Introduction
Ulcerative colitis, a type of inflammatory bowel
disease, is characterized by uncontrolled inflammation in the colon
and rectum, recurrent episodes of bloody diarrhea, cramping,
abdominal pain, mucosal inflammation and injury (1). Considering the pathogenic mechanisms
of ulcerative colitis, it is suggested that deregulation of the
pro-/anti-inflammatory systems and antioxidant system may to be
involved (2). Currently, the
medical treatment of ulcerative colitis relies predominantly on the
use of traditional drugs, including aminosalicylates,
corticosteroids and immunosuppressants. These conventional
therapies for ulcerative colitis fail to successfully induce
remission or prevent relapse, and can also cause various side
effects (3). Therefore, there is
an increasing interest in identifying alternative and more
tolerable treatments for this disease.
Ursolic acid (UA), a natural pentacyclic
triterpenoid carboxylic acid, is abundant in numerous plants,
including apples, blueberries and haw thorn berries, and is the
major component of certain oriental medicinal herbs, which are
widely used in folk medicine (4).
UA is well known to possess various biological activities,
including anti-inflammatory (5,6),
anticancer (7,8), hypoglycemic (9), antioxidant (10–12)
and immunomodulatory activities (13). Previously, UA was reported to be
involved in colitis in a number of publications (4,14,15).
Hawthorn berry extract containing 0.5% UA decreased edema and the
infiltration of neutrophils in the colonic tissues of acetic
acid-treated rats (14). UA
significantly inhibits the production of pro-inflammatory
cytokines, including tumor necrosis factor (TNF)-α, interleukin
(IL)-1β and IL-6, and the expression of cyclooxygenase-2 and
inducible nitric oxide (NO) synthetase in lipopolysacharride
(LPS)-stimulated macrophages and colonic tissues from
2,4,6-trinitrobenzenesulfonic-treated mice (4,15).
UA also exerts an inhibitory effect on the activation of nuclear
factor (NF)-κB in human intestinal epithelial cells and macrophages
(4,15). These results indicate a protective
role of UA in colitis. However, the detailed underlying mechanisms
remain to be fully elucidated.
The present study aimed to investigate the
protective effect of UA in a dextran sulfate sodium (DSS)-induced
mouse model of ulcerative colitis, a widely used ulcerative colitis
model (16), in order to elucidate
the potential mechanisms underlying the anti-inflammatory and
antioxidative activities of UA.
Materials and methods
Reagents
UA was purchased from Shanxi Huike Plant Development
Co., Ltd. (Xi'an, China; purity ≥98%), dissolved in
dimethyl-sulphoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA), and
diluted in normal saline solution. The total quantity of DMSO did
not exceed 1% upon assessment; a dose considered of no significance
in the assays used. DSS was obtained from MP Biomedicals (Solon,
OH, USA) and dissolved in distilled water. Chemiluminescence
reagent was purchased from Pierce (Rockford, IL, USA). Rabbit
anti-NF-κB p65 monoclonal antibody (cat. no. 4764) and rabbit
anti-β-actin polyclonal antibody (cat. no. 4967) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Horseradish peroxidase (HRP)-conjugated goat anti-rabbit
immunoglobulin (Ig)G secondary antibody (cat. no. sc-2004) was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, Texas,
USA).
Animals
A total of 36 male BALB/c mice (7-week-old; 18–22 g)
were obtained from the Experiment Animal Center, Liaoning Medical
University (Jinzhou, China). The mice were allowed to adapt to the
laboratory environment for 1 week prior to experiments. The animals
were housed in plastic cages containing corn chip bedding, and were
maintained on a 12-h light-dark cycle (07:00–19:00 h light cycle;
19:00–07:00 h dark cycle) with a room temperature of 22±1°C and a
humidity of 65–70%. Water and food were available ad
libitum. The animal experiments were approved by the Ethical
and Research Committee of Liaoning Medical University (Jinzhou,
China).
Induction of ulcerative colitis and UA
treatment
DSS was used to induce ulcerative colitis in the
present study. DSS-induced colitis is characterized by the mucosal
infiltration of inflammatory cells, epithelial injury and
ulceration, which are similar to acute and chronic ulcerative
colitis in humans (17). In the
present study, the mice were divided into four groups, as follows
(9 mice/group): Control, DSS, DSS+UA and DSS+sulfasalazine [SFZ; a
reference drug in colitis treatment (2)]. Colitis was induced by the provision
of distilled water containing 5% DSS (w/v) for 7 days ad
libitum, followed by fresh water. The mice in the DSS+UA and
DSS+SFZ groups were then treated with UA (20 mg/kg) or SFZ (100
mg/kg), respectively, by gavage once per day for another 7 days.
The mice in the DSS group were provided with the same volume of
saline by gavage. The mice were assessed daily for behavior, body
weight, presence of blood in stools and stool consistency.
Evaluation of disease activity index
(DAI)
The DAI was calculated for each animal, as reported
previously (18). Briefly, the DAI
was determined by combining the scores assigned for body weight
loss, stool consistency and stool blood, with the mean of the three
values deemed the DAI. A higher DAI score is indicative of more
severe colon damage. Body weight loss was calculated as the
percentage difference between the initial body weight and the final
body weight. Stool blood was determined using the Fecal Occult
Blood Test kit (Baso Diagnostics Inc., Zhuhai, China). The
parameters investigated for the DAI evaluation are listed in
Table I.
 | Table IParameters of disease activity index
evaluation. |
Table I
Parameters of disease activity index
evaluation.
| Score | Weight loss
(%) | Stool
consistency | Blood in stool |
|---|
| 0 | – | Normal | − |
| 1 | 1–5 | Normal | + |
| 2 | 6–10 | Very soft but
formed | ++ |
| 3 | 11–15 | Liquid | +++ |
| 4 | >15 | Liquid | Gross rectal
bleeding |
Measurement of serum IL-1β and TNF-α
Blood samples (0.5 ml/mouse) were collected from all
mice following anesthetization with 0.1 ml phenobarbital
(Sigma-Aldrich) and sacrifice by decapitation. Serum was collected
following centrifugation at 2,000 × g for 10 min at 4°C and
aliquoted. The aliquots (150 μl) were stored at −20°C until
use. The serum levels of IL-1β and TNF-α were measured using ELISA
kits (cat. nos. EK0394 and EK0527, respectively; Wuhan Boster
Biological Engineering Co., Ltd., Wuhan, China), according to the
manufacturer's protocol.
Assessment of pathological damage
For histological analysis, the colons following
collection of blood samples and rinsed with phosphate-buffered
saline (PBS; pH 7.4). From each colon, two 2-cm long segments were
removed from the proximal and distal ends, which were fixed in 4%
paraformaldehyde (Sigma-Aldrich), dehydrated and embedded in
paraffin (Sigma-Aldrich). These colon segments were cut
longitudinally into 4-μm thick sections, which were then
stained with hematoxylin and eosin (H&E; Leica Microsystems,
Inc., Buffalo Grove, IL, USA). The colon sections were interpreted
according to the criteria listed in Table II. The final colon pathological
score was calculated as the average of the scores from the proximal
and distal segments of the mouse colons.
 | Table IIPathological scores of colitis in
colon tissues. |
Table II
Pathological scores of colitis in
colon tissues.
| Score | Pathological
parameter |
|---|
| 0 | Normal tissues |
| 1 | Mild inflammation
in the mucosa with some infiltrating mononuclear cells |
| 2 | Increased level of
inflammation in the mucosa with more infiltrating cells, crypt
glands pulled away from basement membrane, mucin depletion from
goblet cells, epithelium beginning to pull away from mucosa into
lumen |
| 3 | Extensive
infiltrating cells in the mucosa and submucosa area, crypt
abscesses present, increased mucin depletion, epithelial cell
disruption |
| 4 | Extensive
infiltrating cells in the tissue, complete loss of crypts |
Measurement of malondialdehyde (MDA)
content, and superoxide dismutase (SOD) and myeloperoxidase (MPO)
activities
The transverse colon tissues were rinsed and
weighed, and were then placed into tubes with nine volumes of
normal saline. The tissue samples were then homogenized. Following
centrifugation at 3,000 × g for 10 min at 4°C, the MDA content, and
activities of SOD and MPO in the supernatant, were measured using
commercially available kits (cat. nos. A003, A001 and A044,
respectively; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China), according to the manufacturer's protocol.
Western blotting for the protein
expression of NF-κB p65
The nuclear proteins in the transverse colon tissues
were extracted using a kit, according to the manufacturer's
protocol (Wuhan Boster Biological Engineering Co., Ltd.). The
protein concentration was quantified using a bicinchoninic acid
assay (Thermo Fisher Scientifc, Inc., Waltham, MA, USA). The
nuclear protein expression of NF-κB p65 was measured using Western
blotting, in accordance with a previous report (19). Briefly, the nuclear proteins (20
μg) were separated electrophoretically on 4–12% SDS-PAGE
gels, and transferred onto nitrocellulose membranes (Thermo Fisher
Scientific, Inc.). Following 30 min of blocking with 2.5% nonfat
milk, the membranes were incubated with rabbit anti-NF-κB p65
monoclonal antibody (1:1,000) and rabbit anti-β-actin polyclonal
antibody (1:2,000) at 4°C overnight, followed by 1 h incubation
with HRP-conjugated goat anti-rabbit IgG (1:2,000). The membranes
were washed with PBS containing 0.5% Tween 20 (Sigma-Aldrich)
subsequent to each antibody treatment. The membranes were developed
using chemiluminescence reagent and then exposed to X-ray film
(Midwest Scientific, Valley Park, MO, USA). The band intensities
were analyzed using ImageJ software, version 1.5g (https://imagej.nih.gov/ij/). The protein levels of p65
are expressed as the ratio of the band optical intensity to that of
β-actin.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis was performed using SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). One-way
analysis of variance and Dunnett's test were used for group
comparisons. P<0.05 was considered to indicate a statistically
different difference.
Results
Effects of UA on DAI and histological
injury
The administration of 5% DSS in mice for 7 days
resulted in marked clinical and histological signs of colitis.
These mice exhibited loose stools or diarrhea, occult or gross
rectal bleeding, and weight loss. The DAI score of colitis in the
DSS-treated mice was significantly increased with time, and
increased continuously, even following the termination of DSS
treatment (Fig. 1A). Following 7
days of DSS administration, the mice were treated with UA (20 mg/kg
daily) for another 7 days. UA treatment significantly reduced
DSS-induced DAI following 3 days of treatment with UA (day 10), and
this reduction was more marked following 7 days of treatment
(P<0.05; Fig 1A). SFZ, a
well-known prodrug, which releases 5-aminosalicylic acid in the
intestine to disturb the prostaglandin and leukotriene pathways,
has been the most widely-prescribed anti-colitis drug (2). As a reference drug, SFZ (100 mg/kg
daily) also significantly decreased the DSS-induced DAI in the
present study (P<0.05; Fig
1A).
The administration of DSS in the mice for 7 days
caused marked colon tissue damage. H&E staining of the colon
tissues exhibited extensive infiltrating cells, epithelial cell
disruption or the complete loss of crypts in the DSS group
(Fig. 1B). In the DSS group, the
histopathological score was increased to 3.67, which was
significantly increased, as compared with the score of 0 in the
control group (P<0.05; Fig.
1C). UA treatment significantly improved the DSS-induced colon
histological injury, and decreased the pathological score to 1.29
(P<0.05). The reference drug, SFZ, also improved histological
injury, with a pathological score of 2.17 (P<0.05, vs. DSS
group). The results from the DAI and pathological examinations
demonstrated the protective role of UA in colitis.
UA decreases MDA content and MPO
activity, and increases SOD activity in colon tissues
The present study then examined the possible
mechanisms by which UA offers protection from colitis. As UA
exhibits antioxidant activity in certain cell models or cell-free
models (10–12), the present study aimed to determine
whether UA interferes in antioxidant ability in colon tissues. The
content of M DA, a marker of lipid peroxidative damage, and
activity of SOD, an important anti-oxidative enzyme were measured.
As shown in Fig. 2A and B, DSS
administration significantly decreased SOD activity and increased
MDA content in the colon tissues, compared with the control group
(P<0.05). Treatment with UA significantly reversed the
DSS-induced changes in SOD activity and MDA content (P<0.05).
SFZ also induced a significant decrease in DSS-stimulated MDA
content, and a non-significant increase in SOD activity. Hawthorn
berry extract, a source of UA, has been found to decrease
neutrophil infiltration in colon tissues in a colitis model
(14). In the present study, MPO
activity, an important maker of neutrophils, was measured to
examine the effect of UA on neutrophil infiltration. As shown in
Fig. 2C, UA significantly
decreased colonic MPO activity stimulated by DSS administration.
Simultaneously, MPO activity was also reduced by SFZ treatment.
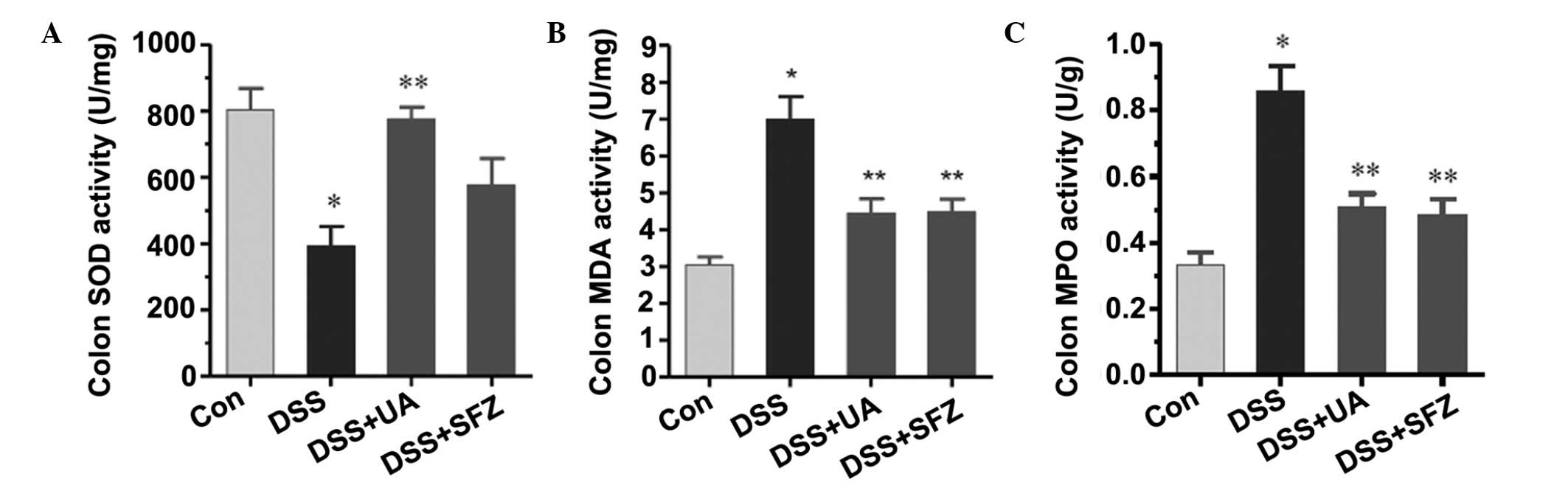 | Figure 2Effects of UA on MDA content, and SOD
and MPO activities in colon tissues. The mice were treated with DSS
for 7 days, and then with UA or SFZ for a further 7 days. Colon
homogenates were used to measure (A) SOD activity, (B) MDA content
and (C) MPO activity. The results are expressed as MDA content, and
SOD and MPO activity per mg colon tissue, as the mean ± standard
error of the mean (n=6–9). *P<0.05, vs. Con group;
**P<0.05, vs. DSS group. UA, ursolic acid; DSS,
dextran sulfate sodium; SFZ, sulfasalazine; MDA, malondialdehyde;
SOD, superoxide dismutase; MPO; myeloperoxidase. |
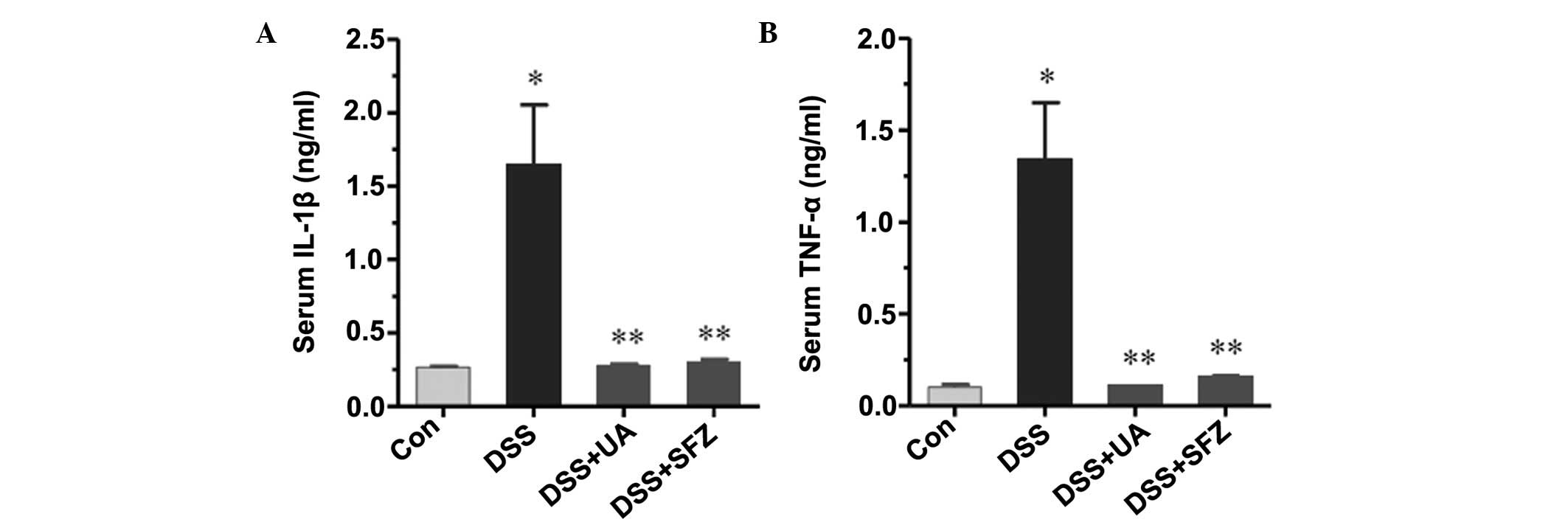
UA decreases the expression of
pro-inflammatory cytokines
DSS induced a marked inflammatory response in the
colon tissues, which was significantly improved by UA treatment.
Thus, the present study examined whether UA decreases the levels of
pro-inflammatory cytokines. The levels of IL-1β and TNF-α cytokines
were measured using ELISA. As shown in Fig. 3, DSS administration significantly
increased the serum level of IL-1β, compared with the control group
(P<0.01). This increase in the level of IL-1β was significantly
decreased by UA or SFZ treatment (P<0.05). Similar to the
results of IL-1β, DSS administration increased the serum level of
TNF-α, which was also significantly decreased by UA or SFZ
treatment.
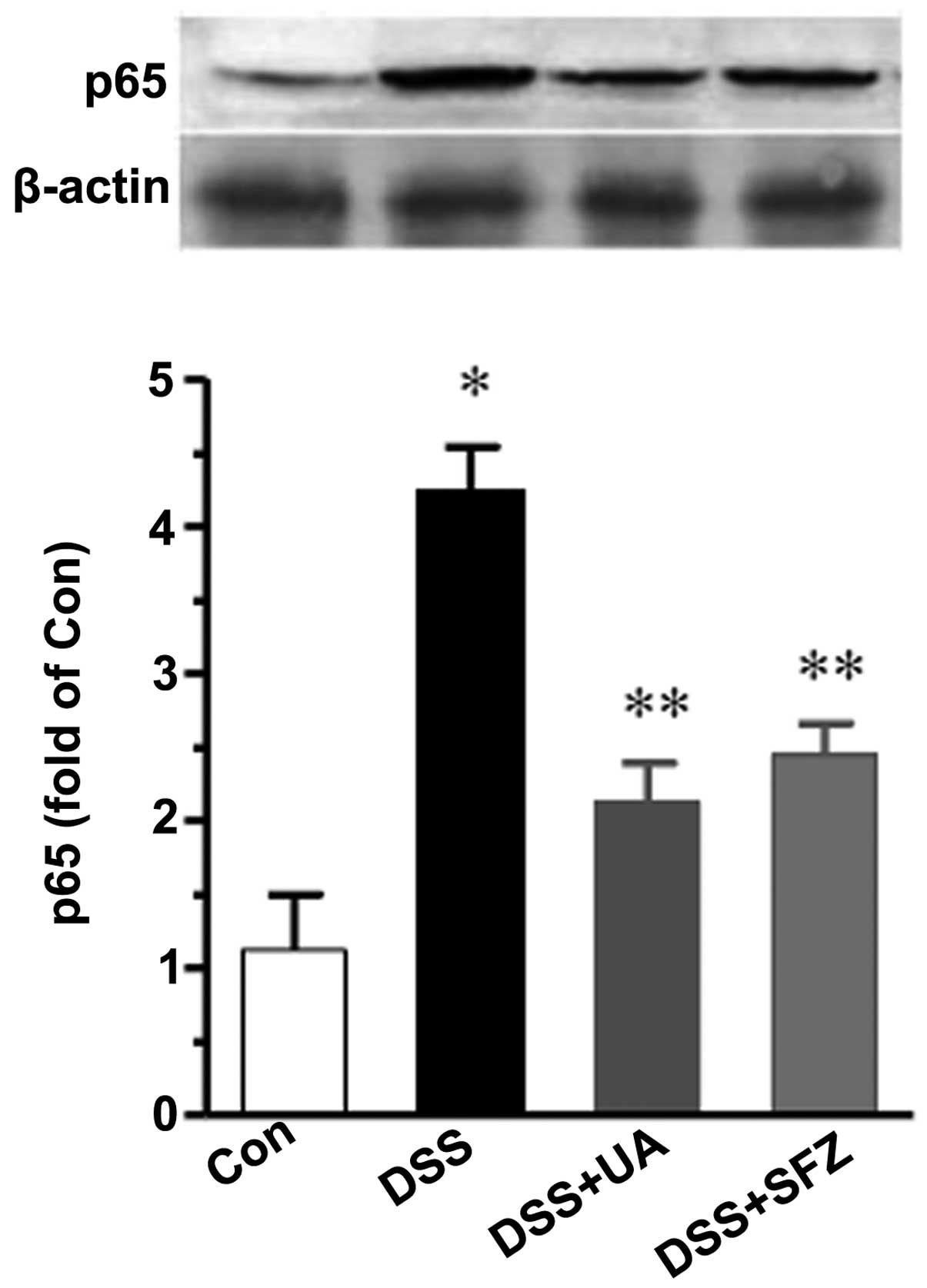
UA decreases the level of NFκB p65
The expression levels of pro-inflammatory factors,
including IL-1β and TNF-α is closely regulated by transcription
factor activity, particularly NF-κB. Thus, the present study
examined whether UA alters the activity of NF-κB in colon tissues
using Western blotting. As shown in Fig. 4, the nuclear level of NF-κB p65 was
markedly increased by DSS, compared with the control group
(P<0.01). Treatment with UA or SFZ resulted in a significant
reduction of p65 (P<0.05).
Discussion
The present study aimed to evaluate the role of UA
in DSS-induced colitis and the underlying mechanisms. The results
showed that UA significantly improved DSS-induced colitis in mice,
which was shown by improvements in body weight, stool consistency,
rectal bleeding and pathologic involvement of colon tissues, and a
decrease in neutrophils infiltration reflected by MPO activity. UA
also decreased serum levels of IL-1β and TNF-α, decreased MDA
content and increased SOD activity in colon. Finally, UA
downregulated DSS-stimulated nuclear expression of transcription
factor NF-κB. These results demonstrated a protective role of UA in
experimental colitis. Antioxidant and anti-inflammatory activity
may underlie the mechanisms of action.
Reactive oxygen species (ROS) is an important factor
involved in ulcerative colitis. Oxidative stress and its consequent
lipid peroxidation can aggravate free radical chain reactions,
disrupt the integrity of intestinal mucosa barrier and activate
pro-inflammatory responses. MDA, a marker of lipid peroxidative
damage, and SOD, an important antioxidative enzyme, have been
reported to increase and decrease in experimental colitis studies,
respectively (20,21). Antioxidative and anti-inflammatory
treatment improved these effects on the MDA level and SOD activity
in experimental ulcerative colitis (20,21).
UA has showed beneficial effects on SOD activity and
MDA content in various cells and animal models. UA pretreatment
significantly reverses H2O2- and
1-Methyl-4-phenylpyridinium-induced impairment in SOD activity, and
decreases MDA formation in PC12 glial cells (22). UA increases SOD in Ehrlich ascites
carcinoma tumor (23). UA has also
demonstrated a neuroprotective effect on D-gal induced
neurotoxicity in mice via, at least in part, increases in the
activity of SOD enzymes with a reduction in MDA content (24). UA has also shown a protective
effect in chronic ethanol-induced oxidative stress in the rat heart
via the improvement of SOD activity (25). In the present study, SOD activity
was decreased and MDA content was increased in the colon tissues of
the DSS group, compared with those of the control group. This
indicated that DSS administration damaged the antioxidative
ability, which may subsequently cause the onset of colitis onset.
This is consistent with previous reports (26,27).
In the present study, UA treatment for 7 days significantly
improved the increased MDA content and decreased SOD activity in
the colon tissues. These results suggested that antioxidative
protection may be an important mechanism for UA in colitis.
Furthermore, the accumulation of ROS in colon tissues, in addition
to directly damaging intestinal epithelial cells, stimulates
inflammatory responses to release pro-inflammatory cytokines,
particularly TNF-α and IL-1β (28,29),
which may cause further damage.
Ulcerative colitis is a type of inflammatory bowel
disease. It is characterized by pro-inflammatory cell infiltration
and pro-inflammatory cytokine release. Several cytokines, including
TNF-α, IL-1β and inducible NO synthetase are crucial components of
these inflammatory pathways (30).
In the present study, the levels of TNF-α and IL-1β were markedly
upregulated during the onset of DSS-induced colitis induction.
Treatment of ulcerative colitis commonly reduces inflammatory cell
infiltration and the levels of serum TNF-α and IL-1β (31,32).
In particular, treatment with TNF-α neutralizing antibody or TNF-α
inhibitory agents significantly improveds colitis (33,34).
Therefore, suppression of these cytokines can offer an alternative
therapy for ulcerative colitis. In the present study, when UA was
applied in the DSS-administrated mice, the serum levels of TNF-α
and IL-1β were significantly decreased. Given the improvement of
clinical features and pathological features by UA, UA may act an
inflammation alleviator in the treatment of ulcerative colitis. Of
note, UA has also been found to decrease various pro-inflammatory
markers, including TNF-α, IL-1β, IL-6, IL-17 and cyclooxygenase-2,
and ameliorate inflammatory injury in experimental nephritis and
hepatitis models (35,36).
In the present study, it was also demonstrated that
UA significantly decreased DSS-stimulated nuclear levels of NF-κB
p65 in colon tissues. NF-κB is a dimeric transcription factor
containing p50 and p65 subunits, and p65 nuclear translocation
represents the activation state of NF-κB. NF-κB activates the
expression of several genes involved in inflammatory process,
including IL-1β and TNF-α. In earlier studies, UA showed a
suppressive effect on NF-κB activity in several disease models. UA
protects against carbon tetrachloride-induced injury in the kidney
and liver via inhibition of NF-κB activities (35,36).
UA also inhibits T cell activation and proliferation via inhibition
of the NF-κB signaling pathway (37). The administration of UA also
decreases the product of lipid peroxidation and decreases the
expression of NF-κB following stroke in mice (38). UA also markedly inhibits serum
levels of TNF-α, IL-6 and IL-1β, decrease MAD levels and attenuates
the protein expression of NF-κB in the lungs of LPS-treated mice
(39). In terms of the
gastrointestinal system, UA has a direct inhibitory effect on NF-κB
activation in intestinal epithelial cells stimulated by TNF-α, and
in peritoneal macrophages stimulated by LPS (4). UA also suppresses NF-κB signaling in
colon cancer cells (7), and other
types of cancer cell (40,41). In the present study, the nuclear
level of NF-κB p65 in the colon tissues of the DSS group was
significantly increased, compared with that of the control group.
The increased nuclear level of NF-κB p65 was significantly reversed
by UA treatment. This result, together with the direct inhibitory
effects of UA on NF-κB signaling in intestinal epithelial cells and
colon cancer cells described above, indicated that NF-κB signaling
system may be important in the induction of colitis, and act an
important mechanism in the protective effects of UA. Although the
cause or subsequent effects of NF-κB suppression and inflammation
improvement remains to be fully elucidated, UA treatment not only
decreased the nuclear expression of NF-κB, but also downregulated
the production of IL-1β and TNF-α, and thereby ameliorated the
severity of the colitis. Therefore, supplementation with UA may be
an effcacious and promising agent in the treatment of ulcerative
colitis.
In conclusion, the present results demonstrated that
UA exerted a beneficial effect on DSS-induced colitis in mice. As a
possible mechanism, UA improved the SOD activity, increased
scavenging of oxidative-free radicals, and downregulated the levels
of cytokines, including TNF-α and IL-1β. The suppression of the
NF-κB transcription system may underlie the mechanism of the
anti-inflammatory ability of UA.
Acknowledgments
This study was supported by the Research Startup
Fund of Liaoning Medical University for Doctors and Teachers with
two and more year-training abroad (grant no. Y2012B014) and the
Youth Science and Technology Startup Fund of the First Affiliated
Hospital of Liaoning Medical University (grant no. FY2012-17).
References
|
1
|
Furrie E, Macfarlane S, Cummings JH and
Macfarlane GT: Systemic antibodies towards mucosal bacteria in
ulcerative colitis and crohn's disease differentially activate the
innate immune response. Gut. 53:91–98. 2004. View Article : Google Scholar
|
|
2
|
Sakthivel KM and Guruvayoorappan C:
Protective effect of Acacia ferruginea against ulcerative colitis
via modulating inflammatory mediators, cytokine profile and NF-κB
signal transduction pathways. J Environ Pathol Toxicol Oncol.
33:83–98. 2014. View Article : Google Scholar
|
|
3
|
Ooi CJ and Sands BE: Treatment of
ulcerative colitis. Curr Opin Gastroenterol. 15:298–301. 1999.
View Article : Google Scholar
|
|
4
|
Chun J, Lee C, Hwang SW, Im JP and Kim JS:
Ursolic acid inhibits nuclear factor-κB signaling in intestinal
epithelial cells and macrophages and attenuates experimental
colitis in mice. Life Sci. 110:23–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baricevic D, Sosa S, Della Loggia R,
Tubaro A, Simonovska B, Krasna A and Zupancic A: Topical
anti-inflammatory activity of Salvia officinalis L. leaves: The
relevance of ursolic acid. J Ethnopharmacol. 75:125–132. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ku CM and Lin JY: Anti-inflammatory
effects of 27 selected terpenoid compounds tested through
modulating Th1/Th2 cytokine secretion profiles using murine primary
splenocytes. Food Chem. 141:1104–1113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Liu L, Qiu H, Zhang X, Guo W, Chen
W, Tian Y, Fu L, Shi D, Cheng J, et al: Ursolic acid simultaneously
targets multiple signaling pathways to suppress proliferation and
induce apoptosis in colon cancer cells. PLoS One. 8:e638722013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang Q, Ji Q, Tang Y, Chen T, Pan G, Hu S,
Bao Y, Peng W and Yin P: Mitochondrial translocation of cofilin-1
promotes apoptosis of gastric cancer BGC-823 cells induced by
ursolic acid. Tumour Biol. 35:2451–2459. 2014. View Article : Google Scholar
|
|
9
|
Alqahtani A, Hamid K, Kam A, Wong KH,
Abdelhak Z, Razmovski-Naumovski V, Chan K, Li KM, Groundwater PW
and Li GQ: The pentacyclic triterpenoids in herbal medicines and
their pharmacological activities in diabetes and diabetic
complications. Curr Med Chem. 20:908–931. 2013.
|
|
10
|
D'Abrosca B, Fiorentino A, Monaco P and
Pacifico S: Radical-scavenging activities of new hydroxylated
ursane triterpenes from cv. Annurca apples. Chem Biodivers.
2:953–958. 2005. View Article : Google Scholar
|
|
11
|
Ali MS, Ibrahim SA, Jalil S and Choudhary
MI: Ursolic acid: A potent inhibitor of superoxides produced in the
cellular system. Phytother Res. 21:558–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
do Nascimento PG, Lemos TL, Bizerra AM,
Arriaga ÂM, Ferreira DA, Santiago GM, Braz-Filho R and Costa JG:
Antibacterial and antioxidant activities of ursolic acid and
derivatives. Molecules. 19:1317–1327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raphael TJ and Kuttan G: Effect of
naturally occurring triterpenoids glycyrrhizic acid, ursolic acid,
oleanolic acid and nomilin on the immune system. Phytomedicine.
10:483–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malekinejad H, Shafie-Irannejad V,
Hobbenaghi R, Tabatabaie SH and Moshtaghion SM: Comparative
protective effect of hawthorn berry hydroalcoholic extract,
atorvastatin and mesalamine on experimentally induced colitis in
rats. J Med Food. 16:593–601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang SE, Jeong JJ, Hyam SR, Han MJ and Kim
DH: Ursolic acid isolated from the seed of Cornus officinalis
ameliorates colitis in mice by inhibiting the binding of
lipopolysaccharide to Toll-like receptor 4 on macrophages. J Agric
Food Chem. 62:9711–9721. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal infammation. Nat
Protoc. 2:541–546. 2007. View Article : Google Scholar
|
|
17
|
Hendrickson BA, Gokhale R and Cho JH:
Clinical aspects and pathophysiology of inflammatory bowel disease.
Clin Microbiol Rev. 15:79–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aldini R, Micucci M, Cevenini M, Fato R,
Bergamini C, Nanni C, Cont M, Camborata C, Spinozzi S, Montagnani
M, et al: Antiinflammatory effect of phytosterols in experimental
murine colitis model: Prevention, induction, remission study. PLoS
One. 9:e1081122014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Wang J, Wang Y, Fu Q, Lei YH, Nie
ZY, Qiu J and Bao TY: Protective effect of exogenous matrix
metalloproteinase-9 on chronic renal failure. Exp Ther Med.
7:329–334. 2014.PubMed/NCBI
|
|
20
|
Tahan G, Aytac E, Aytekin H, Gunduz F,
Dogusoy G, Aydin S, Tahan V and Uzun H: Vitamin E has a dual effect
of anti-inflammatory and antioxidant activities in acetic
acid-induced ulcerative colitis in rats. Can J Surg. 54:333–338.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou YH, Yu JP, Liu YF, Teng XJ, Ming M,
Lv P, An P, Liu SQ and Yu HG: Effects of Ginkgo biloba extract on
infammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in
TNBS-induced colitis in rats. Mediators Inflamm. 5:926422006.
|
|
22
|
Tsai SJ and Yin MC: Antioxidative and
anti-inflammatory protection of oleanolic acid and ursolic acid in
PC12 cells. J Food Sci. 73:H174–H178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saraswati S, Agrawal SS and Alhaider AA:
Ursolic acid inhibits tumor angiogenesis and induces apoptosis
through mitochondrial-dependent pathway in Ehrlich ascites
carcinoma tumor. Chem Biol Interact. 206:153–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu J, Zheng YL, Wu DM, Luo L, Sun DX and
Shan Q: Ursolic acid ameliorates cognition deficits and attenuates
oxidative damage in the brain of senescent mice induced by
D-galactose. Biochem Pharmacol. 74:1078–1090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saravanan R and Pugalendi V: Impact of
ursolic acid on chronic ethanol-induced oxidative stress in the rat
heart. Pharmacol Rep. 58:41–47. 2006.PubMed/NCBI
|
|
26
|
Zhao J, Hong T, Dong M, Meng Y and Mu J:
Protective effect of myricetin in dextran sulphate sodium-induced
murine ulcerative colitis. Mol Med Rep. 7:565–570. 2013.
|
|
27
|
Farombi EO, Adedara IA, Awoyemi OV, Njoku
CR, Micah GO, Esogwa CU, Owumi SE and Olopade JO: Dietary
protocatechuic acid ameliorates dextran sulphate sodium-induced
ulcerative colitis and hepatotoxicity in rats. Food Funct.
7:913–921. 2016. View Article : Google Scholar
|
|
28
|
Lee JH, Lee B, Lee HS, Bae EA, Lee H, Ahn
YT, Lim KS, Huh CS and Kim DH: Lactobacillus suntoryeus inhibits
pro-inflammatory cytokine expression and TLR-4-linked NF-kappaB
activation in experimental colitis. Int J Colorectal Dis.
24:231–237. 2009. View Article : Google Scholar
|
|
29
|
Zhang DK, Yu JJ, Li YM, We i LN, Yu Y,
Feng YH and Wang X: A Picrorhiza kurroa derivative, picroliv,
attenuates the development of dextran-sulfate-sodium-induced
colitis in mice. Mediators Inflamm. 2012:7516292012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oishi M, Tokuhara K, Miki H, Tanaka Y,
Yamaki S, Kaibori M, Yoshizawa K, Yuri T, Yoshigai E, Nishizawa M,
et al: Temporal and spatial dependence of inflammatory biomarkers
and suppression by fluvastatin in dextran sodium sulfate-induced
rat colitis model. Dig Dis Sci. 59:2126–2135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang ZL, Fan HY, Yang MY, Zhang ZK and
Liu K: Therapeutic effect of a hydroxynaphthoquinone fraction on
dextran sulfate sodium-induced ulcerative colitis. World J
Gastroenterol. 20:15310–15318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fakhoury M, Coussa-Charley M, Al-Salami H,
Kahouli I and Prakash S: Use of artificial cell microcapsule
containing thalidomide for treating TNBS-induced Crohn's disease in
mice. Curr Drug Deliv. 11:146–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dharmani P, Leung P and Chadee K: Tumor
necrosis factor-α and Muc2 mucin play major roles in disease onset
and progression in dextran sodium sulphate-induced colitis. PLoS
One. 6:e250582011. View Article : Google Scholar
|
|
34
|
Lv R, Qiao W, Wu Z, Wang Y, Dai S, Liu Q
and Zheng X: Tumor necrosis factor alpha blocking agents as
treatment for ulcerative colitis intolerant or refractory to
conventional medical therapy: A meta-analysis. PLoS One.
9:e866922014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma JQ, Ding J, Xiao ZH and Liu CM: Ursolic
acid ameliorates carbon tetrachloride-induced oxidative DNA damage
and inflammation in mouse kidney by inhibiting the STAT3 and NF-κB
activities. Int Immunopharmacol. 21:389–395. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma JQ, Ding J, Zhang L and Liu CM: Ursolic
acid protects mouse liver against CCl4-induced oxidative stress and
inflammation by the MAPK/NF-κB pathway. Environ Toxicol Pharmacol.
37:975–983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zeng G, Chen J, Liang QH, You WH, Wu HJ
and Xiong XG: Ursolic acid inhibits T-cell activation through
modulating nuclear factor-κB signaling. Chin J Integr Med.
18:34–39. 2012. View Article : Google Scholar
|
|
38
|
Li L, Zhang X, Cui L, Wang L, Liu H, Ji H
and Du Y: Ursolic acid promotes the neuroprotection by activating
Nrf2 pathway after cerebral ischemia in mice. Brain Res.
1497:32–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen X, Wan Y, Zhou T, Li J and Wei Y:
Ursolic acid attenuates lipopolysaccharide-induced acute lung
injury in a mouse model. Immunotherapy. 5:39–47. 2013. View Article : Google Scholar
|
|
40
|
Gai L, Cai N, Wang L, Xu X and Kong X:
Ursolic acid induces apoptosis via Akt/NF-κB signaling suppression
in T24 human bladder cancer cells. Mol Med Rep. 7:1673–1677.
2013.PubMed/NCBI
|
|
41
|
Liu K, Guo L, Miao L, Bao W, Yang J, Li X,
Xi T and Zhao W: Ursolic acid inhibits epithelial-mesenchymal
transition by suppressing the expression of astrocyte-elevated
gene-1 in human nonsmall cell lung cancer A549 cells. Anticancer
Drugs. 24:494–503. 2013. View Article : Google Scholar : PubMed/NCBI
|