Introduction
Intervertebral disc (IVD) herniation is the major
cause of chronic sciatica and lower back pain (1). However, these severe symptoms can be
noticeably relieved in 70% of lumbar disc herniation patients
within 6 weeks of onset, while certain patients demonstrate a
decrease in the size, or disappearance, of the herniated disc by
magnetic resonance imaging (MRI) and computed tomography (CT)
(1–3). This natural resorption is more likely
to occur in extruded types, particularly in the sequestered type,
as the herniated disc is more easily exposed to the epidural
vascular supply by the presence of a tear in the posterior
longitudinal ligament (PLL) (1,4,5). The
mechanism of spontaneous resorption is associated with numerous
factors. It has been shown that infiltrating macrophages and newly
formed vessels promote the progression of spontaneous herniated
disc resorption (6). Tumor
necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), which are
released from macrophages after the onset of disc herniation, are
crucial in herniated disc resorption (7,8).
The apoptosis of IVD cells is increased more in
non-contained disc herniation than in contained disc herniation, in
which the herniated nucleus pulposus penetrates the PLL and is
exposed to the epidural space (9).
p38 mitogen-activated protein kinase (MAPK) is hypothesized to be
closely associated with inflammation and apoptosis (10,11).
Furthermore, increasing evidence suggests that activated p38 MAPK
induces apoptosis in the herniated disc (12,13).
However, the association between p38 MAPK and apoptosis in
herniated disc resorption remains to be clarified, thus the present
study hypothesized that the induction of herniated disc apoptosis
by p38 MAPK activation may be significant in spontaneous
resorption.
In the current study, the occurrence of apoptosis in
disc cells and the expression level of caspase-3 was examined in a
rat model of IVD herniation. In addition, the expressions of TNF-α,
IL-1β, p38 MAPK, and P-p38 (phosphorylated p38) was investigated.
These data may provide further insight into the underlying
mechanism of spontaneous resorption of lumbar disc herniation.
Materials and methods
Animals and materials
All experiments were approved by the Institutional
Animal Care and Use Committee (School of Pharmacy, East China
University of Science and Technology; Shanghai, China). A total of
30 male Sprague-Dawley rats, (weight, 230–300 g) were obtained from
the Shanghai Laboratory Animal Center Laboratory Animal Co., Ltd.
(Shanghai, China). SB203580 (a p38 MAPK inhibitor) was obtained
from Selleck Chemicals Co., Ltd. (Houston, TX, USA). The following
antibodies were used: Rabbit anti-human polyclonal IL-1β (1:1,000;
13082-1-AP; Proteintech Group, Inc., Chicago, IL, USA) and
polyclonal rabbit anti-human P-p38 (1:1,000; GWB-ASB336; GenWay
Biotech, Inc., San Diego, CA, USA), rabbit anti-human polyclonal
p38 MAPK (1:1,000; 33149; Signalway Antibody Co., Ltd., Maryland,
MD, USA), rabbit anti-human polyclonal TNF-α (1:1,000; 17590-1-AP;
Proteintech Group, Inc.) and rabbit anti-human polyclonal caspase-3
(1:1,000; 19677-1-AP; Proteintech Group, Inc.).
Animal model
The 30 rats were divided into control and p38i (p38
MAPK inhibition) groups in a 2:1 ratio. The rats were housed
separately in plastic cages in a pathogen-free environment. The
rats were fed sterile feed (Shanghai SLAC Laboratory Animal Co.,
Ltd., Shanghai, China) and were maintained under a 12 h light/dark
cycle. The control group was subdivided equally into contained and
non-contained groups according to different processing of the
discs. Two coccygeal IVDs, containing the nucleus pulposus, annulus
fibrosus and adjacent cartilage endplates, were obtained under 40
mg/kg intraperitoneal pentobarbital (Sigma-Aldrich, St. Louis, MO,
USA) from the rat tail. In the non-contained group, the cartilage
endplates were punctured with a needle and the harvested disc
material was weighed using the FA1004B Millionth Sophisticated
Analytical Balance (Shanghai Precision Instrument Co., Ltd.,
Shanghai, China) prior to autografting into the back muscle of the
rat. In the contained group, the discs were placed into the back
directly after noting the weight. In the p38i group, rats with
autografted non-contained discs were injected into the peritoneum,
with 10 mg/kg SB203580, daily from days 1 to 28 after surgery. In
the other two groups, rats received an injection of the same
quantity of saline. Five rats from each group were sacrificed by
overdose with pentobarbital sodium (200 mg/kg) for harvested disc
material at weeks 1 and 4 post-surgery.
Tissue processing
After recording the weight of the harvested discs,
one tissue specimen from each rat was prepared for histological
observation, immunohistochemistry and terminal deoxynucleotidyl
transferase dUTP nick end labeling (TUNEL) staining. All samples
were fixed in 10% neutral buffered formalin (YiYan Biological
Technology, Ltd., Shanghai, China) at room temperature overnight
and embedded in paraffin (YiYan Biological Technology, Ltd.). The
tissue specimens were sliced into 5 mm-thick paraffin sections in
the axial plane, using a microtome (BZ-600; BZ Technology Co.,
Ltd., Daventry, UK). Hematoxylin and eosin (H&E; Qianchen
Biological Technology Co., Ltd., Shanghai, China) staining was used
according to the standard method and the morphology of the
harvested discs was examined under a microscope (170BN; Wincom
Company Ltd., Changsha, China). The remaining tissue specimens were
prepared for western blot by mechanically pulverizing the tissue
with a pestle and mortar on ice and homogenizing in
phosphate-buffered saline (PBS; Sigma-Aldrich).
Western blot analysis
Protein concentrations were determined using the
bicinchoninic acid assay (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Proteins (50 µg) were resolved in 10% sodium
dodecyl sulfate-polyacrylamide gels (Beyotime Institute of
Biotechnology, Shanghai, China), then transferred onto
nitrocellulose membranes (GE Healthcare Life Sciences, Uppsala,
Sweden). The membrane was blocked with 5% nonfat milk in
Tris-buffered saline (Cell Signaling Technology, Inc., Danvers, MA,
USA) for 1 h at room temperature and incubated with the IL-1β,
TNF-α, p38 and P-p38 primary antibodies in dilution buffer
(Beyotime Institute of Biotechnology) overnight at 4°C. The
membranes were incubated with the goat anti-rabbit polyclonal
secondary antibody (1:10,000; 110806; Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA), alkaline phosphatase (AP;
Roche Diagnostics, Basel, Switzerland), conjugated with AP
containing nitro-blue tetrazolium
chloride/5-bromo-4-chloro-3-indolyl-phosphate [Meryer (Shanghai)
Chemical Technology Co., Ltd., Shanghai, China] at room temperature
for 10–20 min, and imaged using enhanced chemiluminescence (GE
Healthcare Life Sciences, Piscataway, NJ, USA). The X-ray films
(Kodak, Rochester, NY, USA) were scanned, and then the intensity of
each signal density was measured and analyzed using ImageJ
software, version 1.48 (National Institutes of Health, Bethesda,
MD, USA). β-actin served as the internal control for protein
loading.
Immunohistochemical staining
The tissue specimens were dewaxed with xylene
(Qianchen Biological Technology Co., Ltd.) and rehydrated using a
graded alcohol series. The endogenous peroxidase reactions were
quenched with 3% H2O2 (Qianchen Biological
Technology Co., Ltd.) for 10 min at room temperature. Then,
nonspecific binding was blocked with 5% normal bovine serum albumin
[Meryer (Shanghai) Chemical Technology Co., Ltd.] for 1 h at room
temperature. The specimens were washed three times with PBS after
incubation with the anti-caspase-3 primary antibodies overnight at
4°C. After incubation for 2 h with fluorescent-labeled secondary
antibodies, the specimens were washed another three times with PBS.
Subsequently, for marker staining, the specimens were incubated
with streptavidin-horseradish peroxidase [Meryer (Shanghai)
Chemical Technology Co., Ltd.] at room temperature for 2 h and
subsequently immersed in 3,3′-diaminobenzidine tetrachloride (Roche
Diagnostics) in the dark for 5–10 min. After counterstaining with
hematoxylin, the specimens were dehydrated with a graded series of
alcohol and examined under a light microscope (LB202; Leader
Precision Instrument Co., Ltd., Dongguan, China).
TUNEL staining
The tissue specimens were rehydrated and endogenous
peroxidase reactions were quenched (as mentioned above) and
incubated with proteinase K (Roche Diagnostics) for 15 min at 37°C.
The tissue specimens were then incubated with Equilibration Buffer
(Roche Diagnostics) for 10 min prior to incubation with BrightGreen
Labeling Mix (Roche Diagnostics) and TUNEL (Roche Diagnostics), for
1 h in the dark. Subsequent to three washes with distilled water,
the tissue specimens were examined for apoptosis under a
fluorescence microscope (LF302; Leader Precision Instrument Co.,
Ltd.).
Statistical analysis
Differences in the weight of the relocated discs
were determined by one-way analysis of variance, followed by the
Bonferroni post hoc test for multiple comparisons. Differences
between any two groups were analyzed using the unpaired Student's
t-test or the Mann-Whitney test as appropriate and P<0.05
was considered to indicate a statistically significant difference.
SPSS software, version 18.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis.
Results
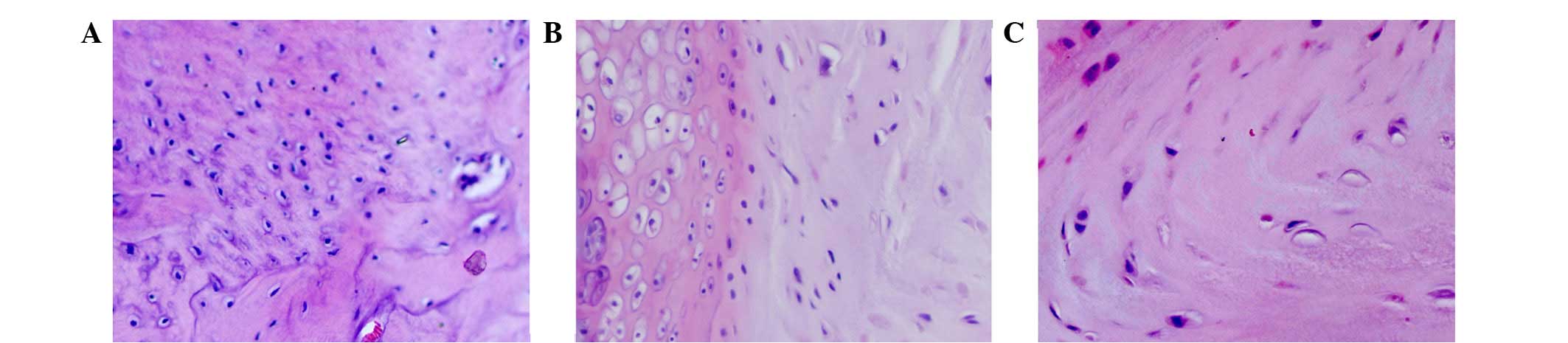
Histological changes
H&E-stained sections were examined under a
microscope. As shown in Fig. 1,
morphological changes in the annulus fibrosus and eosinophilic
staining were not observed in contained disc tissues, but were
apparent in the p38i group and were more evident in the
non-contained group. The extracellular matrix and collagen fibers
were disordered in the non-contained group, and were accompanied by
neovascularization and inflammatory cell infiltration, including
the presence of macrophages in the relocated discs.
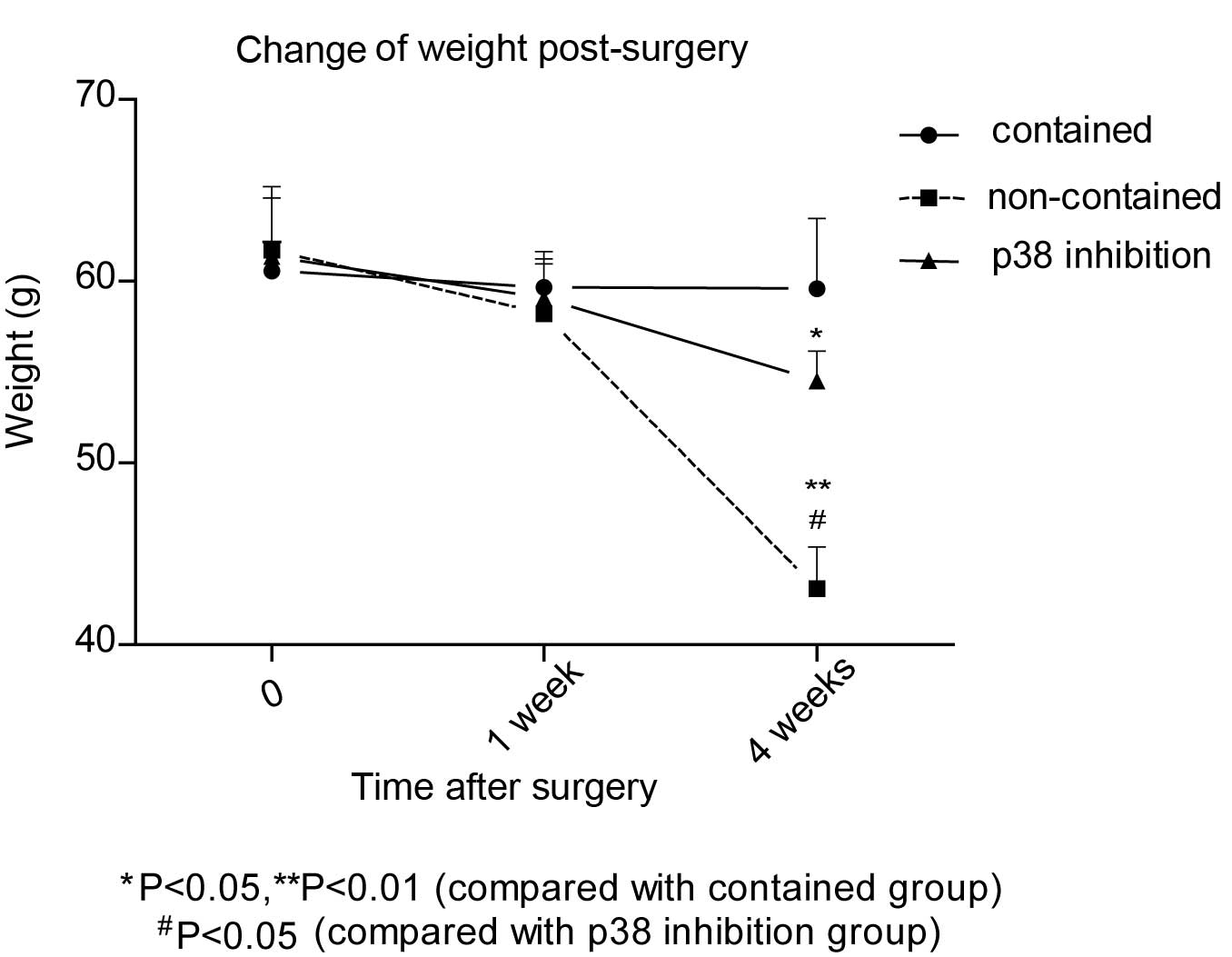
Weight change
The weight of relocated discs was examined prior to
disc cell relocation and following sacrifice. The weight in the
non-contained group decreased significantly at week 4 compared with
week 1 and the time of surgery (P<0.05). However, in the
contained and p38i groups (Fig.
2), there was no significant difference in disc weight between
any of the three time-points. This indicated that the weight of the
relocated disc in the non-contained group decreased more markedly
than the other two groups as time progressed.
TNF-α and IL-1β expression levels
The expression levels of TNF-α and IL-1β were
observed in the three groups by western blot analysis (Fig. 3). The expression levels of TNF-α
and IL-1β were markedly higher in the non-contained and p38i groups
compared with the contained group at week 1, however, at week 4 the
difference was reduced. In the p38i group, the expression levels of
TNF-α and IL-1β decreased gradually over time.
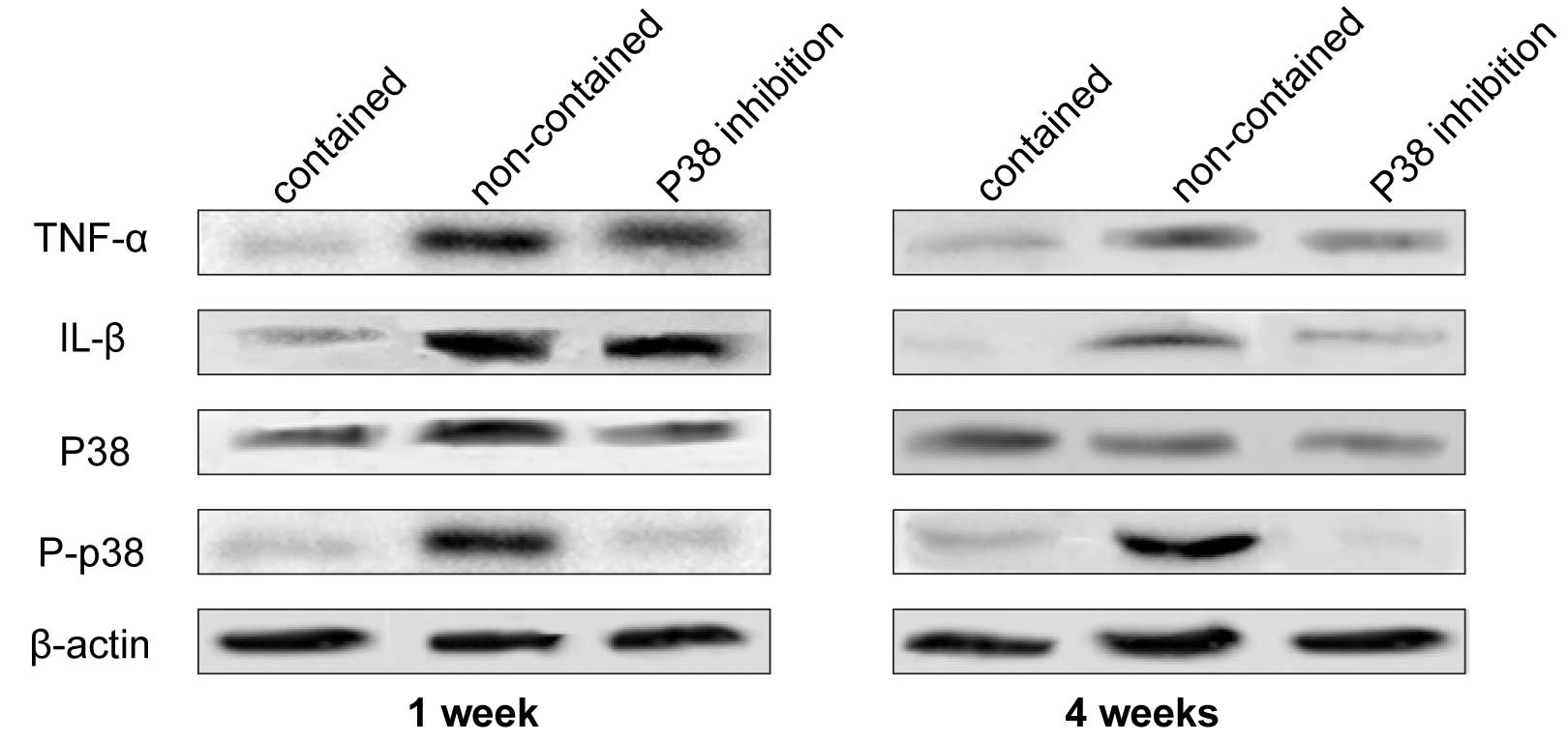 | Figure 3Western blots were generated and
probed for TNF-α, IL-1β, p38 MAPK, and p-p38 MAPK at weeks 1 and 4.
In the non-contained group, TNF-α, IL-1β, and p38 MAPK exhibited
high expression levels, which were not observed in the contained
group. The expression level of p38 MAPK was inhibited in the
presence of SB203580, indicating that TNF-α and IL-1β promoted p38
expression in this condition. Additionally, the expression levels
of TNF-α and IL-1β were suppressed by SB203580 treatment. TNF-α,
tumor necrosis factor-α; IL-1β, interleukin-1β; MAPK,
mitogen-activated protein kinase; P, phosphorylated. |
Activation of p38 MAPK
A high expression level of P-p38 was observed in the
non-contained group, indicating that p38 phosphorylation was
significantly suppressed by p38i. However, there was almost no
difference in the expression intensity of non-P-p38 between all
three groups at weeks 1 and 4 (Fig.
3).
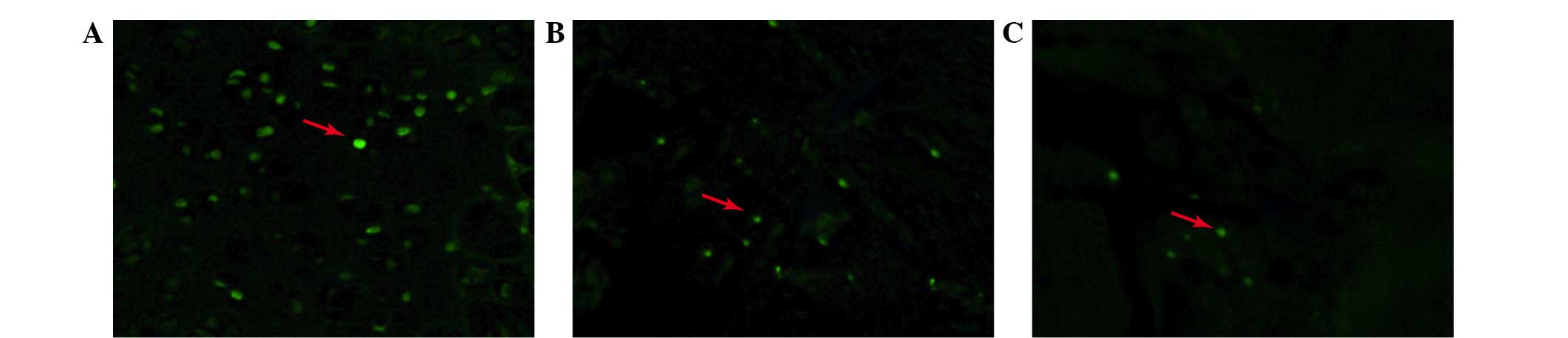
Apoptosis of nucleus pulposus cells
TUNEL staining was performed to detect the presence
of apoptotic cells in the relocated discs. As shown in Fig. 4, the apoptotic percentage differed
between each group and was highest in the non-contained group.
Apoptosis in the p38i group was less than that in the non-contained
group, however, was more than in the contained group.
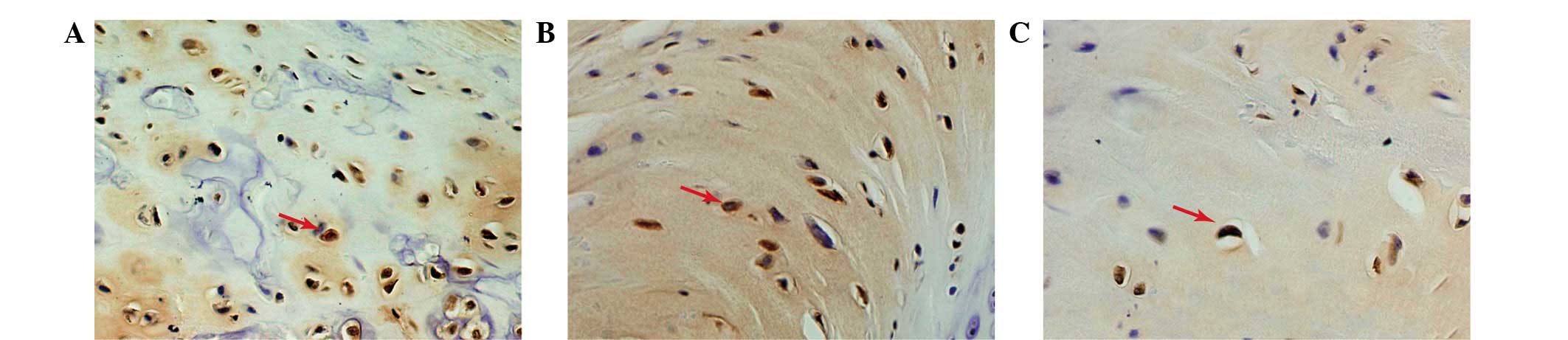
Caspase-3 expression
Immunohistochemical staining was performed to
evaluate the expression of caspase-3 in the relocated discs.
Caspase-3 staining demonstrated different trends in the three
groups (Fig. 5). The majority of
the caspase-3-positive disc cells were observed in the
non-contained group disc tissue. The expression of caspase-3 in the
p38i group was higher than in the contained group.
Discussion
With developments in imaging techniques, such as MRI
and CT, there has been an increase in the reporting of the
disappearance or decreases in size of herniated IVDs (14). Regression is more significant in
extruded and sequestrated herniated discs. Disc migration is a
subtype of disc extrusion, where the herniation is exposed to the
epidural space, as well as transligamentous herniation. Komori
et al (1) identified that
the complete resolution rate was higher in a migration group when
compared with a non-migration group (41 vs. 0%). Various hypotheses
have been proposed to explain the mechanism by which spontaneous
resorption occurs. One hypothesis is that, in disc bulges and
protrusions, the herniation may retract back into the parent disc
(15,16). A second is that dehydration
promotes disc regression due to a higher MRI T2 signal intensity,
as higher regression rates have previously been reported (17,18).
Disc herniation into the epidural space causes an inflammatory
reaction and neovascularization, resulting in the absorption of the
herniated disc by phagocytosis and enzymatic degradation (19). In addition to these hypotheses, the
apoptosis of disc cells has received greater attention, as evidence
indicates that a higher degree of apoptotic disc cells is present
in non-contained discs when compared with contained discs,
suggesting that apoptosis of disc cells may be another mechanism in
spontaneous resorption (20).
In the current study, an experimental rat model of
disc resorption was proposed, which was modified based on a
previously described method (21,22).
The changes in disc weight and morphological structure indicated
that this model appropriately simulated the sequestrated type of
human disc herniation, in which spontaneous resorption is most
likely to occur. In the non-contained model, newly formed vessels
and macrophages easily infiltrated into the exposed disc tissues
when the cartilage endplate was punctured with a needle. However,
the infiltration was not obvious in the contained model, as the
disc tissue is isolated from the blood supply and immune system.
The decrease in weight of the relocated IVD may indicate
spontaneous regression in vivo (8,23),
thus this decrease demonstrated that the needle puncture model
adopted was effective, simple and practical in the mechanistic
investigation of spontaneous regression by simulating extruded and
sequestrated intervertebral disc herniation.
The MAPK signaling pathway family acts as a major
kinase pathway, which regulates numerous physiological activities
in cells, such as inflammation, metabolic balance, and apoptosis
(24). Previous studies have
demonstrated that when the IVD was relocated in vivo, the
autografts induced TNF-α and IL-1β mRNA upregulation, rapidly
followed by macrophage infiltration (25). The phosphorylation of p38 MAPK, an
important component of the MAPK family, was closely associated with
the secretion and accumulation of proinflammatory factors. p38 MAPK
can be activated by TNF-α and IL-1β through numerous signaling
pathways, such as apoptosis-stimulating kinase and transforming
growth factor-β-activated kinase (26–28).
P-p38 MAPK in nucleus pulposus cells was markedly increased after
cells were exposed to TNF-α or IL-1β, and the mRNA expression
levels of TNF-α and IL-1β were downregulated by p38 inhibition
(29,30). Furthermore, IL-1β and TNF-α may
stimulate herniated disc nucleus pulposus cells to produce
prostaglandin E2, IL-6 and matrix metalloproteinase-3 (MMP-3),
which were closely associated with disc degeneration, but were
decreased when p38 MAPK was inhibited. In a previous study, p38
MAPK inhibition increased the ratio of tissue inhibitor of
metalloproteinases metallopeptidase inhibitor 1 to MMP-3 in
vitro when activated by IL-1β or TNF-α, subsequently
influencing the degradation of the extracellular matrix of nucleus
pulposus cells (29).
In the present study, the expression levels of TNF-α
and IL-1β were observed after the IVD was implanted in the
non-contained group, accompanied by the high expression level of
P-p38 MAPK. However, in the contained group, these proinflammatory
factors were almost undetectable, which may have been due to the
resulting isolation between the relocated disc, and the now
segregated blood supply. Furthermore, P-p38 MAPK was expressed at a
moderately low level. In addition, the current study demonstrated
that the P-p38 MAPK was suppressed significantly by SB203580 in the
p38i group. It has been previously shown that p38 MAPK is activated
by TNF-α and IL-1β secretion from macrophages or IVD tissue when
the herniated disc penetrates the PLL (31).
p38 MAPK induces apoptosis through various signaling
pathways. Cai et al (12)
found that the phosphorylation of BimEL, a member of the
Bcl-2 family, on Ser-65 may be a common regulatory point for cell
death induced by the c-Jun N-terminal kinase and p38 MAPK signaling
pathways. Hsu et al (32)
proposed that receptor engagement activates p38α to promote
apoptosis by the induction of Fas ligand (FasL) expression. In
addition, p38 MAPK activation induces the activation of caspases,
such as caspase-3, and apoptosis via the Fas-mediated death pathway
(33). Regarding disc cells,
Rannou et al (13)
suggested that p38 MAPK signaling is crucial in the process of
annulus fibrosus cell apoptosis during mechanical overload.
Apoptotic cells are induced by the Fas-mediated death pathway, in
which p38α MAPK activation increases the expression of Fas and FasL
proteins, as well as caspase activation (33). In herniated discs, the apoptosis of
disc cells following herniation differs depending on the type of
herniation, which is higher in non-contained discs when compared
with contained discs (9). When IVD
cells undergo apoptosis, they are phagocytosed by macrophages and
disc cells, including neighboring cells within cell clusters
(34).
The present study found that the expression ratio of
apoptotic cells and caspase-3 in the non-contained group was
significantly higher than that of the contained group, however,
this advantage was suppressed by p38 MAPK inhibition. Thus,
following activation by TNF-α and IL-1β, P-p38 MAPK induces
apoptosis of disc cells via a mechanism that remains unknown.
In conclusion, p38 MAPK was found to be involved in
the process of spontaneous resorption by the induction of apoptosis
in disc cells in a rat model of IVD herniation. To the best of our
knowledge, this finding is the first direct evidence of the
involvement of p38 MAPK in spontaneous resorption of IVDs.
Acknowledgments
The present study was supported by the National
Natural Science Funds of China (grant no. 81473691).
References
|
1
|
Komori H, Shinomiya K, Nakai O, Yamaura I,
Takeda S and Furuya K: The natural history of herniated nucleus
pulposus with radiculopathy. Spine (Phila Pa 1976). 21:225–229.
1996. View Article : Google Scholar
|
|
2
|
Yu PF, Jiang FD, Liu JT and Jiang H:
Outcomes of conservative treatment for ruptured lumbar disc
herniation. Acta Orthop Belg. 79:726–730. 2013.
|
|
3
|
Haro H: Translational research of
herniated discs: Current status of diagnosis and treatment. J
Orthop Sci. 19:515–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maigne JY, Rime B and Deligne B: Computed
tomographic follow-up study of forty-eight cases of nonoperatively
treated lumbar intervertebral disc herniation. Spine (Phila Pa
1976). 17:1071–1074. 1992. View Article : Google Scholar
|
|
5
|
Ahn SH, Ahn MW and Byun WM: Effect of the
transligamentous extension of lumbar disc herniations on their
regression and the clinical outcome of sciatica. Spine (Phila Pa
1976). 25:475–480. 2000. View Article : Google Scholar
|
|
6
|
Minamide A, Tamaki T, Hashizume H, Yoshida
M, Kawakami M and Hayashi N: Effects of steroid and
lipopolysaccharide on spontaneous resorption of herniated
intervertebral discs: An experimental study in the rabbit. Spine
(Phila Pa 1976). 23:870–876. 1998. View Article : Google Scholar
|
|
7
|
Haro H, Crawford HC, Fingleton B,
Shinomiya K, Spengler DM and Matrisian LM: Matrix
metalloproteinase-7-dependent release of tumor necrosis
factor-alpha in a model of herniated disc resorption. J Clin
Invest. 105:143–150. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshida M, Nakamura T, Sei A, Kikuchi T,
Takagi K and Matsukawa A: Intervertebral disc cells produce tumor
necrosis factor alpha, interleukin-1beta and monocyte
chemoattractant protein-1 immediately after herniation: An
experimental study using a new hernia model. Spine (Phila Pa 1976).
30:55–61. 2005. View Article : Google Scholar
|
|
9
|
Ha KY, Koh IJ, Kirpalani PA, Kim YY, Cho
YK, Khang GS and Han CW: The expression of hypoxia inducible
factor-1alpha and apoptosis in herniated discs. Spine (Phila Pa
1976). 31:1309–1313. 2006. View Article : Google Scholar
|
|
10
|
Lee JC, Laydon JT, McDonnell PC, Gallagher
TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR and
Landvatter SW: A protein kinase involved in the regulation of
inflammatory cytokine biosynthesis. Nature. 372:739–746. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai B, Chang SH, Becker EB, Bonni A and
Xia Z: p38 MAP kinase mediates apoptosis through phosphorylation of
BimEL at Ser-65. J Biol Chem. 281:25215–25222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rannou F, Lee TS, Zhou RH, Chin J, Lotz
JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A and Shyy JY:
Intervertebral disc degeneration: The role of the mitochondrial
pathway in annulus fibrosus cell apoptosis induced by overload. Am
J Pathol. 164:915–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cvetanovich GL, Hsu AR, Frank RM, An HS
and Andersson GB: Spontaneous resorption of a large cervical
herniated nucleus pulposus. Am J Orthop (Belle Mead NJ).
43:E140–E145. 2014.
|
|
15
|
Teplick JG and Haskin ME: Spontaneous
regression of herniated nucleus pulposus. Am J Roentgenol.
145:371–375. 1985. View Article : Google Scholar
|
|
16
|
Sari H, Akarirmak U, Karacan I and Akman
H: Computed tomographic evaluation of lumbar spinal structures
during traction. Physiother Theory Pract. 21:3–11. 2005. View Article : Google Scholar
|
|
17
|
Splendiani A, Puglielli E, De Amicis R,
Barile A, Masciocchi C and Gallucci M: Spontaneous resolution of
lumbar disk herniation: Predictive signs for prognostic evaluation.
Neuroradiology. 46:916–922. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Henmi T, Sairyo K, Nakano S, Kanematsu Y,
Kajikawa T, Katoh S and Goel VK: Natural history of extruded lumbar
intervertebral disc herniation. J Med Invest. 49:40–43.
2002.PubMed/NCBI
|
|
19
|
Rätsep T, Minajeva A and Asser T:
Relationship between neovascularization and degenerative changes in
herniated lumbar intervertebral discs. Eur Spine J. 22:2474–2480.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JB, Kim KW, Han CW and Chang H:
Expression of fas receptor on disc cells in herniated lumbar disc
tissue. Spine (Phila Pa 1976). 26:142–146. 2001. View Article : Google Scholar
|
|
21
|
Meng W, Yonenobu K, Ariga K, Nakase T,
Okuda S, Obata K and Yoshikawa H: Localization of cathepsins G and
L in spontaneous resorption of intervertebral discs in a rat
experimental model. J Musculoskelet Neuronal Interact. 2:171–176.
2001.
|
|
22
|
Geiss A, Larsson K, Rydevik B, Takahashi I
and Olmarker K: Autoimmune properties of nucleus pulposus: An
experimental study in pigs. Spine (Phila Pa 1976). 32:168–173.
2007. View Article : Google Scholar
|
|
23
|
Zhou G, Dai L, Jiang X, Ma Z, Ping J, Li J
and Li X: Effects of human midkine on spontaneous resorption of
herniated intervertebral discs. Int Orthop. 34:103–108. 2010.
View Article : Google Scholar :
|
|
24
|
Muthuswamy R, Jenkins F, Bovbjerg D and
Kalinski P: Synergistic induction of cancer-related
immunosuppression by β2-adrenergic stress mediators and P38MAPK
inflammatory pathway. J Immunother Cancer. 1(Suppl 1): 1912013.
View Article : Google Scholar
|
|
25
|
Takada T, Nishida K, Maeno K, Kakutani K,
Yurube T, Doita M and Kurosaka M: Intervertebral disc and
macrophage interaction induces mechanical hyperalgesia and cytokine
production in a herniated disc model in rats. Arthritis Rheum.
64:2601–2610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kimura N, Matsuo R, Shibuya H, Nakashima K
and Taga T: BMP2-induced apoptosis is mediated by activation of the
TAK1-p38 kinase pathway that is negatively regulated by Smad6. J
Biol Chem. 275:17647–17652. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ichijo H, Nishida E, Irie K, ten Dijke P,
Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K and Gotoh
Y: Induction of apoptosis by ASK1, a mammalian MAPKKK that
activates SAPK/JNK and p38 signaling pathways. Science. 275:90–94.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki K, Hino M, Kutsuna H, Hato F,
Sakamoto C, Takahashi T, Tatsumi N and Kitagawa S: Selective
activation of p38 mitogen-activated protein kinase cascade in human
neutrophils stimulated by IL-1beta. J Immunol. 167:5940–5947. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Studer RK, Aboka AM, Gilbertson LG,
Georgescu H, Sowa G, Vo N and Kang JD: P38 MAPK inhibition in
nucleus pulposus cells: A potential target for treating
intervertebral disc degeneration. Spine (Phila Pa 1976).
32:2827–2833. 2007. View Article : Google Scholar
|
|
30
|
Kakutani K, Pichika R, Yoshikawa T, et al:
P38 MAPK inhibition has a positive effect on human and rabbit
intervertebral disc degeneration: SP49. (C)/Spine Journal Meeting
Abstracts. LWW:1312010.
|
|
31
|
Cheng X, Ni B, Zhang Z, Liu Q, Wang L,
Ding Y and Hu Y: Polyol pathway mediates enhanced degradation of
extracellular matrix via p38 MAPK activation in intervertebral disc
of diabetic rats. Connect Tissue Res. 54:118–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu SC, Gavrilin MA, Tsai MH, Han J and
Lai MZ: P38 mitogen-activated protein kinase is involved in Fas
ligand expression. J Biol Chem. 274:25769–25776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu WH, Cheng YC and Chang LS:
ROS-mediated p38alpha MAPK activation and ERK inactivation
responsible for upregulation of Fas and FasL and autocrine
Fas-mediated cell death in Taiwan cobra phospholipase A (2)-treated
U937 cells. J Cell Physiol. 219:642–651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jones P, Gardner L, Menage J, Williams GT
and Roberts S: Intervertebral disc cells as competent phagocytes in
vitro: Implications for cell death in disc degeneration. Arthritis
Res Ther. 10:R862008. View
Article : Google Scholar : PubMed/NCBI
|