Introduction
The processes of generation and maturity of
cardiomyocytes (CMs) are completed in utero, therefore, CMs
are considered a source of terminally differentiated cells and CMs
are unable regenerate if the heart is injured. For example,
myocardial tissue destroyed following myocardial infarction is
replaced typically by noncontractile scar tissue (i.e., fibrosis),
which leads to congestive heart failure (1,2).
Heart transplantation is currently the gold standard therapy for
patients with end-stage heart failure. However, its application is
limited in clinical practice due to a shortage of donor hearts and
the side effects of immunosuppressive drugs (3,4).
Thus, cell-based cardiac repair has attracted interest as an
alternative method to ameliorate cardiac injury (5,6).
Specifically, significant attention has been paid to embryonic stem
cell (ESC)-based cardiac repair, particularly for infarcted hearts
(5).
Fetal and neonatal CMs can improve the biological
function of damaged hearts (7–9);
however, their use is restricted by the shortage of cell sources
and ethical problems. By contrast, adult stem cells are abundant,
however, whether these have the ability to differentiate into CMs
remains to be elucidated. ESCs are multipotent cells derived from
the inner cell mass of blastocysts, which are able to differentiate
into all cell types of the body (10). They have an unlimited capacity for
self-renewal (11) and
unquestioned cardiomyogenic potential (12,13).
Previous studies using ESC transplantation to repair
infarcted hearts have achieved much acclaim. Xie et al
(14) reported that
undifferentiated human ESCs (hESCs) can be induced towards a
cardiac lineage under a locally injured environment in the heart,
which may provide a potential method for regenerating de
novo myocardium to treat myocardial infarction. Caspi et
al (15) reported that the
transplantation of hESC-derived CMs (hESC-CMs) in rats following
extensive myocardial infarction results in the formation of stable
CM grafts, attenuates the remodeling process, and provides
functional benefit. Numerous studies have demonstrated that ESC-CMs
exhibit a developmentally appropriate program of cardiac gene
expression, as well as the expected electro-physiological and
contractile phenotype (16,17).
It was also demonstrated that, following transplantation into
infarcted rodent hearts, ESC-CMs survive and formed stable cardiac
implants with improved heart contractile function (18–20).
However, immunological rejection and teratoma formation induced by
ESC transplantation are the two most significant challenges
limiting its clinical application (21–23).
To accompany the rapid development of stem cell and
nuclear transfer technologies, nuclear-transferred ESCs (nt-ESCs)
emerged towards the end of the 20th century (24,25).
By combining therapeutic cloning with nuclear transfer technology
and embryonic stem cell technology, and using donor somatic cells
(such as skin cells) as nuclear donor cells and nuclear maturation
in oocytes of other animals as receptor cells, researchers obtained
nt-ESCs with an identical genetic background to that of the donor
(26). An nt-ESC has the same
genetic material as the donor, therefore it is not associated with
immunological rejection following transplantation (26). However, teratoma formation cannot
be avoided due to the multipotent ability of nt-ESCs.
Teratoma formation following ESC transplantation has
achieved substantial attention in the research community. Leor
et al (27) reported that
the transplantation of undifferentiated hESCs and embryoid bodies
(EBs) into infarcted hearts leads to teratoma formation. He et
al (22) reported that,
following the injection of mouse (m)ESCs into the infarcted hearts
of immunosuppressed rats, the incidence of teratomas was ~50% after
1 week and 100% after 4 weeks. Additional studies have demonstrated
that teratoma formation following ESC transplantation is associated
with the immune response, the site of transplantation and the
number of undifferentiated ESCs (23,28,29).
However, to date, few studies have reported teratoma formation
following nt-ESC transplantation.
The aim of the present study was to investigate
teratoma formation from ESCs of different origins (mESCs and
nt-mESCs) and at different stages of maturity [nt-mESCs, nt-mESC-CM
and Percoll-enriched-nt-mESC-CMs (nt-mESC-PE-CMs)]. Infarcted rat
hearts were selected as the experimental model. The tumor incidence
and volume were assessed to compare the tumorigenesis of different
transplanted seed cells. The aim of the present study was to
compare the pluripotency of mESC and nt-mESC by comparing their
tumorigenesis and to observe the influence of differentiation and
enrichment on tumorigenesis of nt-ESC following transplantation in
the infarcted rat heart.
Materials and methods
Reagents
All reagents and chemicals were, unless otherwise
specified, purchased from Beijing Chemical Reagent Company
(Beijing, China).
Undifferentiated nt-mESC and mESC
culture
The mESC ES-D3 lines and nt-ESCs were cultured as
described previously (30).
Briefly, the cells were maintained in an undifferentiated state in
Medium I, containing Dulbecco's modified Eagle's medium (DMEM;
Gibco, Thermo Fisher Scientific, Waltham, MA, USA) with 4.5 g/l
glucose, 15% fetal calf serum (FCS; GE Healthcare Life Sciences,
Logan, UT, USA), 0.1 mM β-mercaptoethanol (Sigma-Aldrich, St.
Louis, MO, USA), 2 mM glutamine and 0.1 mM non-essential amino
acids (NEAAs; Gibco), containing 1,000 U/ml leukemia inhibitory
factor (Chemicon International, Temecula, CA, USA).
Bioreactor expansion of EBs
The nt-mESCs were enzy-matically dissociated using
0.25% trypsin (Gibco) and 0.04% ethylene-diamine tetraacetic acid
(Sigma-Aldrich) at 37°C for 5 min. To amplify the nt-mESCs and
obtain the EBs, 2.5×106 ES-D3 cells were transferred
into a 250 ml slow-turning lateral vessel (STLV; Synthecon, Inc.,
Houston, TX, USA) filled with Medium II, containing DMEM with 4.5
g/l glucose, 20% FCS, 0.1 mM β-mercaptoethanol, 2 mM glutamine and
0.1 mM NEAA. The speed of rotation was 15 rpm for the first 12 h
and was adjusted to 45 rpm for 5 days. Half of the culture medium
was replaced every 2 days.
Adherence culture and ascorbic acid
induction
The cells were cultured for 5 days in the STLV.
Subsequently, the formed EBs were transferred onto gelatin-coated
plates (1–3 EBs/cm2) and cultured in Medium II. EB
differentiation into CMs (nt-mESC-CMs) was induced by adding 5 mg/l
ascorbic acid followed by incubation for 8 days, with medium
containing 5 mg/l ascorbic acid refreshed at days 3, 5 and 7.
Dissociation and Percoll enrichment of
the nt-mESC-CMs
The differentiated cell cultures containing CMs were
washed once with phosphate-buffered saline (PBS), followed by
incubation in PBS containing 1 mg/ml dispase (Roche Diagnostics,
Basel, Switzerland) at 1×106 cells/ml at 37°C for 30
min. The cells were then resuspended in a solution containing 85 mM
KCl, 30 mM K2HPO4 5 mM MgSO4, 1 mM
ethylene-glycol tetraacetic acid, 2 mM Na2ATP, 5 mM
sodium pyruvate, 5 mM creatine, 20 mM taurine and 20 mM glucose,
and incubated at 37°C for 15 min for complete dissociation.
Following dissociation, the cells were centrifuged at 1,500 × g for
30 min at room temperature, resuspended in 3 ml Medium II and
loaded onto a discontinuous Percoll gradient for enrichment of the
CMs. Percoll (GE Healthcare Life Sciences) was diluted in buffer
containing 20 mM HEPES and 150 mM NaCl. The gradient consisted of a
40.5% Percoll layer over a layer of 58.5% Percoll. Following
centrifugation at 1,500 × g for 30 min, the cell layers were
apparent. Fraction V contained the enriched CMs (mESC-PE-CMs),
which were collected for cell transplantation and semi-quantitative
reverse transcription (RT)-polymerase chain reaction (RT-PCR)
analysis. Octamer-binding transcription factor-4 (OCT-4) was used
as a specific marker of undifferentiated mESCs. Mouse fibroblasts
obtained from the embryos (13–14 days old) of Kunming white mice
served as negative controls (Institute of Basic Medical Sciences,
Academy of Military Medical Sciences, Beijing, China). The
second-generation mouse fibroblasts were used as ESC feeder cells.
An RT-PCR kit (Promega Corp., Madison, WI, USA) was used. First,
RNA was extracted with TRIzol (Tiangen Biotech Co., Ltd., Beijing,
China) according to the manufacturer's instructions. The RNA
content was determined by measuring its optical density at 260/280
nm (BD-600 Electrophoresis Densitometer Measurement System
analysis; QHBODA Technology Co., Ltd, Beijing, China). The RT-PCR
reaction was performed in a total volume of 50 μl [template
100 ng, 10× primer mi× (2 μM each) 5 μl, 10× Mutli
HotStart buffer 5 μl, dNTPs 200 μM, Multi HotStart (5
U/μl), ddH2O up to 50 μl] with 1 μg
RNA using a Multiplex PCR Amplification kit [KT109; Tiangen Biotech
(Beijing) Co., Ltd., Beijing China]. RT was performed at 45°C for
45 min, followed by PCR amplification under the following
conditions: Initial denaturation at 95°C for 5 min, followed 35
cycles of amplification with annealing at 53°C, and a final
extension at 72°C for 10 min. The PCR products were separated by 1%
agarose gel electrophoresis and images were captured with a gel
imager (haCmpGel-3200, Beijing Sage Creation, Beijing, China) for
qualitative detection of Oct-4. The following specific primer
sequences were used: Oct-4 forward, 5′-GGA GGA AGC CGA CAA CAA
TGAG-3′ and reverse, 5′-TGGGGGCAGAGGAAAGGATACAG-3′ (331 bp); and
GAPDH forward, 5′-AACGACCCCTTCATTGAC-3′ and reverse,
5′-TCCACGACATACTCAGCAC-3′ (191 bp).
Myocardial infarction model and cell
transplantation
Female Sprague-Dawley rats (8-week-old, weighing
230±20 g) were purchased from the Laboratory Animal Center of the
Chinese Academy of Military Medical Science (Beijing, China). All
animal experiments were performed under the authority of the
Institutional Animal Care and Use Committee of the Chinese Academy
of Military Medical Science (Beijing, China). The study was
approved by the ethics committee of the First Affiliated Hospital
of Dalian Medical University (Dalian, China). The animals were
individually housed in cages at a constant room temperature of
24±2°C under a 12-h light/darkcycle with access to water and rat
chow ad libitum. The rats were then prepared for the
induction of ischemia, as described in detail by Miyahara et
al (31). Briefly, the rats
were anesthetized via an intraperitoneal (i.p) injection of 30
mg/kg sodium pentobarbital (China National Medicine Group, Beijing
Chemical Reagent Company). Subsequently, limb-lead
electrocardiography was performed using an RM6240BD type
multichannel physiological instrument (Chengdu Instrument Factory,
Chengdu, China), and rats were ventilated with a volume-regulated
respirator for the entire duration of the procedure. The surgical
approach to induce ischemia involved a left lateral thoracotomy,
pericardiectomy and identification of the left anterior descending
(LAD) coronary artery. The LAD was ligated with a 6-0 Prolene
suture (Ningbo Medical Needle Co. Ltd., Ningbo, China) 2–3 mm from
its origin between the pulmonary artery conus and the left atrium.
Following ligation, the infarcted area of the left ventricle became
immediately pale and contraction was limited. Typical myocardial
infarction waves were simultaneously visible on the
electrocardiogram. The rats were randomly assigned to receive
either mESCs, nt-mESCs, nt-mESC-CMs or nt-mESC-PE-CMs (n=15 per
group). For administration ~5×106 of each cell type,
resuspended in 100 μl PBS, was injected through a 28 gauge
needle into the center of the infarcted area 5 min after the
induction of myocardial infarction. The injections were verified by
marginal lightening in color of the myocardium as the solution
entered the infarcted wall. Subsequently, the chest was closed. To
avoid graft rejections, all rats received daily i.p. injections of
cyclosporine A (15 mg/kg) and methylpred-nisolone (2 mg/kg).
Evaluation of teratoma incidence and
volume
The rats were sacrificed at 8 weeks after cell
transplantation by anesthesia with sodium pentobarbital (30 mg/kg,
i.p.), left lateral thoracotomy to expose the heart and injection
of 5 ml 10% KCl into the heart. The hearts were rapidly excised,
and the cardiac cavities rinsed in PBS to remove blood and thrombi.
The incidence and volume of teratomas in the transplant area were
calculated in a blinded-manner. Owing to fibrosis, the ventricular
wall was sufficiently thin to permit observation of a teratoma, and
the volume of the teratoma was measured as that, which formed
subcutaneously. Tumor incidence was expressed as a percentage of
the number of samples in each group. Tumor volume was calculated
using the following formula: Tumor long diameter × (tumor short
diameter)2 / 2.
Histology, histochemistry and
immunohistochemistry
The hearts were fixed with 10% formalin for 16 h,
embedded in paraffin and cut into 4-μm thick sections. The
tissue sections were then stained with hematoxylin and eosin for
histological examination. Chondrogenesis differentiation was
identified by immunohistochemical staining of type II collagen
(1:200; Chemicon Incorporated). Prior to immunohistochemistry, the
sections were digested with pepsin at 1 mg/ml in TrisHCl (pH 2.0)
for 10 min at 37°C. For immunohistochemistry, the intrinsic
peroxidase activity was blocked by incubation with 5%
H2O2 in PBS for 30 min following
deparaffinization. Nonspecific antibody binding was blocked with 5%
bovine serum albumin (Medgenics Inc., Philadelphia, PA, USA) in PBS
for 1 h at 37°C. Immunohistochemical staining was performed using
an avidin-biotin technique, followed by visualization with
3,3′-diaminobenzidine tetrahydrochloride dehydrate (0.006%) and
H2O2 (0.003%). The following primary
antibodies were used: Mouse monoclonal anti-type II collagen (cat.
no. MAB8887; Chemicon), mouse monoclonal anti-Nestin (1:200; cat.
no. ab93666; Wuhan Boster Biological Technology, Ltd., Wuhan,
China), as a neural precursor cell marker, and rabbit polyclonal
anti-α-smooth muscle actin (α-SMA; 1:200; cat. no. A2547; Wuhan
Boster Biological Technology, Ltd.), as a smooth muscle cell
marker. Samples were then incubated with secondary antibody, Cy3
goat anti-mouse or rabbit immunoglobulin G (1:50 dilution; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing. China),
at 37°C for 30 min. Blank controls were performed by omitting the
primary antibodies. Following washing with distilled water,
counterstaining was performed with hematoxylin for 1 min. Samples
were observed and images were captured using a microscope (BX51;
Olympus, Tokyo, Japan).
Statistical analysis
The tumor volume data are expressed as the mean ±
standard deviation. Fisher's exact test and two-tailed Student's
t-test were used to evaluate the incidence and volume of teratomas,
respectively. Statistical analysis was performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Differentiation and enrichment of
mESC-CMs
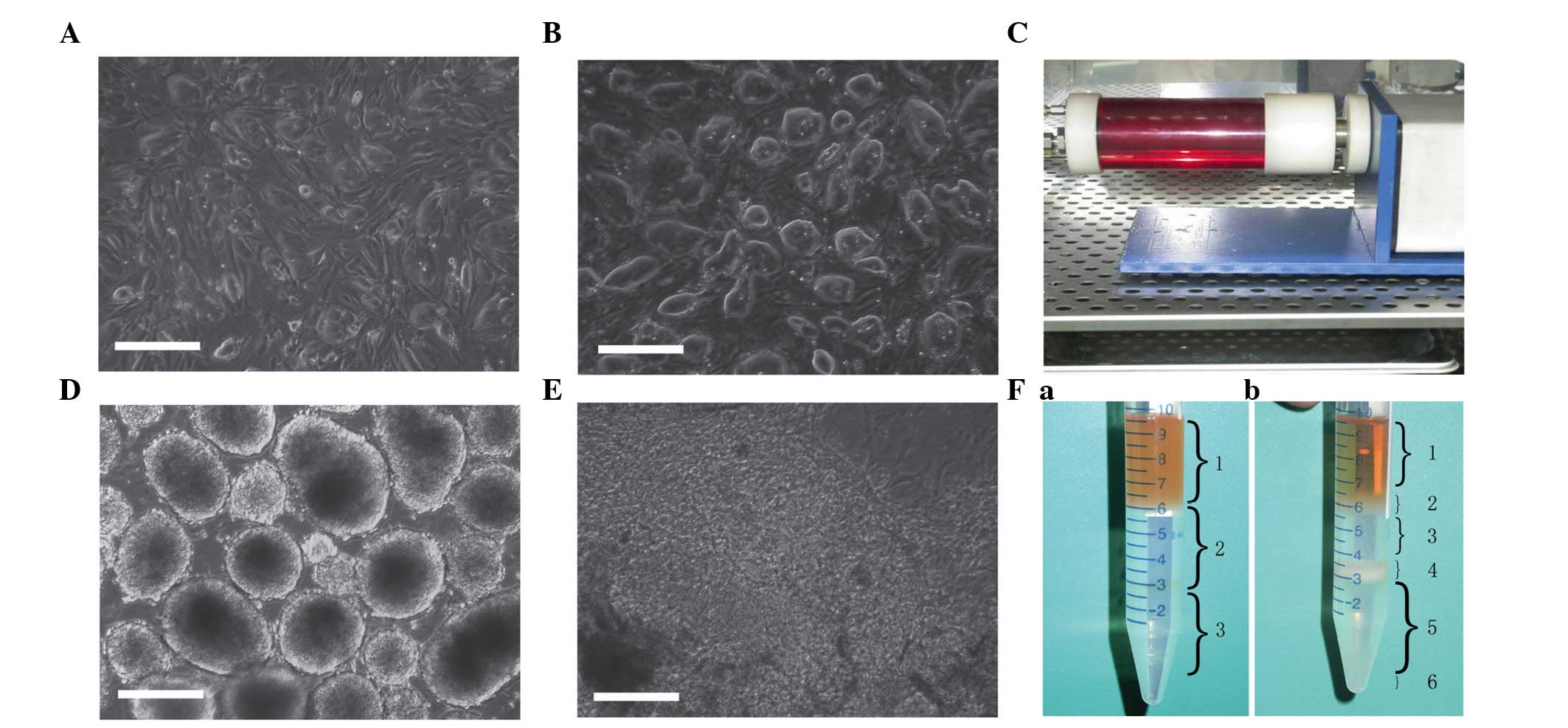
The mESC ES-D3 lines (Fig. 1A) and nt-mESCs (Fig. 1B) were successfully cultured and
the nt-mESCs were expanded to EBs using an STLV bioreactor system
(Fig. 1C). At 5 days
post-expansion, ~4×106 EBs of 100±30 μm diameter
were obtained. The EBs were spherical in shape with an intact
structure and no signs of apoptosis (Fig. 1D).
The EBs were inoculated onto culture plates at low
density to induce differentiation into CMs. The EBs grew in an
attachment pattern and differentiated into beating CMs in the
presence of ascorbic acid. After 5 days, the CMs began to
spontaneously contract (Fig. 1E).
At 14 days post-differentiation, the nt-mESC-CMs became dissociated
and subsequently underwent discontinuous Percoll gradient
separation (Fig. 1Fa). Following
centrifugation, six layers of cells were observed (Fig. 1Fb). As demonstrated previously,
mESC-CMs are predominantly concentrated in fraction 5 (Fig. 1Fb5) (32), therefore, these cells were
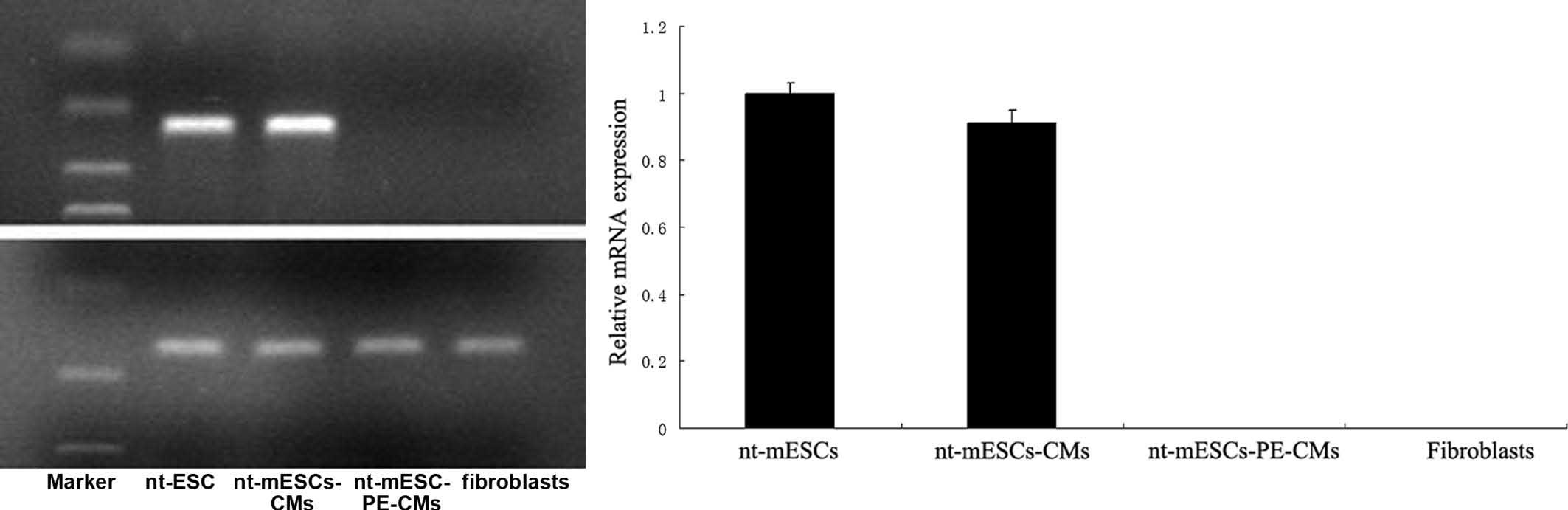
collected for subsequent cell transplantation. The RT-PCR assays
demonstrated that OCT-4, a marker of early-stage ESCs, was
expressed in the nt-mESCs and nt-mESC-CMs, but was absent in the
nt-mESC-PE-CMs (Fig. 2).
Tumor formation is induced by seed cell
transplantation in the infarcted heart
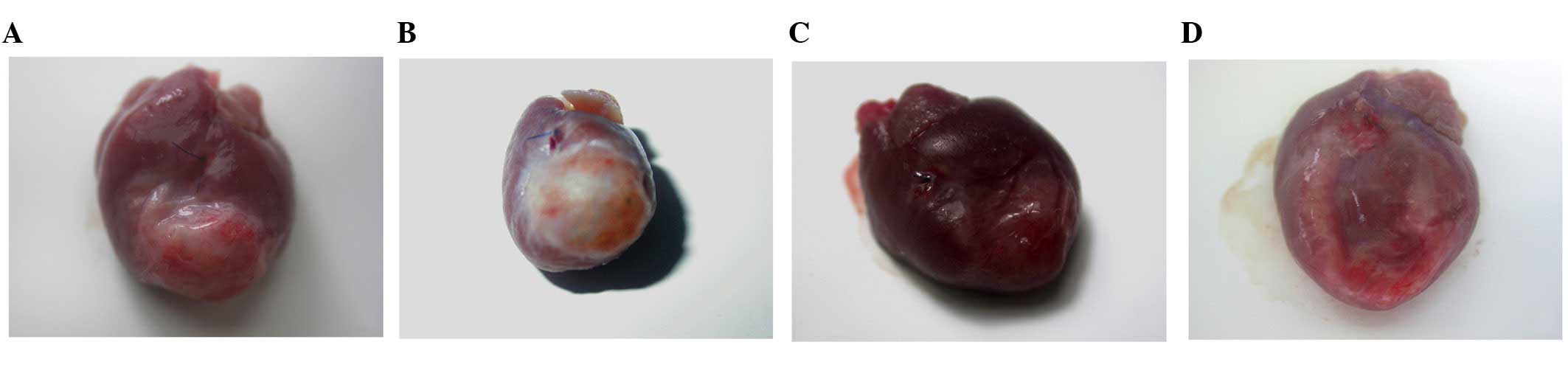
Tumor formation was observed in the infarcted area
of the hearts administered with the mESC, nt-mESC and mESC-CM
grafts (Fig. 3A–C), but not in the
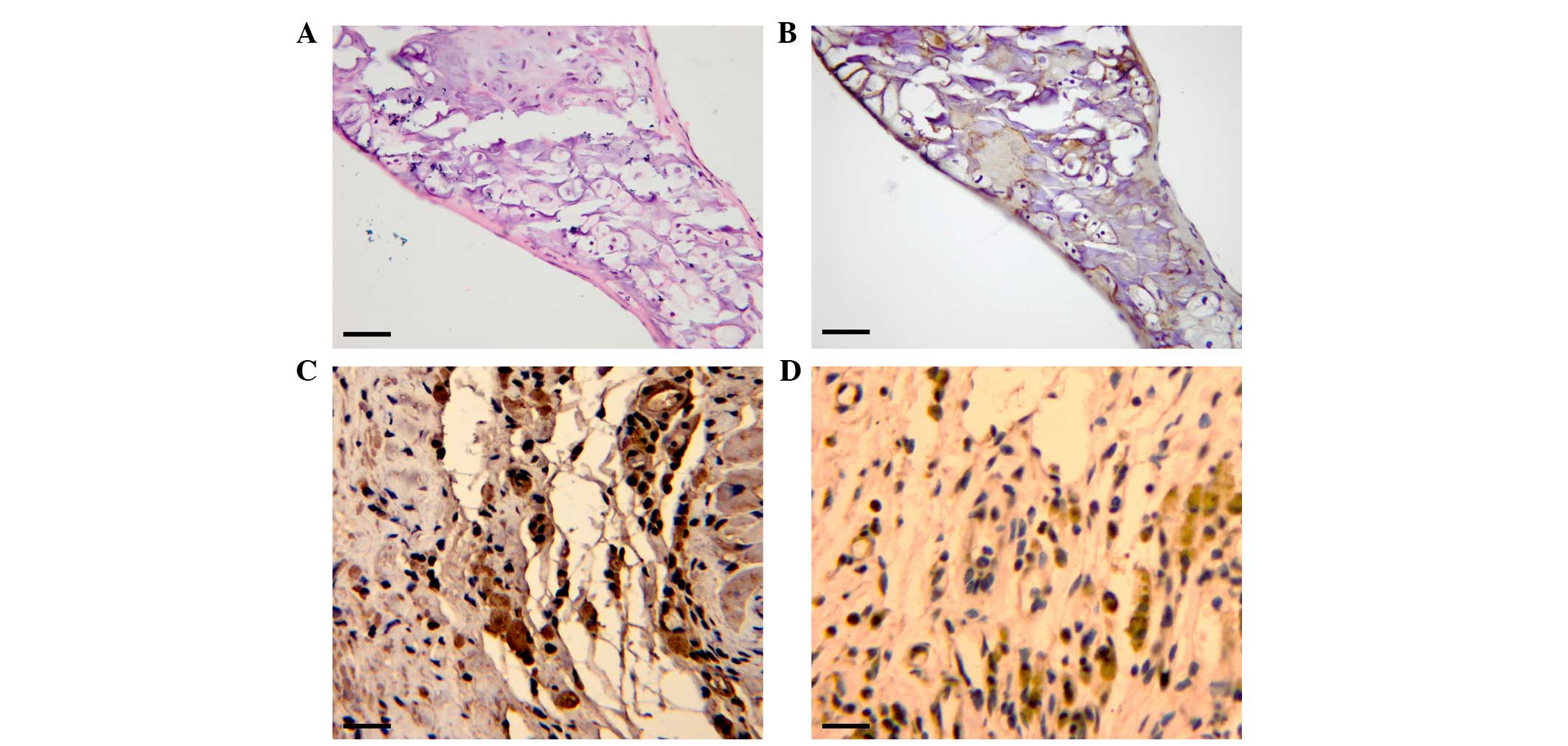
hearts administered with the nt-mESC-PE-CM grafts (Fig. 3D). Hematoxylin and eosin staining
revealed that the formed tumor was entirely different from the
myocardial tissue, and predominantly consisted of cartilage
(Fig. 4A). Immunohistochemistry
indicated that the tumor tissue was positive for type II collagen
(Fig. 4B), α-SMA (Fig. 4C) and nestin (Fig. 4D).
Comparison of tumorigenesis in different
seed cells
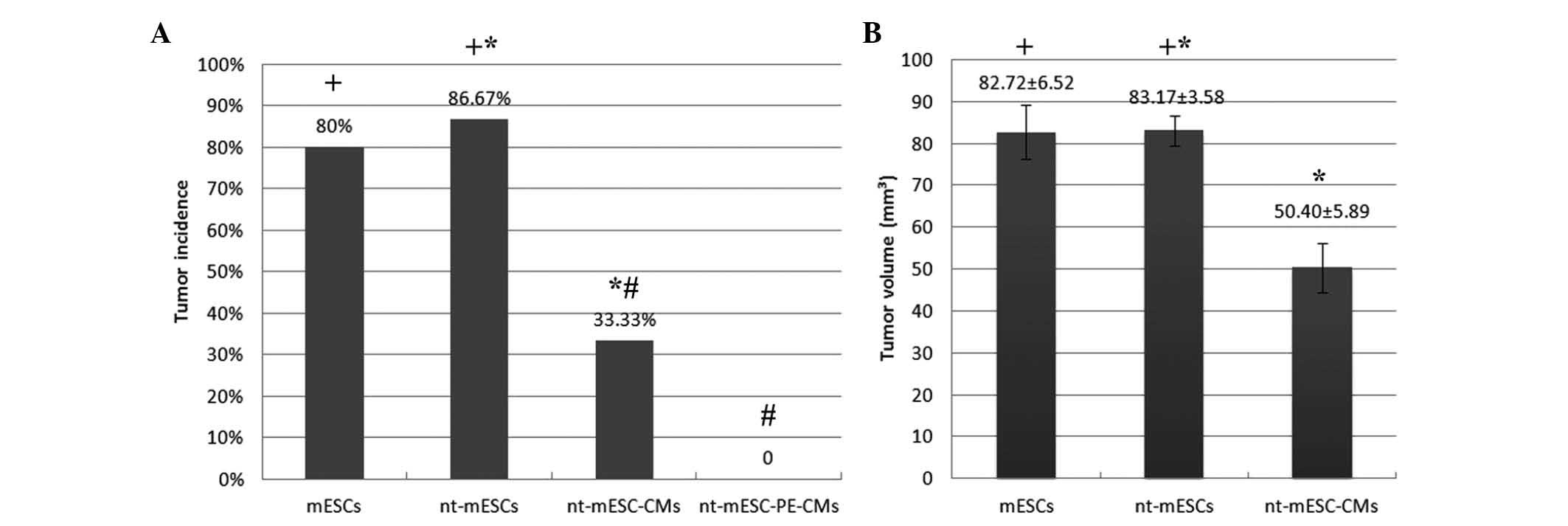
Tumors were observed in 86.67% of the rats that
received nt-mESCs and in 80% of the rats that received mESCs. No
significant difference was observed in tumor incidence between
these two groups (Fig. 5A).
However, tumors were observed in 33.33% of the rats administered
mESC-CMs, which differed significantly from that of the
mESC-treatment group (P<0.05; Fig.
5A). The tumor volumes were 83.17±3.58 and 82.72±6.52
mm3 in the rats administered with nt-mESCs and mESCs,
respectively (Fig. 3A and B and
Fig. 5B). No difference in mean
tumor volume was observed between the two groups. By contrast, a
mean tumor volume of 50.40±5.98 mm3 was observed in the
nt-mESC-CM group, which was significantly reduced, compared with
that in the nt-mESC group (P<0.05; Fig. 3B and C and Fig. 5A). As expected, the infarcted rat
hearts transplanted with nt-mESC-PE-CMs did not exhibit signs of
tumor formation (Figs. 3D and
5A). The incidence of
tumorigenesis and mean tumor volume in the nt-mESC-PE-CM group were
significantly lower, compared with those in the nt-mESC-CM group
(P<0.05; Fig. 3C and D and
Fig. 5).
Discussion
ESCs offer significant promise in regenerative
medicine, however, several critical obstacles must be overcome
prior to the use of ESCs in clinical medicine. Possibly the most
important obstacle from a safety standpoint is the immunogenicity
of ESCs and their tumorigenic ability (22–24).
nt-ESCs are distinctly superior in that these cells do not induce
immunological rejection following transplantation, however, due to
their multipotency, nt-ESCs have been associated with
tumorigenesis. It was reported in our previous study that
tumorigenesis in the infarcted rat heart is eliminated through the
differentiation and enrichment of transplanted mESCs (32). In the same study, it was
demonstrated that mESCs also prevent teratoma formation through
differentiation and enrichment.
In the present study, an established infarcted rat
heart model was used, and it was demonstrated that the
transplantation of mESCs and nt-mESCs resulted in tumor formation.
Considering that no discernible differences were identified in
tumor incidence and volume between these two groups, it was
concluded that nt-mESCs exhibit the same level of tumorige-nicity
as mESCs. Teratoma formation is generally considered to represent
the multipotency of ESCs (33,34).
Therefore, it is appropriate to conclude that nt-mESCs have the
same degree of multipotency as mESCs.
In the present study, pre-differentiated mESC-CMs
induced by ascorbic acid also resulted in tumor formation in the
infarcted hearts. However, the incidence of tumorigenesis and mean
tumor volume in the nt-mESC-CM group were markedly lower than those
observed in the nt-mESC group. The detection of OCT-4, a marker of
undifferentiated mESCs, in the two groups suggested that
undifferentiated nt-mESCs remained in the ascorbic acid-induced
differentiated CMs. This finding is consistent with that of a
previous report concerning mESCs (32) and indicates the importance of
transplantation using differentiated CMs to decrease the incidence
of tumorigenesis. The pre-differentiation of nt-mESCs into CMs
reduces the possibility of differentiation into other cell lineages
and reduces the number of undifferentiated nt-mESCs. By contrast,
the proliferative and self-renewal ability of the remaining
undifferentiated nt-mESCs may be subject to the effects of the
inducer, ascorbic acid, and the microenvironment formed by the
pre-differentiated CMs. This may explain why the incidence of
tumorigenesis resulting from the undifferentiated nt-ESCs decreased
significantly in the implanted areas. This finding was also
suggestive of teratoma formation. Histochemical analysis revealed
that the teratomas that developed in the rat ischemia model in the
present study were formed predominantly of bone and cartilage
tissue.
As demonstrated in a previous study, to obtain
maximally differentiated CMs, the CMs were enriched with ascorbic
acid-induced EBs using Percoll gradient centrifugation (32). No teratomas developed in the rats
injected with nt-mESC-PE-CMs, as expected. Teratoma were derived
from undifferentiated ESCs. RT-PCR analysis demonstrated that OCT-4
was not expressed in the nt-mESC-PE-CMs group; therefore, it was
speculated that the content of undifferentiated nt-mESC in
nt-mESC-PE-CMs was further reduced, even without treatment, through
differentiation and enrichment. Furthermore, no OCT-4 was detected
in this treatment group. Laflamme et al (35) reported that when hESCs are
inductively differentiated into CMs and subsequently enriched with
Percoll, they fail to form teratomas following transplantation into
normal rat hearts, but rather form stable grafts of human
myocardium with a period of 4 weeks. This finding is consistent
with our previous study, which revealed that mESC-PE-CMs were not
associated with teratoma formation following transplantation
(32). It was further demonstrated
in the present study that the nt-mESC-PE-CMs were not associated
with tumorigenesis following transplantation in the ischemic rat
heart model.
In conclusion, the present study demonstrated that
nt-mESCs exhibited the same multipotency as mESCs. In addition,
transplantation of nt-mESC-PE-CMs prevented the formation of
teratomas in an infarcted rat heart model. These findings suggested
that nt-ESCs are an ideal cell resource for myocardial cell
regeneration.
Acknowledgments
The authors would like to thank Professor Changyong
Wang (Department of Tissue Engineering, Institute of Basic Medical
Sciences and Tissue Engineering Research Center, Academy of
Military Medical Sciences, Beijing) for their equipment and
technical assistance.
References
|
1
|
Soonpaa MH and Field LJ: Survey of studies
examining mammalian cardiomyocyte DNA synthesis. Circ Res.
83:15–26. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Y and Weber KT: Infarct scar: A
dynamic tissue. Cardiovasc Res. 46:250–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taylor DO, Edwards LB, Aurora P, Christie
JD, Dobbels F, Kirk R, Rahmel AO, Kucheryavaya AY and Hertz MI:
Registry of the international society for heart and lung
transplantation: Twenty-fifth official adult heart transplant
report-2008. J Heart Lung Transplant. 27:943–956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Augoustides JG and Riha H: Recent progress
in heart failure treatment and heart transplantation. J
Cardiothorac Vasc Anesth. 23:738–748. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laflamme MA and Murry CE: Regenerating the
heart. Nat Biotechnol. 23:845–856. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Laflamme MA, Zbinden S, Epstein SE and
Murry CE: Cell-based therapy for myocardial ischemia and
infarction: Pathophysiological mechanisms. Annu Rev Pathol.
2:307–339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soonpaa MH, Koh GY, Klug MG and Field LJ:
Formation of nascent intercalated disks between grafted fetal
cardiomyocytes and host myocardium. Science. 264:98–101. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reinecke H, Zhang M, Bartosek T and Murry
CE: Survival, integration, and differentiation of cardiomyocyte
grafts: A study in normal and injured rat hearts. Circulation.
100:193–202. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koh GY, Soonpaa MH, Klug MG, Pride HP,
Cooper BJ, Zipes DP and Field LJ: Stable fetal cardiomyocyte grafts
in the hearts of dystrophic mice and dogs. J Clin Invest.
96:2034–2042. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Evans MJ and Kaufman MH: Establishment in
culture of pluripotential cells from mouse embryos. Nature.
292:154–156. 1981. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amit M, Carpenter MK, Inokuma MS, Chiu CP,
Harris CP, Waknitz MA, Itskovitz-Eldor J and Thomson JA: Clonally
derived human embryonic stem cell lines maintain pluripotency and
proliferative potential for prolonged periods of culture. Dev Biol.
227:271–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu C, Police S, Rao N and Carpenter MK:
Characterization and enrichment of cardiomyocytes derived from
human embryonic stem cells. Circ Res. 91:501–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mummery C, Ward-van Oostwaard D,
Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden
M, Opthof T, Pera M, de la Riviere AB, et al: Differentiation of
human embryonic stem cells to cardiomyocytes: Role of coculture
with visceral endoderm-like cells. Circulation. 107:2733–2740.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie CQ, Zhang J, Xiao Y, Zhang L, Mou Y,
Liu X, Akinbami M, Cui T and Chen YE: Transplantation of human
undifferentiated embryonic stem cells into a myocardial infarction
rat model. Stem Cells Dev. 16:25–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Caspi O, Huber I, Kehat I, Habib M, Arbel
G, Gepstein A, Yankelson L, Aronson D, Beyar R and Gepstein L:
Transplantation of human embryonic stem cell-derived cardiomyocytes
improves myocardial performance in infarcted rat hearts. J Am Coll
Cardiol. 50:1884–1893. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Robbins J, Gulick J, Sanchez A, Howles P
and Doetschman T: Mouse embryonic stem cells express the cardiac
myosin heavy chain genes during development in vitro. J Biol Chem.
265:11905–11909. 1990.PubMed/NCBI
|
|
17
|
Maltsev VA, Wobus AM, Rohwedel J, Bader M
and Hescheler J: Cardiomyocytes differentiated in vitro from
embryonic stem cells developmentally express cardiacspecific genes
and ionic currents. Circ Res. 75:233–244. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang Y, Min JY, Rana JS, Ke Q, Cai J, Chen
Y, Morgan JP and Xiao YF: VEGF enhances functional improvement of
postin-farcted hearts by transplantation of ESC-differentiated
cells. J Appl Physiol. 93:1140–1151. 2002. View Article : Google Scholar
|
|
19
|
Laflamme MA, Chen KY, Naumova AV, Muskheli
V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S,
et al: Cardiomyocytes derived from human embryonic stem cells in
pro-survival factors enhance function of infarcted rat hearts. Nat
Biotechnol. 25:1015–1024. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Laake LW, Passier R, Monshouwer-Kloots
J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard
D, Korving J, Tertoolen LG, et al: Human embryonic stem
cell-derived cardiomyocytes survive and mature in the mouse heart
and transiently improve function after myocardial infarction. Stem
Cell Res. 1:9–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Swijnenburg RJ, Tanaka M, Vogel H, Baker
J, Kofidis T, Gunawan F, Lebl DR, Caffarelli AD, de Bruin JL,
Fedoseyeva EV and Robbins RC: Embryonic stem cell immunogenicity
increases upon differentiation after transplantation into ischemic
myocardium. Circulation. 112(9 Suppl): I166–I172. 2005.PubMed/NCBI
|
|
22
|
He Q, Trindade PT, Stumm M, Li J,
Zammaretti P, Bettiol E, Dubois-Dauphin M, Herrmann F, Kalangos A,
Morel D, et al: Fate of undifferentiated mouse embryonic stem cells
within the rat heart: Role of myocardial infarction and immune
suppression. J Cell Mol Med. 13:188–201. 2009. View Article : Google Scholar
|
|
23
|
Nussbaum J, Minami E, Laflamme MA, Virag
JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H and Murry
CE: Transplantation of undifferentiated murine embryonic stem cells
in the heart:Teratoma formation and immune response. Fasbe J.
21:1345–1357. 2007. View Article : Google Scholar
|
|
24
|
Willadsen SM: Nuclear transplantation in
sheep embryos. Nature. 320:63–65. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cibelli JB, Stice SL, Golueke PJ, et al:
Transgenic bovine chimeric offspring produced from somatic
cell-derived stem-like cells. Nat Biotechnol. 16:642–646. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koh CJ and Atala A: Tissue engineering,
stem cells, and cloning: Opportunities for regenerative medicine. J
Am Soc Nephrol. 15:1113–1125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leor J, Gerecht S, Cohen S, Miller L,
Holbova R, Ziskind A, Shachar M, Feinberg MS, Guetta E and
Itskovitz-Eldor J: Human embryonic stem cell transplantation to
repair the infarcted myocardium. Heart. 93:1278–1284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cooke MJ, Stojkovic M and Przyborski SA:
Growth of teratomas derived from human pluripotent stem cells is
influenced by the graft site. Stem Cells Dev. 15:254–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prokhorova TA, Harkness LM, Frandsen U,
Ditzel N, Schrøder HD, Burns JS and Kassem M: Teratoma formation by
human embryonic stem cells is site dependent and enhanced by the
presence of Matrigel. Stem Cells Dev. 18:47–54. 2009. View Article : Google Scholar
|
|
30
|
Toumadje A, Kusumoto K, Parton A, Mericko
P, Dowell L, Ma G, Chen L, Barnes DW and Sato JD: Pluripotent
differentiation in vitro of murine ES-D3 embryonic stem cells. In
Vitro Cell Dev Biol Anim. 39:449–453. 2003. View Article : Google Scholar
|
|
31
|
Miyahara Y, Nagaya N, Kataoka M, Yanagawa
B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, et
al: Monolayered mesenchymal stem cells repair scarred myocardium
after myocardial infarction. Nat Med. 12:459–465. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Q, Fu Q, Zhang Y, Wang H, Liu Z, Zhou
J, Duan C, Wang Y, Wu K and Wang C: Tumourigenesis in the infarcted
rat heart is eliminated through differentiation and enrichment of
the transplanted embryonic stem cells. Eur J Heart Fail.
12:1179–1185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reubinoff BE, Pera MF, Fong CY, Trounson A
and Bongso A: Embryonic stem cell lines from human blastocysts:
Somatic differentiation in vitro. Nat Biotechnol. 18:399–404. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thomson JA, Itskovitz-Eldor J, Shapiro SS,
Waknitz MA, Swiergiel JJ, Marshall VS and Jones JM: Embryonic stem
cell lines derived from human blastocysts. Science. 282:1145–1147.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laflamme MA, Gold J, Xu C, Hassanipour M,
Rosler E, Police S, Muskheli V and Murry CE: Formation of human
myocardium in the rat heart from human embryonic stem cells. Am J
Pathol. 167:663–671. 2005. View Article : Google Scholar : PubMed/NCBI
|