Introduction
Hyperlipidemia refers to abnormally elevated levels
of lipids or lipoproteins in the bloodstream, and is a significant
risk factor associated with numerous diseases, including
ventricular remodeling, cardiac hypertrophy and cardiac dysfunction
(1). A previous study demonstrated
that hyperlipidemia has a direct association with an increase in
the number and size of atheromatous plaques and thus has been
considered as a contributor to atherosclerosis (2). Patients with hyperlipidemia usually
have higher levels of total cholesterol (TC), triglycerides (TG),
and low-density lipoprotein cholesterol (LDL-C), however,
relatively lower levels of high-density lipoprotein cholesterol
(HDL-C) compared with healthy controls (3). High plasma TC, TG or LDL-C levels or
low plasma HDL-C levels may result in damage to vascular
endothelial cells and initiates atherosclerosis (4). By contrast, HDL-C has been generally
accepted to serve a protective role in the suppression of the
formation of atherosclerosis (5).
Thus, clinical control of hyperlipidemia may prevent
atherosclerosis and cardiovascular diseases.
Tanshinone IIA is a pharmacologically active
compound isolated from Salvia miltiorrhiza bunge, and is
known as Danshen in traditional Chinese medicine. Tanshinone IIA
has been used historically in traditional Chinese medicine to
prevent and treat cardiovascular diseases in Asian countries, due
to its putative cardioprotective and anti-atherosclerotic effects.
Numerous experimental and clinical studies have indicated that
Tanshinone IIA possesses a variety of biological activities,
including, but not limited to, vasodilatory, anti-atherosclerosis,
anti-oxidation, anti-inflammatory, anti-hyperlipidemic and
anti-adipogenic effects (6–9).
However, the underlying mechanisms of Tanshinone IIA activity
remain to be determined. In a previous study, Tanshinone IIA
treatment increased HDL-C level and reduced LDL-C levels in
C57BL/6J mice fed with a high fat-diet (9). Additionally, Tanshinone IIA has been
shown to reduce the formation of atherosclerotic plaques without a
significant effect on alterations in the lipid profile (6,10). A
previous study indicated that Tanshinone IIA served an
anti-atherosclerotic effect via the inhibition of Toll-like
receptor 4 and tumor necrosis factor-α expression in EA.hy926 cells
following stimulation with lipopolysaccharides (11). Additionally, protective effects of
Tanshinone IIA have been indicated on apoptosis induced by
H2O2 in EA.hy926 cells (12).
Increasingly, evidence indicates that sterol
regulatory element-binding protein 2 (SREBP-2) and LDL receptor
(LDL-R) are transcription factors involved in the regulation of
cholesterol metabolism (13–15).
Furthermore, there is a binding site for SREBP localized in the
promoter region of proprotein convertase subtilisin/kexin type 9
(Pcsk9) (16) and thus, SREBP
regulates expression of Pcsk9 at the transcriptional level.
Reductions in hepatic cholesterol levels are able to activate the
SREBP signaling pathway to upregulate the expression of Pcsk9 and
LDL-R simultaneously (15,17), and in turn, increase the levels of
LDL particles by reducing LDL-R (18). Conversely, inhibition of SREBP
expression in the liver has been shown to reduce the levels of
Pcsk9 and LDL-R (19). In
addition, a previous study indicated that microRNA (miR)-33, an
intronic miRNA residing in the SREBP-2 gene, serves a crucial role
in the regulation of HDL metabolism, cholesterol efflux and fatty
acid β-oxidation through the modulation of the expression of the
ATP-binding cassette transporter A1 (ABCA1) and G1 (ABCG1)
(20–23). However, in mice, but not in humans,
miR-33 overexpression has been observed to reduce cholesterol
efflux to nascent HDL (24). Thus,
inhibition of endogenous miR-33 levels may possess therapeutic
function to upregulate HDL levels by repressing ABCA1 and ABCG1
expression (25,26). Thus, in the present study,
experiments were conducted to elucidate the potential mechanisms of
Tanshinone IIA activity in vivo on lipid metabolism and the
underlying molecular events.
Materials and methods
Animals and rat hyperlipidemia model
The study was approved by the ethics committee of
Liaoning University of Traditional Chinese Medicine experimental
animal ethics committee (Shenyang, China). A total of 90 male
Sprague-Dawley rats (6 weeks old, 200±10 g) were obtained from
Vital River Laboratories Co., Ltd. (Beijing, China) and housed in a
climate-controlled environment (22±1°C, humidity at 50±5%) with a
12/12 h light/dark cycle and ad libitum access to water. The rats
were randomly divided into control, hyperlipidemia (HLP) and
Tanshinone IIA treatment (TAN) groups, with 30 rats in each group.
The control rats were fed with a regular balanced diet, while the
HLP and TAN groups were fed with a high-fat diet (6% sucrose, 1%
sodium glutamate, 5% yolk powder, 8% peanut oil, 1.5% cholesterol,
0.4% methylthiouracil, 0.2% cholate and 73.3% regular balanced
diet) for 3 months. Following this, the TAN group received 1.2 ml
of sodium Tanshinone IIA sulfonate (Shanghai No. 1 Biochemical
& Pharmaceutical Co., Ltd., Shanghai, China) at a dose of 10
mg/kg by daily intraperitoneal injection for an additional 3
months, whereas the control and HLP groups received the same amount
of phosphate-buffered saline (PBS) for the same period of time.
Collection of sera and lipid
profiling
Following the treatment process, the rats (n=6) were
anesthetized with 10% chloral hydrate and aortic blood was
collected for 2 h at room temperature. The blood samples were then
centrifuged at 4°C, 300 × g for 20 min, and the serum was collected
and stored at −80°C until use. For the lipid profiling, the serum
samples were analyzed using an automatic TBA-120FR biochemical
analyzer (Toshiba Corporation, Tokyo, Japan). Levels of TG, TC,
HDL-C and LDL-C in the serum were measured using the corresponding
kits (all from Sichuan Maker Biotechnology Co., Ltd., Sichuan,
China).
Hematoxylin and eosin (H&E) staining
of liver tissues
Liver tissues were collected (n=3), fixed in 4%
formaldehyde for 24 h at 4°C, dehydrated through an ethanol series
(70–100%), cleared in xylene and embedded in paraffin. The tissue
was cut into 5 µm thick sections and dewaxed in xylene,
rehydrated through a 70–100% ethanol series, and then stained with
hematoxylin, differentiated in 70% acid ethanol and further stained
with eosin. Subsequently, the tissue sections were dehydrated
through 70–100% ethanol, cleared in xylene, and mounted using
Permount Mounting Medium (BioWorld Technology, Inc., Atlanta,
Georgia, USA). The tissue sections were photographed using a
BX51-WIF light microscope linked to a digital charge-coupled device
(CCD) camera (Olympus Corporation, Tokyo, Japan).
Oil Red O staining of liver tissues
For Oil Red O staining, frozen sections were
prepared (6 µm) from liver tissue samples (n=3) and fixed in
50% ethanol. Subsequently, sections were stained with Oil Red O
(Beijing Noble Rider Technology Co., Ltd., Beijing, China) for 8
min and differentiated with 50% ethanol, rinsed with tap water, and
counterstained with hematoxylin. Following a final rinse in tap
water, the sections were mounted with glycerin jelly. The sections
were photographed using the light microscope linked to a digital
CCD camera (Olympus Corporation).
Immunohistochemistry
The expression of SREBP-2, Pcsk9, and LDL-R proteins
was assessed in paraffin-embedded liver tissues. In brief, tissue
sections were dewaxed in xylene, rehydrated through a 70–100%
ethanol series, and then incubated in 0.3%
H2O2 in PBS to block endogenous peroxidase
activity and 10% skim milk in PBS to block non-specific protein
binding of the secondary antibodies. Subsequently, the sections
were incubated with primary polyclonal rabbit anti-rat SREBP-2 (cat
no. 980594w), LDL-R (cat no. YSLS15w), PCSK9 (cat no. bs-6060R)
antibodies (all from BIOSS, Beijing, China). at 4°C overnight, then
washed with PBS three times and incubated with a horseradish
peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody
(1:1,000; 36J00180; Beijing Dingguo Changsheng Biotechnology Co.,
Ltd., Beijing, China) at room temperature for 1 h. The peroxidase
activity was detected using 3, 3′-diaminobenzidine
tetrahydrochloride solution. Following washing and counterstaining
with hematoxylin, the sections were mounted using AquaMount [Hyde
Entrepreneurship (Beijing) Biological Technology Co., Ltd.,
Beijing, China] and photographed and scored using the light
microscope linked to a digital CCD camera (Olympus Corporation).
The area of positive expression of each section was observed at the
low magnification (×10), then 4 randomly selected fields were
captured at high magnification (×40). Exposure conditions were
consistent. Images were semi-quantitatively analysed using
Image-Pro Plus software (Media Cybernetics, Inc., Rockville, MD,
USA). The the integrated optical density of each image was measured
and an average from 4 images was calculated for each section.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from liver tissue (n=3) using
a TRIzol reagent (Takara Biotechnology Co., Ltd., Dalian, China)
and reverse transcribed into cDNA using the miScript II RT kit
(Qiagen GmbH, Hilden, Germany), according to the manufacturer's
instructions. To assess the levels of miR-33, total RNA was
isolated using a miRNeasyMini kit (Qiagen GmbH), amplified using
specific primers to miR-33 and normalized to small U6 RNA (Qiagen
GmbH). The miR-33 transcripts were amplified as follows: Initial
denaturation at 94°C for 10 min; 35 cycles of denaturation at 94°C
for 1 min, annealing at 55°C for 15 sec and extension at 70°C for
15 sec; and a final extension step at 70°C for 5 min. To measure
the levels of gene transcripts, the following rat primers were
used: LDL-R, foward 5′-GGGTTCTTGTCCATCTTCC-3′ and reverse
5′-ACTGGGTTGTCAAAGTTTATG-3′; SREBP-2, forward
5′-GTGGGCTGAGAAGAAAGATG-3′ and reverse 5′-CCAGAGGCAGAAAGGAGA C-3′;
and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward
5′-TGTGTCCGTCGTGGATCTGA-3′ and reverse 5′-TTGCTGTTGAAGTCGCAGGAG-3′.
All primers were designed using Premier 5.0 software (PREMIER
Biosoft, Palo Alto, CA, USA) and custom-synthesized by Takara
Biotechnology Co., Ltd. RT-qPCR was performed in triplicate using a
SYBR Green Master Mix (Takara Biotechnology Co., Ltd.) in an ABI
7500 PCR Sequence Detector (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The transcripts were amplified
by 40 cycles of 95° for 2 min, 95°C for 30 sec and 60°C for 30 sec.
The gene transcripts were normalized to GAPDH. The RNA levels
measured by RT-qPCR were quantatified used the 2−ΔΔCq
method.
Protein extraction and western
blotting
Total cellular protein was extracted from rat liver
tissues (n=3) using radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Shanghai, China) and the
protein concentration was measured using Bicinchoninic Acid Protein
Assay kit (Beijing Dingguo Changsheng Biotechnology Co., Ltd.,
Beijing, China). To measure the expression levels of ABCA1, ABCG1,
SREBP-2, Pcsk9 and LDL-R proteins, 80 µg of protein samples
were separated on a 10% sodium dodecyl sulfate-polyacrylamide
electrophoresis gel (Beijing Solarbio Science and Technology Co.,
Ltd., Beijing, China) and transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% skin, high protein, high calcium milk powder (Inner
Mongolia Yili Industrial Group Co., Ltd., Hohhot, China). The
membranes were then incubated overnight at 37°C with rabbit
anti-rat SREBP-2, LDL-R, PCSK9, ABCA1 (cat no. bs-1627R), ABCG1
(cat no. bs-1231R) and GAPDH (No. bs-2188R) primary antibodies (all
1:300; BIOSS), and then with the HRP-conjugated goat anti-rabbit
secondary antibody (1:200) for 1 h at 37°C. Protein bands were
visualized using an enhanced chemiluminescence kit (Thermo Fisher
Scientific, Inc.) and exposed to X-ray films (Kodak, Rochester, NY,
USA). Protein expression levels were normalized to GAPDH
levels.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Comparisons between the group values were performed by
one-way analysis of variance. Tukey's test was used for multiple
comparisons between the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Tanshinone IIA alterations in lipid
levels in sera and lipid deposition in the liver
In the present study, an in vivo experiment
was performed to assess the effects of Tanshinone IIA on
alterations in the lipid levels in sera and lipid deposition in the
liver. The results indicated that compared with the control rats,
the HLP rats had significantly higher serum levels of TG, TC and
LDL-C, however, significantly lower serum levels of HDL-C
(P<0.05 or P<0.01). Compared with the HLP group, the TAN
group did not exhibit altered lipid profiles (Fig. 1A).
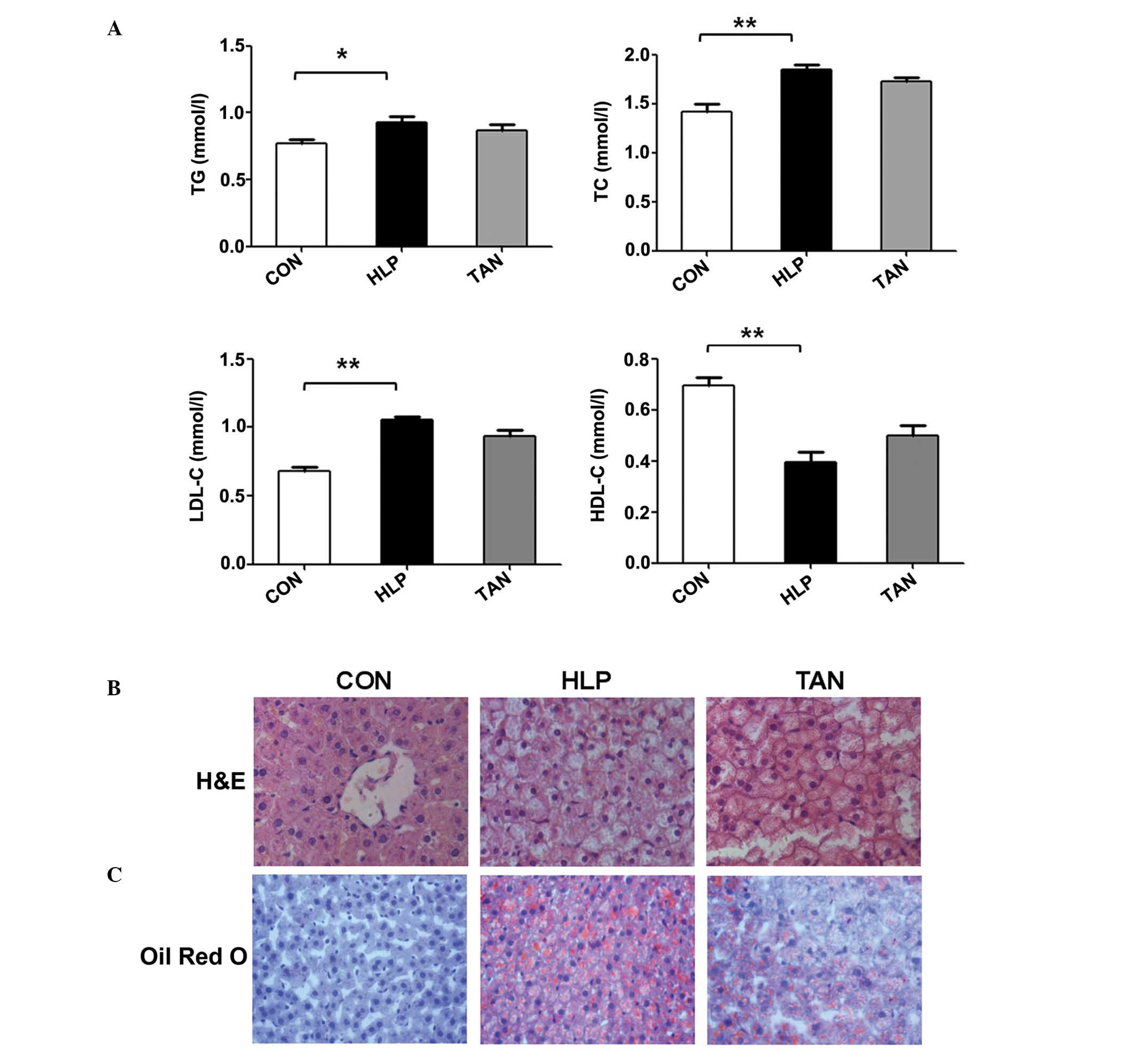 | Figure 1Tanshinone IIA alterations in the
lipid levels in sera and lipid deposition in the liver. (A) TG, TC,
LDL-C and HDL-C serum lipid levels. (B) H&E staining indicating
the alterations in the liver cells, such as lipid droplets. (C) Oil
red O staining showing the lipid droplets in the liver tissue. Data
are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 vs. CON group. TG,
triglycerides; TC, total cholesterol; LDL-C, low-density
lipoprotein-cholesterol; HDL-C, high-density
lipoprotein-cholesterol; H&E, hematoxylin and eosin; CON,
control; HLP, high lipid diet-fed rats; TAN, Tanshinone IIA
treatment of high lipid diet-fed rats. |
Furthermore, H&E stained liver sections
indicated that the hepatic cord is arranged normally in the control
rats and that hepatocytes display normal morphology, with a large
round nucleus in the centre and little accumulation of lipid
droplets in the cytoplasm. There was no infiltration of
inflammatory cells in the lobules. However, in the HLP rats, severe
hepatic steatosis was observed, and hepatocytes were swollen and
round, and the cytoplasm was loose and contained large lipid
droplets (Fig. 1B). In a number of
hepatocytes, the nucleus was located in the periphery, and lipid
vacuoles of varying size and inflammatory cells were observed. In
the TAN rats, the level of hepatic steatosis, the number of lipid
vacuoles and hepatocyte size were all reduced (Fig. 1B). The oil red O staining indicated
that the hepatocytes in the HLP rats had significantly greater
numbers of lipid droplets compared with the control rats, whereas
the number of lipid droplets in hepatocytes was significantly
reduced in the TAN rats (Fig.
1C).
Tanshinone IIA regulation of miR-33a,
ABCA1 and ABCG1 expression in the liver
The expression levels of miR-33a in the livers of
HLP rats was significantly upregulated compared with the control
rats (P<0.01), whereas the expression levels of miR-33a were
significantly reduced in the TAN rats (P<0.01; Fig. 2A), indicating that Tanshinone IIA
was able to control miR-33a expression in the rat liver.
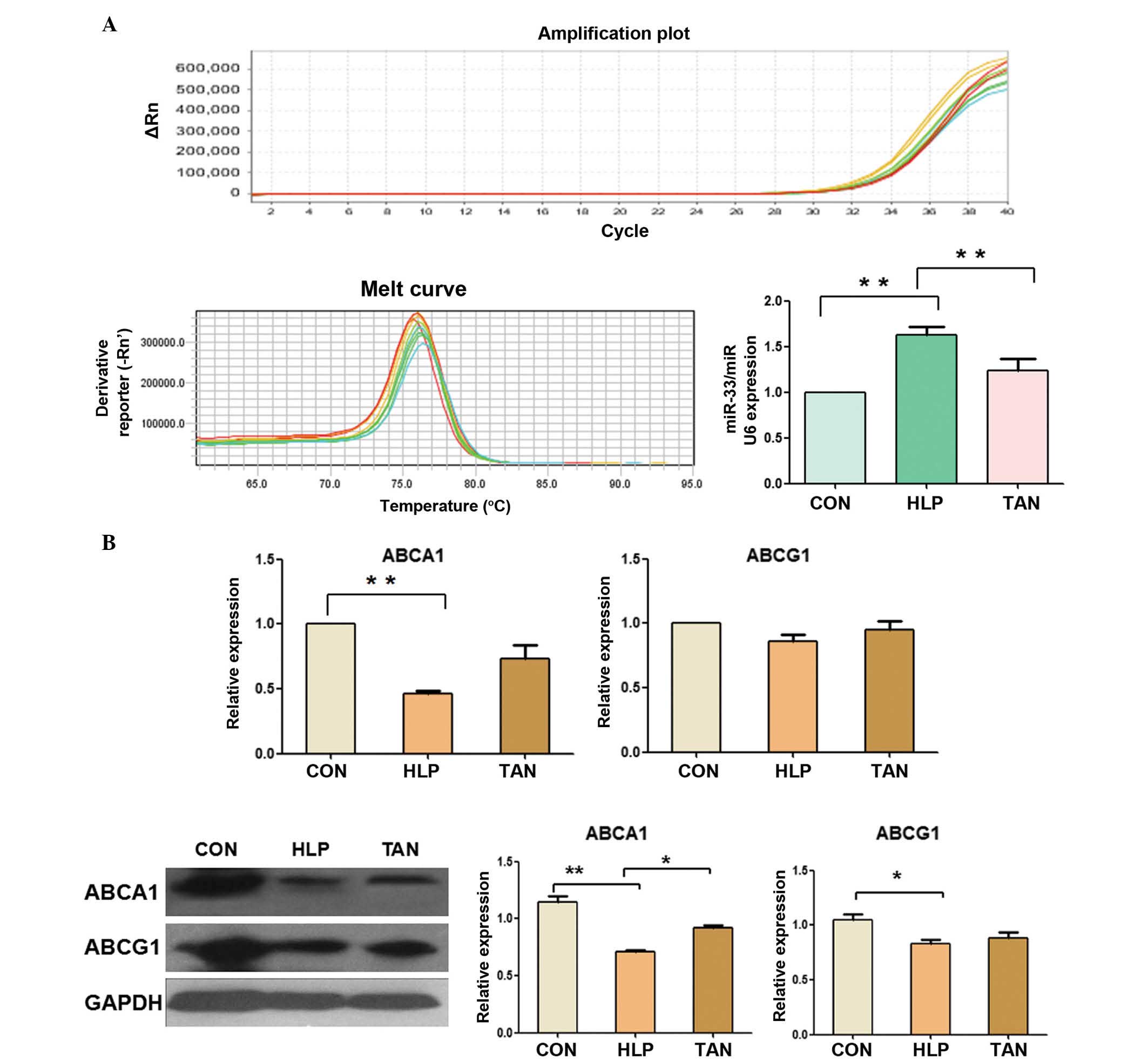 | Figure 2Tanshinone IIA regulation of miR-33a,
ABCA1 and ABCG1 expression in the liver. (A) RT-qPCR measurement of
miR-33a expression in rat liver tissue. (B) RT-qPCR and western
blotting analyses of ABCA1 and ABCG1 expression in liver tissue.
Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01. miR, microRNA;
ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding
cassette transporter G1; RT-qPCR, reverse trancription-polymerase
chain reaction; CON, control; HLP, high lipid diet-fed rats; TAN,
Tanshinone IIA treatment of high lipid diet-fed rats; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Furthermore, expression of ABCA1 mRNA and protein in
the liver of HLP rats was significantly downregulated compared with
the control rats (P<0.01), whereas the expression levels of
ABCA1 protein were significantly higher in the TAN rats compared
with the HLP rats (P<0.05), however, the levels of ABCA1 mRNA
showed no significant alteration (Fig.
2B). In addition, the expression levels of ABCG1 mRNA and
protein showed no significant alterations between the TAN and HLP
rats (Fig. 2B).
Tanshinone IIA regulation of SREBP-2,
Pcsk9 and LDL-R mRNA and protein expression in the liver
The mRNA expression levels of SREBP-2, Pcsk9 and
LDL-R were significantly reduced in the liver tissue of HLP rats
compared with the control rats (P<0.01; Fig. 3A). However, the mRNA expression
levels of SREBP-2 and Pcsk9 were significantly upregulated in the
liver tissue of TAN rats, with no alterations observed in LDL-R
mRNA expression. The western blotting data supported the RT-qPCR
data (Fig. 3B). The
immunohistochemical staining additionally indicated that the liver
tissues from the HLP rats exhibited weak staining for SREBP-2,
Pcsk9 and LDL-R compared with the control rats, whereas the liver
tissues from the TAN rats indicated stronger staining for SREBP-2,
Pcsk9 and LDL-R (P<0.01; Fig.
4).
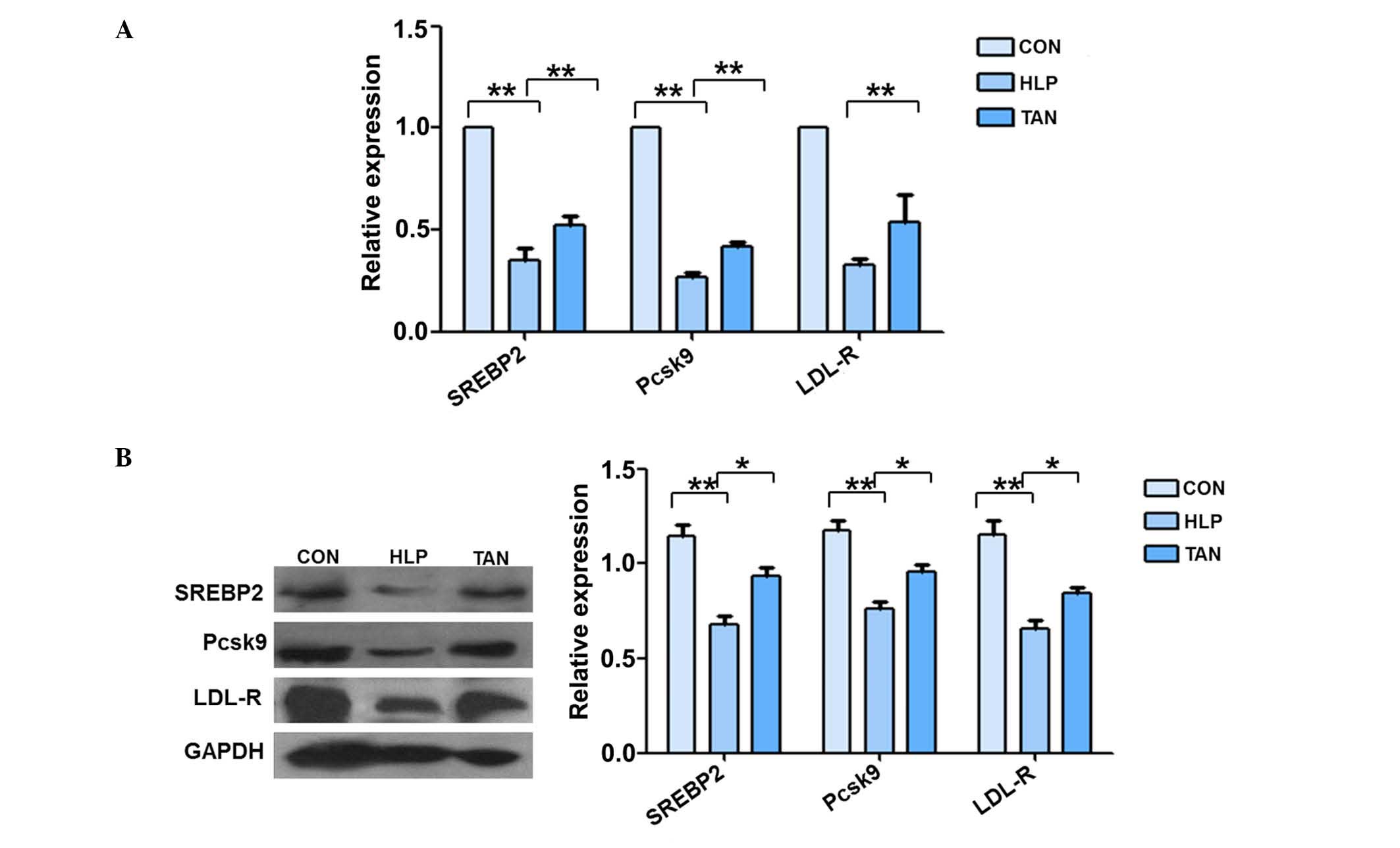 | Figure 3Tanshinone IIA regulation of SREBP-2,
Pcsk9 and LDL-R mRNA and protein expression levels in the liver.
(A) Reverse transcription-polymerase chain reaction measurement of
SREBP-2, Pcsk9 and LDL-R mRNA expression levels in rat liver
tissue. (B) Western blotting analysis of SREBP-2, Pcsk9 and LDL-R
protein expression levels in the liver. Data are presented as the
mean ± standard deviation. *P<0.05,
**P<0.01. SREBP-2, sterol regulatory element-binding
protein 2; Pcsk9, proprotein convertase subtilisin/kexin type 9;
LDL-R, low-density lipoprotein receptor; CON, control; HLP, high
lipid diet-fed rats; TAN, Tanshinone IIA treatment of high lipid
diet-fed rats; GAPDH, .glyceraldehyde 3-phosphate
dehydrogenase. |
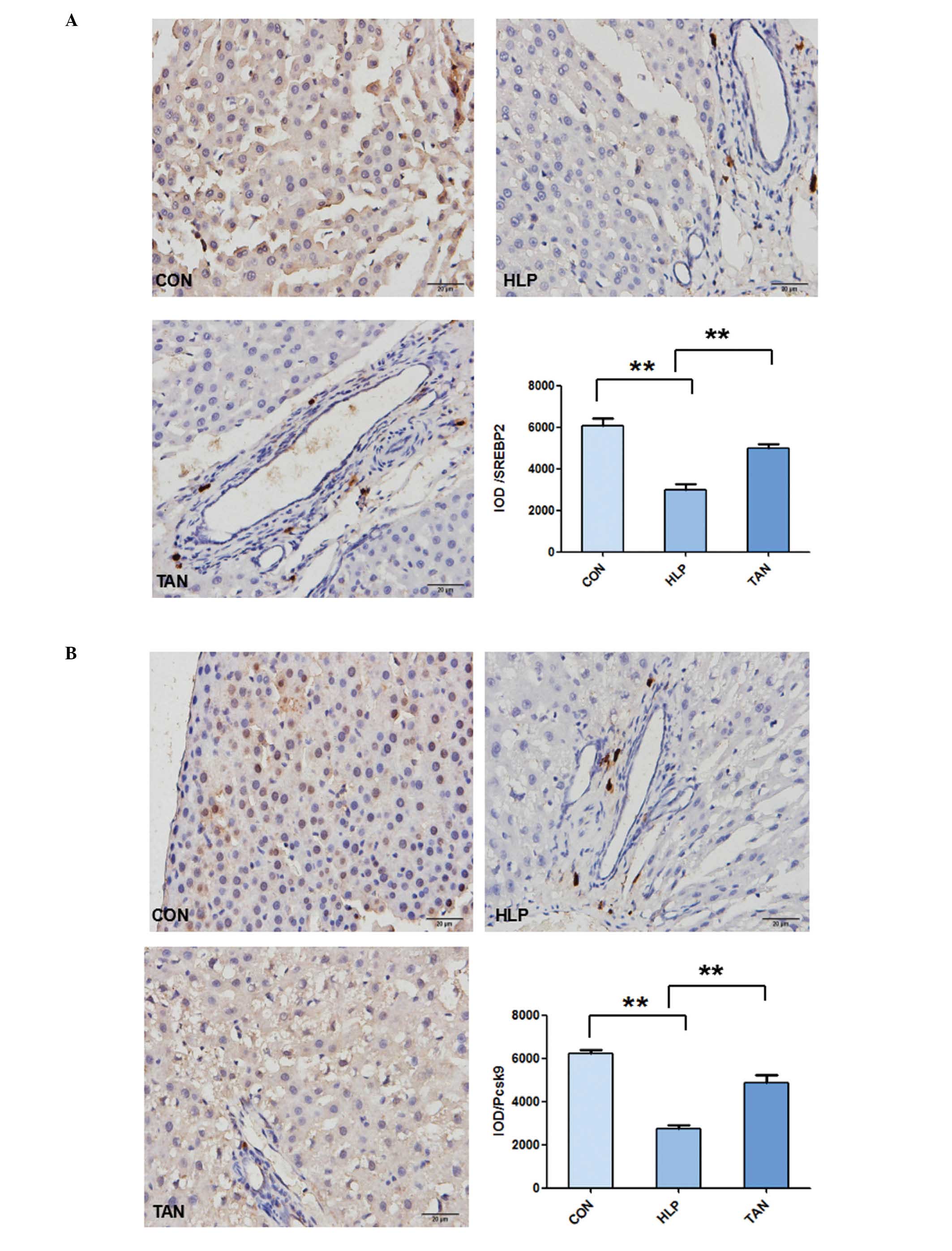 | Figure 4Immunohistochemical detection of
SREBP-2, Pcsk9 and LDL-R expression in the liver tissue. Tissue
from the CON, HLP and TAN groups was collected and stained with
antibodies against SREBP-2, Pcsk9 and LDL-R. (A) SREBP-2 protein
expression in liver tissue. (B) Pcsk9 protein expression in liver
tissue. Data are presented as the mean ± standard deviation.
**P<0.01. SREBP-2, sterol regulatory element-binding
protein 2; Pcsk9, proprotein convertase subtilisin/kexin type 9;
LDL-R, low-density lipoprotein receptor; CON, control; HLP, high
lipid diet-fed rats; TAN, Tanshinone IIA treatment of high lipid
diet-fed rats; IOD, integrated optical density. (C) LDL-R protein
expression in liver tissue. Data are presented as the mean ±
standard deviation. **P<0.01. LDL-R, low-density
lipoprotein receptor; CON, control; HLP, high lipid diet-fed rats;
TAN, Tanshinone IIA treatment of high lipid diet-fed rats; IOD,
integrated optical density. |
Discussion
Tanshinone is an active compound in Danshen, a
traditional Chinese medicine, which has been used historically for
the prevention and treatment of cardiovascular diseases in China.
There are four major lipophilic components of Tanshinone, namely
Tanshinone I, Tanshinone IIA, Tanshinone IIB and crypto Tanshinone.
Of these, Tanshinone IIA is the most abundant derivative and
structural representative of the Tanshinones (7). In the current study, the in
vivo effects of Tanshinone IIA were investigated in a rat model
of hyperlipidemia, which indicated that Tanshinone IIA was able to
reduce lipid deposition in the liver, without altering the blood
lipid profile following a three-month treatment. At the gene level,
Tanshinone IIA inhibited miR-33a expression in the liver and
upregulated ABCA1, ABCG1, SREBP-2 and Pcsk9 expression. These genes
may form a miR-33a/SREBP-2/Pcsk9 signaling pathway to regulate
lipid metabolism (27–29). Thus, the current study indicates
that Tanshinone IIA should be further studied due to their
potential use clinically to prevent or treat fatty liver and
associated diseases.
Fat-enriched diets have been used for decades to
model obesity, hyperlipidemia, dyslipidemia and insulin intolerance
in rodents (30). In the current
study, rats were fed with a high-fat diet for three months to
establish a rat model of hyperlipidemia according to previous
studies (31). Following 3 months
of a high fat diet, a group of rats were treated with Tanshinone
IIA for a further 3 months. The aim of the present study was to
demonstrate Tanshinone IIA activity against hyperlipidemia in
vivo, with the data indicating that Tanshinone IIA is able to
reduce lipid deposition in the liver, however, did not affect the
serum lipid profile. Clinically, lipid deposition in the liver can
result in fatty liver, abnormal liver function or cirrhosis, which
may be due to non-alcoholic fatty liver disease or excessive
alcohol consumption. The prevalence of non-alcoholic fatty liver
disease ranges from 9–36.9% of the population in different parts of
the world (32–34). From use in Chinese clinics, Danshen
has been observed to display no notable side effects; thus,
Tanshinone IIA may have potential in aiding the control of fatty
livers.
Cholesterol is an essential structural component of
the cell membrane in the majority of vertebrates, and its
transportation requires lipoproteins in the human body (35). It is known that LDL is the main
carrier of cholesterol in the blood, and altered serum LDL levels
are considered a major risk factor for cardiovascular disease
(36). LDL-R, a transmembrane
glycoprotein, is the predominant molecule to maintain serum LDL
levels and serves an important role in cholesterol homeostasis
(37,38). When cells accumulate excess
sterols, LDL-R expression on the cell surface will reduced. By
contrast, upon depletion of intracellular cholesterol, cells
express a high level of LDL-R (39). Previous studies have indicated that
Pcsk9 is highly expressed in the liver and contributes to
cholesterol homeostasis by regulating LDL-R levels (39,40).
Pcsk9 mutations have been associated with autosomal dominant
hypercholesterolemia, which is characterized by high LDL levels
(41). In support of this, the
current study indicated that Tanshinone IIA is able to promote
Psck9 expression in liver tissue. miRNAs are a class of non-coding
small RNAs, ~18–22 nucleotides long, which serve an important role
in the regulation of gene expression at the post-transcriptional
level (42,43). Previous studies have demonstrated
that miR-33 is able to regulate cholesterol efflux and HDL
biogenesis by suppressing expression of the ABC transporters, ABCA1
and ABCG1 (39,26). These transporters promote the
efflux of phospholipids and cholesterol to their associated
apolipoprotein, apo-A1, to generate nascent, discoidal HDL
particles, a critical step for the initiation of reverse
cholesterol transport to the liver for excretion (5). A previous study demonstrated that
there are three highly conserved miR-33 binding sites in the
3′-untranslated region of ABCA1, and that miR-33 overexpression
represses the expression of ABCA1 protein in a variety of cells
(20). SREBPs are transcription
factors that bind to the sterol regulatory element DNA sequence,
TCACNCCAC, to regulate the cholesterol biosynthetic pathway
(44). Hepatic cholesterol
deprivation results in SREBP cleavage-activating protein escorting
SREBP to the Golgi apparatus for processing and activation,
followed by transportation to the nucleus and the upregulation of
target gene expressions (45,46).
In the current study, miR-33a expression was observed to be
upregulated, whilst the expression levels of ABCA1 and ABCG1 were
reduced in the liver tissues of HLP rats, which further supports
that ABCA1 and ABCG1 are the miR-33a target genes. Thus, further
studies should investigate whether the effect of Tanshinone IIA is
mediated by suppression of miR-33a expression.
To conclude, the current study indicates that
Tanshinone IIA is able to attenuate lipid deposition in the livers
of hyperlipidemic rats, and modulate the expression of miR-33a and
SREBP2/Pcsk9 signaling pathway proteins. Further studies are
required to investigate the underlying molecular mechanisms and to
assess the potential clinical use of Tanshinone IIA.
Acknowledgments
The current study was supported by the National
Natural Science Foundation of China (grant no. 81202834), National
Program on Key Basic Research Project (973 Program) (grant no.
2013CB531704), Natural Science Foundation of Liaoning Province
(grant no. 2015020394), and Institutions of Higher Learning Talents
Support Program in Liaoning Province (grant no. LR2015041).
References
|
1
|
Chait A and Brunzell JD: Acquired
hyperlipidemia (secondary dyslipoproteinemias). Endocrinol Metab
Clin North Am. 19:259–278. 1990.PubMed/NCBI
|
|
2
|
Williams AD: Hyperlipidaemia and
atherogenesis. Med Hypotheses. 33:213–217. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie Y, He YB, Zhang SX, Pan AQ, Zhang J,
Guan XH, Wang JX and Guo WS: Treatment of combined hyperlipidemia
patients by jiangzhi tongluo soft capsule combined atorvastatin
calcium tablet: A clinical study. Chin J Int Trad Western Med.
34:1059–1063. 2014.In Chinese.
|
|
4
|
Fruebis J, Bird DA, Pattison J and
Palinski W: Extent of antioxidant protection of plasma LDL is not a
predictor of the antiatherogenic effect of antioxidants. J Lipid
Res. 38:2455–2464. 1997.
|
|
5
|
Tall AR, Yvan-Charvet L, Terasaaka N,
Pagler T and Wang N: HDL, ABC transporters and cholesterol efflux:
Implications for the treatment of atherosclerosis. Cell Metab.
7:365–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang F, Wu X, Wang T, Wang P, Li R, Zhang
H, Gao J, Chen S, Bao L, Huang H and Liu P: Tanshinone IIA
attenuates atherosclerotic calcification in rat model by inhibition
of oxidative stress. Vascul Pharmacol. 46:427–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gao S, Liu Z, Li H, Little PJ, Liu P and
Xu S: Cardiovascular actions and therapeutic potential of
Tanshinone IIA. Atherosclerosis. 220:3–10. 2012. View Article : Google Scholar
|
|
8
|
Xu W, Yang J and Wu LM: Cardioprotective
effects of anshinone IIA on myocardial ischemia injury in rats.
Pharmazie. 64:332–336. 2009.PubMed/NCBI
|
|
9
|
Gong Z, Huang C, Sheng X, Zhang Y, Li Q,
Wang MW, Peng L and Zang YQ: The role of Tanshinone IIA in the
treatment of obesity through peroxisome proliferator-activated
receptor gamma antag-onism. Endocrinology. 150:104–113. 2009.
View Article : Google Scholar
|
|
10
|
Tang FT, Cao Y, Wang TQ, Wang LJ, Guo J,
Zhou XS, Xu SW, Liu WH, Liu PQ and Huang HQ: Tanshinone IIA
attenuates atherosclerosis in ApoE (−/−) mice through
down-regulation of scavenger receptor expression. Eur J Pharmacol.
650:275–884. 2011. View Article : Google Scholar
|
|
11
|
Jia LQ, Feng JY, Yang GL, Chen WN and Chen
Y: Effect of Tanshinone IIA on TLR4 and TNF-α of endothelial cells
induced by LPS. Chin J Cell Mol Immunol. 27:733–735. 2011.In
Chinese.
|
|
12
|
Jia LQ, Yang GL, Ren L, Chen WN, Feng JY,
Gao Y, Zhang L, Li XT and Lei P: Tanshinone IIA reduces apoptosis
induced by hydrogen peroxide in the human endothelium-derived
EA.hy926 cells. J Ethnopharmacol. 143:100–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horie T, Ono K, Horiguchi M, Nishi H,
Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga
Y, et al: MicroRNA-33 encoded by an intron of sterol regulatory
element-binding protein 2 (SREBP-2l) regulates HDL in vivo. Proc
Natl Acad Sci USA. 107:17321–17326. 2010. View Article : Google Scholar
|
|
14
|
Dávalos A, Goedeke L, Smibert P, Ramirez
CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U,
Pastor-Pareja JC, et al: miR-33a/b contribute to the regulation of
fatty acid metabolism and insulin signaling. Proc Natl Acad Sci
USA. 108:9232–9237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu CY, Tang ZH, Liu LS and Jiang ZS:
Selecting pharmacological targets of Pcsk9. Chin J Biochem Mol
Biol. 25:991–996. 2009.In Chinese.
|
|
16
|
Costet P, Cariou B, Lambert G, Lalanne F,
Lardeux B, Jarnoux AL, Grefhorst A, Staels B and Krempf M: Hepatic
Pcsk9 expression is regulated by nutritional status via insulin and
sterol regulatory element-binding protein 1c. J Biol Chem.
281:6211–6218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Attie AD: The mystery of Pcsk9.
Arterioscler Thromb Vasc Biol. 24:1337–1339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steinberg D and Witztum JL: Inhibition of
Pcsk9: A powerful weapon for achieving ideal LDL cholesterol
levels. Proc Natl Acad Sci USA. 106:9546–9547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong B, Wu MH, Li H, Kraemer FB, Adeli K,
Seidah NG, Park SW and Liu J: Strong iduction of Pcsk9 gene
expression through HNF1alpha and SREBP-2: Mechanism for the
resistance to LDL-cholesterol lowering effect of statins in
dyslipidemic hamsters. J Lipid Res. 51:1486–1495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Najafi-Shoushtari SH, Kristo F, Li Y,
Shioda T, Cohen DE, Gerszten RE and Näär AM: MicroRNA-33 and the
SREBP host genes cooperate to control cholesterol homeostasi.
Science. 328:1566–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Attie AD: ABCA1: At the nexus of
cholesterol, HDL and atherosclerosis. Trends Biochem Sci.
32:172–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fernández-Hernando C, Ramírez CM, Goedeke
L and Suárez Y: MicroRNAs in metabolic disease. Arterioscler Thromb
Vasc Bio. 33:178–185. 2013. View Article : Google Scholar
|
|
23
|
Gerin I, Clerbaux LA, Haumont O, Lanthier
N, Das AK, Burant CF, Leclercq IA, MacDougald OA and Bommer GT:
Expression of miR-33 froman SREBP-2 intron inhibits cholesterol
export and fatty acid oxidation. J Biol Chem. 285:33652–33661.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fernández-Hernando C, Suárez Y, Rayner KJ
and Moore KJ: MicroRNAs in lipid metabolism. Curr Opin Lipidol.
22:86–92. 2011. View Article : Google Scholar :
|
|
25
|
Lewis GF and Rader DJ: New insights into
the regulation of HDL metabolism and reverse cholesterol transport.
Circ Res. 96:1221–1232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rayner KJ, Sheedy FJ, Esau CC, Hussain FN,
Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y,
et al: Antagonism of miR-33 in mice promotes reverse cholesterol
transport and regression of atherosclerosis. J Clin Invest.
121:2921–2931. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rayner KJ, Esau CC, Hussain FN, McDaniel
AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X,
et al: Inhibition of miR-33a/b in non-human primates raises plasma
HDL and lowera VLDL triglycerides. Nature. 478:404–447. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu W, Zhai C, Zhang X, Zhang H, Jiang M,
Zhou S, Liu Y, Zhao N and Zhao J: Research of the whole
grain-soybean compound package to regulate the cholesterol
metabolism by SREBP-2, LDLR and visfatin. J Hyg Res. 42:196–202.
2013.In Chinese.
|
|
29
|
Xu W, Liu L and Homby D: c-IAP1 binds and
processes Pcsk9 protein: Linking the c-IAP1 in a TNF-α pathway to
Pcsk9-mediated LDLR degradation pathway. Molecules. 17:12086–12101.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mani DN, Bawankule D and Saroj BK:
Hyperlipidemic model: Studying lipid profile in small experimental
animal. Int J Pharmacy Pharm Sci. 4:337–340. 2012.
|
|
31
|
Munshi RP, Joshi SG and Rane BN:
Development of an experimental diet model in rats to study
hyperlipidemia and insulin resistance, markers for coronary heart
disease. Indian J Pharmacol. 46:270–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Omagari K, Kadokawa Y, Masuda J, Egawa I,
Sawa T, Hazama H, Ohba K, Isomoto H, Mizuta Y, Hayashida K, et al:
Fatty liver in non-alcoholic non overweight Japanese adults:
Incidence and clinical characteristics. J Gastroenterol Hepatol.
17:1098–1105. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hilden M, Christoffersen P, Juhl E and
Dalgaard JB: Liver histology in a 'normal' population-examinations
of 503 consecutive fatal traffic casualties. Scand J Gastroenterol.
12:593–597. 1997. View Article : Google Scholar
|
|
34
|
Shen L, Fan JG, Shao Y, Zeng MD, Wang JR,
Luo GH, Li JQ and Chen SY: Prevalence of nonalcoholic fatty liver
among administrative officers in Shanghai: An epidemiological
survey. World J Gastroenterol. 9:1106–1110. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ikonen E: Cellular cholesterol trafficking
and compartmentalization. Nat Rev Mol Cell Biol. 9:125–138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sone H, Tanaka S, Tanaka S, Iimuro S, Oida
K, Yamasaki Y, Oikawa S, Ishibashi S, Katayama S, Ohashi Y, et al:
Serum level of triglycerides is a potent risk factor comparable to
LDL cholesterol for coronary heart disease in Japanese patients
with type 2 diabetes: Subanalysis of the Japan Diabetes
Complications Study (JDCS). J Clin Endocrinol Metab. 96:3448–3456.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brown MS and Goldstein JL: A
receptor-mediated pathway for cholesterol homeostasis. Science.
232:34–47. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sharpe LJ and Brown AJ: Rapamycin
down-regulates LDL-receptor expression independently of SREBP-2.
Biochem Biophys Res Commun. 373:670–674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rayner KJ, Suárez Y, Dávalos A, Parathath
S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ and
Fernández-Hernando C: MiR-33 contributes to the regulation of
cholesterol homeostasis. Science. 328:1570–1573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maxwell KN and Breslow JL:
Adenoviral-mediated expression of Pcsk9 in mice results in a
low-density lipoprotein receptor knockout phenotype. Proc Natl Acad
Sci USA. 101:7100–7105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abifadel M, Varret M, Rabès JP, Allard D,
Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich
D, et al: Mutations in Pcsk9 cause autosomal dominant
hypercholesterolemia. Nat Genet. 34:154–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010.PubMed/NCBI
|
|
43
|
Kato M, de Lencastre A, Pincus Z and Slack
FJ: Dynamic expression of small non-coding RNAs, including novel
microRNAs and piRNAs/21U-RNAs, during Caenorhabditis elegans
development. Genome Biol. 10:R542009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roglans N, Peris C, Verd JC, Alegret M,
Vázquez M, Sánchez RM and Laguna JC: Increase in hepatic expression
of SREBP-2 by gemfibrozil administration to rats. Biochem
Pharmacol. 62:803–809. 2011. View Article : Google Scholar
|
|
45
|
Horton JD and Shimomura I: Sterol
regulatory element-binding proteins: Activators of cholesterol and
fatty acid biosynthesis. Curr Opin Lipidol. 10:143–150. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Horton JD: Sterol regulatory
element-binding proteins: Transcriptional activators of lipid
synthesis. Biochem Soc Trans. 30:1091–1095. 2002. View Article : Google Scholar : PubMed/NCBI
|


















