Introduction
The maintenance of corneal transparency is required
for optimal vision, thus, the cornea must preserve avascularity and
alymphaticity (1). It is widely
recognized that new blood and lymphatic vessels in the cornea are
strong risk factors for corneal transparency. Corneal
neovascularization (CNV), also referred to as angiogenesis or
hemangiogenesis (HA), is the formation of ectopic corneal
vasculature from preexisting vasculature (2). CNV is promoted by a wide variety of
proangiogenic factors, including vascular endothelial growth factor
(VEGF), basic fibroblast growth factor (bFGF), interleukin-6,
platelet-derived growth factor (PDGF), hepatocyte growth factor
(3–7). In addition, recent studies have
demonstrated that anti-angiogenic factors, such as angiostatin and
endostatin, inhibit CNV (3).
Lymphangiogenesis (LG) is the formation of novel
lymphatic vessels from pre-existing vasculature. In contrast to
angiogenesis, research on the lymphatic system has been slowed by a
lack of molecular markers to identify the vessels. The recent
discovery of several lymphatic endothelial molecular markers,
including VEGF receptor-3 (VEGFR-3), lymphatic vessel endothelial
hyaluronan receptor-1 (LYVE-1), and Prox-1, has enabled further
research and progress in the field (8–10).
Signaling via VEGF-C/D and VEGFR3 is a central
pathway for LG (11,12). VEGF-C+/− mice are viable
but suffer from lymphatic deficiency and subsequent lymphedema
(13). Similarly, inhibition of
VEGFR3 signaling during the formation of lymphatic vessels induces
lymphatic regression and lymphedema in mouse embryos and neonates
(14). VEGF-A is widely used for
angiogenesis research as it promotes several processes of
angiogenesis, including proteolytic activities (dissolution of the
membrane of the original vessel), endothelial cell proliferation,
migration, and capillary tube formation. Notably, the requirement
of VEGF in corneal angiogenesis was demonstrated by the inhibition
of NV following stromal implantation of an anti-VEGF blocking
antibody in a rat model (15).
Chemokine (C-X-C motif) ligand 12 (CXCL12) is a
member of the CXC subfamily of chemokine peptides, also known as
stromal cell-derived factor-1 (SDF-1). However, unlike other
chemokines that interact with multiple G-protein coupled receptors,
CXCL12 mediates its effects via its only known receptor, chemokine
(C-X-C motif) receptor 4 (CXCR4). CXCL12 is highly conserved with
99% homology between humans and mice, allowing it to exert effects
across species barriers (16). The
CXCL12/CXCR4 axis is essential for multiple biological processes,
including development, hematopoiesis, organogenesis, and
vascularization (17–20). Furthermore, CXCR4 is highly
expressed in a variety of cancers (21). The interaction between CXCL12 and
CXCR4 is important in tumorigenesis (22) and metastasis (21,23).
AMD3100, a well-known antagonist of CXCR4, reversibly inhibits
interaction of CXCL12 and CXCR4 (24). Previous studies have identified the
CXCL12/CXCR4 axis is associated with NV (25–27).
However, the association between the CXCL12/CXCR4 axis and LG
remains to be elucidated.
The role of the CXCL12 (SDF-1)/CXCR4 axis in
mediating LG in suture-induced CNV remains to elucidated. To
investigate the roles of CXCR4 signaling, the process of
suture-induced CNV was analyzed using mice with suture placement or
CXCR4 antagonist (AMD3100)-treated mice in comparison with
control-treated mice. The present study also investigated whether
VEGF signaling was involved in the molecular signaling pathway
resulting in corneal LG due to placement of a suture.
Materials and methods
Animals and anesthesia
All animal protocols were approved by the local
animal care committee of The First Affiliated Hospital of Harbin
Medical University (Harbin, China), and were in accordance with the
Association for Research in Vision and Ophthalmology Statement for
the Use of Animals in Ophthalmic and Vision Research. A total of 25
male C57BL/6 mice (age, 6–8 weeks) were purchased from the
Changchun Animal Center (Changchun, China). Mice were housed in
three groups (9 mice in control group, and 8 mice in AMD3100 and
suture placement groups each) under a 12-h light/dark cycle at
moderate temperature and humidity with free access to food and
water. Animals were anesthetized with a mixture of ketamine and
xylazine (Zhejiang Jiuxu Pharmaceutical Co., Ltd.; Jinhua, China)
at 120 mg/kg body weight and 20 mg/kg body weight, respectively).
At the end of the experiment, the mice were sacrificed by
CO2 inhalation.
Suture-induced CNV
The mouse model of suture-induced inflammatory CNV
was used as previously described (28). The central cornea was marked with a
2-mm diameter trephine and three 11-0 nylon sutures (Lingqiao,
Ningbo, China) were placed in the intrastromal position. The outer
point of suture placement was selected to be near the limbus, and
the inner point was selected to be near the corneal center
equidistant from the limbus. Sutures were removed after 7 days.
Mice were randomly selected to receive subconjunctival injection of
the CXCR4 antagonist, AMD3100 (1 g/l; Abcam, Cambridge, UK) or
balanced salt solution once a day from day 1 of the suture-induced
model.
Measure of suture-induced CNV
CNV was examined using a slit lamp every other day
following the corneal suture. Measurements of NV were made using a
slit lamp by an ophthalmologist. Vessel growth onto the cornea was
recorded in millimeters on day 7. CNV was quantified by calculating
the wedge-shaped area of vessel growth with the formula: A = C/12 ×
3.1416[r2−(r−l)2], where A is the area, C is
the time (h), l is the radius from the center to the border of
vessel growth, and r is the radius of the cornea (29).
Immunohistochemistry
Corneas were cut and fixed in 10% neutral buffered
formalin (Beijing Yili Fine Chemicals Co., Ltd.; Beijing, China)
for 24 h. Paraffin-embedded tissue sections (4 μm) were
deparaffinized, rehydrated, and treated with 0.3% hydrogen peroxide
in methanol (Beyotime Institute of Biotechnology, Inc., Haimen,
China) for 30 min, to eliminate endogenous peroxidase activity. The
tissue sections were incubated for 60 min at room temperature with
rabbit anti-mouse CXCR4 polyclonal antibody (1:200 dilution; cat.
no. ab2074; Abcam). Following three washes of 3 min with
phosphate-buffered saline (PBS; Beyotime Institute of
Biotechnology, Inc.), a 3,3′-diaminobenzidine detection kit
(PV9000; ZSGB-BIO, Beijing, China) was used for CXCR4 staining,
according to the manufacturer's protocols. Images were captured
with the Leica DM4000B biological microscope equipped with a Leica
DFC 550 digital camera and Leica Application Suite version 4.2.0
software (Leica Microsystems GmbH, Wetzlar, Germany).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocols. Total RNA (400 ng) was
reverse transcribed using the PrimeScript™ RT reagent kit with gDNA
Eraser (Takara Biotechnology Co., Ltd., Dalian, China). qPCR was
performed using SYBR® Premix Ex Taq™ II (Takara
Biotechnology Co., Ltd.) in a LightCycler 480 Real-time PCR system
(Roche Diagnostics, Basel, Switzerland). The thermocycling
conditions were as follows: Initial denaturation step of 95°C for
30 sec; 40 cycles of 95°C for 5 sec and 60°C for 30 sec; followed
by an additional denaturation step of 95°C for 5 sec and 60°C for
60 sec, as a subsequent melt curve analysis to check amplification
specificity. The assays were conducted three times, and were
performed in triplicate. Results were derived from the
2−ΔΔCq method (30) and
glyceraldehyde-3-phosphate dehydrogenase served as an internal
control for normalization. Primers used in the RT-qPCR are
presented in Table I.
 | Table IPrimers used in the reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers used in the reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5′-3′) |
|---|
| GAPDH | F:
GTATTGGGCGCCTGGTCACC |
| R:
CGCTCCTGGAAGATGGTGATGG |
| VEGF-A | F:
ACACGGTGGTGGAAGAAGAG |
| R:
CAAGTCTCCTGGGGACAGAA |
| VEGF-C | F:
CTACAGATGTGGGGGTTGCT |
| R:
GATTGGCAAAACTGATTGTGAC |
| VEGFR-1 | F:
CTGGACTGAGACCAAGCCCAAG |
| R:
GCTCAGATTCATCGTCCTGCAC |
| VEGFR-3 | F:
CTCTGACCTAGTGGAGATCCTG |
| R:
CTTCGGTGATATGTAGAGCTGTG |
| CXCR4 | F:
AGCATGACGGACAAGTACC |
| R:
GATGATATGGACAGCCTTACAC |
| CXCL12 | F:
GAGAGCCACATCGCCAGAG |
| R:
TTTCGGGTCAATGCACACTTG |
Western blot analysis
The corneas were harvested and lysed in ice-cold
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology, Inc.) with the addition of protease inhibitors.
Following separation by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis at 120 V for 2 h, proteins were transferred
onto nitrocellulose membranes (Pall Life Science, Port Washington,
NY, USA). The membranes were incubated in blocking solution of 2%
bovine serum albumin (BSA; Beyotime Institute of Biotechnology,
Inc.) in Tris-buffered saline with Tween-20 (Beyotime Institute of
Biotechnology, Inc.) for 1 h at room temperature, then incubated
with rabbit anti-mouse CXCR4 polyclonal antibody and rabbit
anti-mouse CXCL12 polyclonal antibody (cat. no. ab25117; Abcam) at
1:1,000 dilution overnight at 4°C. Each step was followed by
extensive washing. β-actin was used as loading control (mouse
anti-mouse β-actin monoclonal antibody; cat. no. A00702-100;
1:1,000 dilution; GenScript, Nanjing, China). The membranes were
then incubated with horseradish peroxidase-conjugated rabbit
anti-mouse polyclonal immunoglobulin (Ig)G (1:5,000 dilution; cat.
no. A9044; Sigma-Aldrich, St. Louis, MO, USA) or goat anti-rabbit
polyclonal IgG secondary antibody (cat. no. A0545; 1:5,000 dilution
Sigma-Aldrich) for 1 h at room temperature and developed using an
enhanced chemiluminescence system (GE Healthcare, Little Chalfont,
UK). Images were captured on X-ray film.
Corneal immunofluorescence microscopy
assays and quantification
The immunofluorescence experiments were performed as
previously described (31). The
excised corneas from the CNV assay were rinsed in PBS and fixed in
acetone (Beyotime Institute of Biotechnology, Inc.) for 30 min.
Following washing and blocking with 2% BSA in PBS for 2 h, the
corneas were stained overnight at 4°C, with a rabbit anti-mouse
LYVE-1 antibody (cat. no. ab14917; 1:500 dilution; Abcam) and a rat
anti-mouse cluster of differentiation (CD)31 antibody (1:100; cat.
no. 550274; BD Pharmingen, San Diego, CA, USA). On day 2, the
tissue was washed three times in PBS and stored at 4°C in the dark.
LYVE-1 antibody was detected with an Alexa Fluor®
647-conjugated goat anti-rabbit IgG antibody (1:200; cat. no.
A-21244; Invitrogen; Thermo Fisher Scientific, Inc.) and the CD31
antibody was detected with Alexa Fluor 488®-conjugated
goat anti-rat IgG polyclonal antibody (1:200; cat. no. A-11006
Invitrogen; Thermo Fisher Scientific, Inc.).
The stained whole mounts were analyzed with a
fluorescence microscope (EVOS f1; Thermo Fisher Scientific, Inc.).
Each whole mount picture was quantified using Image J software
version 1.24o (National Institutes of Health, Bethesda, MD, USA)
analysis software as described previously (32). The mean vascularized area of the
suture placement was defined as being 100%; vascularized areas were
then relative to this value (vessel ratio).
Statistical analysis
Statistical analysis was performed by the Student's
t-test using SPSS version 13.0 software (Armonk, NY, USA). Results
were expressed as the mean ± standard error of the mean and
P<0.05 was considered to indicate a statistically significant
difference. Graphs were drawn using GraphPad Prism, version 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Expression levels of CXCL12 and CXCR4 in
a mouse model of suture-induced inflammatory CNV
The present study analyzed CXCL12 and CXCR4 mRNA and
protein expression levels in normal and vascularized corneas by
RT-qPCR, immunohistochemistry and western blot analysis. The
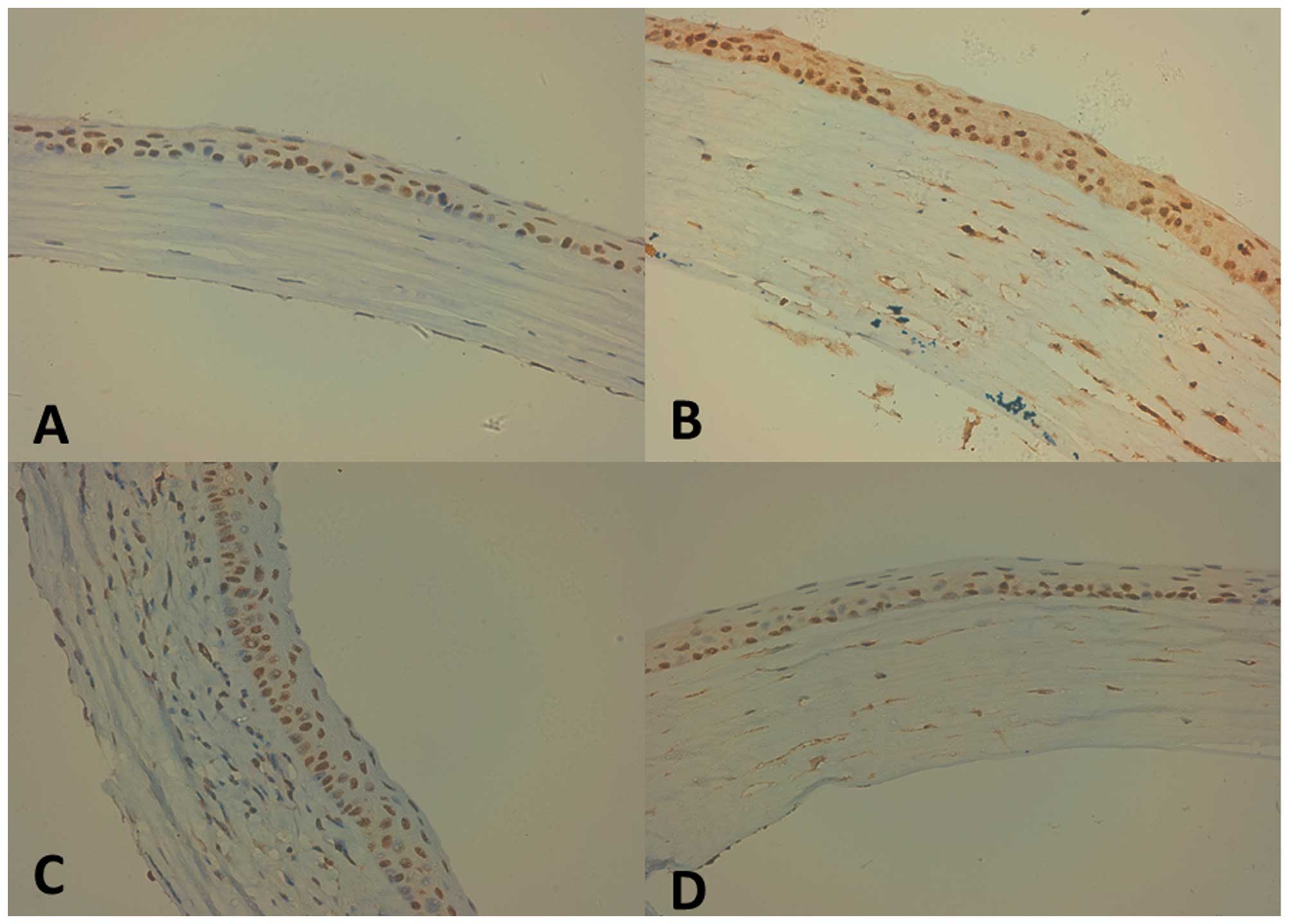
immunohistochemical staining demonstrated CXCR4 was only detected
in epithelial cells in normal corneas (Fig. 1A). Immunohistochemical staining
showed a high expression of CXCR4 in corneas on day 7 following
suture placement in the control group, and a weak expression of
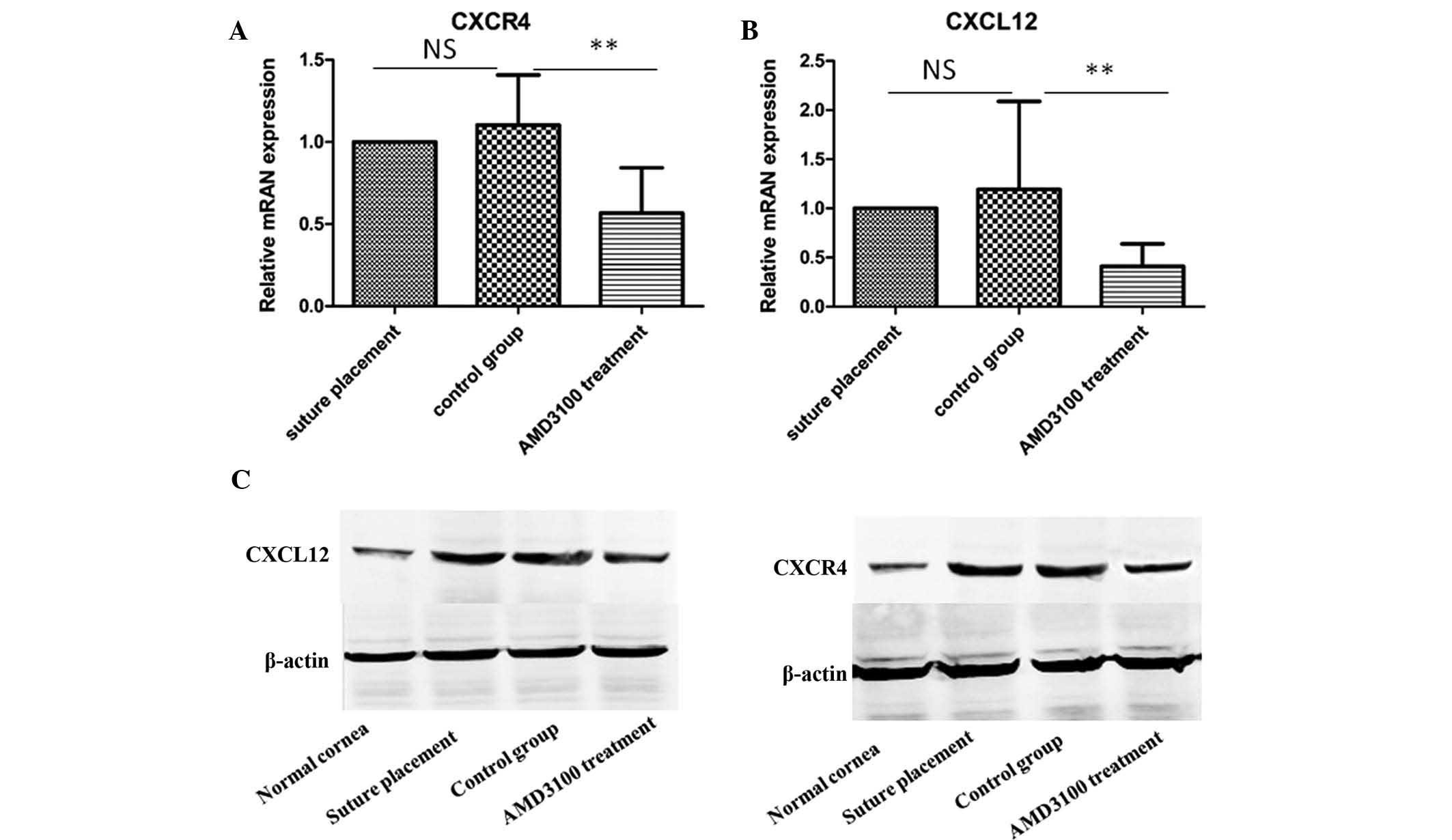
CXCR4 in AMD3100 subconjunctival injection (Fig. 1B–D). CXCL12 and CXCR4 mRNA was
detected at low levels in normal eyes, but was significantly
increased following suture placement and decreased with treatment
with AMD3100 (P<0.01; Fig. 2A and
B). CXCL12 and CXCR4 mRNA expression levels presented no
significant change between the suture placement and control groups
(P>0.05; Fig. 2A and B).
Furthermore, the western blot assay also indicated a marked
upregulation of CXCL12 and CXCR4 expression levels in the suture
placement and control groups, and the downregulation of CXCL12 and
CXCR4 expression levels with AMD3100 treatment (Fig. 2C). The increased mRNA and protein
expression levels of CXCL12 and CXCR4 resulted in further
investigation into whether CXCL12/CXCR4 interactions are involved
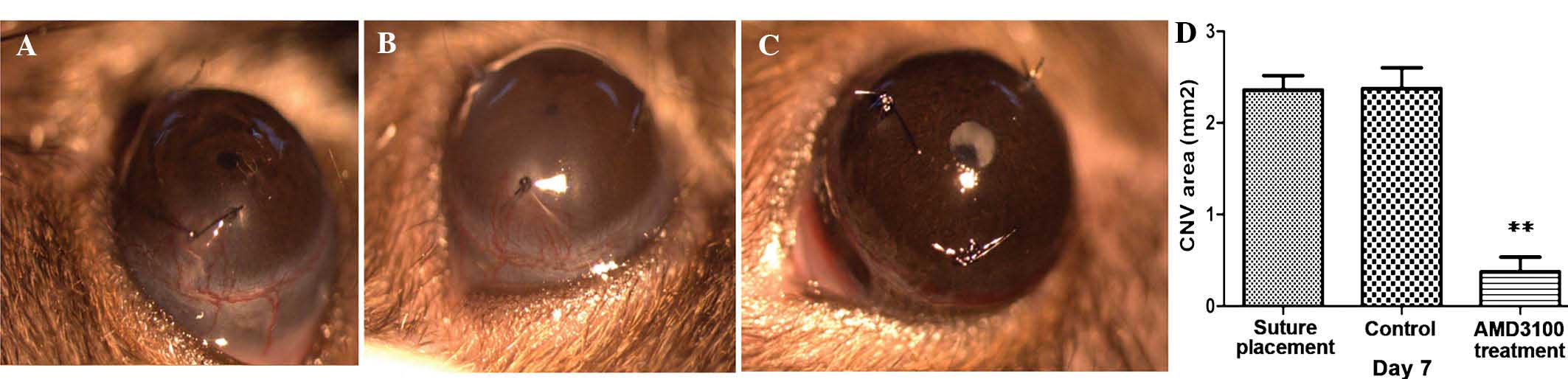
in suture-induced CNV. Slit lamp examinations demonstrated that
untreated corneas were avascular, however, suture placement
markedly increased the vascular areas in corneas and the vascular
areas decreased following AMD3100 treatment (P<0.01; Fig. 3). Slit lamp examination was
consistent with results from the analysis of mRNA and protein
expression levels.
CXCL12/CXCR4 axis regulates CNV
CXCL12 is one of the major chemokines associated
with CXCR4, thus, the present study hypothesized that the
CXCL12/CXCR4 axis regulates NV and LG. To confirm the bioactivity
of CXCL12/CXCR4 on NV, a mice model of suture-induced CNV was
established in vivo. The effect of CNV was evaluated by
comparing the CNV area between mice from the suture placement and
AMD3100 treatment groups on day 7 (Fig. 3). The results demonstrated that the
size of the areas of CNV indicate no significant difference between
the control and the suture placement groups. The area following
AMD3100 treatment was smaller than that in the suture placement and
control groups, suggesting CXCL12/CXCR4 axis regulates CNV
(0.54±0.11, 2.34±0.24 and 2.65±0.32, respectively; Fig. 3A–C). Corneal suture placement
induced a robust CNV response that emerged from day 3 and reached
suture placement sites at day 7 following suture injury (31). The densities of platelet
endothelial cell adhesion molecule-1 (CD31)-positive blood vessels
were detected by immunofluorescence on day 7 (Fig. 4A–C). Quantitative
immunofluorescence and morphometric analyses demonstrated that the
number of blood vessels in mice treated with AMD3100 was
significantly decreased (P<0.01), whereas the number of blood
vessels in the suture placement group showed no changes compared
with balanced salt solution group (P=0.46; Fig. 4J). These results suggest that the
CXCL12/CXCR4 axis is associated with CNV.
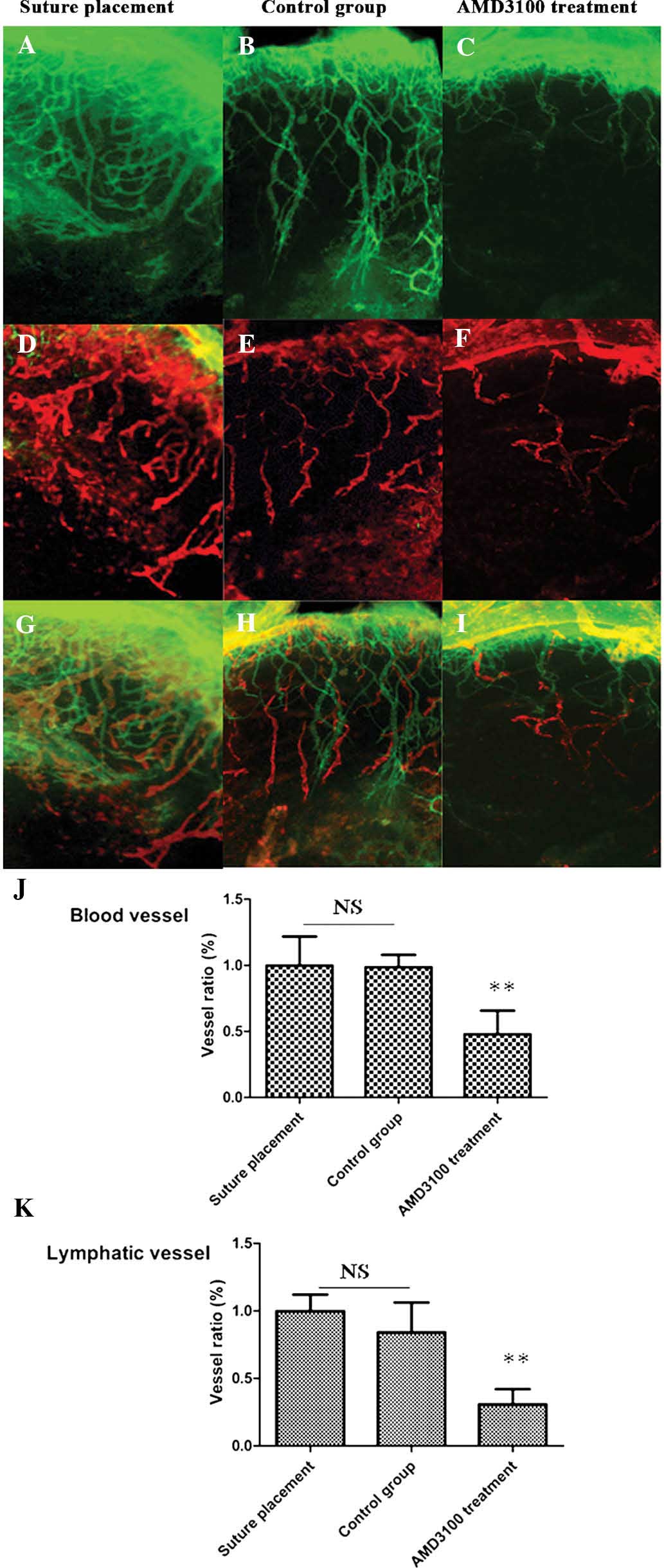 | Figure 4CXCL12/CXCR4 axis regulates CNV and
LG using immunofluorescence. (A–C) Representative images showing
that suture placement on day 7. (D–I) Representative segments of
corneal whole-mounts (blood vessels stained green using CD31/Alexa
Fluor® 488 and lymphatic vessels stained red using
LYVE-1/Alexa Fluor® 647) in eyes treated with balanced
salt solution (B, E and H) or AMD3100 (C, F and I) (magnification,
×40). (J and K) Summarized data showing that areas of CNV and LG
are not significantly different between the control and suture
placement groups. The area of CNV following AMD3100 treatment was
smaller than in the suture placement and control groups. (A, D and
G) n=8 mice, (B, E and H) n=9 mice and (C, F and I) n=8 mice.
**P<0.01 vs. suture placement and control groups. NS,
not significant; CXCL12, chemokine (C-X-C motif) ligand 12; CXCR4,
chemokine (C-X-C motif) receptor 4 CNV, corneal neovascularization;
LG, lymphangiogenesis. |
CXCL12/CXCR4 axis regulates LG
Lymphatic vessels express multiple specific markers,
including LYVE-1, Prox-1, and VEGFR-3 (8–10).
LYVE-1 is specifically expressed on newly formed lymphatic vessels
but not on blood vessels in the mouse corneal model (33). Thus, the present study used LYVE-1
as a specific maker for detection of lymphatic vessels in the
present study. On day 7, the densities of LYVE-1 positive lymphatic
vessels were detected by immunofluorescence. The lymphatic vessels
of suture placement had vascular 'tree-like' structures (Fig. 4D–F). Quantitative
immunofluorescence and morphometric analyses demonstrated that
corneal LG was induced by suture placement and the vessel ratio of
lymphatic vessels in mice treated with AMD3100 were significantly
decreased (P<0.01; Fig. 4K)
compared with the suture placement and control groups. The present
study supports that CXCL12/CXCR4 regulates corneal LG.
CXCL12/CXCR4 pathway is dependent on the
VEGF-A/VEGFR-1 pathway in the regulation of CNV
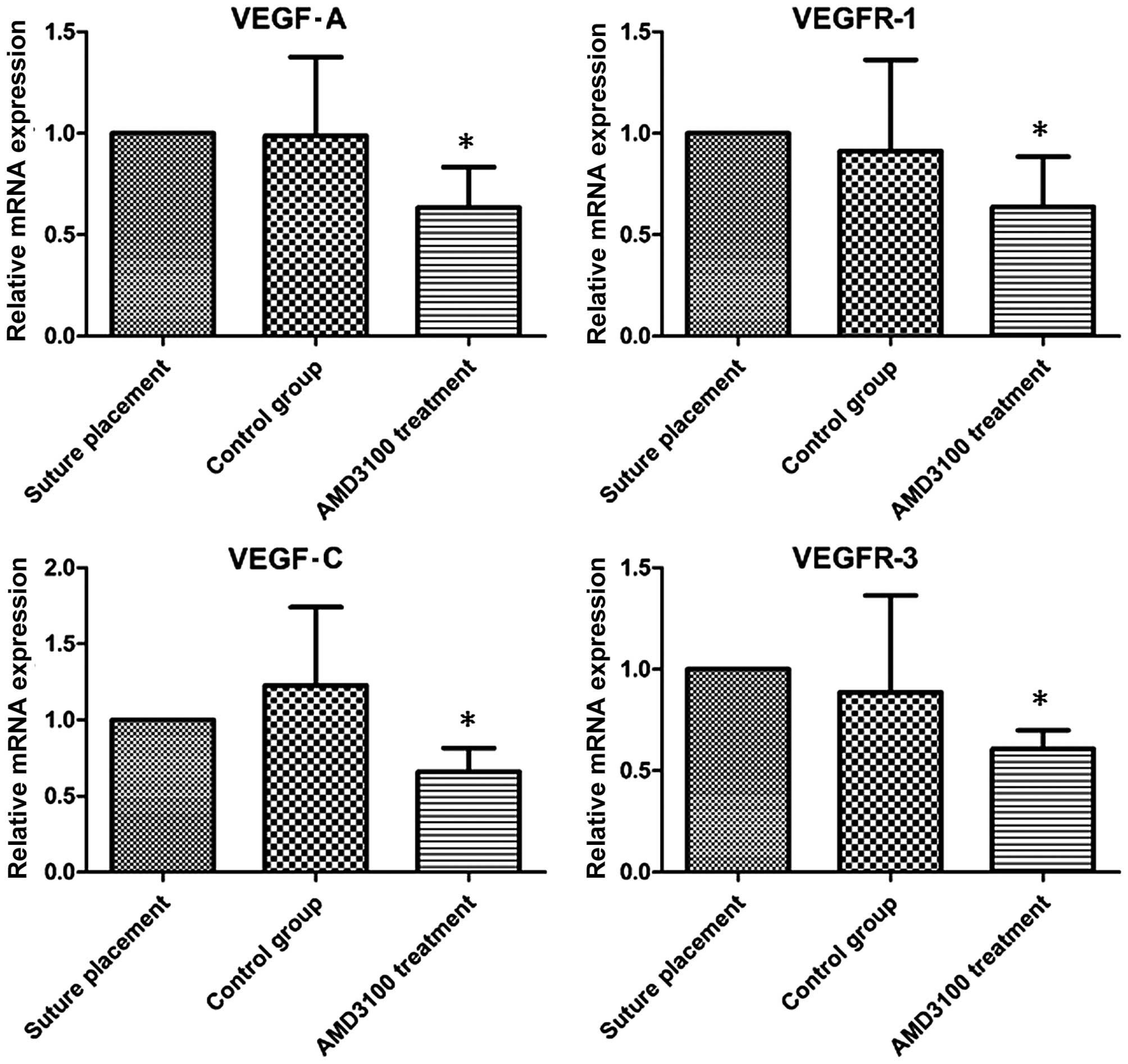
VEGF-A/VEGFR-1 is considered to induce angiogenesis.
To investigate whether VEGF-A/VEGFR-1 was associated with
CXCL12/CXCR4, RT-qPCR for VEGF-A/VEGFR-1 was performed.
VEGF-A/VEGFR-1 mRNA expression levels were upregulated in the
suture-induced CNV and control groups (Fig. 5), whereas VEGF-A/VEGFR-1 expression
levels in the corneas treated with AMD3100 were downregulated. The
results are consistent with the results of CXCL12/CXCR4. Thus,
these results demonstrate that the CXCL12/CXCR4 pathway is
dependent on the VEGF-A/VEGFR-1 pathway in regulating CNV.
CXCL12/CXCR4 pathway is dependent of the
VEGF-C/VEGFR-3 pathway in the regulation of LG
The abovementioned results demonstrated that
CXCL12/CXCR4 mediates LG. However, whether CXCL12/CXCR4 mediation
of LG is associated with VEGF-C/VEGFR-3 remains to be elucidated.
To further investigate the roles of VEGF-C and VEGFR-3 in
suture-induced CNV, RT-qPCR was used to examine mRNA expression
levels. VEGF-C and VEGFR-3 expression levels were significantly
downregulated in mice treated with AMD3100 (P<0.05), whereas the
expression levels of the two factors indicated no significant
difference between the suture-induced and control groups (Fig. 5). Thus, these results demonstrate
that the CXCL12/CXCR4 signaling pathway is dependent on the
VEGF-C/VEGFR-3 pathway.
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that CXCL12/CXCR4 regulates LG in
vivo in a corneal suture-induced mouse model. Giacomini et
al (34) quantified and
compared HA and LG following alkali burn- and suture-induced CNV.
They demonstrated that LG was more marked in the suture-induced
model as compared with the alkali burn model, and the two types
induced similar HA. Thus, the present study used a suture-induced
CNV mouse model.
In the current study, there are low levels of
expression of CXCL12/CXCR4 in normal cornea analyzed by RT-qPCR and
immunohistochemistry. Immunohistochemistry results demonstrated
that CXCR4 was present in normal corneal epithelium. Bourcier et
al (35) detected CXCL12/CXCR4
mRNA expression levels in normal human corneal by in situ
hybridization with similar results to those in the present study.
CXCL12/CXCR4 expression levels in suture-induced CNV was
investigated by immunohistochemical analysis, RT-qPCR and western
blotting. The present study observed SDF-1 and CXCR4 mRNA
overexpression on day 7 in suture-induced CNV, consistent with a
previous study (36). These
findings suggest that suture-induced inflammation increases
CXCL12/CXCR4 expression levels in the cornea, and that CXCL12/CXCR4
is crucial to the induction of inflammatory corneal HA and LG.
AMD3100 is a specific antagonist of CXCR4, with no cross reaction
with other chemokines. According to the immunofluorescence results,
corneal HA and LG were markedly reduced in eyes receiving
subconjunctival injections of AMD3100 compared with the suture
placement group. Subconjunctival injection of AMD3100 was an
effective method to decrease the expression of CXCL12/CXCR4. These
results suggest that CXCL12/CXCR4 was involved in CNV and,
particularly, LG.
Cornea avascularity is maintained by the balance
between angiogenic and anti-angiogenic factor expression (37,38).
Corneal wounding enhances predominantly the expression of
angiogenic factors, including VEGF and bFGF (25,39),
and skewed the balance toward angiogenesis, thus resulting in CNV.
The crucial role of VEGF-A in the pathophysiology of CNV has been
extensively previously reported (15,40,41).
CXCL12 and its receptor CXCR4 promote glioma stem cell-initiated
glioma growth and angiogenesis by stimulating VEGF-A production
(25). Antoniou et al
(42) demonstrated VEGF-A and
SDF-1 mRNA coexpression in angiogenesis as part of the pathogenesis
of idiopathic pulmonary fibrosis. Mirshahi et al (27) demonstrated that SDF-1 induces an
angiogenic effect in vitro and in rabbit corneal pocket.
SDF-1 was associated with a slight increase in VEGF production
(42). In the present study,
VEGF-A/VEGFR-1 mRNA expression levels were upregulated in the
suture-induced and control groups, whereas VEGF-A/VEGFR-1
expression levels were downregulated in corneas treated with
AMD3100. The results are consistent with the results from
investigation of CXCL12/CXCR4, thus, the current study hypothesizes
CXCL12/CXCR4 may enhance CNV by increasing VEGFA/VEGFR-1.
Among the known lymphangiogenic factors, VEGF-C,
which exerts its functions via VEGFR-3, is the most potent and
specific growth factor acting directly on lymphatic vessels.
Although bFGF, angiopoient-1/2, insulin-like growth factor-1/2,
hepatocyte growth factor, and PDGF-BB have been demonstrated to be
prolymphangiogenesis factors, the VEGF-C/VEGFR-3 signaling pathway
is common for a number of lymphangiogenic factors. bFGF has also
been reported to upregulate VEGF-C expression in endothelial cells,
and its lymphangiogenic property is mediated by VEGF-C (43). The present study hypothesized that
CXCL12/CXCR4 activates a signaling pathway dependent on that
triggered by the VEGF-C/VEGFR-3 axis. The present study has
demonstrated that VEGF-C and VEGFR-3 expression were significantly
upregulated in the suture placement and control groups, whereas the
expression levels of the two factors were downregulated in mice
treated with AMD3100. Although it is possible that CXCL12 may also
have indirect effects in promoting LG via inducing other factors,
the VEGF-C/VEGFR-3 pathway and CXCL12/CXCR4 signaling pathways are
involved in LG, and the CXCL12/CXCR4 pathway may depend on
VEGF-C/VEGFR-3 pathway in regulating LG. The present study suggests
that the CXCL12/CXCR4 axis is a potent positive-regulator of
LG.
In conclusion, the results from the present study
demonstrate CXCL12/CXCR4 regulates HA and LG following corneal
suture placement, and provides a novel strategy to inhibit LG.
Notably, the present study is the first to demonstrate evidence
that CXCL12/CXCR4 modulates LG in a corneal suture-induced mouse
model.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81470618), the
Scientific Research Fund of Heilongjiang Provincial Education
Department (no. 12521262) and the Scientific Research Fund of
Heilongjiang Provincial Health Bureau (no. 2011-031).
References
|
1
|
Qazi Y, Wong G, Monson B, Stringham J and
Ambati BK: Corneal transparency: Genesis, maintenance and
dysfunction. Brain Res Bull. 81:198–210. 2010. View Article : Google Scholar
|
|
2
|
Chung ES, Chauhan SK, Jin Y, Nakao S,
Hafezi-Moghadam A, van Rooijen N, Zhang Q, Chen La and Dana R:
Contribution of macrophages to angiogenesis induced by vascular
endothelial growth factor receptor-3-specific ligands. Am J Pathol.
175:1984–1992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ellenberg D, Azar DT, Hallak JA, Tobaigy
F, Han KY, Jain S, Zhou Z and Chang JH: Novel aspects of corneal
angiogenic and lymphangiogenic privilege. Prog Retin Eye Res.
29:208–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hajrasouliha AR, Sadrai Z, Chauhan SK and
Dana R: b-FGF induces corneal blood and lymphatic growth in a
spatially distinct pattern. Cornea. 31:804–809. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ebrahem Q, Minamoto A, Hoppe G, Anand-Apte
B and Sears E: Triamcinolone acetonide inhibits IL-6- and
VEGF-induced angiogenesis downstream of the IL-6 and VEGF
receptors. Invest Ophthalmol Vis Sci. 47:4935–4941. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Cao R, Zhang Y, Jia T, Cao Y and
Wahlberg E: Differential roles of PDGFR-alpha and PDGFR-beta in
angiogenesis and vessel stability. FASEB J. 23:153–163. 2009.
View Article : Google Scholar
|
|
7
|
Xin X, Yang S, Ingle G, Zlot C, Rangell L,
Kowalski J, Schwall R, Ferrara N and Gerritsen ME: Hepatocyte
growth factor enhances vascular endothelial growth factor-induced
angiogenesis in vitro and in vivo. Am J Pathol. 158:1111–1120.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaipainen A, Korhonen J, Mustonen T, van
Hinsbergh VW, Fang GH, Dumont D, Breitman M and Alitalo K:
Expression of the fms-like tyrosine kinase 4 gene becomes
restricted to lymphatic endothelium during development. Proc Natl
Acad Sci USA. 92:3566–3570. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Banerji S, Ni J, Wang SX, Clasper S, Su J,
Tammi R, Jones M and Jackson DG: LYVE-1, a new homologue of the
CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J
Cell Biol. 144:789–801. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilting J, Papoutsi M, Christ B,
Nicolaides KH, von Kaisenberg CS, Borges J, Stark GB, Alitalo K,
Tomarev SI, Niemeyer C and Rössler J: The transcription factor
Prox1 is a marker for lymphatic endothelial cells in normal and
diseased human tissues. FASEB J. 16:1271–1273. 2002.PubMed/NCBI
|
|
11
|
Tammela T and Alitalo K: Molecular
mechanisms and future promise. Cell. 140:460–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jeltsch M, Kaipainen A, Joukov V, Meng X,
Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK and Alitalo K:
Hyperplasia of lymphatic vessels in VEGF-C transgenic mice.
Science. 276:1423–1425. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Karkkainen MJ, Haiko P, Sainio K, Partanen
J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala
H, et al: Vascular endothelial growth factor C is required for
sprouting of the first lymphatic vessels from embryonic veins. Nat
Immunol. 5:74–80. 2004. View
Article : Google Scholar
|
|
14
|
Mäkinen T, Jussila L, Veikkola T, Karpanen
T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H,
Nishikawa S, et al: Inhibition of lymphangiogenesis with resulting
lymphedema in transgenic mice expressing soluble VEGF receptor-3.
Nat Med. 7:199–205. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amano S, Rohan R, Kuroki M, Tolentino M
and Adamis AP: Requirement for vascular endothelial growth factor
in wound- and inflammation-related corneal neovascularization.
Invest Ophthalmol Vis Sci. 39:18–22. 1998.PubMed/NCBI
|
|
16
|
Murphy PM, Baggiolini M, Charo IF, Hébert
CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ and Power CA:
International union of pharmacology. XXII. Nomenclature for
chemokine receptors. Pharmacol Rev. 52:145–176. 2000.PubMed/NCBI
|
|
17
|
Nagasawa T, Hirota S, Tachibana K, et al:
Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in
mice lacking the CXC chemokine PBSF/SDF-1. Nature. 382:635–638.
1996. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou YR, Kottmann AH, Kuroda M, Taniuchi I
and Littman DR: Function of the chemokine receptor CXCR4 in
haematopoiesis and in cerebellar development. Nature. 393:595–599.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma Q, Jones D, Borghesani PR, et al:
Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar
neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad
Sci USA. 95:9448–53. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tachibana K, Hirota S, Iizasa H, Yoshida
H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N,
Nishikawa S, et al: The chemokine receptor CXCR4 is essential for
vascularization of the gastrointestinal tract. Nature. 393:591–594.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burger JA and Kipps TJ: CXCR4: A key
receptor in the crosstalk between tumor cells and their
microenvironment. Blood. 107:1761–1767. 2006. View Article : Google Scholar
|
|
23
|
Chiang AC and Massagué J: Molecular basis
of metastasis. N Engl J Med. 359:2814–2823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Clercq E: The bicyclam AMD3100 story.
Nat Rev Drug Discov. 2:581–587. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salcedo R, Wasserman K, Young HA, et al:
Vascular endothelial growth factor and basic fibroblast growth
factor induce expression of CXCR4 on human endothelial cells: In
vivo neovascularization induced by stromal-derived factor-1alpha.
Am J Pathol. 154:1125–1135. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ping YF, Yao XH, Jiang JY, et al: The
chemokine CXCL12 and its receptor CXCR4 promote glioma stem
cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT
signalling. J Pathol. 224:344–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mirshahi F, Pourtau J, Li H, Muraine M,
Trochon V, Legrand E, Vannier J, Soria J, Vasse M and Soria C:
SDF-1 activity on microvascular endothelial cells: Consequences on
angiogenesis in in vitro and in vivo models. Thromb Res.
99:587–594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cursiefen C, Chen L, Borges LP, Jackson D,
Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ and
Streilein JW: VEGF-A stimulates lymphangiogenesis and
hemangiogenesis in inflammatory neovascularization via macrophage
recruitment. J Clin Invest. 113:1040–1050. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Ma JX, Gao G, Li C, Luo L, Zhang
M, Yang W, Jiang A, Kuang W, Xu L, et al: Plasminogen kringle 5
inhibits alkali-burn-induced corneal neovascularization. Invest
Ophthalmol Vis Sci. 46:4062–4071. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
31
|
Tang XL, Sun JF, Wang XY, Du LL and Liu P:
Blocking neuropilin-2 enhances corneal allograft survival by
selectively inhibiting lymphangiogenesis on vascularized beds. Mol
Vis. 16:2354–2361. 2010.PubMed/NCBI
|
|
32
|
Bock F, Onderka J, Hos D, Horn F, Martus P
and Cursiefen C: Improved semiautomatic method for morphometry of
angiogenesis and lymphangiogenesis in corneal flatmounts. Exp Eye
Res. 87:462–470. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao R, Björndahl MA, Religa P, et al:
PDGF-BB induces intra-tumoral lymphangiogenesis and promotes
lymphatic metastasis. Cancer Cell. 6:333–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giacomini C, Ferrari G, Bignami F and Rama
P: Alkali burn versus suture-induced corneal neovascularization in
C57BL/6 mice: An overview of two common animal models of corneal
neovascularization. Exp Eye Res. 121:1–4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bourcier T, Berbar T, Paquet S, Rondeau N,
Thomas F, Borderie V, Laroche L, Rostène W, Haour F and Lombet A:
Characterization and functionality of CXCR4 chemokine receptor and
SDF-1 in human corneal fibroblasts. Mol Vis. 9:96–102.
2003.PubMed/NCBI
|
|
36
|
Carr AN, Howard BW, Yang HT, Eby-Wilkens
E, Loos P, Varbanov A, Qu A, DeMuth JP, Davis MG and Proia A:
Efficacy of systemic administration of SDF-1 in a model of vascular
insufficiency: Support for an endothelium-dependent mechanism.
Cardiovasc Res. 69:925–935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cursiefen C, Masli S, Ng TF, Dana MR,
Bornstein P, Lawler J and Streilein JW: Roles of thrombospondin-1
and -2 in regulating corneal and iris angiogenesis. Invest
Ophthalmol Vis Sci. 45:1117–1124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao G and Ma J: Tipping the balance for
angiogenic disorders. Drug Discov Today. 7:171–172. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Uno K, Hayashi H, Kuroki M, Uchida H,
Yamauchi Y, Kuroki M and Oshima K: Thrombospondin-1 accelerates
wound healing of corneal epithelia. Biochem Biophys Res Commun.
315:928–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bock F, Onderka J, Dietrich T, Bachmann B,
Kruse FE, Paschke M, Zahn G and Cursiefen C: Bevacizumab as a
potent inhibitor of inflammatory corneal angiogenesis and
lymphangiogenesis. Invest Ophthalmol Vis Sci. 48:2545–2552. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Manzano RP, Peyman GA, Khan P, Carvounis
PE, Kivilcim M, Ren M, Lake JC and Chévez-Barrios P: Inhibition of
experimental corneal neovascularisation by bevacizumab (Avastin).
Br J Ophthalmol. 91:804–807. 2007. View Article : Google Scholar
|
|
42
|
Antoniou KM, Soufla G, Lymbouridou R,
Economidou F, Lasithiotaki I, Manousakis M, Drositis I, Spandidos
DA and Siafakas NM: Expression analysis of angiogenic growth
factors and biological axis CXCL12/CXCR4 axis in idiopathic
pulmonary fibrosis. Connect Tissue Res. 51:71–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chang LK, Garcia-Cardeña G, Farnebo F,
Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J
and Kaipainen A: Dose-dependent response of FGF-2 for
lymphangiogenesis. Proc Natl Acad Sci USA. 101:11658–11663. 2004.
View Article : Google Scholar : PubMed/NCBI
|