Introduction
Osteosarcoma is one of the most commonly diagnosed
malignant bone tumors in the clinic. It is the eighth leading type
of childhood cancer and accounts for ~2.4% of all malignancies in
pediatric patients, and ~20% of all primary bone cancers, thus is a
significant health problem in this age group (1,2).
Osteosarcoma has a high tendency for metastasis, predominantly to
the lungs (particularly the periphery of the lungs). One difficulty
in the treatment of this malignancy is the resistance of
osteosarcoma to conventional chemotherapy (3–5).
Although the diagnosis and treatment of non-metastatic osteosarcoma
has improved, patients with metastasis exhibit poor prognosis, with
a 5-year event-free survival rate less than 20% (6–8). It
is of high priority to elucidate novel methods for the diagnosis
and treatment of early stage osteosarcoma.
The human tripartite motif (TRIM) family has greater
than 77 members, of which the majority of the proteins belong to
the E3 ubiquitin ligases, due to the highly conservative really
interesting new gene (RING) domain. These proteins are involved in
a variety of biological processes, including transcriptional
regulation, membrane repair, cytoskeleton remodeling and
oncogenesis. Certain members of this family, including TRIM13,
TRIM19 and TRIM25 have been demonstrated to exert biological
activity during human tumorigenesis in leukemia, breast and
prostate cancer, through the regulation of transcriptional factors
(9–13). These observations suggest that the
TRIM proteins may serve vital roles in human tumorigenesis.
TRIM59, a surface molecule, has also been
demonstrated to be involved in certain types of human cancer
(14). It has been observed to be
markedly increased in gastric cancer and prominently associated
with the poor outcome of patients (14). The oncogenic features of TRIM59
were first characterized in 2011 (15). Furthermore, TRIM59 has been
identified as a multiple tumor biomarker in human tumorigenesis
(16). The biological activity of
TRIM59 has been observed to be closely associated with the
regulation of P53. TRIM59 interacts with P53, leading to P53
ubiquitination and degradation, and consequently promotes tumor
growth and migration (14). In
addition, TRIM59 was observed to exert its proto-oncogenic function
through interaction with the Ras signaling pathway in a transgenic
mouse model of prostate cancer (17). The exact role of TRIM59 in human
osteosarcoma, however, remains to be fully elucidated.
P53 is a tumor suppressor gene that can be
stimulated by cellular stress, such as oxidative stress. Once
activated, P53 causes cell-cycle arrest, promotes DNA repair or
induces apoptosis through different signaling pathways. Human P53
protein has three domains: The central DNA-binding domain, the
N-terminal transcription-activation domain (TA) and the C-terminal
oligomerization domain (18–20).
Of these domains, the TA domain is important, due to the fact that
it provides the structural basis for other molecules including
murine double minute 2 (MDM2) and MDMX to bind to P53 and regulate
P53 transcription (21,22). P53 is highly inactivated in various
tumor types (14) and numerous
molecules have been reported to promote tumor progression via
regulation of P53.
The current study aimed to investigate the role of
TRIM59 in human osteosarcoma growth and metastasis. Specific small
interfering RNA (siRNA) against TRIM59 and an expression plasmid
for TRIM59 were used for the modulation of TRIM59. In addition, the
expression levels of TRIM59 were determined in human osteosarcoma
tissues and in cultured osteosarcoma cell lines. Furthermore, it
was investigated whether TRIM59 had an effect on P53 expression,
and the potential regulatory mechanisms were discussed.
Materials and methods
Human samples
Samples from 30 patients with osteosarcoma, who had
been admitted to Tianjin Hospital (Tianjin, China) were collected.
Matched adjacent non-cancerous tissues were surgically dissected at
the same time as the cancerous tissues for each case and written
consent was obtained from each patient. Furthermore, all
experiments were conducted in compliance with the official polices
and defined protocols.
Cell culture
The human osteosarcoma cell lines U2OS, MG63 and
MNNG, and the non-cancerous cell line hFOB1.19, were obtained from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The three cancer cell lines were maintained in
Eagle's minimum essential medium (EMEM) media (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and
hFOB1.19 was cultured in Dulbecco's modified Eagle's medium
supplemented with 10% FBS.
Total RNA extraction and cDNA
synthesis
The total RNA from human tissues and cultured cells
were extracted with TRIzol Reagent (Takara Bio, Inc., Otsu, Japan),
according to the manufacturer's instructions. The quality and
concentration of the isolated RNAs were determined by measuring
absorbance with Nanodrop 2000 (Thermo Fisher Scientific, Inc.).
First-strand cDNA (10 ng) was reverse transcribed using the
PrimeScript RT Master Mix Perfect Real Time (Takara Bio, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
All RT-qPCR reactions were performed using an ABI
PRISM 7900 Real-Time System with the SYBR Premix Ex Taq kit (Takara
Bio, Inc.). The cycling protocol was as follows: Initial
denaturation step at 95°C for 2 min, 35 cycles of the three-step
cycling program consisting of 30 sec at 95°C (denaturation), 1 min
at 55°C (primer annealing) and 30 sec at 72°C (elongation), then a
final extension step for 10 min at 72°C. Amplification products
were determined by 1% (w/v) agarose gels (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The primers used are presented in Table
I, and the internal reference gene glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as the internal control. All
quantitative data were normalized to the internal control gene.
Western blot analysis
Total proteins from clinical tissues and cultured
cells were extracted. For human tissues, each sample was cut into
pieces and incubated in lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) with phenyl-methanesulfonyl fluoride
protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA) for 40 min
at 4°C. Subsequent to ultra-centrifugation at 12,000 × g at 4°C for
1 h, the supernatant was used for immunoblot analysis. For cultured
cells, when confluence reached 95%, cells were washed twice with
phosphate-buffered saline (Gibco; Thermo Fisher Scientific, Inc.)
followed by lysis with the lysis buffer (pH 7.5) to generate the
whole protein lysate. Equal amounts of protein (50 μg) were
loaded onto each lane using a 12% sodium dodecyl
sulfate-polyacrylimide gel electrophoresis gel. GAPDH was
synchronously developed as a loading control. The membranes were
blocked in 5% skimmed milk for 1 h at room temperature, and
incubated with rabbit polyclonal TRIM59 (1:1,000; cat. no.
sc-134123), P53 (1:1,000; cat. no. sc-6243) and GAPDH (1:1,000;
cat. no. sc-25778; all purchased from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at 4°C overnight. Subsequent to primary
incubation, the membranes were further incubated with a goat
anti-rabbit IgG (1:200; Santa Cruz Biotechnology, Inc.; cat. no.
sc-45101) at 37°C for 1 h. Immunoreactivity was determined with
enhanced chemiluminescent autoradiography (Thermo Fisher
Scientific, Inc.) and each experiment was repeated at least three
times.
siRNA interference and plasmid
transfection
For knockdown of TRIM59, a specific siRNA was
designed and chemically synthesized by Santa Cruz Biotechnology,
Inc. The pcDNA3.1-TRIM59 expression plasmid was constructed
according to routine protocols. In brief, primers for cloning and
amplifying human TRIM59 gene were designed. The forward primer
sequence was 5′-AAT GCA CAA TTT TGA GGAAG-3′ and the reverse primer
sequence was 5′-TCC ACA CAA ATT CCT TCAAC-3′. The transfection
assay was performed with Lipofectamine 2000 transfection reagent
(Life Technologies; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Subsequent to 6 h of transfection,
the media was replaced with fresh EMEM media with 10% FBS.
Cell proliferation assay
U2OS and MG63 cells were seeded into 96-well plates
(3,000 cells/well) and allowed to grow overnight. Cells were then
transfected with 10 μM siRNA or TRIM59 plasmid, followed by
incubation in EMEM media for an additional 72 h at 37°C. Cell
viability was determined for 5 consecutive days with
3-(4,5-dimethylthiazol-2-yl)-2,5-di-pheny ltetrazoliumbromide (MTT;
Beyotime Institute of Biotechnology) solution. For each monitored
day, 2 mg/ml MTT solution was added to each well. Subsequent to
incubation for 4 h at 37°C, media was discarded and 200 μl
dimethyl sulfoxide (OriGene Technologies, Inc., Rockville, MD, USA)
was added into each well. The plate was shaken for 5 min and the
optical density was measured at a wavelength of 570 nm using the
SpectraMax M5/M5e microplate reader (Molecular Devices LLC,
Sunnyvale, CA, USA).
Transwell assay
The U2OS cell line was cultured in 24-well plates
and transfected with specific TRIM59 siRNA or plasmids. Cells were
harvested 48 h post-transfection in serum-free EMEM as a single
cell suspension. A total of 150 μl cell suspension was
seeded into the upper chamber (Corning Incorporated, Corning, NY,
USA), while the lower chamber was filled with 600 μl EMEM
supplemented with 10% FBS. For the invasion assay, the chamber was
coated with Matrigel (Corning, Inc., Corning, NY, USA) 6 h prior to
seeding cells into the chamber. Subsequent to incubation at 37°C
for 12 h, cells were fixed with ice-cold methanol for 20 min and
stained with 0.1% crystal violet (Beyotime Institute of
Biotechnology) for 5 min. The images were obtained under a Nikon
XP-330C microscope at a magnification of ×200 (Nikon Corporation,
Tokyo, Japan). This assay was repeated a minimum of three times,
each repeat in duplicate.
Statistical analysis
The results were presented as the mean ± standard
deviation. Statistical analysis was conducted with Student's t-test
using the SPSS software, version 18.0 (SPSS, Inc, Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference. All experiments were repeated a minimum of
three times unless otherwise stated.
Results
TRIM59 is overexpressed in human
osteosarcoma tissues and cultured osteosarcoma cells
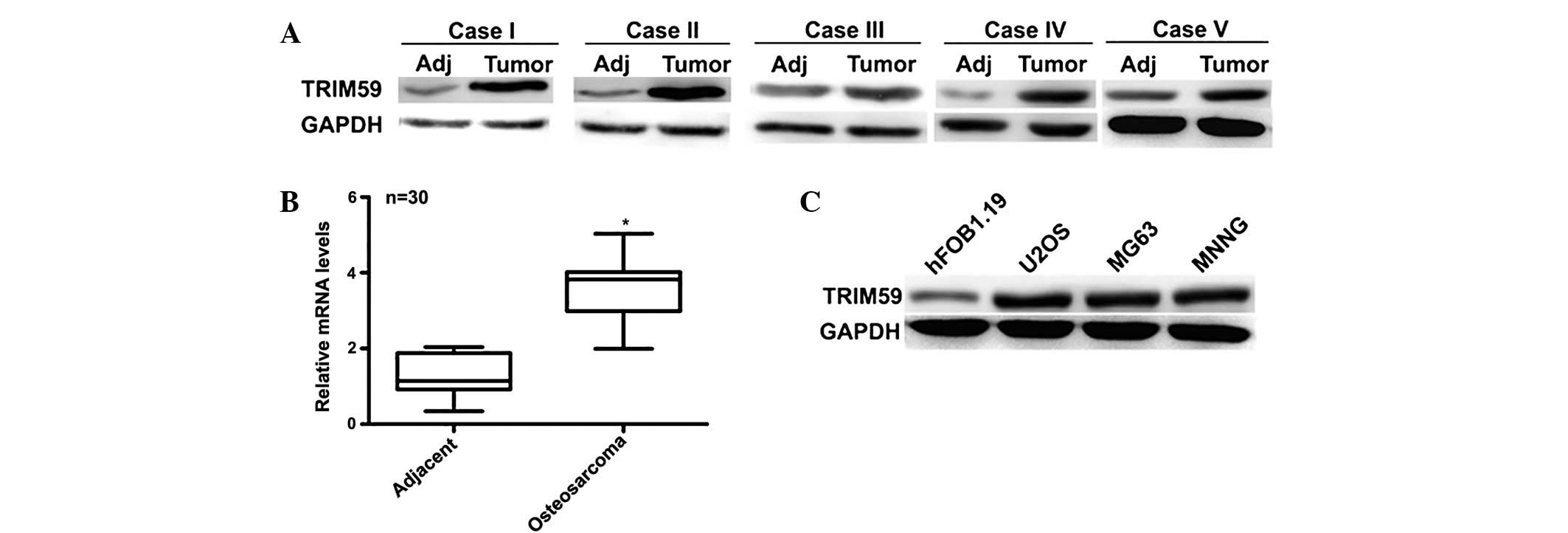
To clarify the role of TRIM59 in osteosarcoma, the
expression of TRIM59 was initially examined in 30 cases of clinical
human osteosarcoma tissues and their adjacent normal tissues.
Western blot analysis indicating that the protein level of TRIM59
was significantly increased in 28 cases of osteosarcoma tissues
compared with the adjacent normal tissues. The remaining 2 cases
were not observed to exhibit significantly different expression
levels of TRIM59 between cancerous and non-cancerous tissues. The
western blots of 5 representative cases that exhibited higher
protein levels of TRIM59 are presented in Fig. 1A. The RT-qPCR assay also indicated
that the average mRNA levels of TRIM59 in the clinical osteosarcoma
tissues were approximately three-fold of that of the adjacent
tissues (n=30; Fig. 1B). U2OS,
MG63 and MNNG cells are three osteosarcoma cell lines that have
been used in previous studies, while the hFOB1.19 cell line is a
commercial human osteoblast cell line, which is
steadily-transfected with SV40. Fig.
1C indicated that the protein levels of TRIM59 in the three
osteosarcoma cell lines were markedly higher than that of the
control non-osteosarcoma cell line hFOB1.19. Among the osteosarcoma
cell lines, U2OS and MG163 cells exhibited the highest protein
levels of TRIM59, making them the optimal cell lines for further
investigation. These data indicated that TRIM59 is highly expressed
in human osteosarcoma tissues and in cultured cell lines.
Transfection efficiency of the specific
siRNA against TRIM59 (siTRIM59) and the TRIM59 expression
plasmid
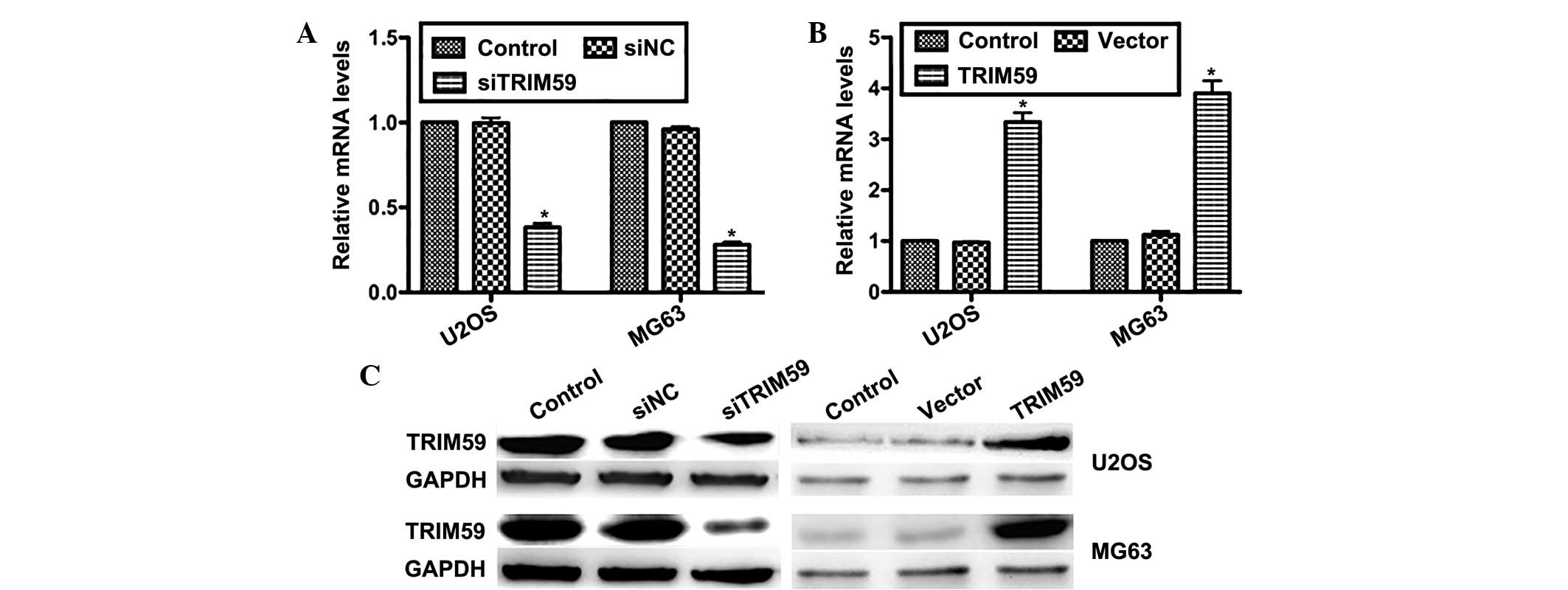
To further examine the role of TRIM59 in
osteosarcoma progression, two osteosarcoma cell lines (U2OS and
MG63) were transfected with specific siRNA against TRIM59
(siTRIM59) and the TRIM59 plasmid. RT-qPCR data demonstrated that
the relative mRNA levels of TRIM59 in siTRIM59-transfected U2OS
cells were reduced by ~60% compared with control cells; while the
levels were reduced by ~70% in siTRIM59-trans-fected MG63 cells
(Fig. 2A). On the contrary, the
mRNA level of TRIM59 was increased by 3.2-fold in U2OS cells and
3.7-fold in MG63 cells, when the pcDNA3.1-TRIM59 plasmid was
transfected (Fig. 2B). The results
of western blot analysis were consistent, indicating that the
protein levels of TRIM59 were notably suppressed by transfection of
siTRIM59, while markedly increased by transfection of the TRIM59
plasmid in the two cell lines (Fig.
2C). These results confirmed that transfection was effective,
and confirmed the efficiency of specific siTRIM59 and the TRIM59
plasmid in the modulation of TRIM59 expression.
TRIM59 increases the cell proliferation
ability in the U2OS and MG63 osteosarcoma cell lines
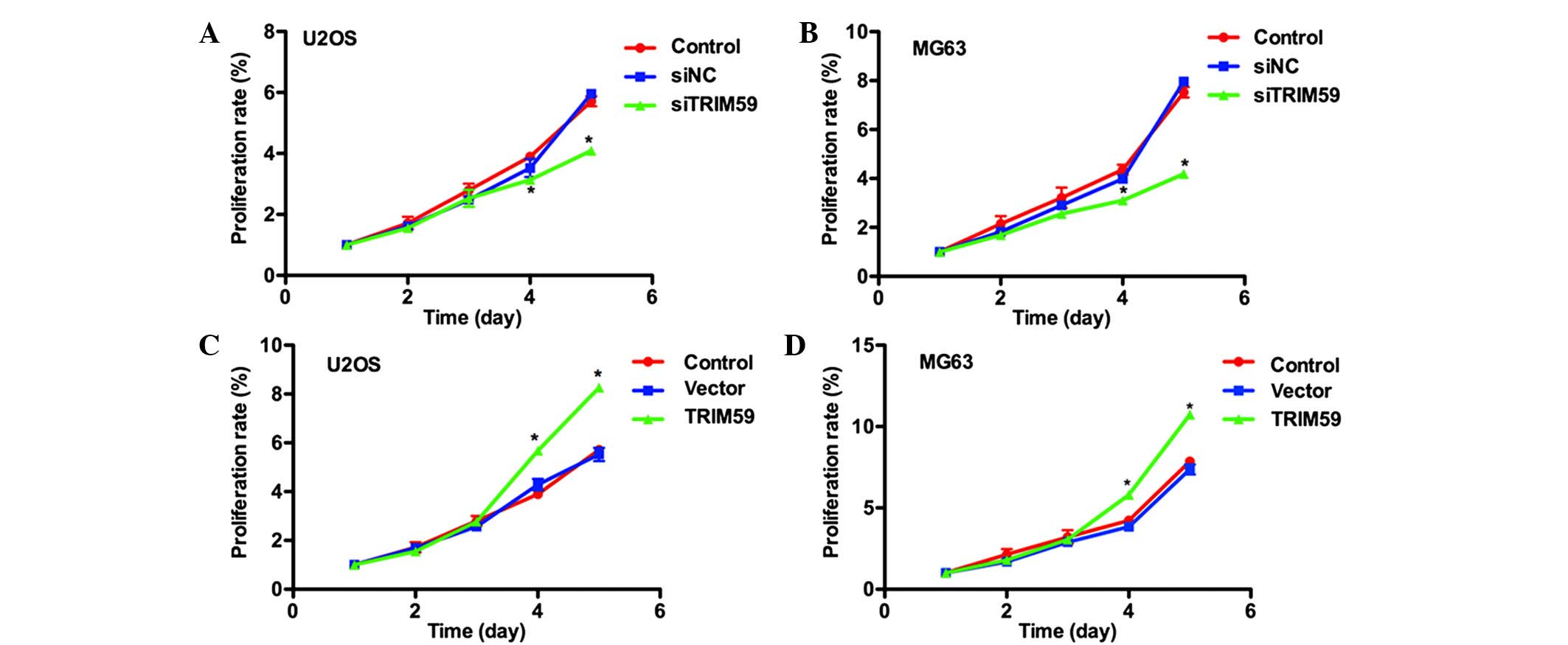
TRIM59 has been reported to function as an activator
of cell proliferation in gastric cancer (14). Thus, the effects of TRIM59
modulation on osteosarcoma cell proliferation were investigated.
The MTT assay indicated that cell proliferative rates were not
significantly different in the first three days among three groups,
only a marginal difference was observed. However, on the fourth and
fifth days, the proliferative rate of siTRIM59-transfected U2OS
cells was significantly reduced, by 12.5% and 23.3%, respectively,
compared with the control U2OS cells (Fig. 3A). Similar results were observed in
the MG63 cells, with significant reductions in the proliferative
rate subsequent to transfection of siTRIM59 (Fig. 3B). By contrast, overexpression of
TRIM59 increased the proliferative rate by 30% in U2OS cells and
27% in MG63 cells on the fourth day. Cell proliferation was further
increased on the fifth day, by 31% and 31.2% in U2OS and MG63
cells, respectively (Fig. 3C and
D). These data suggest that TRIM59 may promote cell
proliferation in osteosarcoma.
TRIM59 promotes cell migration and
invasion in osteosarcoma
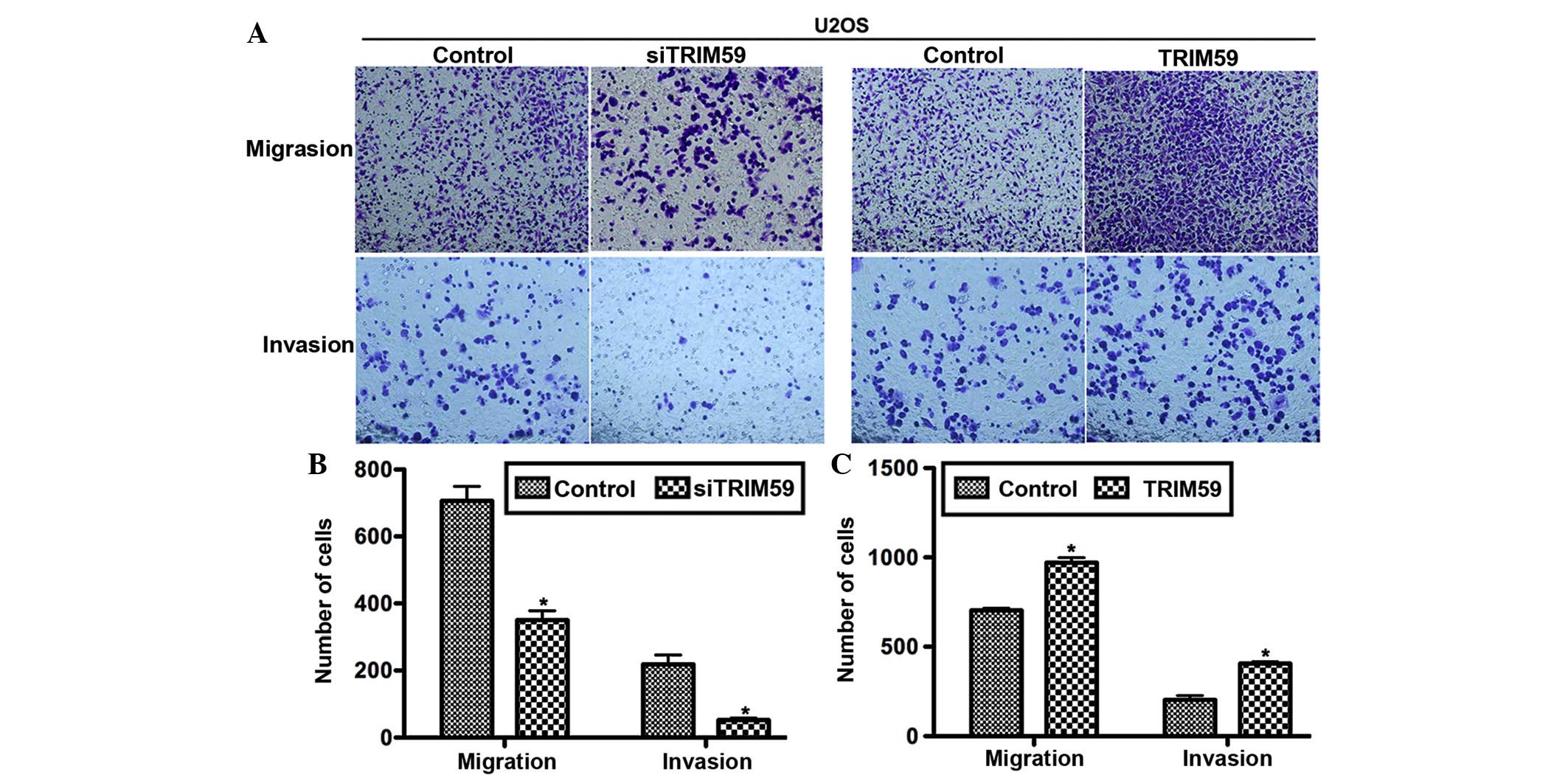
Osteosarcoma has a high tendency to metastasize;
consequently it was investigated whether TRIM59 served a role in
this process. The Transwell assay, together with the subsequent
crystal violet staining, indicated that the number of migrated
cells were markedly different between the experimental and control
groups (Fig. 4A). Counting of the
migrated cells indicated that compared with the corresponding
control group, the number of migrated cells attached to the
permeable membrane was significantly reduced, by ~50% in the
siTRIM59-transfected group, however was significantly increased by
30% in TRIM59-treated U2OS cells (Fig.
4B and C). For the invasion assay, U2OS cells with TRIM59
knockdown exhibited lower abilities, while cells with TRIM59
overexpression exhibited higher abilities to invade through the
Matrigel and migrate through the pores (Fig. 4B and C). These data support the
hypothesis that TRIM59 contributes to cell migration and invasion
in osteosarcoma.
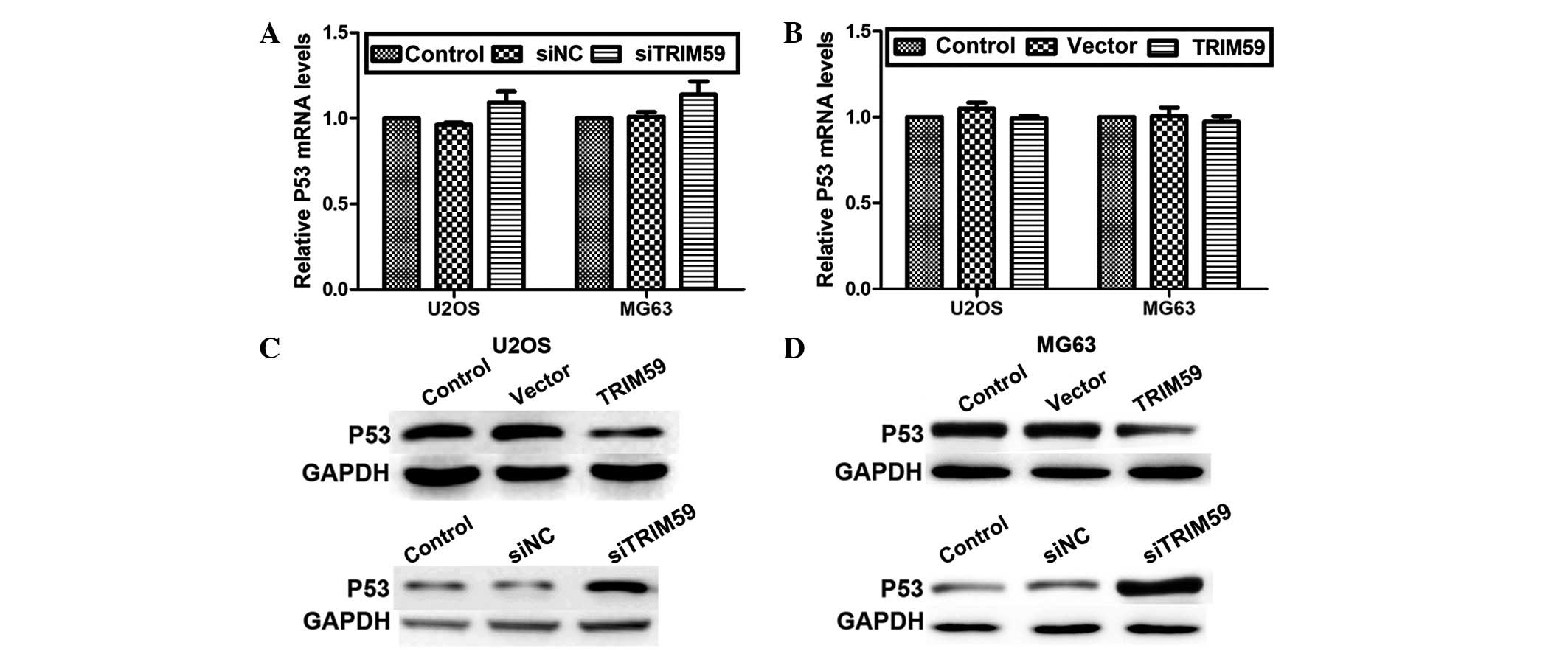
TRIM59 inhibits P53 expression at the
protein level
The tumor suppressor gene P53 works as a “guardian”
of the human genome and mutations of P53 have been reported to
trigger human carcinogenesis (23). Therefore, it was investigated
whether TRIM59 had any effects on P53 expression in osteosarcoma.
The RT-qPCR assay indicated that the mRNA levels of P53 were not
significantly altered in response to knockdown or overexpression of
TRIM59 in the two osteosarcoma cell lines (Fig. 5A and B). However, protein levels of
P53 were markedly altered by modulation of TRIM59. Knockdown of
TRIM59 increased, whereas overexpression of TRIM59 reduced, the
expression of P53 protein in U2OS (Fig. 5C) and MG63 (Fig. 5D) cells. These data suggest that
TRIM59 may regulate P53 expression in osteosarcoma, however only at
a protein level.
Discussion
Osteosarcoma is the fifth most frequent malignancy
among adolescents between 15 and 19 years old, particularly in boys
and African-American children (24,25).
Familial cases, bone dysplasias, Li-Fraumeni syndrome and
Rothmund-Thomson syndrome are the main manifestations of
osteosarcoma observed clinically (26). In the USA, it was reported that one
third of patients suffering from osteosarcoma die each year, with
the majority of these victims being teenagers (27). As a result, further research is
required to aid in the discovery of novel diagnostic and prognostic
biomarkers, and an improved understanding of the mechanisms for
osteosarcoma development.
In the present study, it was demonstrated that
TRIM59 was overexpressed in clinical osteosarcoma tissues at mRNA
and protein levels. By employing RNAi and plasmid transfection
techniques, it was indicated that knockdown of TRIM59 restricted,
whereas overexpression of TRIM59 promoted, the cell proliferative,
migratory and invasive abilities of osteosarcoma U2OS and MG63
cells. Thus it is suggested that TRIM59 is a key mediator of
osteosarcoma progression. However, in vivo data, such as a
mouse model of osteosarcoma, is required in order to further
confirm the critical role of TRIM59 in osteosarcoma
progression.
Notably, it was identified that knockdown of TRIM59
in the two cell lines increased the protein levels of P53, without
significantly affecting the mRNA levels. TRIM59 belongs to the E3
ubiquitin ligase family, which promotes ubiquitin-mediated
degradation of target proteins (28). The regulation of P53 by TRIM59 in
osteosarcoma cells would suggest that TRIM59 mediated osteosarcoma
progression, potentially via regulation of P53 (predominantly by
ubiquitin-mediated degradation). It has also been previously
reported that TRIM59 is upregulated in gastric cancer and promotes
the ubiquitination and degradation of P53 (14). This indicates that ubiquitin ligase
is important in the activity of TRIM59. How TRIM59 regulates the
ubiquitination and degradation of P53 remains unclear. P53 inhibits
tumor development through various approaches including induction of
apoptosis and autophagy (26,29).
MDM2 and its homolog MDMX (also termed MDM4) are the key
suppressors of P53 in multiple tumor types (30,31).
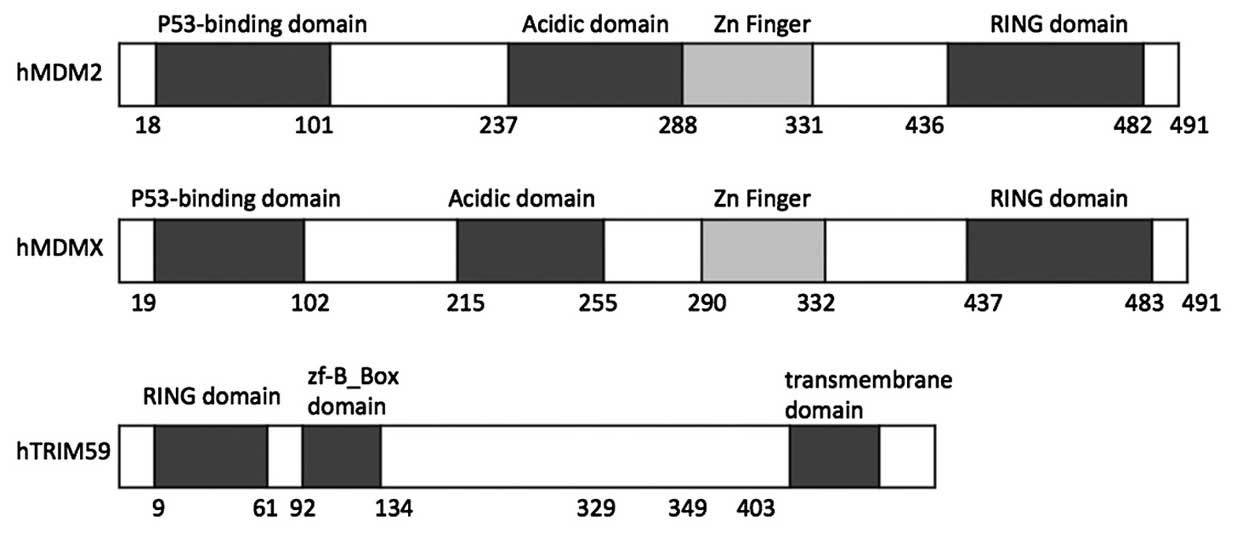
As presented in Fig. 6, the MDM2
N-terminal fragment (18–101 amino acids)/MDMX N-terminal fragment
(19–102 amino acids) has the ability to bind with the P53
transactivation domain peptide (21,32).
In addition, the RING domain of MDMX heterodimerizes with that of
MDM2, leading to the recruitment of E2 ubiquitin-conjugating enzyme
and the final ubiquitination and degradation of P53 (32–34).
One possible mechanism for TRIM59 regulation of P53 is
TRIM59-mediated promotion of P53 degradation through its RING
domain, as observed in MDMX and MDM2. The RING domains of MDM2 and
MDMX are important in their binding with P53, and TRIM59 also has a
RING domain at its N-terminal (Fig.
6). This structural similarity between TRIM59 and MDM2/MDMX
additionally suggests a similar regulatory mechanism for P53.
However, the specific function of the RING domain in TRIM59 remains
to be fully elucidated, thus further investigation is required.
In conclusion, TRIM59 was observed to be upregulated
in clinical osteosarcoma tissues and the cultured osteosarcoma cell
lines U2OS and MG63. Knockdown of TRIM59 with specific siRNA
inhibited the cell proliferative, migratory and invasive abilities
of osteosarcoma cells. By contrast, when TRIM59 was overexpressed
in osteosarcoma cells, increased cell proliferative rates and
migratory abilities were observed. The regulation of P53 by TRIM59
at its protein level may suggest that TRIM59 promotes osteosarcoma
progression by ubiquitination and degradation of P53.
Acknowledgments
The present study was sponsored by the Natural
Science Foundation of China (grant no. 81301596).
References
|
1
|
Messerschmitt PJ, Garcia RM, Abdul-Karim
FW, Greenfield EM and Getty PJ: Osteosarcoma. J Am Acad Orthop
Surg. 17:515–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bacci G, Rocca M, Salone M, Balladelli A,
Ferrari S, Palmerini E, Forni C and Briccoli A: High grade
osteosarcoma of the extremities with lung metastases at
presentation: Treatment with neoadjuvant chemotherapy and
simultaneous resection of primary and metastatic lesions. J Surg
Oncol. 98:415–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bielack SS, Carrle D, Hardes J, Schuck A
and Paulussen M: Bone tumors in adolescents and young adults. Curr
Treat Options Oncol. 9:67–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hughes DP: Strategies for the targeted
delivery of therapeutics for osteosarcoma. Expert Opin Drug Deliv.
6:1311–1321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrari S, Smeland S, Mercuri M, Bertoni
F, Longhi A, Ruggieri P, Alvegard TA, Picci P, Capanna R, Bernini
G, et al: Neoadjuvant chemotherapy with high-dose ifosfamide,
high-dose methotrexate, cisplatin, and doxorubicin for patients
with localized osteosarcoma of the extremity: A joint study by the
Italian and Scandinavian sarcoma groups. J Clin Oncol.
23:8845–8852. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mialou V, Philip T, Kalifa C, Perol D,
Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles
AS and Hartmann O: Metastatic osteosarcoma at diagnosis: Prognostic
factors and long-term outcome-the French pediatric experience.
Cancer. 104:1100–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smeland S, Müller C, Alvegard TA, Wiklund
T, Wiebe T, Björk O, Stenwig AE, Willén H, Holmström T, Follerås G,
et al: Scandinavian sarcoma group osteosarcoma study SSG VIII:
Prognostic factors for outcome and the role of replacement salvage
chemotherapy for poor histological responders. Eur J Cancer.
39:488–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klugbauer S and Rabes HM: The
transcription coactivator HTIF1 and a related protein are fused to
the RET receptor tyrosine kinase in childhood papillary thyroid
carcinomas. Oncogene. 18:4388–4393. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hatakeyama S: TRIM proteins and cancer.
Nat Rev Cancer. 11:792–804. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Thé H, Lavau C, Marchio A, Chomienne C,
Degos L and Dejean A: The PML-RAR alpha fusion mRNA generated by
the t (15;17) translocation in acute promyelocytic leukemia encodes
a functionally altered RAR. Cell. 66:675–684. 1991. View Article : Google Scholar
|
|
12
|
Cambiaghi V, Giuliani V, Lombardi S,
Marinelli C, Toffalorio F and Pelicci PG: TRIM proteins in cancer.
Adv Exp Med Biol. 770:77–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Le Douarin B, Zechel C, Garnier JM, Lutz
Y, Tora L, Pierrat B, Heery D, Gronemeyer H, Chambon P and Losson
R: The N-terminal part of TIF1, a putative mediator of the
ligand-dependent activation function (AF-2) of nuclear receptors,
is fused to B-raf in the oncogenic protein T18. EMBO J.
14:2020–2033. 1995.PubMed/NCBI
|
|
14
|
Zhou Z, Ji Z, Wang Y, Li J, Cao H, Zhu HH
and Gao WQ: TRIM59 Is up-regulated in gastric tumors, promoting
ubiquitination and degradation of p53. Gastroenterology.
147:1043–1054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Valiyeva F, Jiang F, Elmaadawi A, Moussa
M, Yee SP, Raptis L, Izawa JI, Yang BB, Greenberg NM, Wang F and
Xuan JW: Characterization of the oncogenic activity of the novel
TRIM59 gene in mouse cancer models. Mol Cancer Ther. 10:1229–1240.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khatamianfar V, Valiyeva F, Rennie PS, Lu
WY, Yang BB, Bauman GS, Moussa M and Xuan JW: TRIM59, a novel
multiple cancer biomarker for immunohistochemical detection of
tumorigenesis. BMJ Open. 2:pii.e0014102012. View Article : Google Scholar
|
|
17
|
Valiyeva F, Jiang F, Elmaadawi A, Moussa
M, Yee SP, Raptis L, Izawa JI, Yang BB, Greenberg NM, Wang F and
Xuan JW: Characterization of the oncogenic activity of the novel
TRIM59 gene in mouse cancer models. Mol Cancer Ther. 10:1229–1240.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mavinahalli JN, Madhumalar A, Beuerman RW,
Lane DP and Verma C: Differences in the transactivation domains of
p53 family members: A computational study. BMC Genomics. 11(Suppl
1): S52010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Muller PA and Vousden KH: Mutant p53 in
cancer: New functions and therapeutic opportunities. Cancer Cell.
25:304–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sauer M, Bretz AC, Beinoraviciute-Kellner
R, Beitzinger M, Burek C, Rosenwald A, Harms GS and Stiewe T:
C-terminal diversity within the p53 family accounts for differences
in DNA binding and transcriptional activity. Nucleic Acids Res.
36:1900–1912. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kussie PH, Gorina S, Marechal V, Elenbaas
B, Moreau J, Levine AJ and Pavletich NP: Structure of the MDM2
onco-protein bound to the p53 tumor suppressor transactivation
domain. Science. 274:948–953. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Momand J, Zambetti GP, Olson DC, George D
and Levine AJ: The mdm-2 oncogene product forms a complex with the
p53 protein and inhibits p53-mediated transactivation. Cell.
69:1237–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Overholtzer M, Rao PH, Favis R, Lu XY,
Elowitz MB, Barany F, Ladanyi M, Gorlick R and Levine AJ: The
presence of p53 mutations in human osteosarcomas correlates with
high levels of genomic instability. Proc Natl Acad Sci USA.
100:11547–11552. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marina N, Gebhardt M, Teot L and Gorlick
R: Biology and therapeutic advances for pediatric osteosarcoma.
Oncologist. 9:422–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sandberg AA and Bridge JA: Updates on the
cytoge-netics and molecular genetics of bone and soft tissue
tumors: Osteosarcoma and related tumors. Cancer Genet Cytogenet.
145:1–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ottaviani G and Jaffe N: The etiology of
osteosarcoma. Cancer Treat Res. 152:15–32. 2009. View Article : Google Scholar
|
|
27
|
Kim FM, Hayes C, Williams PL, Whitford GM,
Joshipura KJ, Hoover RN and Douglass CW; National Osteosarcoma
Etiology Group: An assessment of bone fluoride and osteosarcoma. J
Dent Res. 90:1171–1176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kondo T, Watanabe M and Hatakeyama S:
TRIM59 interacts with ECSIT and negatively regulates NF-kB and
IRF-3/7-mediated signal pathways. Biochem Biophys Res Commun.
422:501–507. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vousden KH and Prives C: Blinded by the
light: The growing complexity of p53. Cell. 137:413–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pei D, Zhang Y and Zheng J: Regulation of
p53: A collaboration between Mdm2 and Mdmx. Oncotarget. 3:228–235.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang X and Jiang X: Mdm2 and MdmX partner
to regulate p53. FEBS Lett. 586:1390–1396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shvarts A, Steegenga WT, Riteco N, van
Laar T, Dekker P, Bazuine M, van Ham RC, van der Houven van Oordt
W, Hateboer G, van der Eb AJ and Jochemsen AG: MDMX: A novel
p53-binding protein with some functional properties of MDM2. EMBO
J. 15:5349–5357. 1996.PubMed/NCBI
|
|
33
|
Danovi D, Meulmeester E, Pasini D,
Migliorini D, Capra M, Frenk R, de Graaf P, Francoz S, Gasparini P,
Gobbi A, et al: Amplification of Mdmx (or Mdm4) directly
contributes to tumor formation by inhibiting p53 tumor suppressor
activity. Mol Cell Biol. 24:5835–5843. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zdzalik M, Pustelny K, Kedracka-Krok S,
Huben K, Pecak A, Wladyka B, Jankowski S, Dubin A, Potempa J and
Dubin G: Interaction of regulators Mdm2 and Mdmx with transcription
factors p53, p63 and p73. Cell Cycle. 9:4584–4591. 2010. View Article : Google Scholar : PubMed/NCBI
|