Introduction
Cerebral hypoxic-ischaemic insult during the
perinatal period leads to neonatal brain injury, including
encephalopathy, motor and mental deficits, learning disabilities
and epilepsy (1).
Hypoxic-ischaemic brain damage (HIBD) can be caused by brain
lesions and the induction of hippocampal long-term potentiation
through a series of pathophysiological changes in cell metabolic
dysfunction, cerebral blood flow abnormalities, excitatory amino
acid neurotoxicity, and intracellular calcium overload, free
radical (e.g., nitric oxide) accumulation and apoptosis (2,3). The
majority of drug therapies in the clinical arena are based on
maintaining cell activities and delaying neuron death by preventing
apoptosis to improve brain function. However, currently, no
specific treatments are available for HIBD. An oxygen and glucose
deprivation (OGD) in vitro model was used to simplify the
complex brain interaction network in HIBD. Previous studies
demonstrated that mesenchymal stromal cells (MSCs) could
effectively restore learning and memory function in neonatal rats
submitted to hypoxic-ischaemic insult in vivo (4–6), and
these results demonstrated that transplanted MSCs exert
immunomodula-tory effects in the injury microenvironment, and that
the MSCs secreted abundant interleukin-6 (IL-6) when co-cultured
with OGD-injured PC12 cells (7).
These results suggested that the high level of IL-6 expression may
exert a neuroprotective role in neuronal injury. IL-6 is a
pleiotropic cytokine that is able to regulate a variety of cell
functions, including cell proliferation, cell differentiation,
immune defence and the inhibition of apoptosis. Previous evidence
has revealed that IL-6 acts a double-edged sword, having dual
proinflammatory and anti-inflammatory effects. Several studies have
revealed that the expression of IL-6 is markedly increased when the
central nervous system (CNS) is injured or is disease-afflicted,
causing brain injury aggravation (8–10).
However, a previous study (11)
identified that astrocyte and macrophage activation were markedly
decreased in the brains of IL-6 knockout mice following brain
injury. This decrease in immunological reactivity suggested that
IL-6 may have a neuroprotective role in brain injury. Based on
these findings, the present study aimed to identify how MSCs
secrete IL-6, and the physiological role of IL-6 in OGD-injured
PC12 cells.
Materials and methods
Animals
Ten Wistar rats (age, 21 days), including 5 female
and 5 male rats, were purchased from the Animal Experiment Centre
of Daping Hospital affiliated with the Third Military Medical
University (Chongqing, China). The animals were housed under a 12 h
light/dark cycle with food and water freely available [SYXK (Yu)
2012–0015]. The experimental animal procedures were approved by the
Ethics Committee of Chongqing Medical University (Chongqing,
China).
Isolation and culture of MSCs
MSCs were isolated from the bone marrow of
21-day-old Wistar rats. The bone marrow was washed repeatedly with
Dulbecco's modified Eagle's medium (DMEM/F12; Gibco, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in a laminar flow cabinet until
the bone had become bleached and the bone marrow was flushed out.
Subsequently, the fluid containing bone marrow was centrifuged at
10,000 × g at room temperature for 3 min, and the supernatant was
discarded. Then, the bone marrow cells were scattered and plated in
a 60 mm Petri dish. The DMEM/F12 culture medium with 10% foetal
bovine serum (FBS; Gibco, Thermo Fisher Scientific, Inc.) was
changed every 24 h for the first three days, and subsequently the
medium was exchanged every other day for three additional days.
When the MSCs reached 70–80% confluence, the cells were digested
with TrypLE (Gibco; Thermo Fisher Scientific, Inc.) and passaged at
a ratio of 1:2 or 1:3.
Culturing PC12 cells
PC12 cells were obtained from the Cell Bank of
Shanghai Institute of Cell Biology, Chinese Academy of Science
(Shanghai, China). The cells were cultured in DMEM containing 10%
horse serum, 5% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin (all reagents purchased from Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a 5% CO2 incubator. When
the PC12 cells reached 90% confluence, they were digested with
TrypLE (Gibco; Thermo Fisher Scientific, Inc.) and passaged at a
ratio of 1:2 every three to four days.
In vitro OGD model construction and
co-culture configurations
The PC12 cells were seeded and grown to ~90%
confluence in a six-well plate. To establish the OGD model, the
PC12 cell culture medium was changed to Earle's balanced salt
solution (EBSS; GE Healthcare Life Sciences, Logan, Utah, USA)
after the cells had been washed twice with D-Hank's solution (GE
Healthcare Life Sciences). Subsequently, the cells were placed in
an incubator (Thermo Forma 3111; Thermo Fisher Scientific, Inc.)
with 5% O2 and 95% N2 at 37°C for 4 h, after
which the EBSS medium was replaced with normal cell medium. The
OGD-injured PC12 cells were co-cultured with MSCs
(2×106; called the MSC co-culture group) or with normal
PC12 cells (2×106; called the PC12 co-culture group) for
a further 24 h. OGD-injured PC12 cells without co-culture served as
controls. The co-culture methods were performed as previously
described (12), and the PC12 cell
co-culture group without injury was used as a co-culture negative
control.
MSC treatments
To up-regulate Toll-like receptor 2 (TLR2) or
down-regulate NFκB, the MSCs were cultured in the presence of the
TLR2 agonist, peptidoglycan from Staphylococcus aureus
(PGN-SA; 8 μg/ml; InvivoGen, Hong Kong, China) or the NFκB
inhibitor, pyrrolidine dithiocar-bamate (PDTC; 10 μg/ml;
Sigma-Aldrich, St. Louis, MO, USA) for 24 h. Subsequently, the MSCs
were treated with a TLR2- or IL-6-targeting small interfering
(si)RNA, siTLR2 and siIL-6, or recombinant Ad-IL-6 adenovirus to
knock down the expression of TLR2 or to regulate the expression
level of IL-6, respectively, according to our previous studies
(13,14). MSCs treated with red fluorescent
protein (RFG) served as the control. Stable siIL-6-MSCs were used
to investigate the biological function of IL-6, as reported
previously (15). Green
fluorescent protein (GFP)-labelled MSCs were used as a control.
RNA isolation and real-time quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated using an RNA extraction kit
(BioTek Corp., Beijing, China) and reverse-transcribed into cDNA
using a PrimeScript® RT Reagent kit (DRR037A; Takara
Bio, Inc., Shiga, Japan) and a Bio-Rad My Cycler (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's protocol. The single-stranded cDNA was diluted
10-fold, and used as the template. qPCR was performed on a StepOne™
v2.1 Real-Time PCR instrument (Applied Biosystems; Redwood City,
CA, USA) using Real Master mix [SYBR® Green; Tiangen
Biotech (Beijing) Co., Ltd, Beijing, China]. The thermocycling
conditions were as follows: Initial denaturation at 95°C for 30 sec
and denaturation at 95°C for 5 sec, followed by 40 cycles at 60°C
for 30 sec. Melting curve analysis and gel electrophoresis were
performed to ensure that a single PCR product was amplified in each
reaction. The PCR primers were designed using Primer Premier 5.0
software (Premier Biosoft International, Palo Alto, CA, USA). The
ratio of the relative quantity of the target gene to
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), as a reference
gene, was calculated using the 2−ΔΔCq method (16). The primer sequences for GAPDH,
TLR2, NFκB and IL-6 are shown in Table
I.
 | Table IPrimer sequences for polymerase chain
reaction. |
Table I
Primer sequences for polymerase chain
reaction.
| Gene | Primer sequence |
|---|
| GAPDH | F:
5′-CCTGGAGAAACCTGCCAAG-3′ |
| R:
5′-CACAGGAGACAACCTGGTCC-3′ |
| TLR2 | F:
5′-TCTCGGCAACTATGAGTCCC-3′ |
| R:
5′-ATCGGTGAGATCTGCATTCC-3′ |
| NFκB | F:
5′-AGGACTGCCGGGATGGCTTCTAT-3′ |
| R:
5′-GGTCTGGATGCGCTGGCTAATGG-3′ |
| IL-6 | F:
5′-ACAGCCACTGCCTTCCCTAC-3′ |
| R:
5′-TTGCCATTGCACAACTCTTTTC-3′ |
Western blot analysis
The cells were lysed in radioimmuno-precipitation
assay buffer containing phenylmethanesulfonyl fluoride (Bioteke
Co., Ltd., Taiwan, China). The cell lysates were collected, and the
protein concentration was determined using a bicinchoninic acid
protein concentration determination kit (Bioteke Co., Ltd.,
Beijing, China). The cell lysates were purified by 10% sodium
dodecyl sulphate-polyacrylamide gel electrophoresis (Beyotime
Institute of Biotechnology, Beijing, China), with 30–40 μg
total protein loaded per lane. After the proteins were
electrophoresed, they were transferred to polyvi-nylidene fluoride
membranes (Millipore Corp., Billerica, MA, USA). Following blocking
of the membranes with 5% bovine serum antigen in Tris-buffered
saline/Tween-20 (TBST) buffer at room temperature for 1 h, they
were probed overnight with primary antibodies, including mouse
monoclonal anti-B-cell lymphoma-associated X (anti-Bax; cat. no.
sc-7480; 1:1,000; Santa Cruz Biotechnology, Inc., CA, USA), rabbit
polyclonal anti-TLR2 (cat. no. SAB2102440; 1:1,000; Sigma-Aldrich),
rabbit polyclonal anti-NFκB (cat. no. ab12146; 1:500 to 1:1,000;
Abcam, Cambridge, UK), mouse monoclonal anti-IL-6 (cat. no.
MAB5061; 1:500; R&D Systems China Co., Ltd., Shanghai, China),
and mouse monoclonal β-actin (cat. no. sc-47778; 1:100 to 1:1,000;
Santa Cruz Biotechnology, Inc.) at 4°C, and subsequently incubated
with the appropriate secondary antibody conjugated with horseradish
peroxidase (HRP; Santa Cruz Biotechnology, Inc.) at room
temperature for 1 h. The proteins were detected using an enhanced
chemiluminescent (ECL) substrate kit containing chemiluminescent
HRP substrate (Millipore Corp.), and protein levels were recorded
using an ECL Imaging System (G:BOX; Syngene UK, Cambridge, UK).
Enzyme-linked immunosorbent assay
(ELISA)
The levels of IL-6 cytokine released into the
culture media of the different treatment groups were measured with
an ELISA kit (Beijing 4A Biotech Co., Ltd., Beijing, China),
according to the manufacturer's protocol. Background values were
also analysed as a control. The optical density absorbance was
measured at a wavelength of 450 nm. The values were calculated
based on a constructed standard curve, and the assay was performed
in triplicate.
Whole-cell patch-clamp recordings
The resting membrane potential (RMP) of cells was
measured with a whole-cell patch clamp, as previously reported
(4). Briefly, 30 mm glass
coverslips containing PC12 cells, following co-culture with
GFP-labelled MSCs or siIL-6-MSCs, were placed in an acrylic chamber
under a TE-2000U fluorescence inverted microscope (Nikon, Tokyo,
Japan). The membrane potential was amplified using a Multiclamp
700B amplifier, and a Digidata 1322 interface (Axon Instruments,
Foster City, CA, USA) was used for acquisition and off-line
analysis. Between seven and ten intact cells in each group were
selected to record the RMP, and all the recordings were performed
at room temperature and completed within 1 h following the removal
of the cells from the incubator.
Statistical analysis
All the data are expressed as the mean ±standard
error of the mean. Significant differences between samples were
examined by repeated measurements of analysis of variance (ANOVA)
with one-way ANOVA, followed by Duncan's multiple-range test.
P<0.05 was considered to indicate a statistically significant
value.
Results
MSCs down-regulate the expression level
of the apoptosis factor, Bax, and up-regulate the expression level
of IL-6 in OGD-injured PC12 cells
To evaluate the effects of MSCs on the OGD-injured
PC12 cells, an in vitro co-culture system of MSCs and
OGD-injured PC12 cells was established. Bax protein expression in
the OGD-injured PC12 cells, which were co-cultured with the same
quantity of uninjured PC12 cells or MSCs, was analysed by western
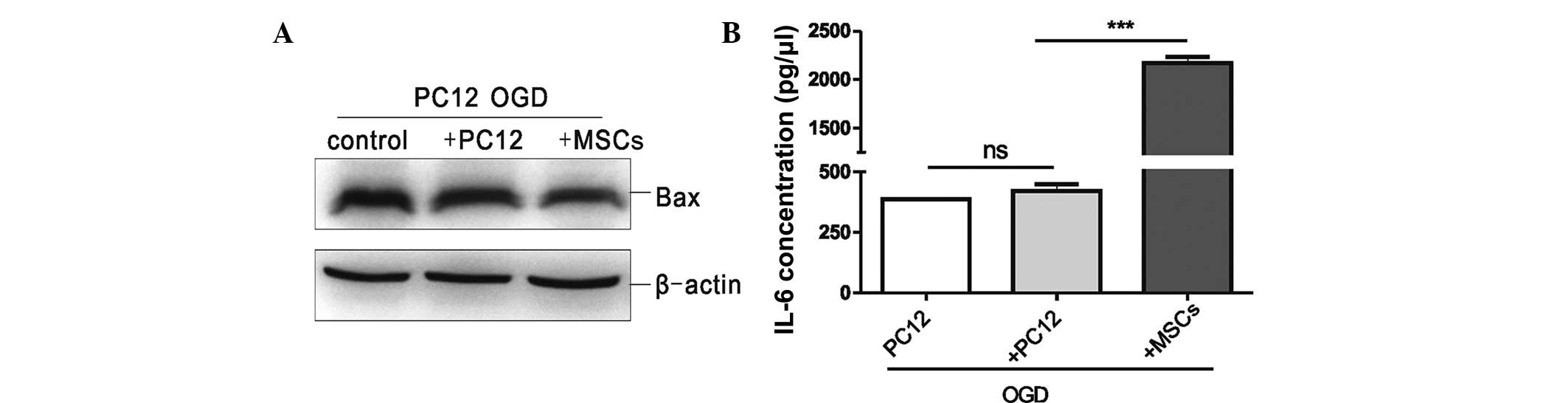
blot analysis. As shown in Fig.
1A, the protein expression level of Bax in the MSC co-culture
group was the lowest, that in the uninjured PC12 cell co-culture
group was at an intermediate level, and that in the OGD-injured
PC12 cell group was the highest. These data demonstrated that
co-culture with MSCs was able to promote anti-apoptotic effects on
the OGD-injured PC12 cells. The supernatants of the three
above-mentioned groups were collected to assess IL-6 expression by
ELISA; as shown in Fig. 1B, IL-6
secretion in the MSC co-culture group was significantly higher
compared with that in the uninjured PC12 cell co-culture group
(P<0.001), whereas no significant difference in IL-6 secretion
was observed between the uninjured PC12 cell co-culture group and
the OGD-injured PC12 cell group. These data demonstrated that IL-6
was secreted primarily by MSCs, which may regulate the
anti-apoptotic effect on the OGD-injured PC12 cells.
Protein expression levels of TLR2, NFκB
and IL-6 are altered following PGN-SA and siTLR2 treatment of
MSCs
To investigate whether IL-6 expression was regulated
via the TLR2/NFκB signalling pathway, the MSCs were treated with
PGN-SA or siTLR2. The changes in the expression levels of TLR2,
NFκB and IL-6 were detected by RT-qPCR and western blot analysis.
As shown in Fig. 2A, when compared
with the control group, the mRNA expression levels of TLR2
(P<0.001), NFκB (P<0.01) and IL-6 (P<0.05) were
signifi-cantly increased following PGN-SA treatment. The changes in
TLR2, NFκB and IL-6 protein expression detected by western blot
analysis were identical with those changes in mRNA expression
(Fig. 2B), suggesting that
treatment with 8 μg/ml PGN-SA not only increased the
expression level of TLR2 efficiently, but also up-regulated the
expression of NFκB and IL-6. Subsequently, the MSCs were infected
with siTLR2 adenovirus; compared with the RFP group, the mRNA and
protein expression levels of TLR2 (P<0.01), NFκB (P<0.05) and
IL-6 (P<0.001) were significantly decreased in the siTLR2 group
(Fig. 2C and D). The above results
demonstrated that the down-regulation of NFκB and IL-6 expression
levels may be associated with reduced levels of TLR2, and further
suggested that MSCs may regulate the secretion of IL-6 via the
TLR2/NFκB/p65 signalling pathway.
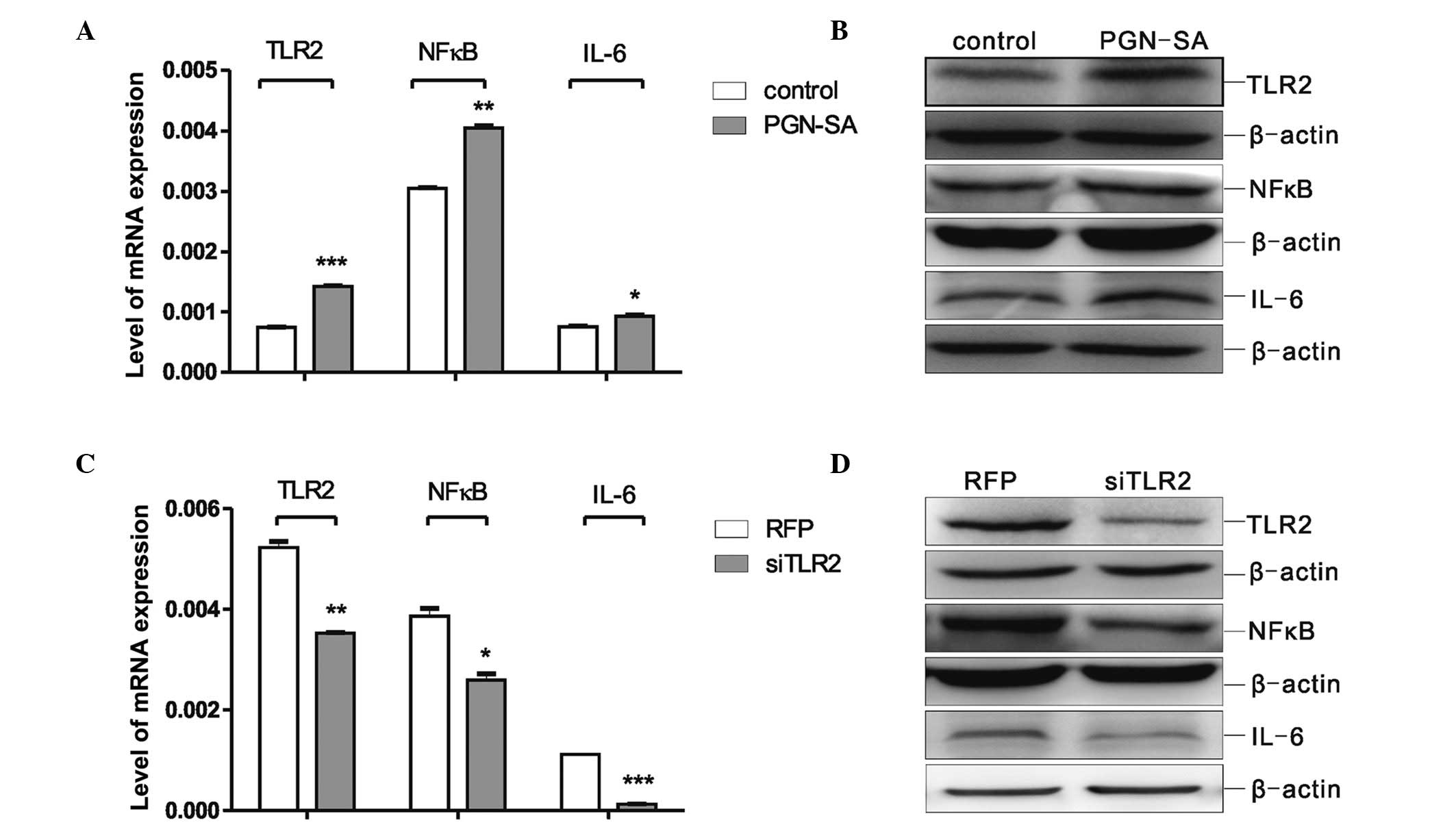 | Figure 2PGN-SA and siTLR2 caused changes in
the TLR2/NFκB pathway and IL-6, respectively, in MSCs. (A) RT-qPCR
was used to assess mRNA levels of TLR2, NFκB and IL-6 expression in
MSCs after being treated with PGN-SA. The values are expressed as
the mean ± standard error of the mean (*P<0.05,
**P<0.01, ***P<0.001 vs. the respective
control group cultured without PGN-SA). (B) Western blot analysis
of the protein levels of TLR2, NFκB and IL-6 in MSCs following
treatment with PGN-SA. (C) Real-time PCR was used to assess mRNA
levels of TLR2, NFκB and IL-6 expression in MSCs after being
treated with siTLR2. The values are expressed as the mean ±
standard error of the mean (*P<0.05,
**P<0.01, ***P<0.001 vs. the respective
control RFP group). (D) Western blot analysis of the protein levels
of TLR2, NFκB and IL-6 in MSCs following treatment with siTLR2. For
the western blot analyses (B and D), the data were obtained from at
least three independent experiments, analysed and normalised
against β-actin. MSCs, mesenchymal stromal cells; RT-qPCR,
real-time quantitative polymerase chain reaction; TLR2, Toll-like
receptor 2; IL-6, interleukin-6; NFκB, nuclear factor κB; PGN-SA,
peptidoglycan from Staphylococcus aureus; siTLR2, Toll-like
receptor 2 small interfering RNA; RFP, red fluorescent protein. |
The levels of NFκB and IL-6 protein
expression are down-regulated by the NFκB inhibitor, PDTC
To evaluate whether IL-6 is released from the MSCs
via the TLR2/NFκB pathway, MSCs were also treated with the NFκB
inhibitor, PDTC. Following treatment of the MSCs with 10
μg/ml PDTC for 48 h, the protein levels of NFκB, IL-6 and
TLR2 were assessed by western blot analysis, and the quantification
of the results is shown in Fig. 3.
Significant decreases in the expression levels of NFκB (P<0.001)
and IL-6 (P<0.005) were observed following PDTC treatment
compared with the control group. However, the expression levels of
TLR2 were not significantly different between the PDTC group and
the control group (Fig. 3A and B).
This finding suggested that the inhibition of NFκB expression was
able to decrease IL-6 expression, although it did not affect TLR2
expression.
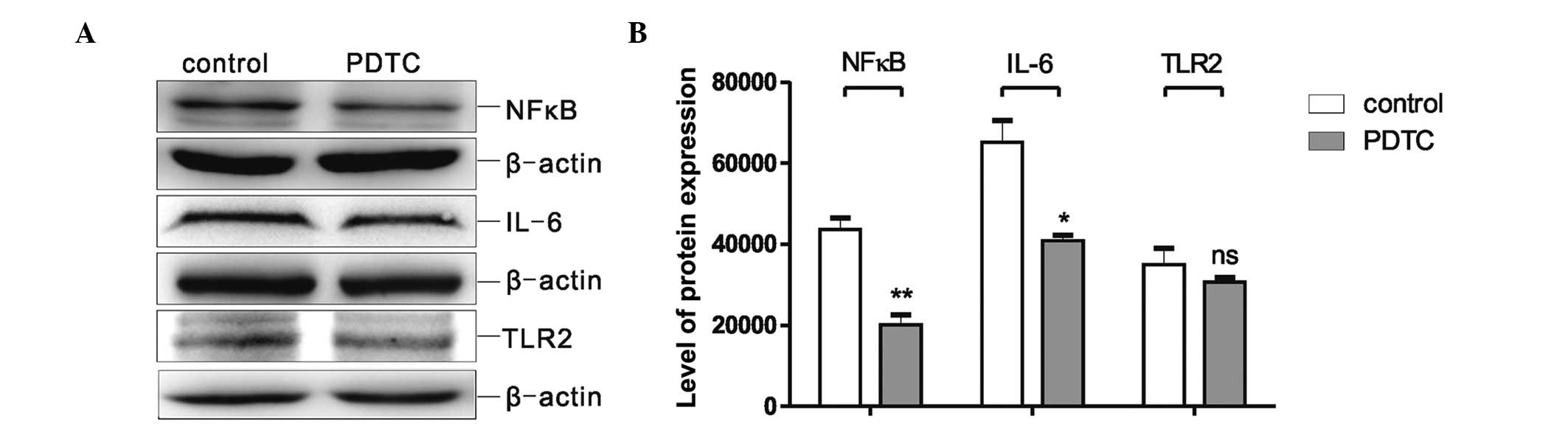 | Figure 3Protein expression levels of NFκB,
IL-6 and TLR2 were assessed in MSCs following treatment with the
NFκB inhibitor, PDTC. (A) Western blot analysis of NFκB, IL-6 and
TLR2 protein expression in MSCs following treatment with 10
μg/ml PDTC. (B) Quantification (grey intensity analysis) of
the western blot results. The data were obtained from at least
three independent experiments, analysed and normalised against
β-actin. The values are expressed as the mean ± standard error of
the mean (*P<0.05, **P<0.01 vs. the
respective control group). MSCs, mesenchymal stromal cells; TLR2,
Toll-like receptor 2; NFκB, nuclear factor κB; IL-6, interleukin-6;
PDTC, pyrrolidine dithiocarbamate. |
siIL-6 and Ad-IL-6 alter IL-6 expression,
but do not affect protein expression in the TLR2/NFκB pathway
To investigate whether IL-6 may regulate via a
feedback process the TLR2/NFκB signalling pathway, MSCs were
treated with Ad-IL-6 or siIL-6, and the expression levels of IL-6,
TLR2 and NFκB were analysed by RT-qPCR and western blot analysis.
The data in Fig. 4A and B
demonstrate that IL-6 expression was markedly increased in the MSCs
treated with Ad-IL-6 (P<0.001) compared with that of the RFP
group, whereas the expression of TLR2 and NFκB did not change.
Subsequently, the MSCs were infected with siIL-6, and as expected,
the IL-6 expression level correspondingly decreased (P<0.001);
however, siIL-6 infection did not reduce the expression levels of
TLR2 or NFκB (Fig. 4C and D).
These findings suggested that siIL-6 and Ad-IL-6 were able to
change the expression level of IL-6, but did not affect the
expression levels of TLR2 or NFκB in the MSCs.
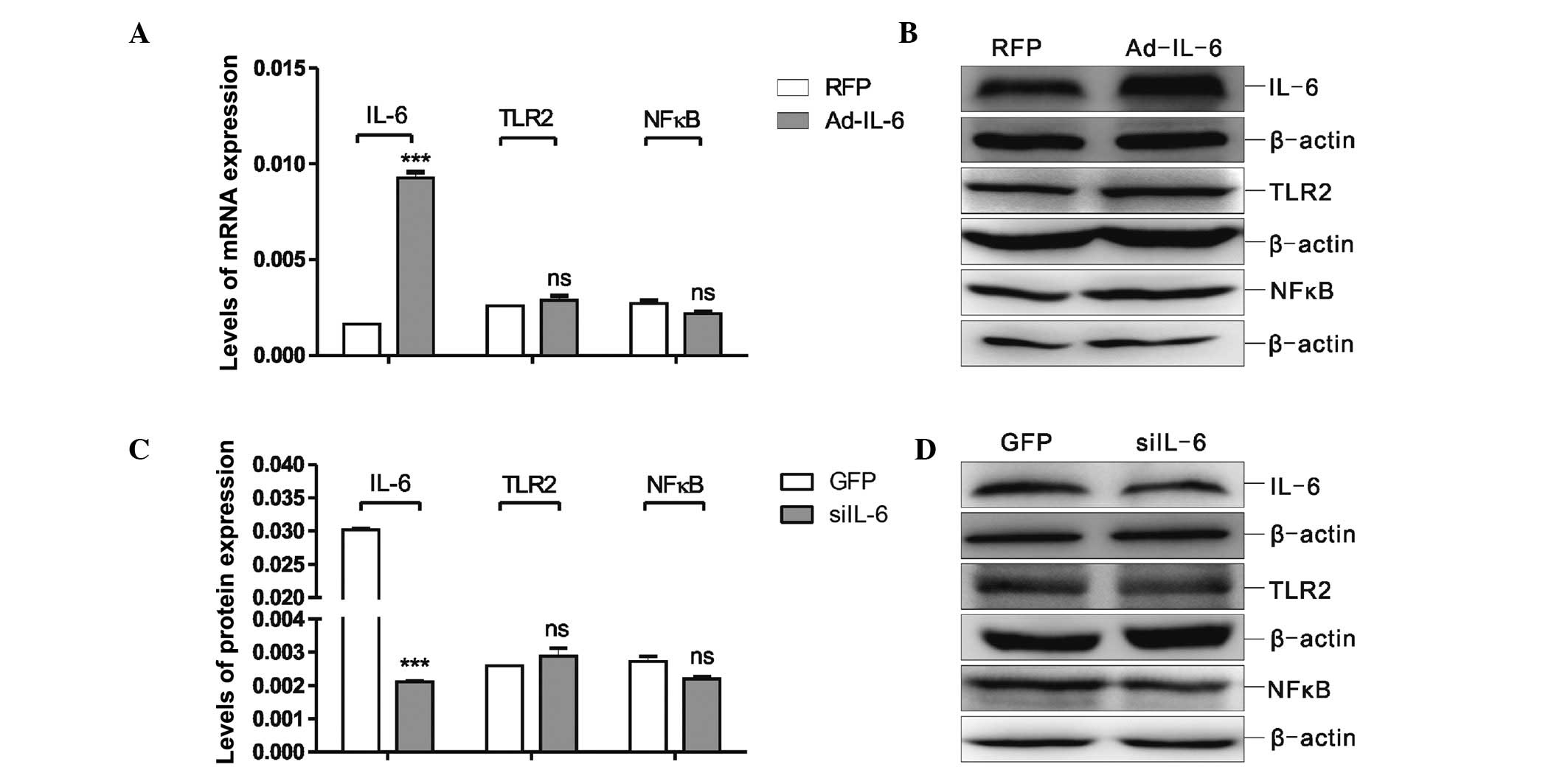 | Figure 4Determination of the changes in the
TLR2/NFκB/IL-6 pathway in MSCs following treatment with Ad-IL-6 or
siIL-6. (A) RT-qPCR analysis of the mRNA expression levels of IL-6,
TLR2 and NFκB in MSCs following treatment with Ad-IL-6. The values
are expressed as the mean ± standard error of the mean.
***P<0.001 vs. the RFP group. (B) Western blot
analysis of IL-6, TLR2 and NFκB protein levels in MSCs following
treatment with Ad-IL-6. (C) RT-qPCR analysis of the mRNA expression
levels of IL-6, TLR2 and NFκB in MSCs following treatment with
siIL-6. The values are expressed as the mean ± standard error of
the mean. ***P<0.001 vs. the RFP group. (D) Western
blot analysis of IL-6, TLR2 and NFκB protein levels in MSCs
following treatment with siIL-6. For the western blot analyses (B
and D), the data were obtained from at least three independent
experiments, analysed and normalised against β-actin. MSCs,
mesenchymal stromal cells; NFκB, nuclear factor κB; IL-6,
interleukin-6; Ad-IL-6, IL-6 adenovirus; RFP, red fluorescent
protein; siIL-6, IL-6 small interfering RNA; GFP, green fluorescent
protein; TLR2, Toll-like receptor 2. |
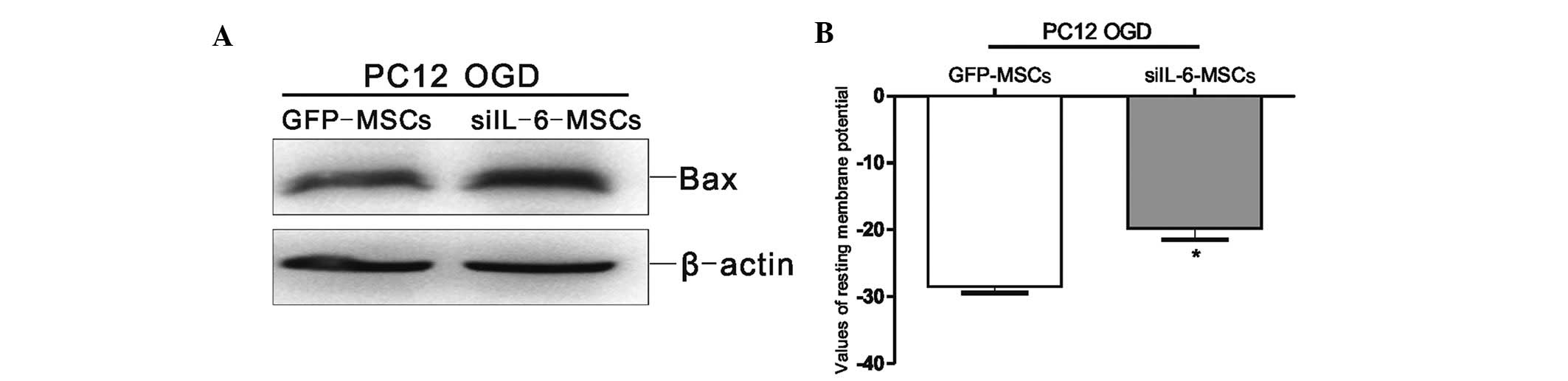
siIL-6-MSCs induce Bax expression and
affect the RMP in the OGD-injured PC12 cells
To evaluate the biological function of IL-6 secreted
from MSCs co-cultured with the OGD-injured PC12 cells, the MSC cell
line was screened for stable siIL-6 expression (15). The protein expression level of Bax
was markedly increased in the OGD-injured PC12 cells co-cultured
with the siIL-6-MSCs compared with the GFP-MSC group (Fig. 5A). The whole-cell patch-clamp
recordings revealed that the RMP of the siIL-6-MSC group
experienced a greater increase compared with that of the GFP-MSC
group (P<0.05; Fig. 5B). These
findings indicated that endogenous IL-6 from MSCs improved the
restorative function of the OGD-injured PC12 cells.
Discussion
A previous study (11) has demonstrated that transplanted
MSCs are not only able to ameliorate newly sustained brain damage,
but are also able to improve the long-term prognosis in
vivo. Transplanted MSCs have neuroprotective effects on the
treatment of CNS injury, including traumatic brain injury and
stroke, as well as on animal models of spinal cord injury. Previous
studies (4,5,17) by
our group have shown that MSCs can be induced and differentiated
into neurons to promote the recovery of nerve function in HIBD
rats. A further study (7)
indicated that MSCs may secrete large quantities of IL-6 and IL-10,
and that the levels of these cytokines may change the injury
microenvironment and reduce H2O2-induced
apoptosis. These results suggested that transplanted MSCs may have
direct or indirect immunomodulatory effects on the injury
microenvironment. In the present study of MSCs co-cultured with
OGD-injured PC12 cells, it was demonstrated that the expression
level of Bax in the MSC co-cultured group was significantly lower
compared with that in the PC12 co-cultured and control groups,
suggesting that MSCs have neuroprotective effects on OGD-injured
PC12 cells.
An ELISA assay of the supernatants revealed that
IL-6 secretion in the MSC co-culture group was significantly higher
compared with that in the PC12 cell co-culture group, indicating
that IL-6 be involved in the neuroprotective effects of MSCs and in
microenvironment regulation. IL-6 is a secreted protein that
consists of 184 amino acids, which is widely present in the human
body, specifically in T cells, B cells, glial cells, fibroblasts,
epithelial cells and certain tumour cells (18,19).
IL-6 is able to generate an immune response, and has a role in
macrophage and astrocyte activation during CNS injury. Under normal
physiological conditions, the expression level of IL-6 in the brain
is extremely low. However, when the CNS is injured or becomes
disease-afflicted, the level of IL-6 is significantly increased to
induce the release of tumour necrosis factor-α (TNF-α) and IL-1β,
leading brain injury aggravation. However, Erta et al
(11), in studying IL-6 knockout
mice following brain injury, demonstrated that astrocyte and
macrophage activation markedly decreased in the injured brain, and
that the immunological reaction was reduced, suggesting that IL-6
is a key regulatory cytokine during brain injury. By contrast,
another study (20) revealed that
a high expression of IL-6 could reduce brain ischaemic injury,
demonstrating that IL-6 also exerted neuroprotective effects. These
results demonstrated that IL-6 exerts dual proinflammatory and
anti-inflammatory effects. To evaluate the biological function of
IL-6 secreted from MSCs co-cultured with OGD-injured PC12 cells, an
MSC cell line was screened in the present study with stable siIL-6
expression. When the expression level of IL-6 decreased, the
expression level of Bax increased and the RMP was induced. Bax is
an apoptotic factor, and the increase in Bax expression levels
indicated that apoptosis of the OGD-injured PC12 cells was
aggravated. The RMP is the membrane potential of a nerve cell in an
unstimulated state, and is known as the resting potential or
transmembrane resting potential. The RMP threshold of neurons
represents their viability and transmitting function. The
whole-cell patch-clamp recordings revealed that the RMP threshold
of the OGD-injured PC12 cells following co-culture with siIL-6-MSCs
experienced a greater increase compared with that of the GFP-MSC
co-culture group. These findings indicated that the decrease in the
expression levels of IL-6 in the MSCs reduced the capability of the
OGD-injured PC12 cells to recover, thereby suggesting that
endogenous IL-6 secreted from MSCs improved the functional
restoration of OGD-injured PC12 cells.
The present study suggested that endogenous IL-6
secreted from MSCs had neuroprotective effects; however, the
mechanism by which the MSCs secreted IL-6, and the signalling
pathway(s) that are involved, have yet to be fully elucidated. In
this preliminary study, the expression levels of TLR2 and IL-6 were
shown to be increased when the PC12 cells were subjected to OGD
injury. In the CNS, TLRs are not only key components of the innate
immune system, but they also are associated with nerve degeneration
and tissue damage (21,22). TLR2 is a member of the TLR family
that is highly expressed in the microglia of the CNS. NFκB is
activated by TLR2 via the myeloid differentiation primary response
gene 88 (MyD88) pathway in the presence of interleukin-1
receptor-associated kinase (IRAK)-1 and IRAK-4, inducing the
production of cytokines and chemical factors, including IL-1, IL-6
and TNF (23–25). In the current study, to investigate
whether endogenous IL-6 secretion from MSCs was also regulated via
the TLR2/NFκB signalling pathway, the MSCs were treated with the
TLR2 agonist, PGN-SA. Compared with the control group, the
expression levels of TLR2, NFκB and IL-6 increased following PGN-SA
treatment. After the MSCs had been treated with siTLR2 adenovirus,
the expression levels of TLR2, NFκB and IL-6 markedly decreased.
These results demonstrated that TLR2 expression in MSCs directly
affected the expression levels of NFκB and IL-6. NFκB is an
important factor in the TLR2 signalling pathway, and exerts a key
role in the induction of trans-shipment in the innate immune system
(26–28). The NFκB inhibitor, PDTC, was also
used in the present study to treat MSCs. Following treatment of the
MSCs with 10 μg/ml PDTC for 48 h, a significant decrease in
the expression level of IL-6 in the MSCs was observed. However, the
expression level of TLR2 was not significantly different compared
with untreated MSCs. These data further demonstrated that
endogenous IL-6 secreted from MSCs was regulated via the TLR2/NFκB
signalling pathway.
At this stage, it had not been established whether
IL-6 feedback regulated the expression levels of TLR2 and NFκB. To
investigate this hypothesis, MSCs were treated with Ad-IL-6
adenovirus and siIL-6, and Ad-IL-6 and siIL-6 were revealed to
change the expression level of IL-6, but not affect the expression
levels of TLR2 or NFκB in the MSCs. These data suggested that
endogenous IL-6 secreted from MSCs was regulated by the TLR2/NFκB
signalling pathway, although IL-6 did not regulate the expression
levels of TLR2 and NFκB via a feedback mechanism. Therefore,
further studies should focus on the biological effects of
endogenous IL-6 from MSCs on the process of HIBD treatment.
In conclusion, the release of IL-6 from MSCs was
regulated via the TLR2/NFκB signalling pathway. Endogenous IL-6
secreted from MSCs was able to reduce the levels of apoptosis and
to improve the functional restoration of OGD-injured PC12 cells
following co-culture with MSCs. These findings represent a novel
immunomodulatory effect of the neural injury microenvironment
during MSC cytotherapy.
Abbreviations:
|
MSCs
|
mesenchymal stromal cells
|
|
HIBD
|
hypoxic-ischaemic brain damage
|
|
IL-6
|
interleukin-6
|
|
PC12 cells
|
pheochromocytoma cells
|
|
OGD
|
oxygen-glucose deprivation
|
|
TLR2
|
Toll-like receptor 2
|
|
NFκB
|
nuclear factor κB
|
|
Bax
|
B-cell lymphoma-associated X
|
|
RMP
|
resting membrane potential
|
|
PDTC
|
pyrrolidine dithiocarbamate
|
|
PGN-SA
|
peptidoglycan from Staphylococcus
aureus
|
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81271385 and 81300522)
and the Stem Cell Therapy for Special Study of Children's Hospital
of Chongqing Medical University (no. SCT-201203).
References
|
1
|
Calvert JW and Zhang JH: Pathophysiology
of an hypoxic-ischemic insult during the perinatal period. Neurol
Res. 27:246–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khot S and Tirschwell DL: Long-term
neurological complications after hypoxic-ischemic encephalopathy.
Semin Neurol. 26:422–431. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kristián T: Metabolic stages, mitochondria
and calcium in hypoxic/ischemic brain damage. Cell Calcium.
36:221–233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bi Y, Gong M, Zhang X, Zhang X, Jiang W,
Zhang Y, Chen J, Liu Y, He TC and Li T: Pre-activation of retinoid
signaling facilitates neuronal differentiation of mesenchymal stem
cells. Dev Growth Differ. 52:419–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu Y, Zhang X, Dai Y, Shu C, Qu P, Liu
YX, Yang L and Li TY: Effects of bone marrow mesenchymal stem cells
on learning and memory functional recovery in neonatal rats with
hypoxic-ischemic brain damage. Zhonghua Er Ke Za Zhi. 46:648–653.
2008.In Chinese. PubMed/NCBI
|
|
6
|
Phinney DG and Isakova I: Plasticity and
therapeutic potential of mesenchymal stem cells in the nervous
system. Curr Pharm Des. 11:1255–1265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Yang Y, Bi Y, Gong M, Jiang W,
Wei X, Li T and Chen J: Mesenchymal stromal cell neuroprotection of
hydrogen peroxide-challenged pheochromocytoma cells through
reducing apoptosis and releasing cytokines. Cytotherapy.
14:954–966. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Winter CD, Pringle AK, Clough GF and
Church MK: Raised paren-chymal interleukin-6 levels correlate with
improved outcome after traumatic brain injury. Brain. 127:315–320.
2004. View Article : Google Scholar
|
|
9
|
Hüll M, Strauss S, Berger M, Volk B and
Bauer J: The participation of interleukin-6, a stress-inducible
cytokine, in the pathogenesis of Alzheimer's disease. Behav Brain
Res. 78:37–41. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu Z, Sweeney DD, Netzeband JG and Gruol
DL: Chronic interleukin-6 alters NMDA receptor-mediated membrane
responses and enhances neurotoxicity in developing CNS neurons. J
Neurosci. 18:10445–10456. 1998.PubMed/NCBI
|
|
11
|
Erta M, Quintana A and Hidalgo J:
Interleukin-6, a major cytokine in the central nervous system. Int
J Biol Sci. 8:1254–1266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen J, Ng CP, Rowlands DK, Xu PH, Gao JY,
Chung YW and Chan HC: Interaction between enteric epithelial cells
and Peyer's patch lymphocytes in response to Shigella
lipopolysaccharide: Effect on nitric oxide and IL-6 release. World
J Gastroenterol. 12:3895–3900. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Gong M, Bi Y, Jiang W, Yu Q, Li
TY and Chen J: Construction and identification of recombinant
adenovirus vector containing siRNA of rat TLR2 gene. Xi Bao Yu Fen
Zi Mian Yi Xue Za Zhi. 28:144–147. 2012.In Chinese. PubMed/NCBI
|
|
14
|
Liu JJ, Zhang YJ, Bi Y, Gong M, Li T-y and
Chen J: Construction and preliminary identification of rat
interleukin-6 recombinant adenovirus. Immunol J. 30:151–155.
2014.
|
|
15
|
He M-l, Liu J-j, Gu Y, L T-y and Chen J:
Effects of inhibiting secretion of mesenchymal stem cells
originated interleukin-6 on oxygen glucose deprivation injured PC12
cells. J Shanghai Jiaotong Uni (Sci). 34:1435–1447. 2014.
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Gong M, Bi Y, Jiang W, Zhang Y, Chen L,
Hou N, Liu Y, Wei X, Chen J and Li T: Immortalized mesenchymal stem
cells: An alternative to primary mesenchymal stem cells in neuronal
differentiation and neuroregeneration associated studies. J Biomed
Sci. 18:872011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kishimoto T: Interleukin-6: Discovery of a
pleiotropic cytokine. Arthritis Res Ther. 8(Suppl 2): S22006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kishimoto T: IL-6: From its discovery to
clinical applications. Int Immunol. 22:347–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Woodcock T and Morganti-Kossmann MC: The
role of markers of inflammation in traumatic brain injury. Front
Neurol. 4:182013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arroyo DS, Soria JA, Gaviglio EA,
Rodriguez-Galan MC and Iribarren P: Toll-like receptors are key
players in neurodegeneration. Int Immunopharmacol. 11:1415–1421.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YC, Lin S and Yang QW: Toll-like
receptors in cerebral ischemic inflammatory injury. J
Neuroinflammation. 8:1342011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vogel SN, Fitzgerald KA and Fenton MJ:
TLRs: Differential adapter utilization by toll-like receptors
mediates TLR-specific patterns of gene expression. Mol Interv.
3:466–477. 2003. View Article : Google Scholar
|
|
24
|
Marsh BJ, Williams-Karnesky RL and
Stenzel-Poore MP: Toll-like receptor signaling in endogenous
neuroprotection and stroke. Neuroscience. 158:1007–1020. 2009.
View Article : Google Scholar :
|
|
25
|
Crack PJ and Bray PJ: Toll-like receptors
in the brain and their potential roles in neuropathology. Immunol
Cell Biol. 85:476–480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mohamed MR and McFadden G: NFκB
inhibitors: Strategies from poxviruses. Cell Cycle. 8:3125–3132.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar
|
|
28
|
Akhmatova NK, Egorova NB, Kurbatova EA and
Akhmatov EA: Activation of innate immunity by bacterial ligands of
toll-like receptors. Front Immunol. 5:892014. View Article : Google Scholar : PubMed/NCBI
|