Introduction
Vascular calcification (VC) is associated with
aging, hypertension, diabetes, chronic kidney diseases (CKD) and
cardiovascular diseases (1,2).
Furthermore, it is a crucial risk factor, contributing to increased
rates of cardiovascular morbidity and mortality worldwide (1,2).
Increasing evidence indicates that VC is an actively regulated
process, similar to bone formation, including deposition of
minerals in the vessel wall, and that vascular smooth muscle cells
(VSMCs) undergo osteogenic differentiation with production of
bone-associated biomarkers, such as osteopontin (OPN) and
osteoprotegerin (OPG), are key in VC (3–6).
Therefore, considering its clinical significance and complex
underlying mechanisms, it is important to develop novel and
efficient therapeutic strategies for preventing and inhibiting
VC.
Mesenchymal stem cells (MSCs) that are extracted
from bone marrow are defined as multipotent cells due to their
ability to differentiate into various cell types, including VSMCs
in certain conditions; however, their immunomodulatory and
paracrine capabilities may present the greatest potential for
therapeutic function in response to local environmental cues
(7–9). Previous studies have been conducted
to elucidate the two characteristics of MSCs for their application
in cardiovascular disease therapy (10). Although this cell population
promotes VC by differentiating into osteogenic cells in direct
contact with calcified cells (11), their most notable therapeutic
impacts are vascular regeneration and injury healing (12,13).
Additionally, MSCs promote angiogenesis via a paracrine mechanism
in vivo and in vitro (12,14).
Therefore, it is important to analyze the anti-inflammatory,
immunomodulatory and paracrine properties of MSCs on the process of
VC using a cell-cell indirect co-culture system.
Wingless-type MMTV integration site family (Wnt)
ligands encode highly conservative cysteine-rich, secreted
glycoproteins and function via the activation of two intracellular
signaling pathways described as canonical (β-catenin dependent) and
noncanonical (β-catenin independent) signaling pathways (15). Activation of Wnt/β-catenin
signaling pathways is key in promoting VC by stimulating smooth
muscle cell (SMC) differentiation into osteoblast-like cells
(15,16). Receptor tyrosine kinase-like orphan
receptor 2 (Ror2) is an orphan receptor tyrosine kinase that
regulates osteoblastic cell proliferation and differentiation
together with the Wnt/β-catenin signaling pathway (17,18).
Additionally, noncanonical wingless-type MMTV integration site
family, number 5A (Wnt5a) activates Wnt/Ror2 signaling, and
enhances Wnt/β-catenin signaling during MSC osteogenic
differentiation (19,20). Furthermore, a previous study
indicated that increased expression of Wnt5a was correlated with VC
(11). Although the cooperation
among the Wnt/Ror2 and Wnt/β-catenin signals during VC remain to be
elucidated, previous studies have suggested that the Wnt signaling
pathways, composed of Wnt5a and β-catenin or Ror2, may control the
development of VC by regulating cell proliferation, differentiation
and apoptosis.
The present study aimed to investigate whether MSCs
prevent osteogenic differentiation and inhibit the development of
VC in VSMCs. The effects of indirect co-culturing of MSCs with
VSMCs in an OS medium on the modulation of bone-associated
biomarkers and the Wnt signaling pathways were evaluated using a
Transwell insert system, in vitro.
Materials and methods
Rat bone marrow MSC isolation and
culture
Bone marrow MSCs were isolated from male rats (age,
3 weeks; Animal Center, Tongji Medical College, Wuhan, China) by
whole marrow direct adherence (21). Rats were housed together, fed a
normal diet and maintained under a light/dark cycle. The adherent
cells were cultured in complete medium containing Hyclone 90% low
glucose Dulbecco's modified Eagle's medium (L-DMEM; GE Healthcare
Life Sciences, Logan, UT, USA), Gibco 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml
penicillin and 100 U/ml streptomycin (Hyclone; GE Healthcare Life
Sciences) in 25-cm2 plastic culture flasks at 37°C in a
5% CO2 supplemented incubator. The culture medium was
replaced every 3–4 days. The adherent cells were treated with
trypsin (GE Healthcare Life Sciences) and expanded at 80–90%
confluence. Cells at passages 3 and 4 were selected and used in the
experiments. The present study was approved by the ethics committee
of Huazhong University of Science and Technology (Wuhan, China) and
the animals were treated according to the Guide for the Care and
Use of Laboratory Animals.
Transwell co-culture system
A 12-well Transwell insert system (pore size, 0.4
µm; Corning Incorporated, Corning, NY, USA) was used to
prevent direct contact between the cells. A-10 VSMCs (American Type
Culture Collection, Manassas, VA, USA) were seeded in the lower
layers of the Transwell plates at 106 cells/well, while,
MSCs were seeded in the upper layers of the Transwell plates at a
ratio of 1:1. All cells were incubated at 37°C in 5% CO2
with the normal experimental medium (NEM), which contained high
glucose DMEM (GE Healthcare Life Sciences) supplemented with 2%
FBS, 100 U/ml penicillin and 100 U/ml streptomycin. Calcified SMCs
were induced using osteogenic medium (OS), which contained NEM
supplemented with 0.1 µM dexamethasone (Sigma-Aldrich, St.
Louis, MO, USA), 10 mM sodium β-glycerol-phosphate (Sigma-Aldrich)
and 0.05 mM ascorbic acid-2-phosphate (Sigma-Aldrich). Cells were
divided into four groups as follows: SMC group (VSMCs cultured with
NEM); SMC + OS group (VSMCs cultured with OS); SMC + MSC group
(co-culture group cultured with NEM); and SMC + MSC + OS group
(co-culture group cultured with OS). In addition, all cells were
incubated at 37°C in a 5% CO2 supplemented incubator.
After 14 days, cells from the four groups were harvested from the
lower layers and underwent calcium quantitative testing, alkaline
phosphatase (AKP) activity assay, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
Identification of MSCs
MSCs at passage 3 were collected and washed twice
with phosphate-buffered saline (PBS). The cell suspensions were
respectively incubated with antibodies (eBioScience, Inc., San
Diego, CA, USA) against cluster of differentiation (CD)29, CD90 and
CD45 in 4°C, in the dark for 30 min. The fluorescence intensity of
the cells was measured by flow cytometry (BD Biosciences, Franklin
Lakes, NJ, USA) and the results were analyzed by BD CellQuest™ Pro
software (version 5.1; BD Biosciences).
Von Kossa staining
After cells in the SMC and SMC + OS groups were
incubated for 14 days, VSMCs in the lower layers of the Transwell
plates were used for von Kossa staining to observe the mineral
deposits. The VSMCs were gently washed three times with PBS and
fixed in 4% paraformaldehyde (Wuhan Boster Biological Technology
Ltd., Wuhan, China) for 30 min at 4°C. Double-distilled water
(ddH2O) was subsequently used for diluting the
paraformaldehyde and rinsing (three times) between each step. The
cells were exposed under ultraviolet light for 45 min subsequent to
staining with 5% silver nitrate (Sigma-Aldrich) and immersed in 5%
sodium thiosulfate (Sigma-Aldrich) for 10 min to remove unreduced
silver. Following rinsing, cells were stained with 1% neutral red
(Sigma-Aldrich) for another 5 min. Following washing with
ddH2O, the plates were dried for observation under a
microscope.
AKP activity assay
VSMCs from each of the four groups were harvested in
cell lysis buffer (Amresco, Solon, OH, USA) following rinsing with
PBS. BCA protein assay kit (Shanghai Biyuntian Biotechnology, Co.,
Ltd., Shanghai, China) was used to evaluate the total cellular
protein. The activity of intracellular AKP was analyzed using an
AKP assay kit (Nanjing Jiancheng Bioengineering Research Institute,
Nanjing, China), according to the manufacturer's protocols. All
samples were measured by an automatic plate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Calcium content analysis
The calcium content of each of the four groups was
analyzed by calcium colorimetric assay kit (Nanjing Jiancheng
Bioengineering Research Institute), which utilizes the chromogenic
composite between calcium and o-cresolphthalein. The assay
was performed following the manufacturer's protocols and all
samples were analyzed by an atomic absorption spectrophotometer
(Agilent Technologies, Inc., Santa Clara, CA, USA).
RT-qPCR
Total RNA from the above-mentioned four groups was
isolated using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocols. The RNA was reverse
transcribed into cDNA with the cDNA Syntheses kit (Invitrogen;
Thermo Fisher Scientific, Inc.). RT-qPCR was conducted using an ABI
PRISM 7900 sequence detector system (Applied Biosystems; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
protocols. β-actin served as an endogenous control. PCR reactions
were performed using SYBR Green/Fluorescein qPCR Master mix (2X;
Thermo Fisher Scientific, Inc.), cDNA and primers. The expression
level of the specific gene (the quantity of the target normalized
to the endogenous control gene) was calculated using the
comparative Cq method, 2−ΔΔCq. The primer sequences for
RT-qPCR were as follows: Sense, 5′-CAA CAG CCG CTT CAA CTCC-3′ and
antisense, 5′-TGA CAT AGC AGC ACC AGTGA-3′ for rat Wnt5a; sense,
5′-ACA ATT TTC AGG ATG ACG ACCA-3′ and antisense, 5′-GTG ATT CGG
TTT TCA ATC TCCC-3′ for rat Ror2; sense, 5′-AAC ACT CAG ATG CTG TAG
CCA-3′ and antisense, 5′-TCT TGC TTA AAG TCA TCC GTT-3′ for rat
β-catenin; sense, 5′-GCT GTT CTA TTC CGA ATG TCT-3′ and antisense,
5′-CAC CAA TGT CCA GTC CGAGA-3′ for rat OPN; sense, 5′-ACA GTT TGC
CTG GGA CCAAA-3′ and antisense, 5′-CGT TGC ACA CTG CTT TCACA-3′ for
rat OPG; and sense, 5′-CAC GAT GGA GGG GCC GGA CTC ATC-3′ and
antisense, 5′-TAA AGA CCT CTA TGC CAA CAC AGT-3′ for β-actin.
Western blot analysis
Cells from each of the four groups were lysed with a
buffer comprising 50 mM Tris, 5 mM EDTA, 1 mM EDT, 1 mM ethylene
glycol tetraacetic acid, 150 mM NaCl, 1% Triton X-100, 25 mM sodium
pyrophosphate, 1 mM NaF, 1 mM β-glycerophosphate, 0.1 mM sodium
orthovanadate, 1 mM phenylmethanesulfonyl fluoride, 2 µg/ml
leupeptin and 10 µg/ml aprotinin (Amresco) for 20 min on
ice. Total protein contents were measured with a BCA assay kit
according to the manufacturer's protocols. The protein lysate was
separated on SDS-PAGE gels (Amresco) and electroblotted onto
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with 5% non-fat dried milk in
Tris-buffered saline (TBS) and TBS and Tween-20 (TBST) buffer, the
membrane was incubated overnight at 4°C with primary antibodies as
follows: Rabbit polyclonal anti-Wnt5a (1:10,000; cat. no. ab72583;
Abcam, Cambridge, MA, USA), rabbit polyclonal anti-Ror2 (1:500,
cat. no. YT4165; ImmunoWay Biotechnology, Co., Newark, DE, USA),
rabbit polyclonal anti-β-catenin (1:1,000; cat. no. 9562; Cell
Signaling Technology, Inc., Danvers, MA, USA) and mouse polyclonal
anti-β-actin (cat. no. KM9001; 1:8,000; Sanjian, Tianjin, China)
The membrane was washed four times with TBST, and further incubated
with horseradish peroxidase (HRP)-conjugated goat anti-mouse (cat.
no. ab20043; 1:10,000; Abcam) and goat anti-rabbit (cat. no.
ab97051; 1:5,000; Abcam) secondary antibodies. Following four
washes with TBST, the immunoblots were visualized using the
chemiluminescence detection system (Bio-Rad Laboratories, Inc.).
The protein loading was normalized by re-incubating the same
membrane with anti-β-actin (cat. no. KM9001; Sanjian, Tianjin,
China) at a dilution of 1:10,000.
Statistical analysis
Data were analyzed using SPSS software (version
17.0; SPSS, Inc., Chicago, IL, USA). Results are presented as the
mean ± standard error of the mean and P<0.05 was considered to
indicate a statistically significant difference.
Results
MSC characteristics
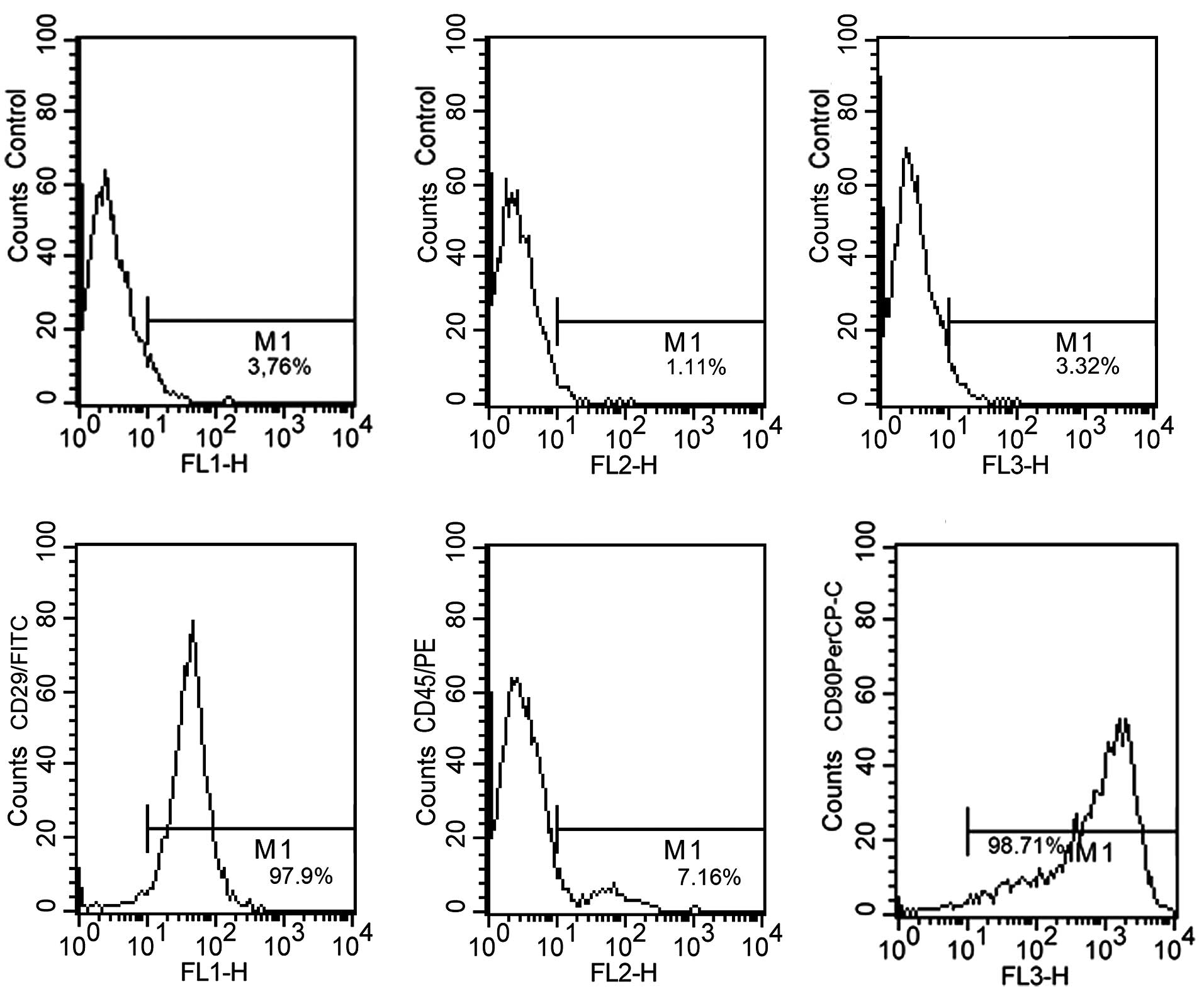
MSCs at passage 3 were analyzed by flow cytometry.
The results indicate that the MSCs were positive for CD29 (97.9%)
and CD90 (98.71%), and negative for CD45 (7.16%); thus these cells
demonstrated expression levels characteristic of MSCs (Fig. 1).
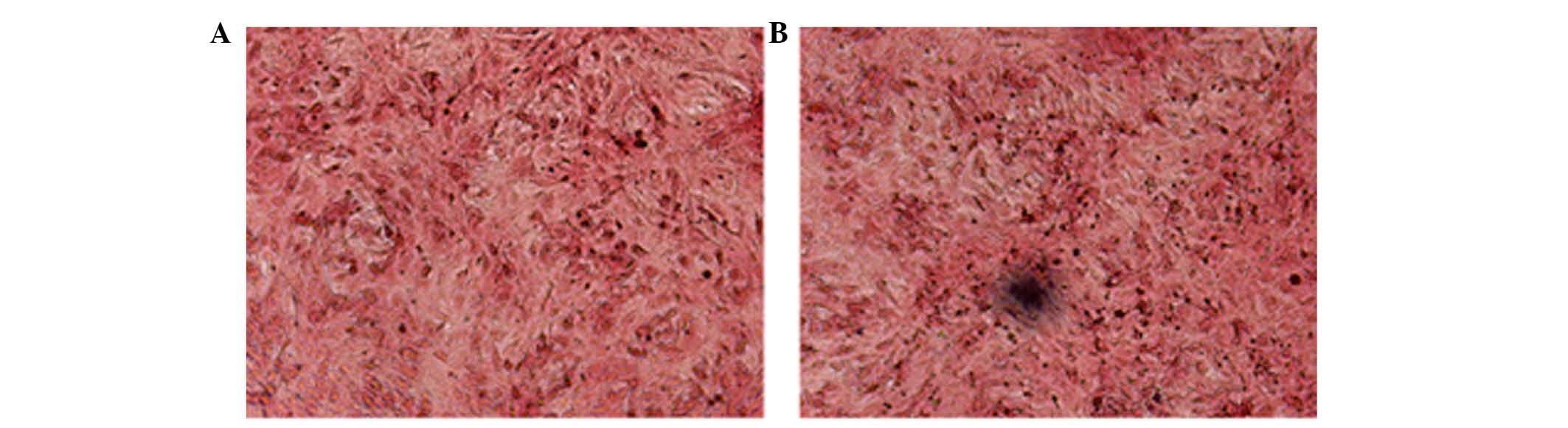
Von Kossa staining for VSMCs
Von Kossa staining was used to evaluate whether
VSMCs transformed into osteogenic cells (Fig. 2). Black calcified nodules were
observed microscopically in the VSMCs cultured with OS. However, no
mineral deposits were observed in the control group incubated with
NEM (Fig. 2). This indicated that
the OS induced VSMCs to differentiate into the osteogenic
phenotype.
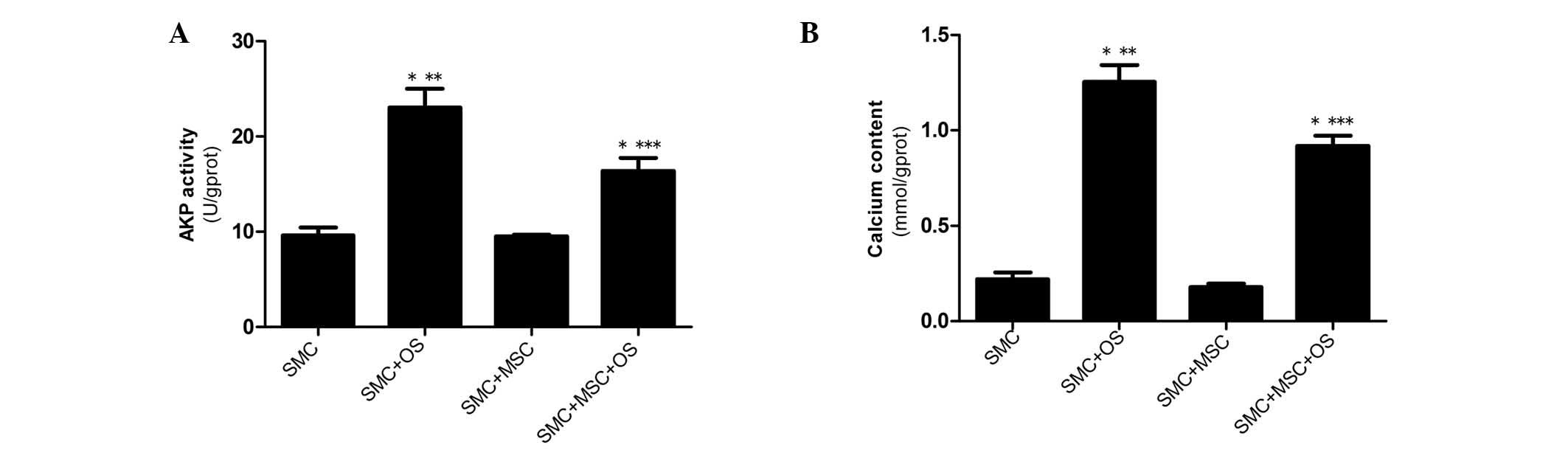
AKP activity and calcium content of
VSMCs
The AKP activity and calcium content in VSMCs were
determined to estimate the progression of calcification. The
results demonstrate that following culture for 14 days, AKP
activity and calcium content were significantly increased in the
SMC + OS and SMC + MSC + OS groups compared with the SMC group
(P<0.001), and reached the highest levels in the SMC + OS group
(P<0.001). Furthermore, the expression levels of osteogenic
markers were significantly decreased in the SMC + MSC + OS group
compared with the SMC + OS group (P<0.001). No change in AKP
activity or calcium content was observed between cells in the SMC
and the SMC + MSC groups (Fig. 3A and
B).
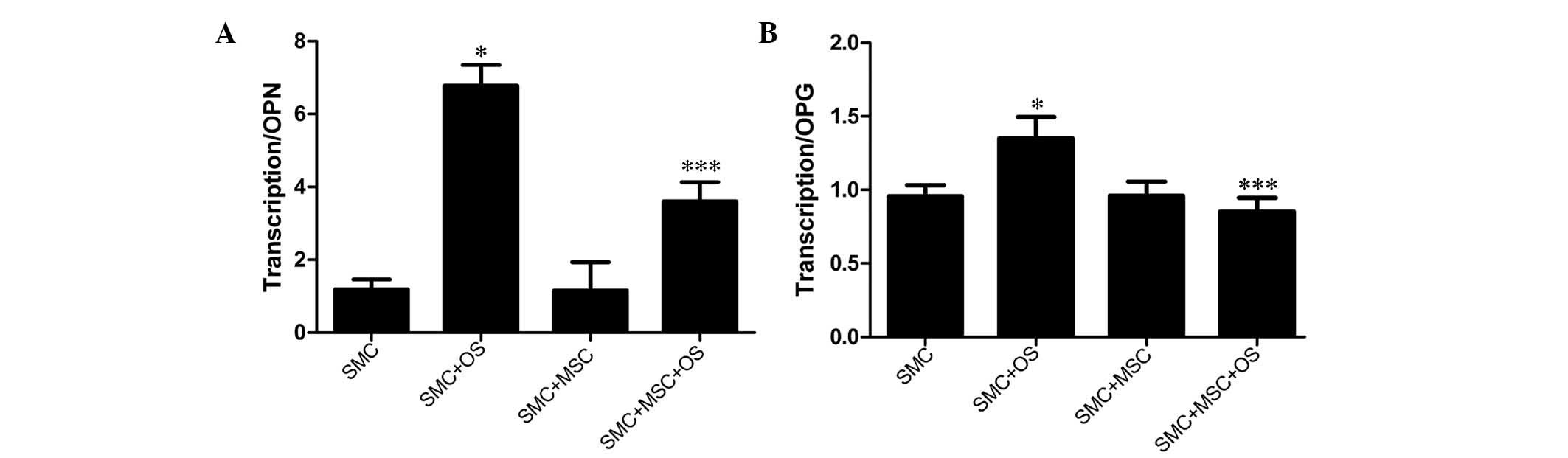
Indirect contact between SMCs and MSCs
reduces the mRNA levels of OPG and OPN
mRNA expression levels of OPG and OPN were evaluated
and negative expression was observed in the normal VSMCs by RT-qPCR
(3,4). Expression levels were significantly
increased in the SMC + OS group compared with the SMC group
(P<0.01), indicating that the VSMCs have a potential to
trans-differentiate into osteoblast-like cells in the presence of
OS. Furthermore, compared with the SMC + OS group, the expression
levels of OPG and OPN were significantly reduced in the SMC + MSC +
OS group (P<0.01; Fig. 4).
Wnt signaling pathways are downregulated
when SMCs indirectly interacted with MSCs
The mRNA and protein expression levels of Wnt5a,
Ror2 and β-catenin, which are known to modulate osteogenic
differentiation, were evaluated by RT-qPCR and western blot
analysis. Comprehensive analysis of the results indicated that
compared with the SMC group, Wnt5a, Ror2 and β-catenin expression
levels were significantly upregulated in the SMC + OS group
(P<0.001), demonstrating that the Wnt signals were activated by
OS. Furthermore, the expression levels of Wnt5a, Ror2 and β-catenin
were downregulated in the SMC + MSC + OS group compared with the
SMC + OS groups (P<0.001), indicating that the Wnt signaling
pathways were downregulated when direct interaction with MSCs
induced VSMC osteogenic differentiation (Fig. 5).
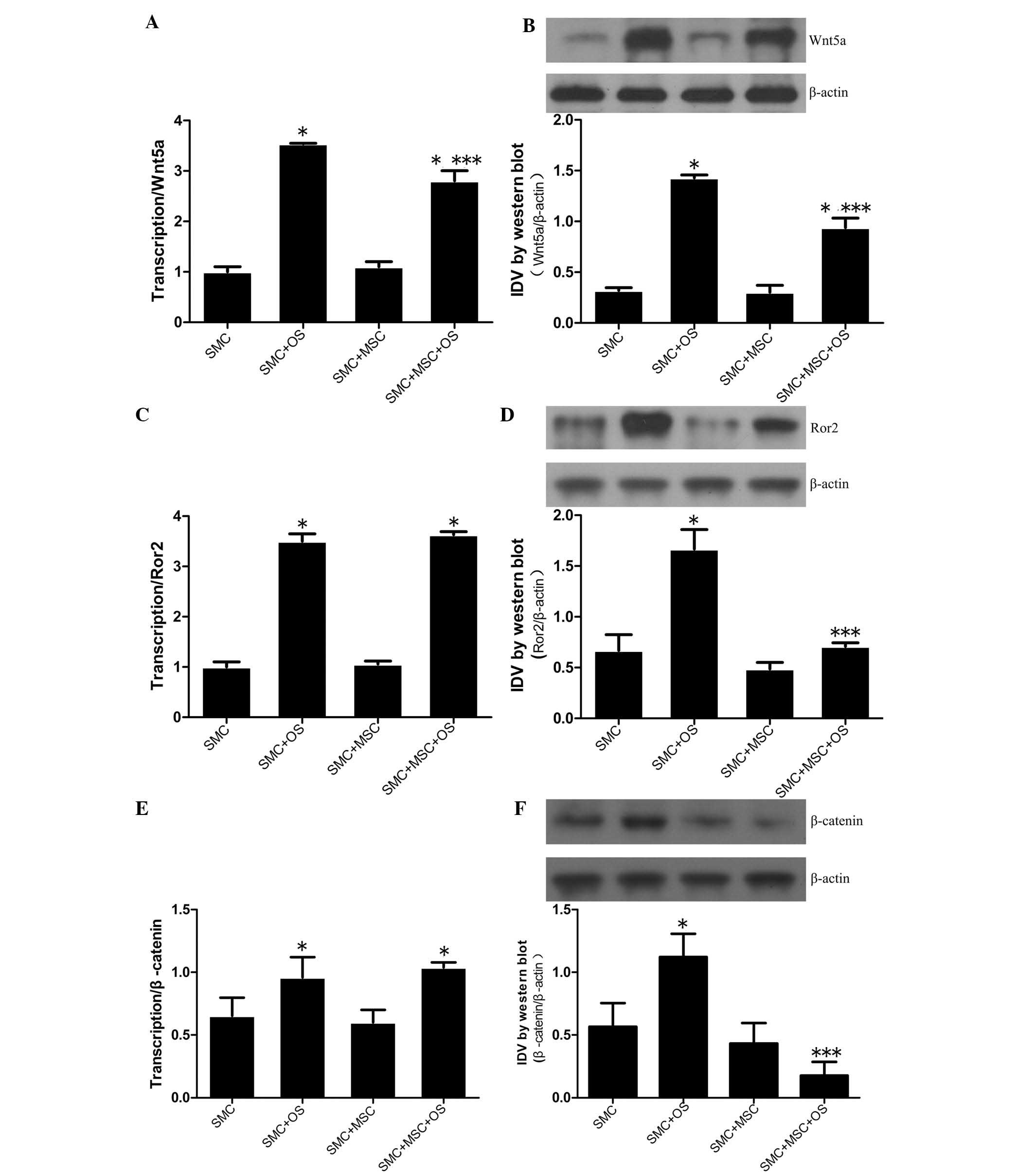 | Figure 5Wnt5a, Ror2, β-catenin expression in
VSMCs. VSMCs were indirectly co-cultured in the presence of absence
of MSCs at a ratio of 1:1 for 14 days, and further tests were
conducted on the VSMCs in the four groups. The mRNA and protein
expression levels of (A and B) Wnt5a, (C and D) Ror2 and (E and F)
β-catenin were measured by RT-qPCR and western blot analysis. The
mRNA expression levels of Wnt5a, Ror2 and β-catenin were
significantly increased in the groups of SMC + OS and SMC + MSC +
OS when compared with the SMC group. No difference in Ror2 and
β-catenin mRNA expression levels were detected between SMC + OS and
SMC + MSC + OS. Compared with the SMC group, the protein levels of
Wnt5a, Ror2 and β-catenin were markedly increased in the SMC + OS
group, but reduced in the SMC + MSC + OS group.
*P<0.001 vs. SMC group; ***P<0.01 vs.
SMC + OS group. SMC, smooth muscle cell; VSMC, vascular SMC; OS,
osteogenic medum; mRNA, messenger RNA; IDV, integrated density
value. |
Discussion
Previous studies have reported that MSCs may be cell
therapy candidates for vascular regeneration and angiogenesis due
to their capacity to directly migrate to sites of injury,
differentiate into residential cell lineages, and secrete
biochemical factors for immunomodulation and paracrine action
(7–9,12).
The current study analyzes the anti-inflammatory, immunomodulatory
and paracrine properties of MSCs on the process of VC. In order to
eliminate direct mechanical stimulation, a cell-cell indirect
co-culture system was constructed in vitro. Results from the
present study indicate that indirect contact with MSCs inhibits VC.
Furthermore, the expression levels of OPG and OPN mRNA were reduced
and the Wnt signaling pathways involved were downregulated during
VC.
The present study demonstrates that VSMCs
trans-differentiate into osteoblast-like cells following OS
induction, and promote the development of VC, which was indicated
by von Kossa staining, AKP activity and calcium content. However,
the severity of calcification was controlled by indirect contact
with MSCs. It is now widely accepted that MSCs isolated from bone
marrow possess marked therapeutic potential for tissue repair and
wound healing via cell transplantation or indirect mechanical
stimulation (22–25). However, there is a limited number
of studies that focus on the effects of MSCs in the treatment of
VC. A recent study (26)
demonstrated that MSCs are involved in VC as they are directed to
the vascular lesions where they undergo osteogenic differentiation.
Kramann et al (27)
implanted bone marrow-derived MSCs intraperitoneally into two rat
models of CKD with VC and demonstrated that MSCs underwent
osteogenic differentiation, which contributed to VC. Wang et
al (28) demonstrated that
transforming growth factor-β released from injured aortas recruited
MSCs into the aortic lesions, and contributed to the development of
VC in a low-density lipoprotein receptor-deficient mouse model that
was fed a high-fat Western diet. A previous study demonstrated that
MSCs trans-differentiate into osteoblast-like cells depending on
the number of MSCs in direct contact with calcified VSMCs (11). Data from the above-mentioned
studies indicate that MSCs promote the development of VC, when MSCs
are recruited into injured lesions, via an underlying osteogenic
differentiation mechanism. However, the results presented here
demonstrate that MSCs exhibit therapeutic potential for treatment
of VC via reducing AKP activity and calcium content in VSMCs. It is
possible that the cell-cell indirect co-culture system may
determine the effects of MSCs in VC. These results indicate that
MSC-induced paracrine and immunomodulatory effects are pivotal
underlying mechanisms for VC therapeutic strategies.
Bone-associated biomarkers in VSMCs, such as OPG and
OPN, can be secreted by VSMCs that undergo osteogenic
differentiation (3,4). The present study evaluated the mRNA
expression levels of these biomarkers. OPG is a soluble member of
the tumor necrosis factor receptor family (5), and previous studies have demonstrated
that increased OPG levels are associated with VC and mortality in
CKD patients (29,30). OPN is a glycoprotein, which is
expressed in calcified vessels (3,4,6) and
clinical trials have indicated that OPN is an effective prognostic
biomarker of coronary calcification (6). Furthermore, OPG and OPN levels are
correlated with the severity of vascular mineralization (6,29).
In the current study, the expression levels of OPG and OPN mRNA
were observed to be significantly increased in the SMC + OS group
compared with the SMC group (P<0.01). By contrast, following
indirect interaction with MSCs, the levels of OPG and OPN were
significantly reduced (P<0.01), indicating that the extent of
calcification may be increased by an underlying mechanism promoting
VSMC osteogenic differentiation, and modulating OPG and OPN mRNA
expression levels. Indirect contact with MSCs may prevent
calcification with MSC-induced paracrine and immunomodulatory
effects, as well as decreased expression levels of OPG and OPN
mRNA.
The effects of MSCs on the expression of Wnt-family
members, which are known to modulate the osteogenic
differentiation, were also evaluated. Numerous studies have
demonstrated that the Wnt signaling pathway is key in the
differentiation of osteoblasts (15,16,31).
Canonical and noncanonical signaling pathways interact and
crosstalk by binding to unrelated receptors or co-receptors
(15,16). It is well known that the
β-catenin-dependent signaling pathway regulates osteogenic
transdifferentiation and promotes vascular calcification (15,16,32).
By contrast, there is limited evidence that indicates the effects
of noncanonical Wnt5a/Ror2 signaling in the process of VC. Xin
et al (11) demonstrated
that Wnt5a protein expression was associated with the severity of
calcification and Ror2 mRNA expression levels were decreased when
cells underwent osteogenic differentiation. However, other previous
studies indicate that the activation of Wnt5a and Ror2 signals is
associated with osteogenic differentiation. Bolzoni et al
(33) demonstrated that the
osteogenic differentiation of human MSCs (hMSCs) increased the
expression level of Ror2 and that activation of the noncanonical
Wnt5a signaling pathway also increases hMSC osteogenic
differentiation. Huh et al (34) reported that arginine promotes
osteogenesis in hMSCs, and increases the expression of Wnt5a via
Wnt and nuclear factor of activated T-cells signaling. Liu et
al (17) and Billiard et
al (18) indicated that Ror2
is a modulator of osteogenesis and that increased levels of Ror2 in
hMSCs may promote formation of mineralization in the extracellular
matrix.
In the present study, canonical and noncanonical
signaling pathways were demonstrated to be involved in the process
of VC. The expression levels of β-catenin, Wnt5a and Ror2 are
undetectable in normal VSMCs, but increase as VSMCs differentiate
into the osteogenic phenotype (11,32,33).
The present study demonstrated that the Wnt5a/Ror2 signaling
pathway inhibits Wnt/β-catenin signaling in vitro. However,
a previous study indicated a dual signaling capacity for Wnt5a in
regulating Wnt/β-catenin signaling (19). Wnt5a either inhibits or activates
the Wnt/β-catenin signaling pathway depending on its expression
level and/or the receptors it is bound to. In addition, previous
studies have demonstrated that Ror2 is able to bind to Wnt5a, as
well as to canonical Wnt signals and mediate the action of
β-catenin (18,35). To the best of our knowledge, there
is no direct evidence that demonstrates the underlying mechanisms
of crosstalk between Wnt5a, Ror2 and β-catenin; however, Ror2 is
notably associated with VC. In addition, the current study
demonstrates that MSCs have the capacity to downregulate Wnt5a,
Ror2, and β-catenin expression in vitro by increasing their
immunomodulatory and paracrine capacities. Thus, during the VC
phase, MSCs produce factors that contribute to the prevention of
the process of VC and down-regulate canonical and noncanonical Wnt
ligands.
In conclusion, the results of the present study
demonstrate that VSMCs trans-differentiate into osteoblast-like
cells by promoting the expression of Wnt5a, Ror2 and β-catenin. In
addition, the immunomodulatory and paracrine capacities of MSCs may
be associated with the prevention of VSMC osteogenic
differentiation and the inhibition of VC via modulating
bone-associated biomarkers and downregulating Wnt signaling
pathways. This is notable for the development of stem cell-based
therapeutic strategies for VC. Further studies are required that
focus on determining which local mechanisms or biochemical factors
maximize the inhibition of VC by MSCs. Furthermore, the crosstalk
between Wnt5a, β-catenin, and Ror2 signals and the key molecular
mechanisms that regulate them, remain to be elucidated.
References
|
1
|
Wu M, Rementer C and Giachelli CM:
Vascular calcification: An update on mechanisms and challenges in
treatment. Calcif Tissue Int. 93:365–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
London GM: Mechanisms of arterial
calcifications and consequences for cardiovascular function. Kidney
Int Suppl (2011). 3:442–445. 2013. View Article : Google Scholar
|
|
3
|
Evrard S, Delanaye P, Kamel S, Cristol JP
and Cavalier E; SFBC/SN joined working group on vascular
calcifications: Vascular calcification: From pathophysiology to
biomarkers. Clin Chim Acta. 438:401–414. 2015. View Article : Google Scholar
|
|
4
|
McCarty MF and DiNicolantonio JJ: The
molecular biology and pathophysiology of vascular calcification.
Postgrad Med. 126:54–64. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El Hadj Othmane T, Speer G, Fekete B,
Szabó T, Egresits J, Fodor E, Kiss I, Nemcsik J, Szabó A, Németh Z,
et al: Osteoprotegerin: Regulator, protector and marker. Orv Hetil.
149:1971–1980. 2008.In Hungarian. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohamadpour AH, Abdolrahmani L, Mirzaei H,
Sahebkar A, Moohebati M, Ghorbani M, Ferns GA and Ghayour-Mobarhan
M: Serum osteopontin concentrations in relation to coronary artery
disease. Arch Med Res. 46:112–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murphy MB, Moncivais K and Caplan AI:
Mesenchymal stem cells: Environmentally responsive therapeutics for
regenerative medicine. Exp Mol Med. 45:e542013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yagi H, Soto-Gutierrez A, Parekkadan B,
Kitagawa Y, Tompkins RG, Kobayashi N and Yarmush ML: Mesenchymal
stem cells: Mechanisms of immunomodulation and homing. Cell
Transplant. 19:667–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang YK and Chen CS: Cell adhesion and
mechanical stimulation in the regulation of mesenchymal stem cell
differentiation. J Cell Mol Med. 17:823–832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chou SH, Lin SZ, Kuo WW, Pai P, Lin JY,
Lai CH, Kuo CH, Lin KH, Tsai FJ and Huang CY: Mesenchymal stem cell
insights: Prospects in cardiovascular therapy. Cell Transplant.
23:513–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xin H, Xin F, Zhou S and Guan S: The
Wnt5a/Ror2 pathway is associated with determination of the
differentiation fate of bone marrow mesenchymal stem cells in
vascular calcification. Int J Mol Med. 31:583–588. 2013.PubMed/NCBI
|
|
12
|
Watt SM, Gullo F, van der Garde M,
Markeson D, Camicia R, Khoo CP and Zwaginga JJ: The angiogenic
properties of mesenchymal stem/stromal cells and their therapeutic
potential. Br Med Bull. 108:25–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L and Xu Q: Stem/Progenitor cells in
vascular regeneration. Arterioscler Thromb Vasc Biol. 34:1114–1119.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SW, Houge M, Brown M, Davis ME and
Yoon YS: Cultured human bone marrow-derived CD31(+)
cells are effective for cardiac and vascular repair through
enhanced angiogenic, adhesion, and anti-inflammatory effects. J Am
Coll Cardiol. 64:1681–1694. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marinou K, Christodoulides C, Antoniades C
and Koutsilieris M: Wnt signaling in cardiovascular physiology.
Trends Endocrinol Metab. 23:628–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mill C and George SJ: Wnt signalling in
smooth muscle cells and its role in cardiovascular disorders.
Cardiovasc Res. 95:233–40. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Bodine PV and Billiard J: Ror2, a
novel modulator of osteogenesis. J Musculoskelet Neuronal Interact.
7:323–324. 2007.PubMed/NCBI
|
|
18
|
Billiard J, Way DS, Seestaller-Wehr LM,
Moran RA, Mangine A and Bodine PV: The orphan receptor tyrosine
kinase Ror2 modulates canonical Wnt signaling in osteoblastic
cells. Mol Endocrinol. 19:90–101. 2005. View Article : Google Scholar
|
|
19
|
van Amerongen R, Fuerer C, Mizutani M and
Nusse R: Wnt5a can both activate and repress Wnt/β-catenin
signaling during mouse embryonic development. Dev Biol.
369:101–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maeda K, Kobayashi Y, Udagawa N, Uehara S,
Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, et
al: Wnt5a-Ror2 signaling between osteoblast-lineage cells and
osteoclast precursors enhances osteoclastogenesis. Nat Med.
18:405–412. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Zhang Y and Qi G: Evaluation of
isolation methods and culture conditions for rat bone marrow
mesenchymal stem cells. Cytotechnology. 65:323–334. 2013.
View Article : Google Scholar :
|
|
22
|
Yan J, Tie G, Xu TY, Cecchini K and
Messina LM: Mesenchymal stem cells as a treatment for peripheral
arterial disease: Current status and potential impact of type II
diabetes on their therapeutic efficacy. Stem Cell Rev. 9:360–372.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee
IH, Lin WS, Wu CH, Lin WY and Cheng SM: Mesenchymal stem cells from
human umbilical cord express preferentially secreted factors
related to neuroprotection, neurogenesis, and angiogenesis. PLoS
One. 8:e726042013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
King A, Balaji S, Keswani SG and
Crombleholme TM: The role of stem cells in wound angiogenesis. Adv
Wound Care (New Rochelle). 3:614–625. 2014. View Article : Google Scholar
|
|
25
|
Burlacu A, Grigorescu G, Rosca AM, Preda
MB and Simionescu M: Factors secreted by mesenchymal stem cells and
endothelial progenitor cells have complementary effects on
angiogenesis in vitro. Stem Cells Dev. 22:643–653. 2013. View Article : Google Scholar :
|
|
26
|
Pal SN and Golledge J: Osteo-progenitors
in vascular calcification: A circulating cell theory. J Atheroscler
Thromb. 18:551–559. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kramann R, Kunter U, Brandenburg VM,
Leisten I, Ehling J, Klinkhammer BM, Knüchel R, Floege J and
Schneider RK: Osteogenesis of heterotopically transplanted
mesenchymal stromal cells in rat models of chronic kidney disease.
J Bone Miner Res. 28:2523–2534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang W, Li C, Pang L, Shi C, Guo F, Chen
A, Cao X and Wan M: Mesenchymal stem cells recruited by active TGFβ
contribute to osteogenic vascular calcification. Stem Cells Dev.
23:1392–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Montañez-Barragán A, Gómez-Barrera I,
Sanchez-Niño MD, Ucero AC, González-Espinoza L and Ortiz A:
Osteoprotegerin and kidney disease. J Nephrol. 2014.Epub ahead of
print. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morena M, Dupuy AM, Jaussent I, Vernhet H,
Gahide G, Klouche K, Bargnoux AS, Delcourt C, Canaud B and Cristol
JP: A cut-off value of plasma osteoprotegerin level may predict the
presence of coronary artery calcifications in chronic kidney
disease patients. Nephrol Dial Transplant. 24:3389–3397. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guan S, Wang Z, Xin F and Xin H: Wnt5a is
associated with the differentiation of bone marrow mesenchymal stem
cells in vascular calcification by connecting with different
receptors. Mol Med Rep. 10:1985–1991. 2014.PubMed/NCBI
|
|
32
|
Montes de Oca A, Guerrero F,
Martinez-Moreno JM, Madueño JA, Herencia C, Peralta A, Almaden Y,
Lopez I, Aguilera-Tejero E, Gundlach K, et al: Magnesium inhibits
Wnt/β-catenin activity and reverses the osteogenic transformation
of vascular smooth muscle cells. PLoS One. 9:e895252014. View Article : Google Scholar
|
|
33
|
Bolzoni M, Donofrio G, Storti P, Guasco D,
Toscani D, Lazzaretti M, Bonomini S, Agnelli L, Capocefalo A, Dalla
Palma B, et al: Myeloma cells inhibit non-canonical wnt co-receptor
ror2 expression in human bone marrow osteoprogenitor cells: Effect
of wnt5a/ror2 pathway activation on the osteogenic differentiation
impairment induced by myeloma cells. Leukemia. 27:451–463. 2013.
View Article : Google Scholar
|
|
34
|
Huh JE, Choi JY, Shin YO, Park DS, Kang
JW, Nam D, Choi DY and Lee JD: Arginine enhances osteoblastogenesis
and inhibits adipogenesis through the regulation of Wnt and NFATc
signaling in human mesenchymal stem cells. Int J Mol Sci.
15:13010–13029. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cai SX, Liu AR, He HL, Chen QH, Yang Y,
Guo FM, Huang YZ, Liu L and Qiu HB: Stable genetic alterations of
β-catenin and ROR2 regulate the Wnt pathway, affect the fate of
MSCs. J Cell Physiol. 229:791–800. 2014. View Article : Google Scholar : PubMed/NCBI
|