Introduction
Epstein-Barr virus (EBV) infection contributes to
human carcinogenesis, particularly in the development of lymphoma,
nasopharyngeal cancer (NPC) and gastric cancer (1). EBV is a γ-herpes virus and infects B
cells of the immune system and epithelial cells. EBV latently
persists in the host B cells for life (2) and thus, EBV is estimated to infect
>90% of the population worldwide (2). In a small number of infected cell
populations, EBV infection can transform B cells to lymphoma cells
(3) or epithelial cells to
nasopharyngeal cancer cells (4).
Furthermore, ~10% of gastric cancer worldwide was associated with
EBV infection and this subtype of gastric cancer is termed
EBV-associated gastric cancer (EBVaGC) (5,6).
However, the molecular mechanism by which EBV infection causes
these malignancies remains to be defined.
Molecularly, the BamHI-A rightward frame 1
(BARF1) is an EBV gene that is expressed early in the EBV lytic
cycle and shares 38% primary amino acid sequence homology with the
bcl-2 proto-oncogene product (7).
The constitutive expression of BARF1 protein was able to
immortalize lymphoblast cells and prolong cell survival (8). The carcinogenic activity of the
oncogene c-myc is obligatory for the development of Burkitt
lymphoma and previous studies suggested that BARF1 expression is
required to inhibit c-myc-induced apoptosis and exhibits a synergic
role in mediating the effect of Bcl-2 and c-myc during B cell
transformation (9–13). BARF1 protein is also involved in
the regulation of microvessel density (MVD) and micro-lymphatic
vessel density (MLVD) (14,15).
Furthermore, MVD and MLVD are strongly associated with cancer
metastasis and with the survival of cancer patients (14). In addition, during EBV latency,
there are >8 EBV encoded proteins and several non-coding RNAs
expressed in cells (e.g., two EBV encoded small RNAs, termed EBER1
and EBER2), nuclear antigens and membrane proteins (16). Latent membrane protein 1 (LMP1) and
LMP2A are two EBV latent membrane proteins, which function as
constitutively active receptors independent of ligand binding and
manipulate the signaling pathways for B cell activation and
differentiation in order to sustain the long-life of EBV-positive
cells (17). The carboxyl terminus
of LMP1 contains consensus tumor necrosis factor
receptor-associated factor (TRAF)-binding domains, which can
constitutively activate signal transducers and activators of
transcription (STAT), janus kinase (JNK), and nuclear factor
(NF)-κB pathways for cell survival and growth (18). Although, the oncogenic role of LMP1
is well established, its roles in microvessel and micro-lymphatic
vessel formation are less clear. Thus, the present study assessed
the EBV infection in NPC and gastric cancer tissue samples, and
then analyzed the levels of MVD and MLVD to identify their
association with the clinicopathological features of the patients.
This study may provide a novel insight into the oncogenic role of
EBV in NPC and gastric cancer.
Patients and methods
Patients and tissue samples
A total of 600 gastric cancer and 75 NPC tissues
were collected. All tumor tissue specimens were histologically
confirmed and retrieved from the Department of Pathology, The
Shandong Provincial Institute of Cancer Prevention and Research
(Jinan, China) and The Affiliated Hospital of Jining Medical
College (Jining, China) between 2008 and 2012. The present study
was approved by the Institutional review boards of The Shandong
Provincial Institute of Cancer Prevention and Research and The
Affiliated Hospital of Jining Medical College. All patients
provided informed consent to participate in this study.
Tissue specimens from each patient were fixed in 10%
buffered formalin and embedded in paraffin (Shijiazhuang Chemical
Technology Co., Ltd., Shijiazhuang, China), and then cut into
serial sections (5 µm). One of the consecutive sections was
stained with hematoxylin and eosin (H&E; Shanghai Biological
Technology Co., Ltd., Shanghai, China) for confirmation of
diagnosis, while others were subjected to in situ
hybridization to detect EBV RNA or immunohisto-chemistry to detect
the expression of LMP1, BARF1, vascular endothelial growth factor-C
(VEGF-C), lymphatic vessel endothelial hyaluronan receptor-1
(LYVE-1) and CD34.
In situ hybridization and
immunohistochemistry
For in situ hybridization, an ISH-5022 EBER
kit was used (Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd.; OriGene Technologies, Inc., Beijing, China). A labeled
oligonucleotide probe complementary to EBER1 was used to detect the
EBER1 as positive REBV infection. Briefly, tissue sections were
deparaffinized and rehydrated, and then hybridized to a labeled
probe according to the manufacturer's instructions. Tissue sections
without probe hybridization were used as negative controls. For
immunohistochemistry, tissue sections were deparaffinized by 2
times incubation in xylenol for 10 min at room temperature and
dehydrated in a series of ethanol (100, 75 and 50%). Sections were
then incubated in 3% H2O2 for 10 min at room
temperature and subsequently subjected twice to antigen repair in
0.01 M acid buffer (pH 6.0) using a microwave at 92–98°C for 5 min,
with a 10 min break between incubations. Sections were washed in
phosphate-buffered saline (PBS), incubated with normal goat serum
for 15 min at room temperature, and further incubated with primary
antibodies at 4°C overnight. The primary antibodies used were
rabbit monoclonal anti-LMP1 (cat. no. ZM-0386), rabbit monoclonal
anti-BHRF1 (cat. no. ZA-0627), rabbit monoclonal anti-VEGF-C (cat.
no. ZA-0266), mouse monoclonal anti-LYVE-1 (cat. no. ZA-0483) and
mouse monoclonal anti-CD34 (cat. no. ZM-0046) (dilution used for
all was 1:200; Zhongshan Golden Bridge Biotechnology Co., Ltd.;
OriGene Technologies, Inc.). The sections were washed 3 times with
PBS and incubated with biotinylated anti-mouse (cat. no. BA1001) or
anti-rabbit (cat. no. BA1003) secondary antibodies (dilution used
for all was 1:200; Boster Biological Technology, Ltd., Beijing,
China) for 30 min at room temperature, and subsequently visualized
by incubation of tissue sections with a 3,3′-diaminobenzidine
solution (Zhongshan Golden Bridge Biotechnology Co., Ltd.; OriGene
Technologies, Inc.). Sections were counterstained with H&E,
mounted with a coverslip and visualized using the Olympus CX23
light microscope (Olympus Corporation, Tokyo, Japan).
Review and scoring of stained tissue
sections
All tissue samples were reviewed and scored blindly
by two pathologists, and the pathology of each tissue section was
confirmed. To assess the in situ hybridization data,
negative controls were indicated as the sections without any
staining, and positive controls were indicated as the stained
sections with appropriate nuclear localization. To score
immunohistochemical data, the two pathologists reviewed at least
ten x400 fields and counted staining intensity and percentage of
positive cells vs. total cells. The staining intensity was judged
as no staining, −; light brown, +; brown, ++; and strong brown,
+++. The percentage of positive cells <10% was considered as
negative and the detailed information is as follows: <10%, −;
11–25%, +; 26–50%, ++; >50%, +++. These two scores were added
together to form a total score, high (score ≤2+) vs. low expression
of a protein.
Quantitative measurement of MVD and MLVD
levels in tissue specimens
MVD and MLVD were visualized using immunostaining of
the tissue sections with CD34 and LYE-1 antibodies, respectively,
to perform morphometric analysis. The stained sections were
reviewed and scored by two pathologists in a blind manner, under a
CX23 light microscope. Briefly, the pathologists reviewed the
sections under a ×100 magnification and then selected 5 high power
fields (×400 magnification) to capture images. CD34 immunostaining
was used to visualize microvascular endothelial cells, while LYVE-1
is specifically expressed in microlymphatic endothelial cells.
Under the CX23 light microscope, any brown colored endothelial
cells or microvascular endothelial cells observed in a cluster was
counted as one microvessel. Their branches were also counted as a
microvessel as long as the structures were not connected. However,
if the lumen had >8 red blood cells or the vessel had a muscular
and vascular wall, this vessel was not counted. MVD was calculated
as the mean number of microvessels from five ×200 microscopic
fields. Furthermore, micro-lymphatic vessels were labeled by
anti-LYVE-1 antibody and reviewed and counted as same as MVD.
Statistical analysis
All statistical analyses were performed using SPSS
software, version 18.0 (SPSS Inc., Chicago, IL, USA). All data were
analyzed using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference. Count data were
presented as frequency and percentage, and analyzed by
χ2 analysis or Fisher's exact test. Measurement data
were presented as the mean/median ± standard deviation. Data with
normal distribution were analyzed by an F-test or t-test. Data with
uncommon distribution were analyzed by a non-parametric test or
Wilcoxon rank test.
Results
EBV infection is associated with
clinicopathological characteristics of patients
EBV infection has been linked to EBVaGC and NPC
(5,19); thus, in situ hybridization
was performed to detect EBV-encoded small RNA 1 (EBER1) as the
indication of EBV infection. Among the 600 gastric cancer tissue
specimens, 30 positive cases of EBV infection were identified
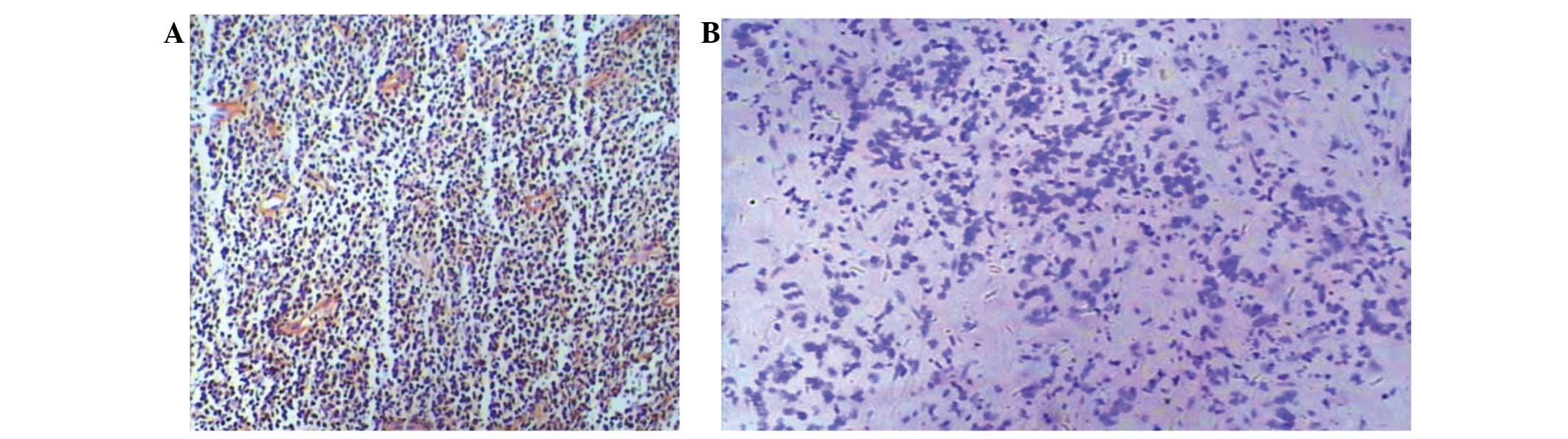
(Fig. 1A and Table I). Compared with negative staining,
ISH positive particles had deep purple blue staining present in the
nuclei (Fig. 1B). EBV infection
was shown to be correlated with the age of the patient (P=0.073),
tumor differentiation (P<0.0001), tumor location (P<0.0001)
and lymph node metastasis (P<0.0001; Table I). The data suggest that EBV
infection only occurs in a small percentage of gastric cancers
(5%).
 | Table IAssociation of EBV infection with
clinicopathological characteristics from patients with gastric
cancer. |
Table I
Association of EBV infection with
clinicopathological characteristics from patients with gastric
cancer.
| Factor | No. of cases
(n=600) | No. of EBV-positive
cases (n=30) | No. of EBV-negative
cases (n=570) | χ2 | P-value |
|---|
| Gender | | | | | 0.049 |
| Male | 384 | 25 | 359 | 3.87 | |
| Female | 216 | 5 | 211 | | |
| Age (years) | | | | | 0.073 |
| <45 | 32 | 4 | 32 | 2.24 | |
| 45–60 | 223 | 13 | 210 | | |
| >60 | 345 | 13 | 334 | | |
| Tumor
differentiation | | | | | <0.0001 |
| Well | 68 | 8 | 60 | 15.69 | |
| Moderate | 124 | 12 | 112 | | |
| Poor | 408 | 10 | 398 | | |
| Tumor location | | | | | <0.0001 |
| Gastric cardia
region | 102 | 12 | 90 | 14.99 | |
| Gastric body
region | 139 | 10 | 129 | | |
| Gastric antral
region | 359 | 8 | 351 | | |
| Lymph node
metastasis | | | | | 0.0001 |
| Yes | 580 | 21 | 559 | 40.39 | |
| No | 20 | 9 | 11 | | |
Furthermore, in 75 patients with NPC, 61 patients
were positive for EBV infection (Table II). EBV infection was not shown to
be correlated with any of the clinicopathological characteristics
investigated (Table II).
 | Table IIAssociation of EBV infection with
clinicopathological characteristics of patients with nasopharingeal
carcinoma. |
Table II
Association of EBV infection with
clinicopathological characteristics of patients with nasopharingeal
carcinoma.
| Factor | No. of cases
(n=75) | No. of EBV-positive
cases (n=61) | No. of
EBV-negativecases (n=14) | χ2 | P-value |
|---|
| Gender | | | | | 0.79 |
| Male | 59 | 50 | 9 | 0.070 | |
| Female | 16 | 11 | 5 | | |
| Age (years) | | | | | 0.311 |
| <45 | 17 | 14 | 3 | 2.333 | |
| 45–60 | 36 | 35 | 1 | | |
| >60 | 22 | 11 | 11 | | |
| Pathology | | | | | |
| Keratinizing
squamous cell carcinoma | 8 | 7 | 10 | 0.016 | 0.90 |
| Non-keratinizing
carcinoma | 67 | 54 | 24 | | |
Expression of the EBV-associated proteins
LMP1 and BHFR1 is correlated with MLVD rather than MVD in patients
with EBVaGC
Expression of the EBV-associated proteins LMP1 and
BHFR1 and markers of MVD and MLVD (CD34 and LYVE-1) was then
further analyzed in the tissue specimens. Morphometric image
analysis of CD34 and LYVE-1 was used to visualize MVD and MLVD. The
data showed that MVD and MLVD were not associated with TNM stage
and lymph node metastasis in patients with EBVaGC (Table III). In the 30 patients with
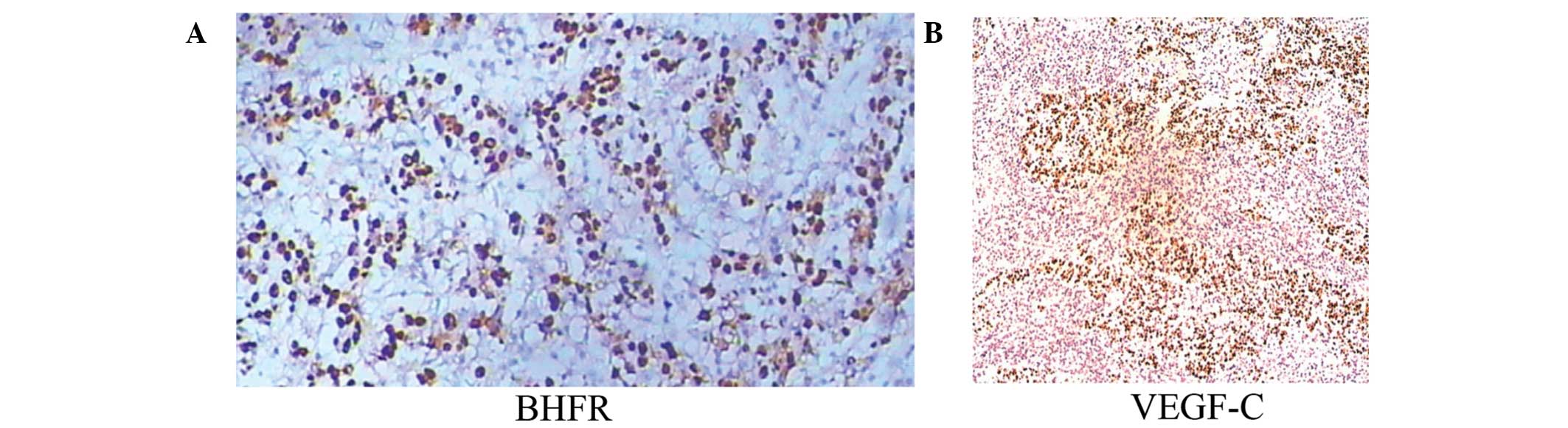
EBVaGC, only one case showed weak LMP1 expression, but 17 cases
(56.7%) showed BARF1 expression (Fig.
2 and Table IV). BARF1
expression was significantly associated with lymph node metastasis
of EBVaGC. Among the 30 patients with EBVaGC, 18 patients (60%)
were VEGF-C positive (data not shown). The expression of VEGF-C was
not associated with lymph node metastasis. BARF1 expression was
associated with MLVD but not MVD. These data suggest that EBV could
infect lymphatic vessels and induce micro-lymphatic vessel
formation. Expression of VEGF-C was associated with MVD and MLVD
(data not shown).
 | Table IIIAssociation of MVD and MLVD levels
with clinicopathological characteristics of patients with
Epstein-Barr-associated gastric cancer. |
Table III
Association of MVD and MLVD levels
with clinicopathological characteristics of patients with
Epstein-Barr-associated gastric cancer.
| Factor | n | MVD | P-value | MLVD | P-value |
|---|
| Gender | | | 0.408 | | 1.000 |
| Male | 25 | 32±2.3 | | 3.1±0.9 | |
| Female | 5 | 33±3.1 | | 3.1±1.4 | |
| TNM stage | | | 0.402 | | 0.128 |
| I/II | 10 | 32±3.1 | | 2.5±0.9 | |
| III/IV | 20 | 33±3.0 | | 3.5±1.9 | |
| Lymph node
metastasis | | | 0.111 | | 1.000 |
| Negative | 9 | 32±3.4 | | 3.2±0.9 | |
| Positive | 21 | 34±2.9 | | 3.0±1.7 | |
 | Table IVAssociation of BARF1 and VEGF-C
expression with MVD and MLVD level in Epstein-Barr-associated
gastric cancer. |
Table IV
Association of BARF1 and VEGF-C
expression with MVD and MLVD level in Epstein-Barr-associated
gastric cancer.
| Expression | n | MVD | P-value | MLVD | P-value |
|---|
| BARF1 | | | 1.000 | | 0.000 |
| Negative | 13 | 29±3.1 | | 1.0±0.6 | |
| Positive | 17 | 29±2.3 | | 3.4±0.9 | |
| VEGF-C | | | 0.000 | | 0.000 |
| Negative | 12 | 23±4.8 | | 1.1±0.8 | |
| Positive | 18 | 31± 4.1 | | 3.1±0.4 | |
Expression of the EBV-associated proteins
LMP1 and BHFR1 was correlated with MLVD rather than MVD in patients
with NPC
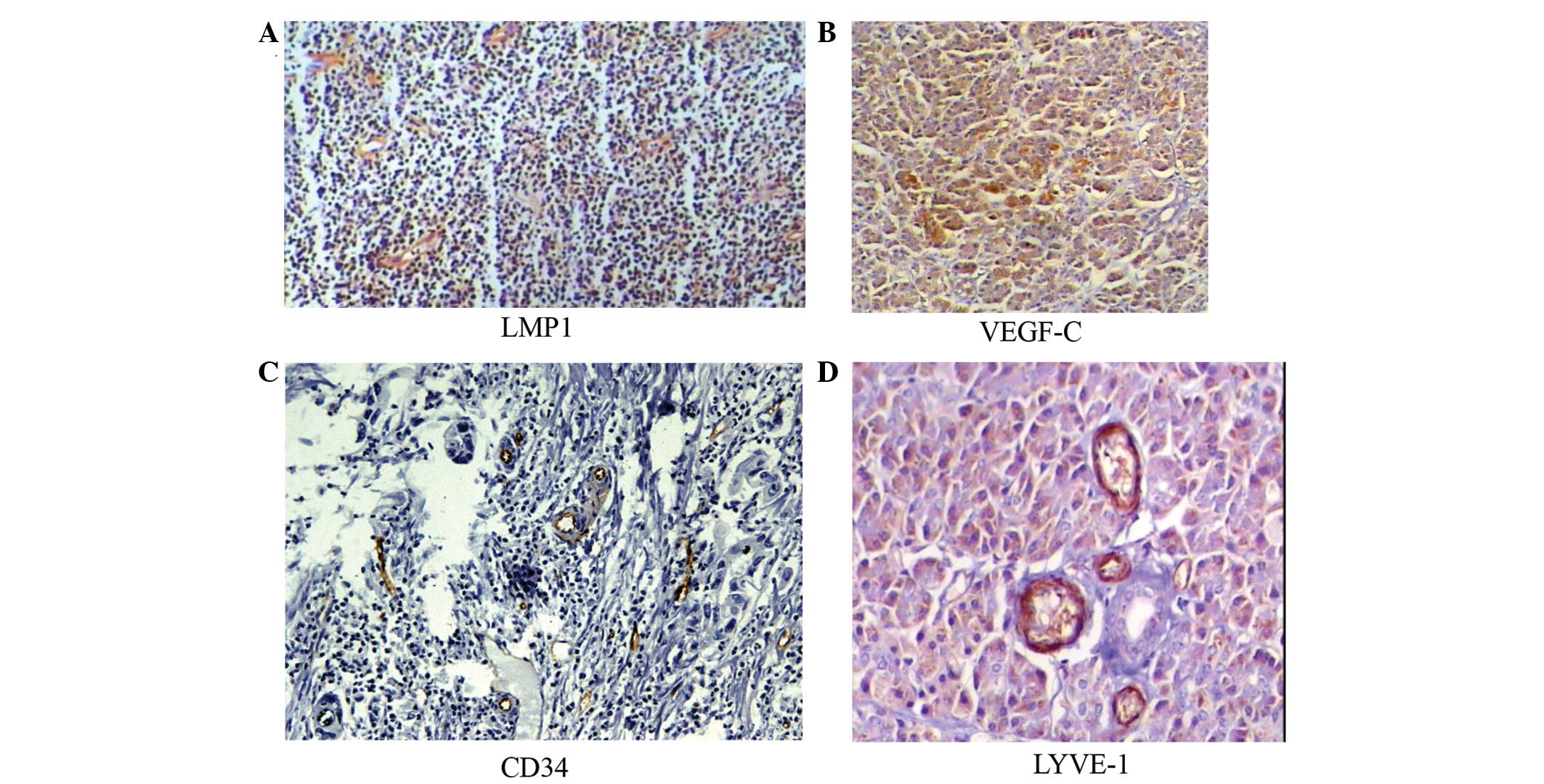
Among 61 EBV positive NPC patients, there were 38
cases that were LMP1-positive (62.3%, Fig. 3). LMP1 expression was associated
with TNM stage (P=0.021) and lymph node metastasis (P=0.046). By
contrast, BARF1 was only expressed in 8 cases (13.3%) and BARF1
expression was not identified to be associated with the analyzed
factors. Moreover, VEGF-C was expressed in 52 cases (85.2%) and
VEGF-C expression was associated with lymph node metastasis
(P<0.0001). The MVD level was not shown to be significantly
different between LMP1-positive and -negative cases (Tables V and VI). However, MLVD in the LMP1-positive
group was significantly higher than the LMP1-negative group. This
suggests that LMP1 may contribute to micro-lymphatic formation. MVD
and MLVD in the VEGF-C-positive group were higher than in the
negative group suggesting that VEGF-C may contribute to microvessel
and micro-lymphatic formation in NPC.
 | Table VAssociation of BHRF1, LMP1 and VGGF-C
expression with the clinicopathological data of patients. |
Table V
Association of BHRF1, LMP1 and VGGF-C
expression with the clinicopathological data of patients.
| Factor | n | LMP1 expression
| VEGF-C expression
| BHRF1 expression
|
|---|
| Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Gender | | | | 0.931 | | | 0.893 | | | 0.914 |
| Male | 50 | 20 | 30 | | 7 | 43 | | 43 | 7 | |
| Female | 11 | 3 | 8 | | 2 | 9 | | 10 | 1 | |
| TNM stage | | | | 0.021 | | | 0.31 | | | 0.943 |
| I/II | 27 | 18 | 9 | | 6 | 21 | | 23 | 4 | |
| III/IV | 34 | 5 | 29 | | 3 | 31 | | 30 | 4 | |
| LN metastasis | | | | 0.046 | | | 0 | | | 0.752 |
| Negative | 14 | 10 | 2 | | 7 | 7 | | 12 | 2 | |
| Positive | 47 | 13 | 36 | | 2 | 45 | | 41 | 6 | |
 | Table VIAssociation of LMP1 and VEGF-C
expression with MVD and MLVD levels in nasopharyngeal
carcinoma. |
Table VI
Association of LMP1 and VEGF-C
expression with MVD and MLVD levels in nasopharyngeal
carcinoma.
| Expression | n | MVD | P-value | MLVD | P-value |
|---|
| LMP1 | | | 1.000 | | 0.046 |
| Negative | 23 | 59±3.1 | | 5.0±2.0 | |
| Positive | 38 | 59±5.3 | | 6.4±2.9 | |
| VEGF-C | | | 0.000 | | 0.000 |
| Negative | 9 | 53±4.8 | | 4.1±1.8 | |
| Positive | 52 | 61±4.1 | | 8.1±3.0 | |
Discussion
EBVaGC is a recently identified cancer type that may
be caused by EBV infection (19).
The present study further demonstrated that EBV infection
contributes to the development of a small percentage (5%) of
gastric cancers, although previous studies have shown that 10% of
worldwide gastric cancers were EBVaGC (6,20).
By contrast, the frequency of EBV infection in NPC was notably
higher, effecting 85% of the patients observed. Although the
frequency of EBV infection in EBVaGC and NPC was different, the
molecular mechanism of EBV infection in different types of cancer
may be the same. Thus, in this study expression of the
EBV-associated proteins LMP1 and BARF1 was further assessed in NPC
and EBVaGC tissue specimens for association with
clinicopathological data and with MVD and MLVD. It was demonstrated
that BARF1 expression was associated with lymph node metastasis and
MLVD in patients with EBVaGC. In NPC, LMP1 expression was
associated with TNM stage (P=0.011) and lymph node metastasis
(P=0.041). Only 13.3% cases were BARF1 positive and MLVD was
significantly higher in LMP1-positive cases than in LMP1-negative
cases. The data from the current study indicate that although EBV
infection is involved in the development of gastric cancer and NPC,
expression of EBV-associated proteins LMP1 and BARF1 may have
differential functions during the tumorigenesis of these two types
of cancer.
Generally, primary EBV infection occurs via the oral
route and establishes a lifelong virus carrier state, termed latent
infection (21). In latent
infection, infected cells only express a limited set of viral
genes, but can provide a survival advantage to the infected cell
(22). The latent infection can be
further divided into different subgroups due to specific viral
proteins (23). EBVaGC is
considered as a latency I EBV infection, while NPC can be both
latency I and II EBV infections (24,25).
Latency I infection is characterized by expression of EBV nuclear
antigen 1 (EBNA1), EBER1 and 2, and BamHI-A rightward
transcripts (BART) (4,26). In addition to latency I
transcripts, latency II infection can also express latent membrane
protein 1 (LMP1) (4,26). Our current data are consistent with
previous findings (4,26). Only one patient with weak LMP1
expression was identified in the 30 EBVaGC cases. By contrast, 38
NPC tissues in these 61 NPCs exhibited LMP1 expression. This
confirmed that the latency of EBV infection between EBVaGC and NPC
was different. Therefore, due to the different expression of viral
proteins, oncogenic mechanisms of EBV in EBVaGC and NPC may
differ.
Furthermore, tumor metastasis is the leading cause
of cancer-related mortality (27,28).
A greater understanding of the molecular mechanism underlying tumor
metastasis may aid the development of effective cancer therapies.
The initial site of cancer metastasis is usually the regional lymph
nodes (29,30). Clinical and experimental data
suggested that lymphoangiogenesis can greatly facilitate the
migration of tumor cells into the lymph nodes (31–33).
The present study demonstrated that MLVD was associated with NPC
lymph node metastasis and that EBV infection was associated with
MLVD. The association between EBV infection and MLVD was consistent
in EBVaGC and NPC. These data suggest that EBV infection may have a
common effect on the regulation of lymphoangiogenesis, although the
exact molecular mechanisms remain unknown. Further investigation is
required to clarify how EBV infection contributes to an increase in
MLVD.
EBV-positive epithelial malignancies show selective
and abundant expression of a viral gene that encodes BARF1 protein
(34). BARF1 expression was
usually low in lymphomas, but more frequent in EBV-associated
carcinomas. BARF1 may function as an oncogene in NPC, parallel to
the more widely investigated viral protein LMP1 (35). In EBV-positive gastric cancer,
BARF1 was expressed in the absence of LMP1, possibly functioning as
the predominant EBV oncogene in this disease (36). BARF1 expression was able to
immortalize and transform epithelial cells of different origins by
acting as a mitogenic growth factor, inducing cyclin-D expression
and upregulating anti-apoptotic Bcl-2, and in turn stimulating host
cell growth and survival (37). In
the current study, 13% of NPCs were positive for BARF1, whereas
BARF1 was expressed in 56.7% of EBVaGCs. This finding suggests that
BARF1 and LMP1 may have redundant functions in promoting
tumorigenesis in gastric cancer and in NPC.
The current study does have certain limitations. For
example, it is only a proof-of-principle descriptive study and
additional mechanistic data are required to support the current
findings. It remains to be determined how these two viral proteins
function differentially in these two types of human cancer or
whether EBV is involved in the development of EBV-positive gastric
cancer since EBV is estimated to infect >90% of the worldwide
population and EBV infection may be just bystander in these gastric
types of cancer.
Acknowledgments
This study was partially supported by the 973
Projects of China (grant no. 2011CB504302) and the Natural Science
Foundation of China (grant no. 30872318). The authors would also
like to acknowledge the valuable comments from other members of the
laboratories.
References
|
1
|
Morales-Sánchez A and Fuentes-Pananá EM:
Human viruses and cancer. Viruses. 6:4047–4079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu X, Yang Z, Chen Q, Yu L, Liang S, Lü H
and Qiu Z: Comparison of clinical characteristics of chronic cough
due to non-acid and acid gastroesophageal reflux. Clin Respir J.
9:196–202. 2015. View Article : Google Scholar
|
|
3
|
Ok CY, Papathomas TG, Medeiros LJ and
Young KH: EBV-positive diffuse large B-cell lymphoma of the
elderly. Blood. 122:328–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Young LS and Rickinson AB: Epstein-Barr
virus: 40 years on. Nat Rev Cancer. 4:757–768. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tokunaga M, Land CE, Uemura Y, Tokudome T,
Tanaka S and Sato E: Epstein-Barr virus in gastric carcinoma. Am J
Pathol. 143:1250–1254. 1993.PubMed/NCBI
|
|
6
|
Takada K: Epstein-Barr virus and gastric
carcinoma. Mol Pathol. 53:255–261. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pfitzner AJ, Tsai EC, Strominger JL and
Speck SH: Isolation and characterization of cDNA clones
corresponding to transcripts from the Bam HI H and F regions of the
Epstein-Barr virus genome. J Virol. 61:2902–2909. 1987.PubMed/NCBI
|
|
8
|
Kelly GL, Long HM, Stylianou J, Thomas WA,
Leese A, Bell AI, Bornkamm GW, Mautner J, Rickinson AB and Rowe M:
An Epstein-Barr virus anti-apoptotic protein constitutively
expressed in transformed cells and implicated in burkitt
lymphomagenesis: The Wp/BHRF1 link. PLoS Pathog. 5:e10003412009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Taub R, Kirsch I, Morton C, Lenoir G, Swan
D, Tronick S, Aaronson S and Leder P: Translocation of the c-myc
gene into the immunoglobulin heavy chain locus in human Burkitt
lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci USA.
79:7837–7841. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adams JM, Harris AW, Pinkert CA, Corcoran
LM, Alexander WS, Cory S, Palmiter RD and Brinster RL: The c-myc
oncogene driven by immunoglobulin enhancers induces lymphoid
malignancy in transgenic mice. Nature. 318:533–538. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Magrath I: The pathogenesis of Burkitt's
lymphoma. Adv Cancer Res. 55:133–270. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strasser A, Harris AW, Bath ML and Cory S:
Novel primitive lymphoid tumours induced in transgenic mice by
cooperation between myc and bcl-2. Nature. 348:331–333. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beverly LJ and Varmus HE: MYC-induced
myeloid leukemo-genesis is accelerated by all six members of the
antiapoptotic BCL family. Oncogene. 28:1274–1279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee KM, Danuser R, Stein JV, Graham D,
Nibbs RJ and Graham GJ: The chemokine receptors ACKR2 and CCR2
reciprocally regulate lymphatic vessel density. EMBO J.
33:2564–2580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hlatky L, Hahnfeldt P and Folkman J:
Clinical application of antiangiogenic therapy: Microvessel
density, what it does and doesn't tell us. J Natl Cancer Inst.
94:883–893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yates JL, Warren N and Sugden B: Stable
replication of plasmids derived from Epstein-Barr virus in various
mammalian cells. Nature. 313:812–815. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gires O, Zimber-Strobl U, Gonnella R,
Ueffing M, Marschall G, Zeidler R, Pich D and Hammerschmidt W:
Latent membrane protein 1 of Epstein-Barr virus mimics a
constitutively active receptor molecule. EMBO J. 16:6131–6140.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dawson CW, Port RJ and Young LS: The role
of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the
pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol.
22:144–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burke AP, Yen TS, Shekitka KM and Sobin
LH: Lymphoepithelial carcinoma of the stomach with Epstein-Barr
virus demonstrated by polymerase chain reaction. Mod Pathol.
3:377–380. 1990.PubMed/NCBI
|
|
20
|
Shibata D and Weiss LM: Epstein-Barr
virus-associated gastric adenocarcinoma. Am J Pathol. 140:769–774.
1992.PubMed/NCBI
|
|
21
|
Shannon-Lowe C and Rowe M: Epstein Barr
virus entry; kissing and conjugation. Curr Opin Virol. 4:78–84.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsao SW, Tsang CM, Pang PS, Zhang G, Chen
H and Lo KW: The biology of EBV infection in human epithelial
cells. Semin Cancer Biol. 22:137–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Woellmer A and Hammerschmidt W:
Epstein-Barr virus and host cell methylation: Regulation of
latency, replication and virus reactivation. Curr Opin Virol.
3:260–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen JN, He D, Tang F and Shao CK:
Epstein-Barr virus-associated gastric carcinoma: A newly defined
entity. J Clin Gastroenterol. 46:262–271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Frappier L: Role of EBNA1 in NPC
tumourigenesis. Semin Cancer Biol. 22:154–161. 2012. View Article : Google Scholar
|
|
26
|
Robertson KD, Manns A, Swinnen LJ, Zong
JC, Gulley ML and Ambinder RF: CpG methylation of the major
Epstein-Barr virus latency promoter in Burkitt's lymphoma and
Hodgkin's disease. Blood. 88:3129–3136. 1996.PubMed/NCBI
|
|
27
|
Lauria R, Perrone F, Carlomagno C, De
Laurentiis M, Morabito A, Gallo C, Varriale E, Pettinato G, Panico
L, Petrella G, et al: The prognostic value of lymphatic and blood
vessel invasion in operable breast cancer. Cancer. 76:1772–1778.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kyzas PA, Geleff S, Batistatou A, Agnantis
NJ and Stefanou D: Evidence for lymphangiogenesis and its
prognostic implications in head and neck squamous cell carcinoma. J
Pathol. 206:170–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chua B, Ung O, Taylor R and Boyages J:
Frequency and predictors of axillary lymph node metastases in
invasive breast cancer. ANZ J Surg. 71:723–728. 2001. View Article : Google Scholar
|
|
30
|
Cunnick GH, Jiang WG, Gomez KF and Mansel
RE: Lymphangiogenesis and breast cancer metastasis. Histol
Histopathol. 17:863–870. 2002.PubMed/NCBI
|
|
31
|
Skobe M, Hawighorst T, Jackson DG, Prevo
R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K and Detmar
M: Induction of tumor lymphangiogenesis by VEGF-C promotes breast
cancer metastasis. Nat Med. 7:192–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He Y, Karpanen T and Alitalo K: Role of
lymphangiogenic factors in tumor metastasis. Biochim Biophys Acta.
1654:3–12. 2004.PubMed/NCBI
|
|
33
|
Salven P, Mustjoki S, Alitalo R, Alitalo K
and Rafii S: VEGFR-3 and CD133 identify a population of CD34+
lymphatic/vascular endothelial precursor cells. Blood. 101:168–172.
2003. View Article : Google Scholar
|
|
34
|
Brink AA, Vervoort MB, Middeldorp JM,
Meijer CJ and van den Brule AJ: Nucleic acid sequence-based
amplification, a new method for analysis of spliced and unspliced
Epstein-Barr virus latent transcripts, and its comparison with
reverse transcriptase PCR. J Clin Microbiol. 36:3164–3169.
1998.PubMed/NCBI
|
|
35
|
Zheng H, Li LL, Hu DS, Deng XY and Cao Y:
Role of Epstein-Barr virus encoded latent membrane protein 1 in the
carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol.
4:185–196. 2007.PubMed/NCBI
|
|
36
|
zur Hausen A, Brink AA, Craanen ME,
Middeldorp JM, Meijer CJ and van den Brule AJ: Unique transcription
pattern of Epstein-Barr virus (EBV) in EBV-carrying gastric
adeno-carcinomas: Expression of the transforming BARF1 gene. Cancer
Res. 60:2745–2748. 2000.PubMed/NCBI
|
|
37
|
Chang MS, Kim DH, Roh JK, Middeldorp JM,
Kim YS, Kim S, Han S, Kim CW, Lee BL, Kim WH and Woo JH:
Epstein-Barr virus-encoded BARF1 promotes proliferation of gastric
carcinoma cells through regulation of NF-kB. J Virol.
87:10515–10523. 2013. View Article : Google Scholar : PubMed/NCBI
|