in myenteric neurons than in the submucosal
neurons (4,10). Regeneration of enteric neurons and
glial cells is possible, since tissue-specific progenitor cells
have been identified (16,17), and mice studies have shown
regeneration of neurons following intestinal damage (18). However, the magnitude of myenteric
neuronal loss was the same as that determined in previous studies
(4,10), even though the present rats were
kept alive for 16 weeks longer.
The difference in body weight between groups started
at week 23, and was not observed in the short-term studies that
ended at week 16 and 18 (4,10),
which suggests that the damage that occurs during the treatment
eventually leads to malnutrition. The enteric nervous system (ENS)
has an impact on digestion and absorption of nutrients,
particularly carbohydrates and fat (19,20),
and an increased fat content in the feces following buserelin
treatment has been observed (10).
Enteric neuropathy may thus lead to malnutrition in rats as well as
in patients (3,9). Sex hormones stimulate increased food
intake, and low plasma estradiol levels have been shown to result
in reduced food intake (21). In a
previous study, no difference in body weight was identified;
however, plasma levels of estradiol were elevated and the uterine
muscle layer was thickened following buserelin treatment (10). The unaffected myometrium thickness
in the present study suggests normalized sex hormone levels
following treatment cessation, and the negative effects of
neuropathy on intestinal nutrient uptake cannot be counteracted by
increased appetite and food intake secondary to elevated sex
hormone levels, as an assumed explanation to the reduced body
weight in the present long-term follow-up.
In previous studies, buserelin caused a loss of
myenteric neurons with signs of apoptosis, but without signs of
muscle degeneration and increased eosinophils (4,10).
In previous studies, the number of T-lymphocytes was only increased
in the ganglia and around nerve fibers (4,11).
This indicates that neuronal loss is the initial damage, and the
other findings are secondary. Degenerative neuropathy may result in
secondary, and not causal, tissue inflammation. A variety of
diseases in the human GI tract are accompanied by eosinophilia,
which is an un-specific condition, without obvious consequences
(22). Mucosal eosinophilia is
associated with hypersensitivity (23), but this has not been shown for
mural forms (24). T-lymphocytes
are involved in the development and activity of inflammatory bowel
disease (25), and antibodies
against CD3 have been used to prevent immunological diseases
(26). Although a number of
patients with ENS diseases express an increased number of
T-lymphocytes in the bowel wall, and in these patients
T-lymphocytes have been shown in close relation to ganglia, their
role in enteric neuropathy is not defined (10,27).
The increased number of T-lymphocytes in the fundus, albeit normal
numbers of neurons in the present study, may be explained by a
dynamic process over time, and when examining the rats at a
specific time point, the process may be at different stages in
different bowel segments. The increased number of T-lymphocytes may
have been proceeded by reversible neuronal damage. The thinner
muscle layer in the intestines could be explained by less neuronal
stimulation and less peristalsis, resulting in atrophy of the
muscle.
The clinical course of CIPO and ED is a slow
propagation of the disease over time with worsened GI functions
(3,9,27).
These conditions show varying histopathological changes with
preserved submucosal neurons and affected myenteric ganglia
(3,9,27),
similar to the rat model. Enteric neuropathies may be accompanied
with muscle hypertrophy or muscle atrophy (27). It is of great interest to examine
the long-term effects of enteric neuropathy in a well-characterized
and standardized rat model, as it is difficult to study
neuropathies in humans due to the rarity of the diseases and poor
sensitivity of GI function tests. In addition, examination of ENS
demands a full-thickness biopsy obtained through surgery.
Furthermore, there are different etiologies to the ENS damage, and
different co-morbidities and life style habits that also have an
effect on these patients. Several confounding factors are therefore
at hand in clinical studies, which obscure the results rendering it
difficult to determine which are primary etiological effects, and
which are secondary to the evoked disturbances. Taken together, all
clinical studies performed on buserelin-induced intestinal damage
in humans, and experimental studies in rats, suggest that this
neuropathy may be classified as a primary degenerative or
inflammatory neuropathy (3,4,9–11).
In conclusion, long-term follow-up of
buserelin-induced enteric neuropathy in a rat model shows
irreversible loss of myenteric neurons. Secondary to this, the
histopathological GI changes were aggravated over time and the rats
presented with thinner muscle layers, and increased numbers of
eosinophils and T-lymphocytes. In addition, the body weight gain is
reduced over time. Future mechanistic studies are necessary in
order to explain the pathophysiology underlying enteric neuropathy
and the associated changes.
Acknowledgments
The King Gustaf V and Queen Victoria Free Mason's
Foundation, Development of Region Skåne, the Bengt Ihre Foundation,
the Lundström Foundation, and the Royal Physiographic Society in
Lund. The authors would like to thank Anna Themner Persson for her
technical assistance.
References
|
1
|
Naor Z: Signalling by G-protein-coupled
receptor (GPCR): Studies on the GnRH receptor. Front
Neuroendocrinol. 30:10–29. 2009. View Article : Google Scholar
|
|
2
|
Huang W, Yao B, Sun L, Pu R, Wang L and
Zhang R: Immunohistochemical and in situ hybridization studies of
gonadotropin releasing hormone (GnRH) and its receptor in rat
digestive tract. Life Sci. 68:1727–1734. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohlsson B, Veress B, Janciauskiene S,
Montgomery A, Haglund M and Wallma rk A: Chronic intestinal
pseudo-obstruction due to buserelin-induced formation of anti-GnRH
antibodies. Gastroenterology. 132:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sand E, Voss U, Hammar O, Alm R,
Fredrikson GN, Ohlsson B and Ekblad E: Gonadotropin-releasing
hormone analog buserelin causes neuronal loss in rat
gastrointestinal tract. Cell Tissue Res. 351:521–534. 2013.
View Article : Google Scholar
|
|
5
|
Chen L, He HX, Sun XD, Zhao J, Liu LH,
Huang WQ and Zhang RQ: Expression of gonadotropin-releasing hormone
receptor and effect of gonadotropin-releasing hormone analogue on
proliferation of cultured gastric smooth muscle cells of rats.
World J Gastroenterol. 10:1780–1784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aguilar-Rojas A and Huerta-Reyes M: Human
gonadotropin-releasing hormone receptor-activated cellular
functions and signaling pathways in extra-pituitary tissues and
cancer cells (Review). Oncol Rep. 22:981–990. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ducker TE, Boss JW, Altug SA, Mehrabian H,
Dekeratry DR, Clench MH and Mathias JR: Luteinizing hormone and
human chorionic gonadotropin fragment the migrating myoelectric
complex in rat small intestine. Neurogastroenterol Motil. 8:95–100.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sand E, Voss U, Ohlsson B and Ekblad E:
Luteinizing hormone receptors are expressed in rat myenteric
neurons and mediate neuronal loss. Auton Neurosci. 193:104–107.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cordeddu L, Bergvall M, Sand E, Roth B,
Papadaki E, Li L, Damato M and Ohlsson B: A description of cases
with severe abdominal complaints after treatment with
gonadotropin-releasing hormone analogs. Scand J Gastroenterol.
16:1–9. 2015.
|
|
10
|
Sand E, Roth B, Weström B, Bonn P, Ekblad
E and Ohlsson B: Structural and functional consequences of
buserelin-induced enteric neuropathy in rat. BMC Gastroenterology.
14:2092014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohlsson B, Sand E and Veress B:
Ganglioneuritis is common in rats with enteric neuropathy due to
buserelin treatment. Regul Pept. 190–191:43–45. 2014. View Article : Google Scholar
|
|
12
|
Sasson R, Rimon E, Dantes A, Cohen T,
Shinder V, Land-Bracha A and Amsterdam A: Gonadotrophin-induced
gene regulation in human granulosa cells obtained from IVF
patients. Modulation of steroidogenic genes, cytoskeletal genes and
genes coding for apoptotic signalling and protein kinases. Mol Hum
Reprod. 10:299–311. 2014. View Article : Google Scholar
|
|
13
|
Trindade CR, Camargos AF and Pereira FE:
The effect of buserelin acetate on the uterus of adult rats:
Morphological aspects. Clin Exp Obstet Gynecol. 35:198–201.
2008.PubMed/NCBI
|
|
14
|
Sand E, Themner-Persson A and Ekblad E:
Mast cells reduce survival of myenteric neurons in culture.
Neuropharmacology. 56:522–530. 2009. View Article : Google Scholar
|
|
15
|
Sand E, Themner-Persson A and Ekblad E:
Infiltration of mast cells in rat colon is a consequence of
ischemia/reperfusion. Dig Dis Sci. 53:3158–3169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawaguchi J, Nichols J, Gierl MS, Faial T
and Smith A: Isolation and propagation of enteric neural crest
progenitor cells from mouse embryonic stem cells and embryos.
Development. 137:693–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heanue TA and Pachnis V: Prospective
identification and isolation of enteric nervous system progenitors
using Sox2. Stem Cells. 29:128–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Laranjeira C, Sandgren K, Kessaris N,
Richardson W, Potocnik A, Vanden Berghe P and Pachnis V: Glial
cells in the mouse enteric nervous system can undergo neurogenesis
in response to injury. J Clin Invest. 121:3412–3424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ratnayake WM and Galli C: Fat and fatty
acid terminology, methods of analysis and fat digestion and
metabolism: A background review paper. Ann Nutr Metab. 55:8–43.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mourad FH and Saadé NE: Neural regulation
of intestinal nutrient absorption. Prog Neurobiol. 95:149–162.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hirschberg AL: Sex hormones, appetite and
eating behaviour in women. Maturitas. 71:248–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yantiss RK: Eosinophils in the GI tract:
How many is too many and what do they mean? Mod Pathol. (28 Suppl
1): S7–S21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rothenberg ME: Eosinophilic
gastrointestinal disorders (EGID). J Allergy Clin Immunol.
113:11–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yun MY, Cho YU, Park IS, Choi SK, Kim SJ,
Shin SH and Kim KR: Eosinophilic gastroenteritis presenting as
small bowel obstrction: A case report and review of the literature.
World J Gastroenterol. 13:1758–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Forster K, Goethel A, Chan CW, Zanello G,
Streutker C and Croitoru K: An oral CD3-specific antibody
suppresses T-cell-induced colitis and alters cytokine responses to
T-cell activation in mice. Gastroenterology. 143:1298–1307. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ochi H, Abraham M, Ishikawa H, Frenkel D,
Yang K, Basso A, Wu H, Chen ML, Gandhi R, Miller A, et al: New
immunosup-pressive approaches: Oral administration of CD3-specific
antibody to treat autoimmunity. J Neurol Sci. 274:9–12. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Knowles CH, De Giorgio R, Kapur RP, Bruder
E, Farrugia G, Geboes K, Gershon MD, Hutson J, Lindberg G, Martin
JE, et al: Gastrointestinal neuromuscular pathology: Guidelines for
histological techniques and reporting on behalf of the gastro 2009
International working group. Acta Neuropathol. 118:271–301. 2009.
View Article : Google Scholar : PubMed/NCBI
|