Introduction
Alzheimer's disease (AD) remains the most severe
form of neurodegenerative disease, and is characterized by a
decline in memory performance and other cognitive abilities. It is
estimated that ~25,000,000 individuals are affected worldwide,
particularly in the elderly population (1). At present, there is no cure for AD,
however, certain symptomatic therapeutics are available (2–4).
Although the etiology of AD remains to be fully elucidated, there
is a general consensus in favor of the plaque hypothesis. The
hallmark appearance of amyloid plaques and intracellular
neurofibrillary tangles of Tau are reported to contribute to neuron
loss in AD (5,6). The formation of β-amyloid plaques in
AD patients affects neuronal synaptic plasticity in the early
phase, and progressively leads to cell death in the later phase
(7–9). Therefore, plaque formation and
synaptic plasticity, particularly in the hippocampus, which is a
region responsible for memory formation, are critical indices in
evaluating anti-AD efficacy.
The use of Chinese medicines has a long-term history
in clinical practice, and has been suggested to offer potential in
improving memory (10,11). Among the effective herbs, ginseng
is considered to promote the health of middle-aged and elderly
populations (12). Ginseng has
been used as an adaptogenic herb in traditional Chinese medicine
for >2,000 years, and the long-term application of ginseng
improves the ability to combat stress, trauma, anxiety and fatigue
(13). Additional pharmacological
activities, including in the prevention of cancer and
neurodegenerative diseases, have also been reported (14,15).
Ginsenosides, the active compounds of the Panax species,
have been widely investigated in basic and clinical settings. A
substantial number of ginsenosides have been found to improve the
decline in memory induced by lipopolysaccharide or okadaic acid
(16–19). However, the effects of ginsenosides
on memory decline induced by genetic interruption have not been
reported, particularly its mechanisms. A previous study suggested
that ginsenoside Rg1 is able to pass through blood-brain barrier to
distribute in the cortex and hippocampus (20,21).
In the present study, an AD transgenic mouse model
(APPswe/PSEN1dE9) was used to investigate the effects of
ginsenoside Rg1 on memory, and to examine its underlying
mechanisms. In combination with other evidence (22,23),
the present study hypothesized that ginsenoside Rg1 is a candidate
memory enhancer, not only in age-related and drug-induced memory
decline, but also in the genetic AD model. The present study may
provide novel evidence to suggest a therapeutic effect of
ginsenoside Rg1 on AD.
Materials and methods
Animals
APP/PS1 mice (n=80; B6C3-Tg) were obtained from the
Jackson Laboratory (Farmington, CT, USA) and were bred amongst the
colony. The offspring were genotyped using primers for APP and PS1
(Sangon Biotech Co., Ltd., Shanghai, China), which were as follows:
Sense, 5′-GAC TGA CCA CTC GAC CAG GTT CTG-3′ and antisense, 5′-CTT
GTA AGT TGG ATT CTC ATA TCCG-3′ for APP; sense, 5′-AAT AGA GAA CGG
CAG GAGCA-3′ and antisense, 5′-GCC ATG AGG GCA CTA ATCAT-3′ for PS1
reference; and sense, 5′-CCT CTT TGT GAC TAT GTG GAC TGA TGT CGG-3′
and antisense, 5′-GTG GAT AAC CCC TCC CCC AGC CTA GACC-3′ also for
PS1, which distinguishes AD. C57 BL/6J mice (n=30) were purchased
from the Animal Center of the Chinese Academy of Sciences
(Shanghai, China). The mice (male; age, 6 months; weight, 30 g)
used in the experiments were housed together in a 12 h light/dark
cycle at 22±3°C, with food and water ad libitum. All
experimental procedures were approved by the ethics committee of
Weifang Medical University (Weifang, China).
Ginsenoside Rg1 treatment
APP/PS1 mice were chronically administered with Rg1
(Sigma-Aldrich, St. Louis, MO, USA) by intraperitoneal injection,
at concentrations of 0.1, 1 or 10 mg/kg once each day for 30 days
consecutively. This concentration range was selected based on
previous publications (22,24).
The control mice received the same volume of saline. During the
drug administration, diet, water intake and body weights were
monitored. At 30 days post-administration, behavioral,
electrophysiological and biochemical experiments were
performed.
Electrophysiological experiments
After 30 days, at least 4 mice from each group were
sacrificed by decapitation. From each group, 4–8 slices were
prepared. Acute hippocampal slices (300 μm) were prepared
following decapitation in cutting solution (Beyotime Institute of
Biotechnology, Haimen, China). The components of the cutting
solution were as follows: 124 mM NaCl, 26 mM NaHCO3, 10
mM D-glucose, 3 mM KCl, 1.25 mM KH2PO4, 5 mM
MgSO4 and 3.4 mM CaCl2. The slices were then
transferred to an interface recording chamber (BSC-ZT; Warner
Instruments LLC, Hamden, CT, USA) and exposed to a warm, humidified
atmosphere of 95% O2/5% CO2 and continuously
perfused (for ~4 h) with oxygenated and preheated (32±0.5°C)
artificial cerebrospinal fluid (aCSF; Beyotime Institute of
Biotechnology, Inc.) comprising 110 mM NaCl, 5 mM KCl, 2.5 mM
CaCl2, 1.5 mM MgSO4, 1.24 mM
KH2PO4, 10 mM D-glucose and 27.4 mM
NaHCO3. The aCSF flow speed was adjusted to 1.4 ml/min.
Following a 2 h recovery period, the field-excitatory postsynaptic
potential (fEPSP), elicited by stimulation of the Schaffer
collateral pathway with twisted nichrome wires (Warner Instruments
LLC), was recorded. The input-output and paired-pulse facilitation
at 30, 50 and 100 msec intervals were assessed. Long-term
potentiation was induced using a θ-burst stimulation (TBS)
protocol. Long-term depression (LTD) was induced by low-frequency
stimulation (LFS).
ELISA
To quantify levels the of β-amyloid 1–42, the
hippo-campus from four sacrificed mice from each of the groups were
homogenized in homogenization buffer (5 M guanidine HCl/50 mM
Tris-HCl; Beyotime Institute of Biotechnology) and centrifuged at
10,000 × g for 10 min at 4°C. The protein concentrations of the
supernatants were determined using a Bicinchoninic Acid (BCA) Assay
kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
supernatant fractions were analyzed using a β-amyloid 1–42 ELISA
kit (cat no. KHB3441; Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The absorbance was
determined for each well at 450 nm using a microplate reader
(Fluoroskan Ascent™; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from the hippocampus using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Reverse transcription was performed using Moloney murine leukemia
virus reverse transcriptase (Promega, Madison, WI, USA). RNA purity
was defined by optical density (OD)260/OD280
on a Fluoroskan Ascent™ microplate reader. qPCR was performed to
quantify the expression of APP in the hippocampus, using a
quantitative thermal cycler (Mastercyclerep realplex; Eppendorf,
Hamburg, Germany). The system included 2 μl cDNA, 2
μl dNTPs, 2 μl MgCl2 and ddH2O
to 25 μl. The thermocycling conditions were as follows:
Initial denaturation, 5 min at 95°C; and 30 cycles of denaturation
at 30 sec at 95°C, annealing at 58°C for 30 sec and extension at
72°C for 30 sec. The relative expression values were calculated as
a ratio of target cDNA to β-actin and the expression of target
genes was calculated by 2−ΔΔCq (25). The primers used in qPCR were
obtained from Sangon Biotech Co., Ltd. as follows: APP, sense
5′-TGC TGG CAG AAC CCC AGA TCG-3′ and antisense 5′-TTC TGG ATG GTC
ACT GGC TGG-3′; β-actin sense 5-ATG AGG TAG TCT GTC AGGT-3 and
antisense 5-ATG GAT GAC GAT ATC GCT-3.
Western blot analysis
The whole hippocampus homogenates were obtained and
lysed, and the protein concentrations were measured using a BCA
protein assay kit (Thermo Fisher Scientific, Inc.), as described
above. Equivalent quantities of proteins (20 μg) were
processed for 12% SDS-PAGE (Beyotime Institute of Biotechnology)
and western blot analysis. The proteins were transferred to
nitrocellulose membranes (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) using a wet transfer and the membranes were blocked in 5%
nonfat milk for 2 h and washed three times in phosphate-buffered
saline with Tween 20 (PBST). The membrane was incubated with
primary antibodies overnight at 4°C, as follows: Rabbit poly-clonal
BDNF (1:1,000; EMD Millipore, Billerica, MA, USA; cat. no.
AB1534SP), rabbit actin (1:10,000; EMD Millipore; cat. no.
MAB1501), rabbit monoclonal phosphorylated (p)-TrkB (1:3,000; Cell
Signaling Technology, Inc., Danvers, MA, USA; cat. no. 4619),
rabbit monoclonal Trk B (1:3,000; Cell Signaling Technology, Inc.;
cat. no. 4607), rabbit poly-clonal p-Tau (1:3,000; Cell Signaling
Technology, Inc.; cat. no. 11834), rabbit monoclonal Tau (1:3,000;
Cell Signaling Technology, Inc.; cat. no. 4019), C-terminal
fragments (CTFs; 1:1,000; EMD Millipore; cat. no. AB5352), rabbit
polyclonal postsynaptic density protein 95 (PSD-95; 1:3,000; Cell
Signaling Technology, Inc.; cat. no. 2507) and rabbit polyclonal
synaptophysin (1:3,000; Cell Signaling Technology, Inc.; cat. no.
4329). Following incubation with primary antibodies, the membranes
were washed with PBST 3 times for 10 min and then incubated with
the mouse anti-rabbit monoclonal secondary antibody (1:10,000; Cell
Signaling Technology, Inc.; cat. no. 5127) for 2 h at room
temperature. Protein levels were quantified by densitometry
analysis using Quantity One software (version 4.5.2; Bio-Rad
Laboratories, Inc.).
Fear conditioning
The fear conditioning experiment was performed, as
previously described (26). The
mice were handled daily for 5 days consecutively prior to training.
On the training day, the mice were placed in the fear-conditioning
chamber and allowed 5 min for exploration. Subsequently, three
tone-footshock pairings, separated by 1 min intervals were
delivered to the animals. The footshocks were 0.70 mA for 2 sec and
a tone of 85 dB 2 kHz for 30 sec. The mice were retained in the
training chamber for another 30 sec, following which they were
transferred to their home cages. A context assessment (5 min) was
performed 24 h post-training. On day 3, the animals were subjected
to a tone test in the same conditioning chamber, which was modified
by a change in the color of the walls. The freezing level (5 min)
in this altered context was measured (moving frequency, <25
msec), and a tone (85 dB; 2 kHz) was delivered for 1 min to measure
freezing to tone. The frequency of freezing was recorded using
FreezeFrame software (version 3; Coulbourn Instruments, Holliston,
MA, USA) and analyzed using FreezeView software (version 3;
Coulbourn Instruments). In each group, there were five animals. The
percentage of time in which the animal froze was calculated.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. All statistical analyses were performed using one-way
analysis of variance with GraphPad Prism 6.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). Bonferroni's correction with a
post-hoc t-test was performed to compare the differences
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Chronic treatment with ginsenoside Rg1
ameliorates long-term memory in AD model mice
In the present study, long-term memory was measured
using a fear conditioning experiment. Ginsenoside Rg1 was
administered to the mice at a range of doses (10, 1 and 0.1 mg/kg)
for 30 days. The dietary intake, drinking and body weights of the
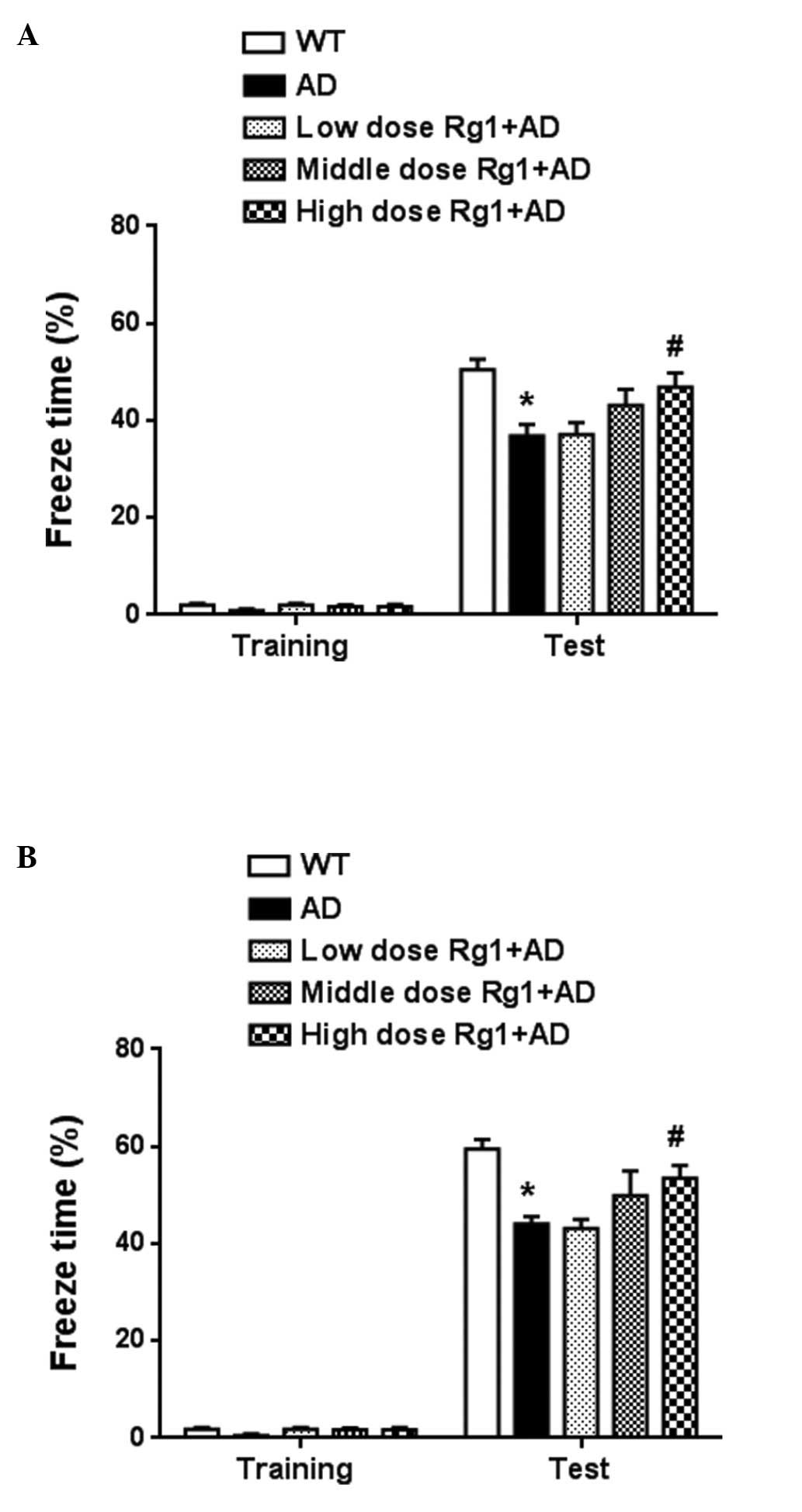
animals were unaffected during the drug treatment. As shown in
Fig. 1A, context memory was
markedly improved following treatment with 10 mg/kg ginsenoside Rg1
(P<0.05). The intermediate dose showed improved memory, but
without statistical significance (P>0.05). No significant effect
was observed following treatment with the low dose of ginsenoside
Rg1. Tone memory was also measured. As shown in Fig. 1B, treatment with ginsenoside Rg1 at
the dose of 10 mg/kg improved tone memory (P<0.05). The
intermediate dose of ginsenoside Rg1 also had an ameliorating
effect. These results confirmed that ginsenoside Rg1 improved
long-term memory in the transgenic AD model.
Chronic treatment with ginsenoside Rg1
reverses LTP deficit in the AD model
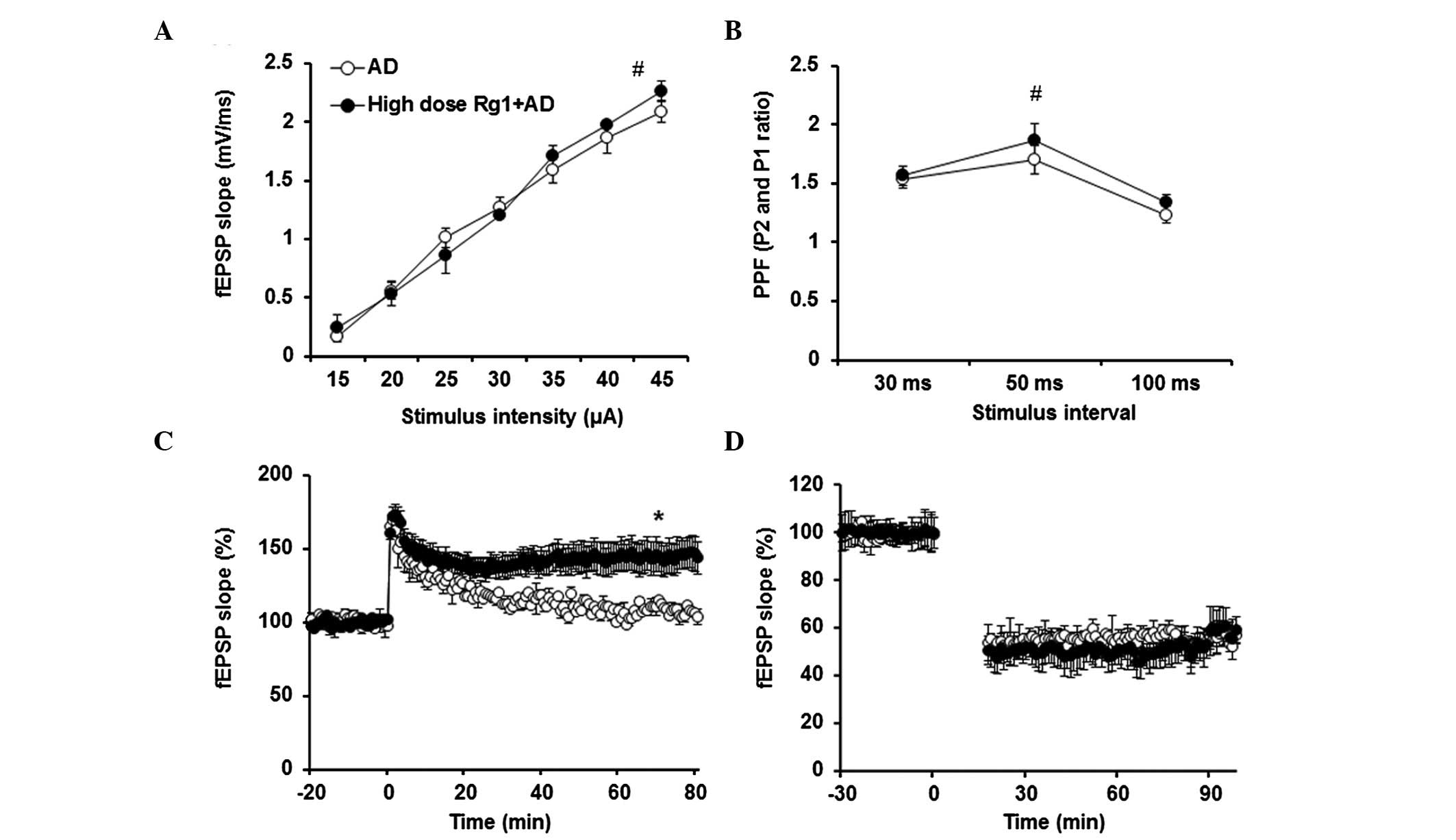
To confirm the effect of ginsenoside Rg1 on
hippocampal synaptic transmission and plasticity, the fEPSPs at
Schaffer collateral-CA1 synapses were measured. As shown in
Fig. 2A and B, ginsenoside Rg1 had
no effect on the input-output. Paired-pulse facilitation was also
unaffected by ginsenoside Rg1 treatment (P>0.05). The induction
of LTP by TBS was impaired in the slices obtained from the AD mice
(Fig. 2C). However, following
ginsenoside Rg1 treatment, TBS-LTP was ameliorated (P<0.05),
compared with in the AD model. LFS-LTD was not affected by
ginsenoside Rg1 treatment (Fig.
2D).
Chronic treatment with ginsenoside Rg1
attenuates the expression of AD-associated proteins
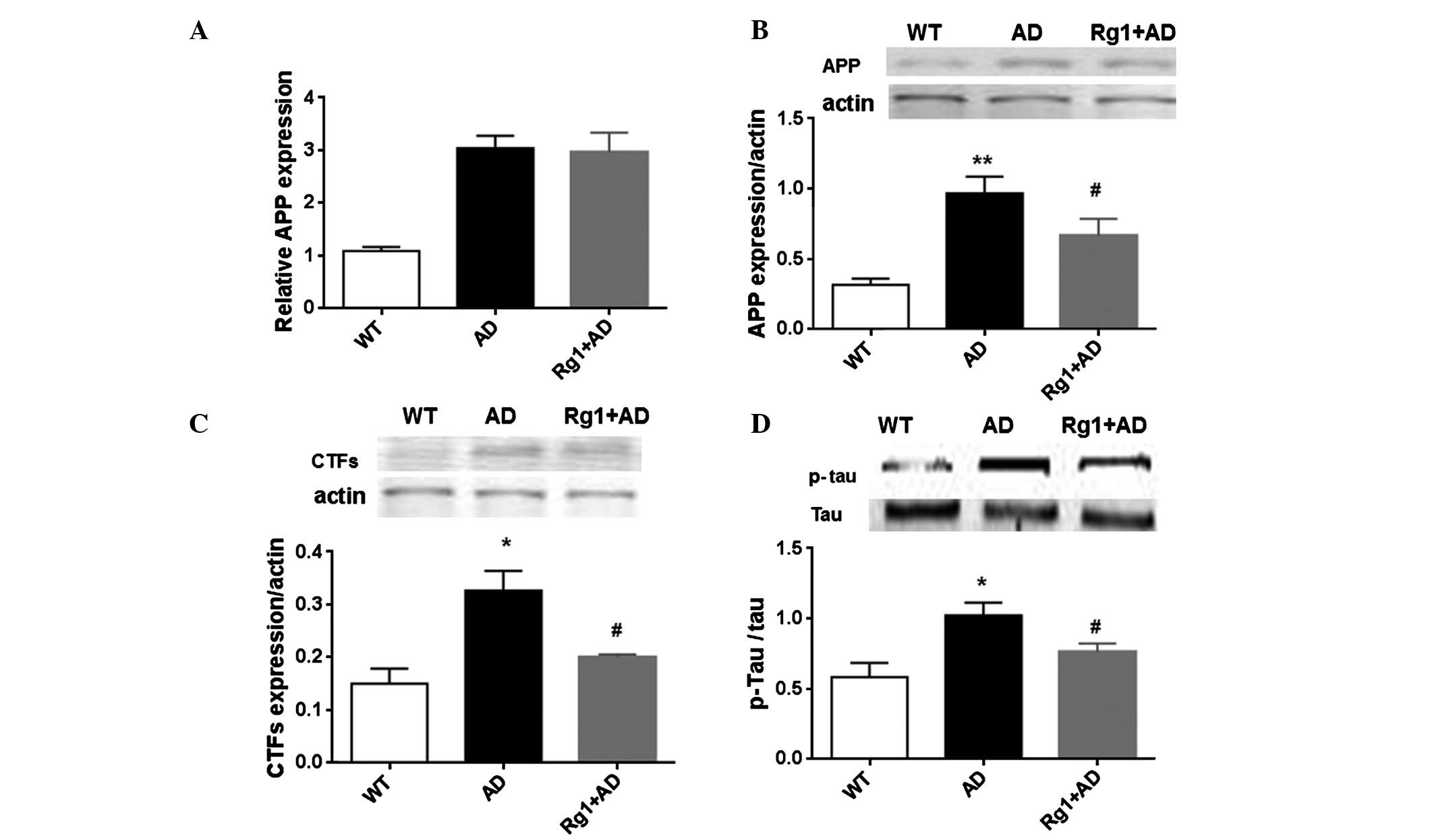
The expression levels of APP and PS1 in the
hippocampus were measured using RT-qPCR and Western blot analyses.
Compared with the wild-type mice, the mRNA expression of APP
increased ~3-fold in the AD model mice (Fig. 3A; P<0.05). Ginsenoside Rg1 did
not alter the mRNA expression levels of APP. The protein levels
were also determined. As shown in Fig.
3B, the protein level of APP also appeared to be enhanced in
the AD model mice, compared with the wild-type mice. Of note,
ginsenoside Rg1 decreased the protein levels following 1 month of
treatment (P<0.05). In addition, the present study detected the
expression of CTFs. In the model mice, the expression of CTFs was
significantly increased (P<0.05), however, the expression was
reduced by ginsenoside Rg1 treatment (P<0.05; Fig. 3C). The expression of p-Tau was also
measured. Compared with the wild-type mice, the expression of p-Tau
was increased in the model mice (P<0.05). Following treatment
with ginsenoside Rg1, the protein level was also attenuated
(P<0.05; Fig. 3D). The level of
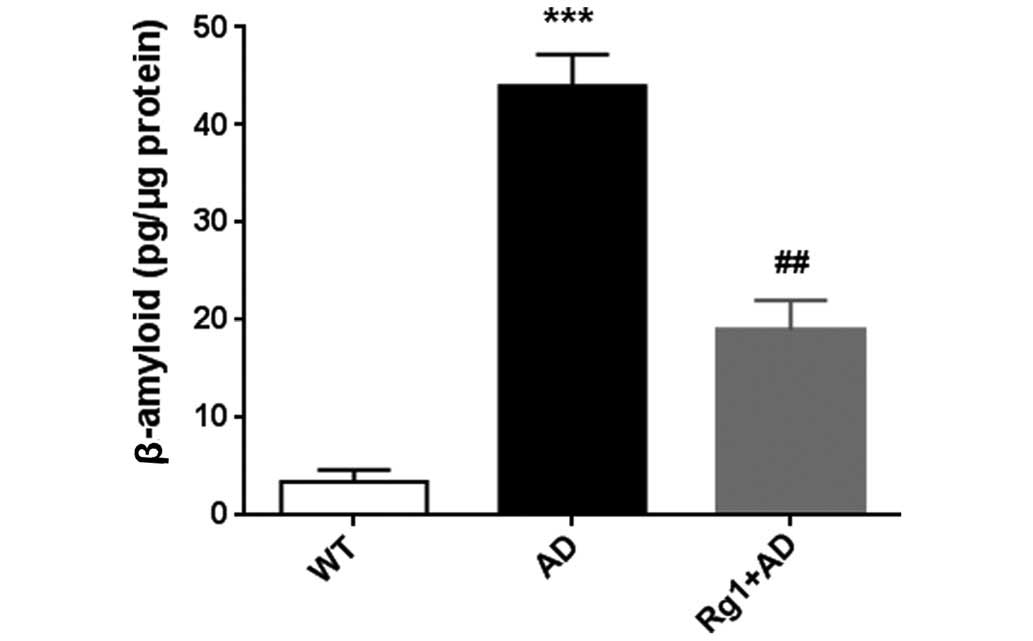
β-amyloid 1–42 was reduced following treatment with ginsenoside Rg1
(Fig. 4). These results suggested
that ginsenoside Rg1 treatment ameliorated the accumulation of
AD-associated proteins in the AD model mice.
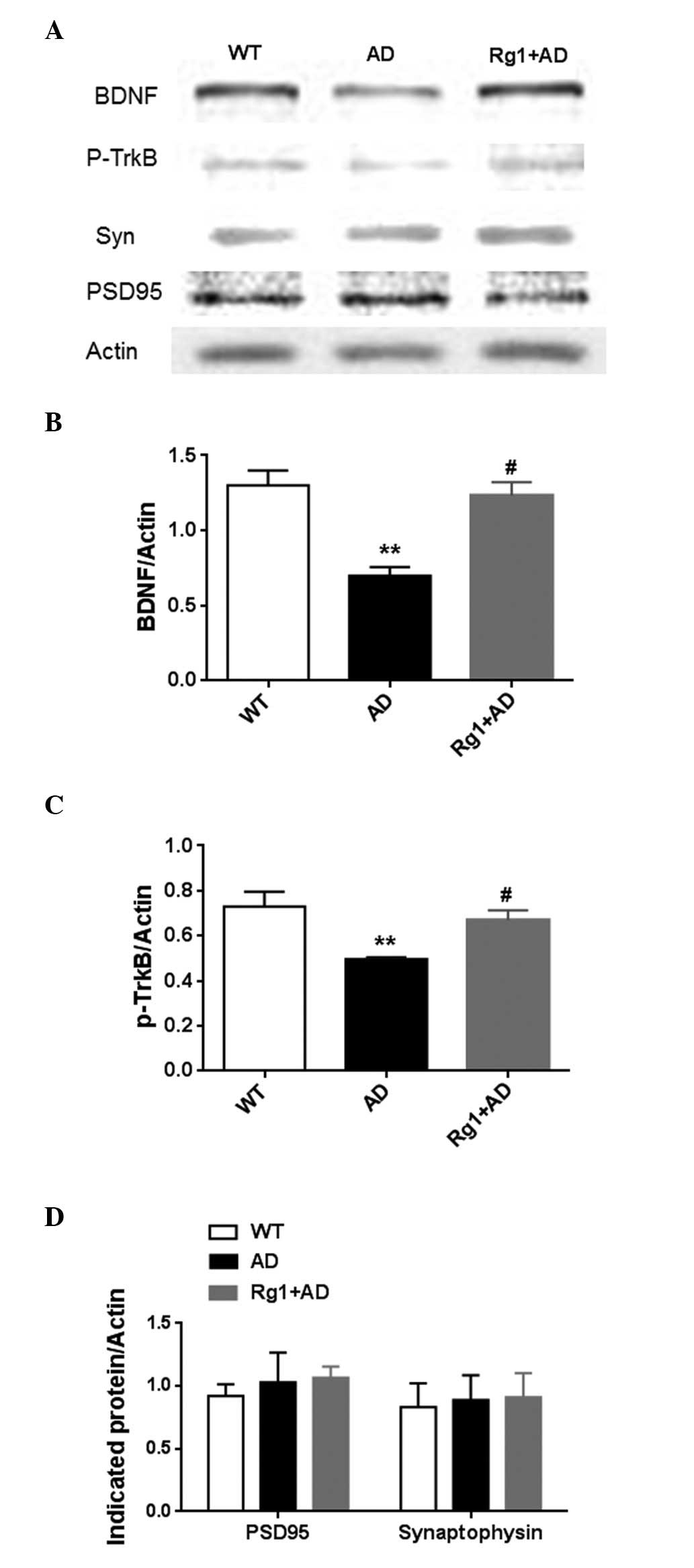
Chronic treatment with ginsenoside Rg1
improves activation of the BDNF-TrkB pathway in AD model mice
Synaptic-associated proteins in the hippocampus were
also measured in the present study, including BDNF, p-TrkB,
synaptophysin and PSD-95. As shown in Fig. 5A and B, the expression of BDNF
increased following treatment with ginsenoside Rg1 (P<0.05).
Correspondingly, the level of p-TrkB was also upregulated following
treatment with ginsenoside Rg1 (P<0.05; Fig. 5C). By contrast, no effects were
observed on the presynaptic marker, synaptophsin or post-synaptic
marker, PSD-95 (Fig. 5D) following
treatment with ginsenoside Rg1. These results indicated that
ginsenoside Rg1 may have improved plasticity, but did not alter
basal synapses in the AD model.
Discussion
In the present study, it was demonstrated that
ginsenoside Rg1 treatment improved memory and hippocampal LTP in
the AD model. The expression levels of AD-associated proteins were
attenuated, and the BDNF-TrkB pathway was improved following
ginsenoside Rg1 treatment.
Ginsenoside Rg1 ameliorates long-term
memory in pathological disease models
A series of studies have reported that ginsenosides
improve memory in exogenous toxin-induced memory deficits (16–19,27,28).
In addition, ginsenosides also improve memory in aging or aged
animals (23,29,30).
These data indicate that ginsenosides are effective in improving
memory. In the present study, ginsenoside Rg1 was selected as the
target therapeutic drug, and a transgenic AD model was used to
screen the effective doses. The results of the present and previous
studies demonstrated that the APP/PS1 transgenic mice exhibited a
decline in memory performance at 6 months of age (25). In the present study,
intraperitoneal injection of Rg1 at concentrations between 1 and 10
mg/kg was selected, based on previous publications (22,24).
As shown by Zhang et al (22), this dose range is normal in mice
and rats, following conversion from humans. Chronic treatment for 1
month with 10 mg/kg ginsenoside Rg1 significantly ameliorated
long-term memory. Although the low dose of ginsenoside Rg1 (0.1
mg/kg) did not cause amelioration in the AD model, the middle dose
(1 mg/kg) demonstrated a protective effect. These results showed a
dose-dependent effect of ginsenoside Rg1 on memory. Due to the
chemical structure of ginsenoside Rg1, effective technology to
improve its capacity to pass through the blood brain barrier is
urgently required. Although β-amyloid peptide 1–42-induced
functional loss is ameliorated by ginsenoside Rg1 application
(24,31), the present study demonstrated a
similar effect of ginsenoside Rg1 using a transgenic AD model and
fear conditioning experiment. The present study also aimed to
clarify the potential mechanisms underlying the memory improvement
observed following ginsenoside Rg1 treatment. As no commercial
ginsenoside Rg1 injection is available, an effective dose range for
oral application requires screening for clinical practice.
Ginsenoside Rg1 facilitates the clearance
of AD-associated proteins
Amyloid plaques are considered to be a detrimental
toxin, contributing to the impairment of hippocampal synaptic
plasticity and to hippocampal cell death (7–9). In
the APP/PS1 transgenic mice, APP was overexpressed, and led to an
increase in the accumulation of amyloid 1–42 in the hippocampus. In
addition, the AD protein, p-Tau, was enhanced at 6 months of age.
These abnormalities caused by the overexpression of APP and PS1 may
be responsible for the subsequent memory decline. In ginsenoside
Rg1-treated mice, the expression levels of APP and PS1 were
unaffected. However, the accumulation of p-Tau and amyloid 1–42 in
the hippocampus were significantly reduced. The decreases in p-Tau
and amyloid 1–42 may be caused by two factors. Protein synthesis
may have been inhibited by ginsenoside Rg1 treatment. This
possibility is supported by a previous study, which showed that
ginsenoside Rg1 inhibits amyloid generation through regulation of
the transcription or translation of BACE1 (32), or via inhibition of γ-secretase
activity (33). The activity of
the protein degradation system was enhanced following treatment
with ginsenoside Rg1, leading to the degradation of β-amyloid,
however, further clarification of this is required.
The majority of previous studies have focused on the
amelioration of ginsenoside Rg1 in the later stage of AD.
Ginsenoside Rg1 may prevent against β-amyloid plague accumulation
to inhibit apoptosis (34–36).
Ginsenoside Rg1 ameliorates synaptic
plasticity in the AD mice model
In addition to the clearance of AD-associated
proteins, ginsenoside Rg1 also facilitated the recovery of
long-term potentiation. Initially, ginsenoside Rg1 treatment did
not affect basal synaptic transmission, in terms of input-output
and paired-pulse facilitation. These results suggested that
ginsenoside Rg1 did not affect basal synaptic transmission, either
presynaptically or postsynaptically. These physiological data were
consistent with the unaltered expression levels of PSD-95 and
synaptophysin. By contrast, plasticity was enhanced following
ginsenoside Rg1 treatment in the AD model. The effects on LTP may
be due to the clearance of AD-associated proteins. The present
study found that the expression of BDNF was upregulated by
ginsenoside Rg1 treatment and, correspondingly, p-TrkB was
activated following ginsenoside Rg1 treatment. Therefore,
activation of the BDNF-TrkB pathway may contribute to the recovery
of LTP in the transgenic AD model. In a senescence-accelerated
mouse prone 8 model, the levels of BDNF are also improved following
treatment with ginsenoside Rg1 (37). These findings indicate the general
pharmacological activity of ginsenoside Rg1 in the AD model. In
addition, other synaptic plasticity-associated proteins, including
NR1 and NR2B, are reported to be upregulated in the AD model to
increase memory (38). How
ginsenoside Rg1 functions in the hippocampus remains to be fully
elucidated and, although the present study did not distinguish the
potential target, estrogen receptors have been implicated (39).
In the present study, data indicating memory
amelioration following ginsenoside Rg1 treatment were obtained in a
transgenic AD model. Clearance of AD-associated proteins and
activation of the BDNF-TrkB pathway may contribute to the effect of
ginsenoside Rg1 on hippocampal LTP. These results suggested that
ginsenoside Rg1 may be a potential memory enhancer in the
transgenic AD model.
References
|
1
|
Qiu C, Kivipelto M and von Strauss E:
Epidemiology of Alzheimer's disease: Occurrence, determinants and
strategies toward intervention. Dialogues Clin Neurosci.
11:111–128. 2009.
|
|
2
|
Salloway S: Current and future treatments
for Alzheimer's disease. CNS Spectr. 14(8 Suppl 7): 4–7; discussion
16–18. 2009.PubMed/NCBI
|
|
3
|
Yiannopoulou KG and Papageorgiou SG:
Current and future treatments for Alzheimer's disease. Ther Adv
Neurol Disord. 6:19–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aisen PS, Cummings J and Schneider LS:
Symptomatic and nonamyloid/tau based pharmacologic treatment for
Alzheimer disease. Cold Spring Harb Perspect Med. 2:a0063952012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
LaFerla FM, Green KN and Oddo S:
Intracellular amyloid-beta in Alzheimer's disease. Nat Rev
Neurosci. 8:499–509. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li M, Chen L, Lee DH, Yu LC and Zhang Y:
The role of intracellular amyloid beta in Alzheimer's disease. Prog
Neurobiol. 83:131–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carter J and Lippa CF: Beta-amyloid,
neuronal death and Alzheimer's disease. Curr Mol Med. 1:733–737.
2001. View Article : Google Scholar
|
|
8
|
Oakley H, Cole SL, Logan S, Maus E, Shao
P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik
L, et al: Intraneuronal beta-amyloid aggregates, neurodegeneration
and neuron loss in transgenic mice with five familial Alzheimer's
disease mutations: Potential factors in amyloid plaque formation. J
Neurosci. 26:10129–10140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chapman PF, White GL, Jones MW,
Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA,
Bliss TV, Hyman BT, et al: Impaired synaptic plasticity and
learning in aged amyloid precursor protein transgenic mice. Nat
Neurosci. 2:271–276. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hung IC, Chang SS, Chang PC, Lee CC and
Chen CY: Memory enhancement by traditional Chinese medicine? J
Biomol Struct Dyn. 31:1411–1439. 2013. View Article : Google Scholar
|
|
11
|
May BH, Lu C, Lu Y, Zhang AL and Xue CC:
Chinese herbs for memory disorders: A review and systematic
analysis of classical herbal literature. J Acupunct Meridian Stud.
6:2–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Zhang J, Zheng K, Shen H and Chen
X: Long-term ginsenoside Rg1 supplementation improves age-related
cognitive decline by promoting synaptic plasticity associated
protein expression in C57BL/6J mice. J Gerontol A Biol Sci Med Sci.
69:282–294. 2014. View Article : Google Scholar
|
|
13
|
Gillis CN: Panax ginseng pharmacology: A
nitric oxide link? Biochem Pharmacol. 54:1–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee CH and Kim JH: A review on the
medicinal potentials of ginseng and ginsenosides on cardiovascular
diseases. J Ginseng Res. 38:161–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nah SY: Ginseng ginsenoside pharmacology
in the nervous system: Involvement in the regulation of ion
channels and receptors. Frontiers in physiology. 5:982014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee B, Sur B, Park J, Kim SH, Kwon S, Yeom
M, Shim I, Lee H and Hahm DH: Ginsenoside rg3 alleviates
lipopolysaccharide-induced learning and memory impairments by
anti-inflammatory activity in rats. Biomol Ther (Seoul).
21:381–390. 2013. View Article : Google Scholar
|
|
17
|
Song XY, Hu JF, Chu SF, Zhang Z, Xu S,
Yuan YH, Han N, Liu Y, Niu F, He X and Chen NH: Ginsenoside Rg1
attenuates okadaic acid induced spatial memory impairment by the
GSK3beta/tau signaling pathway and the Abeta formation prevention
in rats. Eur J Pharmacol. 710:29–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chu S, Gu J, Feng L, Liu J, Zhang M, Jia
X, Liu M and Yao D: Ginsenoside Rg5 improves cognitive dysfunction
and beta-amyloid deposition in STZ-induced memory impaired rats via
attenuating neuroinflammatory responses. Int Immunopharmacol.
19:317–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Kan H, Yin Y, Wu W, Hu W, Wang M
and Li W and Li W: Protective effects of ginsenoside Rg1 on chronic
restraint stress induced learning and memory impairments in male
mice. Pharmacol Biochem Behav. 120:73–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nah JJ, Hahn JH, Chung S, Choi S, Kim YI
and Nah SY: Effect of ginsenosides, active components of ginseng,
on capsaicin-induced pain-related behavior. Neuropharmacology.
39:2180–2184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Long W, Zhang SC, Wen L, Mu L, Yang F and
Chen G: In vivo distribution and pharmacokinetics of multiple
active components from Danshen and Sanqi and their combination via
inner ear administration. J Ethnopharmacol. 156:199–208. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Wang J, Xing Y, Gong L, Li H, Wu
Z, Li Y, Wang J, Wang Y, Dong L and Li S: Effects of ginsenoside
Rg1 or 17β-estradiol on a cognitively impaired, ovariectomized rat
model of Alzheimer's disease. Neuroscience. 220:191–200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu G, Wang Y, Li J and Wang J: Chronic
treatment with ginsenoside Rg1 promotes memory and hippocampal
long-term potentiation in middle-aged mice. Neuroscience.
292:81–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quan Q, Wang J, Li X and Wang Y:
Ginsenoside Rg1 decreases Aβ(1–42) level by upregulating PPARgamma
and IDE expression in the hippocampus of a rat model of Alzheimer's
disease. PloS One. 8:e591552013. View Article : Google Scholar
|
|
25
|
Hong X, Liu J, Zhu G, Zhuang Y, Suo H,
Wang P, Huang D, Xu J, Huang Y, Yu M, et al: Parkin overexpression
ameliorates hippocampal long-term potentiation and β-amyloid load
in an Alzheimer's disease mouse model. Hum Mol Genet. 23:1056–1072.
2014. View Article : Google Scholar
|
|
26
|
Zhu G, Liu Y, Wang Y, Bi X and Baudry M:
Different patterns of electrical activity lead to long-term
potentiation by activating different intracellular pathways. J
Neurosci. 35:621–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu J, Yan X, Li L, Zhu Y, Qin K, Zhou L,
Sun D, Zhang X, Ye R and Zhao G: Ginsennoside rd attenuates
cognitive dysfunction in a rat model of Alzheimer's disease.
Neurochem Res. 37:2738–2747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiu J, Li W, Feng SH, Wang M and He ZY:
Ginsenoside Rh2 promotes nonamyloidgenic cleavage of amyloid
precursor protein via a cholesterol-dependent pathway. Genet Mol
Res. 13:3586–3598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao H, Li Q, Pei X, Zhang Z, Yang R, Wang
J and Li Y: Long-term ginsenoside administration prevents memory
impairment in aged C57BL/6J mice by up-regulating the synaptic
plasticity-related proteins in hippocampus. Behav Brain Res.
201:311–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao HF, Li Q and Li Y: Long-term
ginsenoside administration prevents memory loss in aged female
C57BL/6J mice by modulating the redox status and up-regulating the
plasticity-related proteins in hippocampus. Neuroscience.
183:189–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang XY, Chen J and Zhang JT: Effect of
ginsenoside Rg1 on learning and memory impairment induced by
beta-amyloid peptide (25–35) and its mechanism of action. Yao Xue
Xue Bao. 36:1–4. 2001.In Chinese.
|
|
32
|
Chen LM, Lin ZY, Zhu YG, Lin N, Zhang J,
Pan XD and Chen XC: Ginsenoside Rg1 attenuates β-amyloid generation
via suppressing PPARgamma-regulated BACE1 activity in N2a-APP695
cells. Eur J Pharmacol. 675:15–21. 2012. View Article : Google Scholar
|
|
33
|
Fang F, Chen X, Huang T, Lue LF, Luddy JS
and Yan SS: Multi-faced neuroprotective effects of Ginsenoside Rg1
in an Alzheimer mouse model. Biochim Biophys Acta. 1822:286–292.
2012. View Article : Google Scholar :
|
|
34
|
Wang YH and Du GH: Ginsenoside Rg1
inhibits beta-secretase activity in vitro and protects against
Abeta-induced cytotoxicity in PC12 cells. J Asian Nat Prod Res.
11:604–612. 2009. View Article : Google Scholar
|
|
35
|
Shi C, Zheng DD, Fang L, Wu F, Kwong WH
and Xu J: Ginsenoside Rg1 promotes nonamyloidgenic cleavage of APP
via estrogen receptor signaling to MAPK/ERK and PI3K/Akt. Biochim
Biophys Acta. 1820:453–460. 2012. View Article : Google Scholar
|
|
36
|
Li X, Zhang X, Yuan H and Quan Q:
Experimental research on effect of gensenoside Rg1 on expressions
of P-Tau and caspase-3 in brain slices from AD model rats. Zhongguo
Zhong Yao Za Zhi. 35:369–372. 2010.In Chinese. PubMed/NCBI
|
|
37
|
Shi YQ, Huang TW, Chen LM, Pan XD, Zhang
J, Zhu YG and Chen XC: Ginsenoside Rg1 attenuates amyloid-beta
content, regulates PKA/CREB activity and improves cognitive
performance in SAMP8 mice. J Alzheimer's Dis. 19:977–989. 2010.
|
|
38
|
Li X, Liu Y, Zhang X, Yuan H and Quan Q:
Effect of ginsenoside Rg1 on expressions of phosphory protein tau
and N-methyl-D-aspartate receptor subunits NR1 and NR2B in rat
brain slice model of Alzheimer's disease. Zhongguo Zhong Yao Za
Zhi. 35:3339–3343. 2010.In Chinese.
|
|
39
|
Shi C, Na N, Zhu X and Xu J: Estrogenic
effect of ginsenoside Rg1 on APP processing in post-menopausal
platelets. Platelets. 24:51–62. 2013. View Article : Google Scholar
|