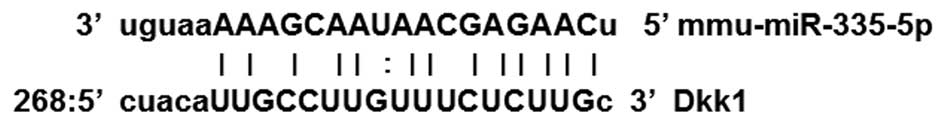Introduction
Diabetes and osteoporosis are common metabolic
diseases, and are closely related. The effect of diabetes on bone
metabolism is predominantly characterized by increased bone
resorption, reduced bone formation and bone mineral content, and is
prone to fracture, leading to osteoporosis (1). MicroRNAs (miRs) are a class of small,
non-coding RNAs, 22–24 nucleotides in length, which
post-transcriptionally regulate gene expression through
specifically binding to the 3′-untranslated region (UTR) of target
mRNAs and inducing either translational repression or mRNA cleavage
(2–4). miRs are present in almost all
eukaryotes, regulating approximately one-third of protein-coding
genes and participating in various pathophysiological processes,
including proliferation, differentiation, apoptosis, immunology,
metabolism, growth and development (5–8).
miR-335-5p is encoded by the genomic region of
chromosome 7q32.2 in humans (9).
It has been shown that miR-335-5p suppresses the invasion and
metastasis of tumor cells and regulate cytoskeleton dynamics in
mouse oocytes (10,11). miR-335-5p is highly expressed in
MC3T3-E1 osteoblasts and promotes their differentiation via
downregulating the expression of dickkopf-1 (DKK1), which was
demonstrated by Zhang et al (10) using a luciferase reporter assay as
well as loss- and gain-of-function studies. As an inhibitor of the
Wnt signaling pathway, DKK1 has key roles in several pathogenic
processes, for example, cancer of the pancreas, stomach, liver,
breast and cervix (12).
miR-335-5p has been shown to influence osteoblast functions through
the MAPK, FAK and ErbB signaling pathways (11). However, the effects of miR-335-5p
and DKK1 on osteoblast apoptosis induced by high glucose (HG) and
the underlying molecular mechanisms have remained elusive.
In the present study, the expression levels of
miR-335-5p in MC3T3-E1 osteoblasts cultured under HG conditions
were detected, and the effects of miR-335-5p overexpression on
HG-induced apoptosis of osteoblasts as well as the expression
levels of DKK1 in these cells were assessed.
Materials and methods
Cell line and culture
The MC3T3-E1 osteoblast cell line was purchased from
Shanghai Fuxiang Biotechnology Co., Ltd. (Shanghai, China). Cells
were cultured in α-modified essential medium (α-MEM; Hyclone,
Logan, UT, USA), supplemented with 10% fetal bovine serum (FBS;
Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin/streptomycin antibiotics (Gibco). These cells were
divided into the following groups: i) The control group, in which
cells were cultured with 5.5 mmol/l glucose (Sigma-Aldrich, St.
Louis, MO, USA); ii) the HG group, in which cells were cultured
with 22.0 mmol/l glucose; iii) the miR-335-5p overexpression group,
in which cells were transfected with agomir-335-5p
(5′-UCAAGAGCAAUAACGAAAAAUGU-3′; synthesized by Shanghai GenePharma
Co., Ltd., Shanghai, China) to overexpress miR-335-5p and then
cultured with 22.0 mmol/l glucose; and iv) the transfection control
group, in which cells were transfected with agomir negative control
(NC; 5′-UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT-3′; Shanghai
GenePharma Co., Ltd.) and cultured with 22.0 mmol/l glucose. Cells
in the transfection groups were first transfected with the optimal
doses of agomir-335-5p (100 pmol and 5 pmol for six-well and
96-well plates, respectively) for 48 h using Lipofectamine 2000
(Invitrogen, Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions, and then cultured in α-MEM containing
glucose for seven days.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen). Reverse transcription was performed with the
Superscript First-Strand Synthesis System (Invitrogen). qPCR was
performed using the Supermix PreMix (SYBR Green; Invitrogen). The
PCR amplification mixture contained 5 µl SYBR Green, 0.4
µl forward primer, 0.4 µl reverse primer, 2 µl
cDNA and 2.2 µl DEPC water. Sequences of the primers used
are listed in Table I. PCR thermal
cycling was performed on a Bio-Rad T100 thermo cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) as follows: Denaturation at
95°C for 5 min, followed by 40 cycles of 95°C for 15 sec, 58°C for
20 sec and 72°C for 30 sec, and a final extension at 72°C for 10
min. U6 and GAPDH were used as the controls for miR and mRNA
detection, respectively. The relative expression levels of target
RNAs were calculated using the 2−ΔΔCq method (13).
 | Table IPrimer sequences for
reverse-transcription quantitative polymerase chain reaction. |
Table I
Primer sequences for
reverse-transcription quantitative polymerase chain reaction.
| Gene | Sequences |
|---|
| miR-335-5p | Forward:
5′-GCGTCAAGAGCAATAACG-3′ |
| Reverse:
5′-GTGCAGGGTCCGAGGT-3′ |
| DKK1 | Forward:
5′-CTCATCAATTCCAACGCGATCA-3′ |
| Reverse:
5′-GCCCTCATAGAGAACTCCCG-3′ |
| U6 | Forward:
5′-TGCAGAGGATCTAATT-3′ |
| Reverse:
5′-GAAAGACCAGTCCAAGTCC-3′ |
| GAPDH | Forward:
5′-AGGTCGGTGTGAACGGATTTG-3′ |
| Reverse:
5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
Flow cytometry
Cell apoptosis was detected using flow cytometry. In
brief, ~1×106 cells were harvested and re-suspended in 1
ml binding buffer [10 mM HEPES/NaOH (pH 7.4), 140 mM NaCl, 2.5 mM
CaCl2; Cube Biotech, Monheim, Germany]. An Annexin
V-FITC/PI Apoptosis Detection kit (Invitrogen) was used. These
cells were stained with 20 µl Annexin V-fluorescein
isothiocyanate and 25 µl propidium iodide for 15 min at room
temperature for 15 min. The fluorescence signals were detected
using a flow cytometer (EPICS Altra; Beckman Coulter, Miami, FL,
USA).
Prediction of miR-335-5p binding site in
DKK1
Prediction of the binding site for miR-335-5p in
DKK1 was performed using a combination of the following
computational algorithms: Targetscan (http://www.targetscan.org/), microRNA (http://www.microrna.org) and miRBase Targets
(http://www.mirbase.org/). As shown in Fig. 1., bioinformatics analysis revealed
that the 3′UTR of DKK1 mRNA contains a sequence which may be
targeted by miR-335-5p. miR-335-5p has been confirmed to directly
target DKK1 in MC3T3-E1 osteoblasts by a previous study (10).
Western blot analysis
Cells were lysed with radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Haimen, Jiangsu,
China). After centrifugation at 4,795 × g for 15 min, the protein
concentration in the supernatant was determined. A total of 1
µl supernatant was added to 96-well plates and then 19
µl PBS and 200 µl BCA working fluid (A:B=50:1,BCA
Protein Assay kit; Beyotime Institute of Biotechnology) was added
to each well and then incubated at 37°C for 30 min. The OD value
(A562 value) of each well was detected using a Bio-Rad 680 enzyme
standard instrument (Bio-Rad Laboratories, Inc.), and then drawing
a standard curve to calculate the protein concentration.
Subsequently, 20 µg protein was separated by 12% sodium
dodecyl sulfate polyacrylamide gel (Beyotime Institute of
Biotechnology) electrophoresis and then electronically transferred
onto a polyvinylidene difluoride membrane (Beyotime Institute of
Biotechnology). After blocking with 5% bovine serum albumin at 37°C
for 2 h, the membrane was incubated with mouse monoclonal anti-DKK1
antibody (cat. no. ab61275; Abcam, Cambridge, UK; 1:200 dilution),
rabbit polyclonal anti-caspase-3 antibody (cat. no. 9662; Cell
Signaling Technology, Beverly, MA, USA; 1:500 dilution) or rabbit
polyclonal GAPDH (cat. no. 10494-1-AP; Proteintech, Wuhan, China;
1:500 dilution) at 4°C overnight. Subsequently, the membrane was
incubated with horseradish peroxidase (HRP)-labeled goat
anti-rabbit IgG (Beyotime Institute of Biotechnology; cat. no.
A0208; 1:2,000 dilution) and HRP-labeled goat anti-mouse IgG
(Beyotime Institute of Biotechnology; cat. no. A0216; 1:2,000
dilution) secondary antibodies at 4°C for 2 h. The blots were
visualized using enhanced chemiluminescence reagent (ECL Western
Blotting Substrate kit; Pierce Biotechnology, Inc., Rockford, IL,
USA). The Gel Doc XR imaging system (Bio-Rad Laboratories, Inc.)
was used to capture images of the blots and Fusion FX5 software
(Vilber, Lourmat, Marne-la-Vallée, France) was used for
densitometric analysis.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis was performed using the SPSS 19.0
software (International Business Machines, Armonk, NY, USA).
Student's t-test was used for pair-wise comparison, and one-way
analysis of variance was used for multiple comparisons. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
HG decreases miR-335-5p in MC3T3-E1
osteoblasts, while not affecting DKK1 mRNA
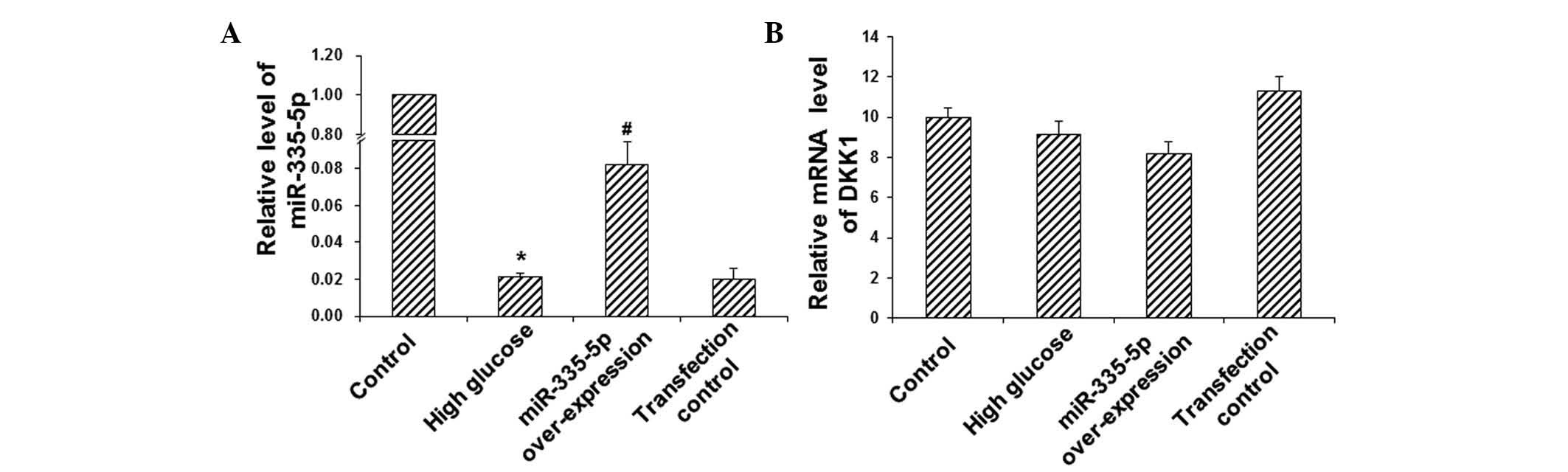
To investigate the expression levels of miR-335-5p
and DKK1 mRNA in MC3T3-E1 osteoblasts, RT-qPCR analysis was
performed. Compared with the control group, the expression of
miR-335-5p was significantly downregulated in the HG group
(P<0.05) (Fig. 2A).
Transfection with miR-335-5p led to a significant elevation of the
expression levels of miR-335-5p in these cells cultured under HG
conditions (P<0.05) (Fig. 2A).
However no significant differences were observed in the mRNA
expression levels of DKK1 between these groups (P>0.05)
(Fig. 2B). These results suggested
that miR-335-5p is significantly decreased in MC3T3-E1 osteoblasts
cultured under HG conditions, and that transfection with miR-335-5p
mimics efficiently increased miR-335-5p levels. As expected, the
mRNA levels of DKK1 were not affected by miR-335-5p, as the latter
interferes with the translation of DKK1 mRNA but not with DKK1 gene
transcription.
miR-335-5p mimics inhibit HG-induced
apoptosis of MC3T3-E1 osteoblasts
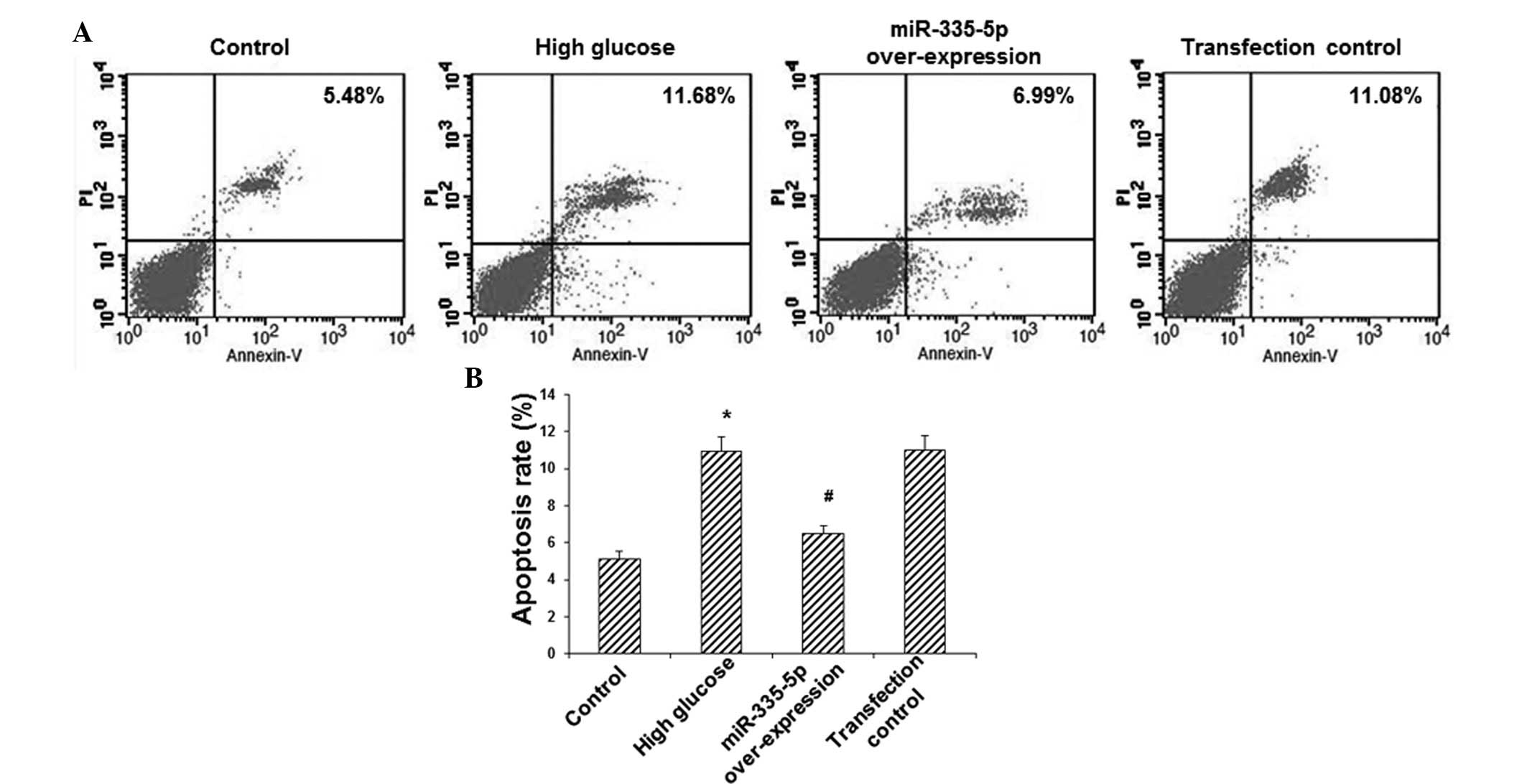
To investigate the effects of miR-335-5p on MC3T3-E1
osteoblast apoptosis, Annexin V/PI double staining and flow
cytometric analysis were utilized. The results showed that,
compared with the control group, the apoptotic rate in the HG group
was increased by >2-fold (P<0.05) (Fig. 3). However, overexpression of
miR-335-5p significantly decreased the apoptotic rate in these
model cells by ~40% (P<0.05) (Fig.
3). These results indicated that miR-335-5p overexpression
inhibited HG-induced apoptosis in MC3T3-E1 osteoblasts.
miR-335-5p mimics reduce HG-induced
increases in the protein expression of DKK1 and caspase-3 in
MC3T3-E1 osteoblasts
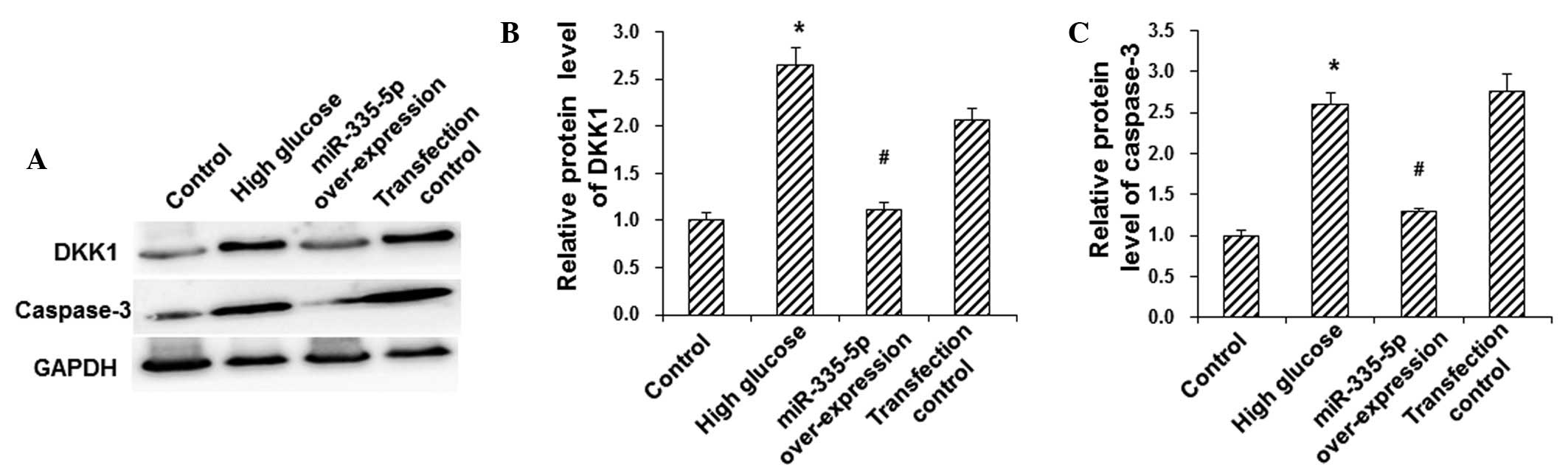
To investigate the protein expression levels of DKK1
and the apoptotic protein caspase-3 in MC3T3-E1 osteoblasts,
western blot analysis was performed. Compared with the control
group, the protein expression levels of DKK1 and caspase-3 were
significantly elevated in the HG group (P<0.05), and were be
significantly downregulated following overexpression of miR-335-5p
in these model cells (P<0.05) (Fig.
4). These results suggested that miR-335-5p reduced the
HG-induced protein expression of DKK1 and caspase-3 in MC3T3-E1
osteoblasts, which may have contributed to the inhibition of
HG-induced apoptosis in these cells.
Discussion
Diabetes mellitus and osteoporosis are common
metabolic diseases, and the incidence of diabetic osteoporosis has
been increasing over the past few years (14). Osteoblasts are the primary
functional cells in bone formation, which synthesize and secrete
bone matrix. Osteoblasts have key roles in the mineralization and
remodeling processes of bone. The dysfunction and/or loss of
osteoblasts leads to increases in bone resorption and relative
decreases in bone formation, resulting in a decrease of bone mass
and an increase of the risk of bone fracture as well as
osteoporosis (15).
miR-335-5p, a sub-type of miR-335, is encoded by
chromosome 7q32.2 in humans. It has been shown that miR-335-5p is
involved in the regulation of various pathophysiological processes
(16–20). Li et al (16) have reported that miR-335-5p
regulates the invasion and metastasis of gastric cancer cells.
Furthermore, Cui et al (17) indicated that miR-335-5p regulates
cytoskeletal dynamics in oocytes. In addition, miR-335-5p has been
found to influence the function of white adipose tissues in
patients with diabetes (18) and
during the aging process (19),
and to be a promising therapeutic target for wet age-associated
macular degeneration (20).
Besides, miR-335-5p is highly expressed in MC3T3-E1 osteoblasts,
which promotes cell differentiation by downregulating DKK1
expression (10). The present
study revealed that, compared with the control group, the apoptotic
rate and the expression levels of caspase-3 were significantly
increased in the HG group, which was consistent with the findings
of a previous study (21).
Furthermore, the expression of miR-335-5p in the HG group was
significantly lower than that in the control group, indicating that
miR-335-5p may regulate osteoblast function. DKK1 is an inhibitor
of the Wnt signaling pathway. While Wnt/β-catenin signaling
promotes the differentiation of mesenchymal stem cells into
osteoblasts and stimulates the proliferation and maturation of
osteoblasts (22–24), DKK1 overexpression decreases the
levels of β-catenin and subsequently inhibits osteoblast
differentiation and induces apoptosis (24,25).
Target searches using computational algorithms have predicted that
DKK1 mRNA is a target of miR-335-5p with specific binding sites in
its 3′-UTR. The results of the present study indicated that the
protein but not the mRNA expression levels of DKK1 were
significantly elevated in the HG group compared with those in the
control group, which may be attributed to the post-transcriptional
regulation of miRNAs.
To verify whether miR-335-5p affects the function of
MC3T3-E1 osteoblasts by regulating the expression levels of DKK1,
these cells were subjected to vector-mediated overexpression of
miR-335-5p. Of note, transfection with miR-335-5p significantly
reduced the HG-induced increases of the apoptotic rate as well as
the protein expression of DKK1 and caspase-3 in MC3T3-E1 cells.
These results indicated that miR-335-5p may inhibit the apoptosis
of osteoblasts through downregulating the protein expression levels
of DKK1.
In conclusion, the present study demonstrated that
HG conditions reduced the expression of miR-335-5p in MC3T3-E1
osteoblasts and thereby upregulated the protein expression levels
of DKK1, ultimately inducing the cellular apoptosis. However,
overexpression of miR-335-5p partly reversed HG-induced DKK1
overexpression and osteoblast apoptosis. These findings provided a
basis for the upregulation of miR-335-5p as a means of prevention
and treatment of diabetic osteoporosis.
Acknowledgments
The present study was supported by the National Key
Clinical Specialties Construction Program of China and the Science
Foundation of Chongqing Municipal Heath Bureau (grant no.
2012-2-041).
References
|
1
|
Wittrant Y, Gorin Y, Woodruff K, Horn D,
Abboud HE, Mohan S and Abboud-Werner SL: High d(+)glucose
concentration inhibits RANKL-induced osteoclastogenesis. Bone.
42:1122–1130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 1:R17–R29. 2006. View Article : Google Scholar
|
|
4
|
Huntzinger E and Izaurralde E: Gene
silencing by microRNAs: Contributions of translational repression
and mRNA decay. Nat Rev Genet. 12:99–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ciesla M, Skrzypek K, Kozakowska M, Loboda
A, Jozkowicz A and Dulak J: MicroRNAs as biomarkers of disease
onset. Anal Bioanal Chem. 401:2051–2061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song L and Tuan RS: MicroRNAs and cell
differentiation in mammalian development. Birth Defects Res C
Embryo Today. 78:140–149. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davis BN and Hata A: Regulation of
MicroRNA Biogenesis: A miRiad of mechanisms. Cell Commun Signal.
7:182009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stern-Ginossar N, Elefant N, Zimmermann A,
Wolf DG, Saleh N, Biton M, Horwitz E, Prokocimer Z, Prichard M,
Hahn G, et al: Host immune system gene targeting by a viral miRNA.
Science. 317:376–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shu M, Zheng X, Wu S, Lu H, Leng T, Zhu W,
Zhou Y, Ou Y, Lin X, Lin Y, et al: Targeting oncogenic miR-335
inhibits growth and invasion of malignant astrocytoma cells. Mol
Cancer. 10:592011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Tu Q, Bonewald LF, He X, Stein G,
Lian J and Chen J: Effects of miR-335-5p in modulating osteogenic
differentiation by specifically downregulating Wnt antagonist DKK1.
J Bone Miner Res. 26:1953–1963. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Z, Wang B, Cao X, Du T and Zhang S:
Prediction of micro-RNA related to change of mouse osteoblast
function under simulated microgravity with bioinformatics method.
Space Med Med Eng. 25:407–411. 2012.In Chinese.
|
|
12
|
Sato N, Yamabuki T, Takano A, Koinuma J,
Aragaki M, Masuda K, Ishikawa N, Kohno N, Ito H, Miyamoto M, et al:
Wnt inhibitor Dickkopf-1 as a target for passive cancer
immunotherapy. Cancer Res. 70:5326–5336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Jackuliak P and Payer J: Osteoporosis,
fractures, and diabetes. Int J Endocrinol. 2014:8206152014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marie PJ: Osteoblast dysfunctions in bone
diseases: From cellular and molecular mechanisms to therapeutic
strategies. Cell Mol Life Sci. 72:1347–1361. 2015. View Article : Google Scholar
|
|
16
|
Li H, Xie S, Liu M, Chen Z, Liu X, Wang L,
Li D and Zhou Y: The clinical significance of downregulation of
mir-124-3p, mir-146a-5p, mir-155-5p and mir-335-5p in gastric
cancer tumorigenesis. Int J Oncol. 45:197–208. 2014.PubMed/NCBI
|
|
17
|
Cui XS, Sun SC, Kang YK and Kim NH:
Involvement of microRNA-335-5p in cytoskeleton dynamics in mouse
oocytes. Reprod Fertil Dev. 25:691–699. 2013. View Article : Google Scholar
|
|
18
|
Oger F, Gheeraert C, Mogilenko D, Benomar
Y, Molendi-Coste O, Bouchaert E, Caron S, Dombrowicz D, Pattou F,
Duez H, et al: Cell-specific dysregulation of microRNA expression
in obese white adipose tissue. J Clin Endocrinol Metab.
99:2821–2833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Zhu X, Cui J, Chen P, Wang S and
Wang J: Differential expressions of microRNA between young and
senescent endothelial cells. Chin Med J. 92:2205–2209. 2012.In
Chinense.
|
|
20
|
Ertekin S, Yıldırım O, Dinç E, Ayaz L,
Fidanci SB and Tamer L: Evaluation of circulating miRNAs in wet
age-related macular degeneration. Mol Vis. 20:1057–1066.
2014.PubMed/NCBI
|
|
21
|
Wang XJ, Feng ZP, Deng HC, Jiang R and Du
J: Effect of AP-1 on apoptosis of MC3T3-E1 osteoblast induced by
high-glucose. Zhong Hua Gu Zhi Shu Song He Gu Kuang Yan Ji Bing Za
Zhi. 7:258–262. 2014.In Chinese.
|
|
22
|
Li J, Sarosi I, Cattley RC, Pretorius J,
Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, et al:
Dkk1-mediated inhibition of Wnt signaling in bone results in
osteopenia. Bone. 39:754–766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morvan F, Boulukos K, Clément-Lacroix P,
Roman Roman S, Suc-Royer I, Vayssière B, Ammann P, Martin P, Pinho
S, Pognonec P, et al: Deletion of a single allele of the Dkk1 gene
leads to an increase in bone formation and bone mass. J Bone Miner
Res. 21:934–945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiang YW, Barlogie B, Rudikoff S and
Shaughnessy JD Jr: Dkk1-induced inhibition of Wnt signaling in
osteoblast differentiation is an underlying mechanism of bone loss
in multiple myeloma. Bone. 42:669–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang LF, Bai D and Han XL: Progress in
Dickkopf-1-mediated bone metabolism. J Clin Rehabil Tissue Eng Res.
17:337–341. 2013.In Chinese.
|