Introduction
Non-Hodgkin's lymphoma (NHL) is a type of
malignancy, which originates from lymphatic cells (1). NHL can be divided into two groups
according to the derivation, namely B cell type and T cell type,
among which the B cell type accounts for 90% (2). Diffuse large B cell lymphoma (DLBCL)
is one of the most life threatening types of malignancy due to its
lack of symptoms in the early period and the lack of efficient
therapeutic strategies, leading to poor prognoses (3). Therefore, there is an urgent
requirement to identify novel biomarkers for early detection and
the prediction of prognosis.
Long non-coding RNAs (lncRNAs), with a size >200
nt, have been an increased area of focus since their
identification. Although they were previously considered a 'waste',
lncRNA has a specific mechanism in several types of disease,
particularly in malignant tumors. Hox transcript antisense
intergenic RNA (HOTAIR), has an oncogenic role in several types of
cancer, including colorectal cancer (4,5),
lung cancer (6–8) and pancreatic cancer (9). However, the role of HOTAIR in DLBCL
has not been investigated. In the present study, it was found that
HOTAIR was upregulated in DLBCL and was correlated with an invasive
phenotype and poor prognosis. Furthermore, it was demonstrated that
silencing HOTAIR inhibited cell proliferation, promoted cell
apoptosis and induced cell cycle arrest, possibly through the
phosphoinositide 3-kinase (PI3K/AKT/nuclear factor(NF)-κB
pathway.
Materials and methods
Tissue sample collection and cell line
preparation
The present study included 50 lymph node samples
from patients diagnosed with DLBCL and 20 individuals with reactive
lymph nodes as controls. All samples were collected at The
Department of Oncology, Dongying People's Hospital (Dongying,
China), between July 2006 and December 2011. All samples were
snap-frozen and stored in liquid nitrogen following collection. All
the patients were diagnosed and confirmed to have DLBCL by
histological examination, and any patients who had received
preoperative radiotherapy or chemotherapy were excluded. Follow-up
data were obtained by reviewing outpatient charts or through
correspondence with the patients. The overall survival (OS) was
defined as the time interval between the date of diagnosis and the
end of the follow-up, or the date at which the patient succumbed to
mortality. The present study was approved by the Research Ethics
Committee of Dongying People's Hospital, and informed consent was
obtained from each of the participants.
Cell lines and culture
The RCK-8, OCL-LY-10, OCL-LY-7, SU-DHL-6 and
SU-DHL-4 DLBCL cell lines were purchased from American Type Culture
Collection (Manassas, VA, USA). The cells were cultured in RPMI
1640 supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), in a humidified 5%
CO2 incubator at 37°C. Analysis results from normal
tissues served as the control for comparison (10).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA from tissue samples and cells at ~80–90%
confluency were isolated using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and the steps were performed according to
the manufacturer's protocol. The acquired RNA was quantified using
ultraviolet spectrophotometry (NanoDrop 2000; Thermo Fisher
Scientific, Inc.). The quantified RNA was then reverse transcribed
into cDNA using the ExScriptRT-PCR kit (Takara Bio, Inc., Otsu,
Japan), according to the manufacturer's protocol. qPCR was then
performed using an ABI ViiA™ 7 PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) to verify the expression levels of
HOTAIR. The expression of GAPDH was also determined and used as an
internal control. The thermocycling conditions were as follows:
95°C for 30 sec; followed by 40 cycles of denaturation at 95°C for
3 sec and annealing at 60°C for 30 sec. The primer sequences
(obtained from Sangon Biotech Co., Ltd., Shanghai, China) were as
follows: HOTAIR, forward 5′-GGT AGA AAA AGC AAC CAC GAAGC-3′ and
reverse 5′-ACA TAA ACC TCT GTC TGT GAG TGC C-3′; GAP DH, forward
5′-CCC CGC TAC TCC TCC TCC TAAG-3′ and reverse 5′-TCC ACG ACC AGT
TGT CCA TTCC-3′. The relative mRNA expression of each gene was
calculated using the comparative quantification cycle (Cq)
(2−ΔΔCq) method (11).
Cell transfection assay
RCK-8 cells were selected for the knockdown
experiment as they demonstrated the highest basal expression level.
The RCK-8 cells (confluency, 30–50%) were transfected with either
50 nM HOTAIR-targeting small interfering (si)RNA or scrambled
negative controls (GenePharma, Shanghai, China) using Lipofectamine
RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.), according to
manufacturer′s protocol. The two HOTAIR RNAi sequences were as
follows: 5′-TAA CAA GAC CAG AGA GCT G-3′ (HOTAIR-si1) and 5′-GAA
CGG GAG TAC AGA GAG A-3′ (HOTAIR-si2). The scramble sequence was as
follows: 5′-UUC UCC GAA CGU GUC ACG UTT -3′. After 24 h of
incubation in a humidified atmosphere with 5% CO2 at
37°C, the RNA was isolated, and the efficiency of HOTAIR knockdown
was determined using qPCR.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assays were used to evaluate
cell proliferation, according to the manufacturer's protocol.
Briefly, the cells (3×103/well) were seeded into 96-well
plates in triplicate and incubated in a 5% CO2
humidified atmosphere at 37°C. At specific time points (24, 48 and
72 h), the cells were incubated with 10 µl CCK-8 solution
for 2 h at 37°C. The absorbance was measured at a wavelength of 450
nm on a Gen5 microplate reader (BioTek, Winooski, VT, USA). The
experiments were repeated in triplicate three times.
Analysis of cell cycle and apoptosis
The HOTAIR RNAi-transfected cells were harvested at
a confluency of ~80–90% and washed twice with phosphate-buffered
saline (PBS), following which they were fixed with pre-cooled 70%
ethanol at 4°C overnight, and washed twice with PBS. The cells were
then resuspended in 500 µl PBS, treated with RNase A (50
µg/ml; Beyotime Institute of Biotechnology, Haimen, China)
and stained with propidium iodide (25 µg/ml; Beyotime
Institute of Biotechnology) for 30 min at 37°C. The distribution of
cell-cycle phases were determined using ModFit software (version
4.1; BD Biosciences, Franklin Lakes, NJ, USA). For the analysis of
apoptosis, the cells were harvested at 70–80% confluence and
incubated with reagent containing Annexin V-fluorescein
isothiocyanate and propidium iodide (BD Biosciences) for 15 min in
the dark at room temperature. The apoptotic cells were analyzed
using a FACS Calibur flow cytometer (BD Biosciences).
Western blot analysis
The transfected cells were lysed in
radio-immunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) and, after 48 h, phenylmethanesulfonyl fluoride
(Beyotime Institute of Biotechnology) was added and protein
concentrations were determined using a standard Bradford assay
(Beyotime Institute of Biotechnology). Equal quantities of proteins
(20 µg) from each cell line were subjected to western blot
analysis. The total proteins were fractionated using SDS-PAGE
(Beyotime Institute of Biotechnology) and transferred onto a
polyvinylidene fluoride membrane (Beyotime Institute of
Biotechnology). The membranes were blocked in milk and then
incubated with the indicated primary antibodies at 4°C overnight.
The membranes were then incubated with secondary antibodies at room
temperature for 1 h, and detection was performed using a
chemiluminescence detection system (ImageQuant™ LAS 4000; GE
Healthcare Life Sciences). The data were adjusted against the
loading control using β-actin. The primary antibodies used for
western blot analyses were as follows: Mouse monoclonal anti-total
PI3K (1:1,000; cat. no. sc-365404), rabbit polyclonal
anti-PI3K-p110 (1:1,000; cat. no. sc-130211), mouse monoclonal
anti-total AKT (1:1,000; cat. no. sc-81434), rabbit polyclonal
anti-phosphorylated (p)-AKT (Thr308; 1:1,000; cat. no. sc-16646-R),
rabbit polyclonal anti-NF-κB p65 (1:1,000; cat. no. sc-372) and
rabbit polyclonal anti-p-NF-κB p65 (Ser536; 1:1,000; sc-101752).
The secondary antibodies used were goat anti-rabbit (1:5,000; cat.
no. sc-2004) and goat anti-mouse (1:5,000; cat. no. sc-2060). All
of the antibodies were obtained from Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA).
Statistical analysis
All statistical analysis was performed using SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). Continuous data were
analyzed using an independent t-test, whereas categorical
data were analyzed using a χ2 test or Fisher's exact
method. Kaplan-Meier analysis was used to compare the OS curves in
the different groups. A Cox proportional hazards model was used to
examine the significance of the effects of different variables on
OS in multivariate analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of HOTAIR are
significantly upregulated in DLBCL
To examine the role of HOTAIR in DLBCL, the present
study first examined the expression levels of HOTAIR in tissues
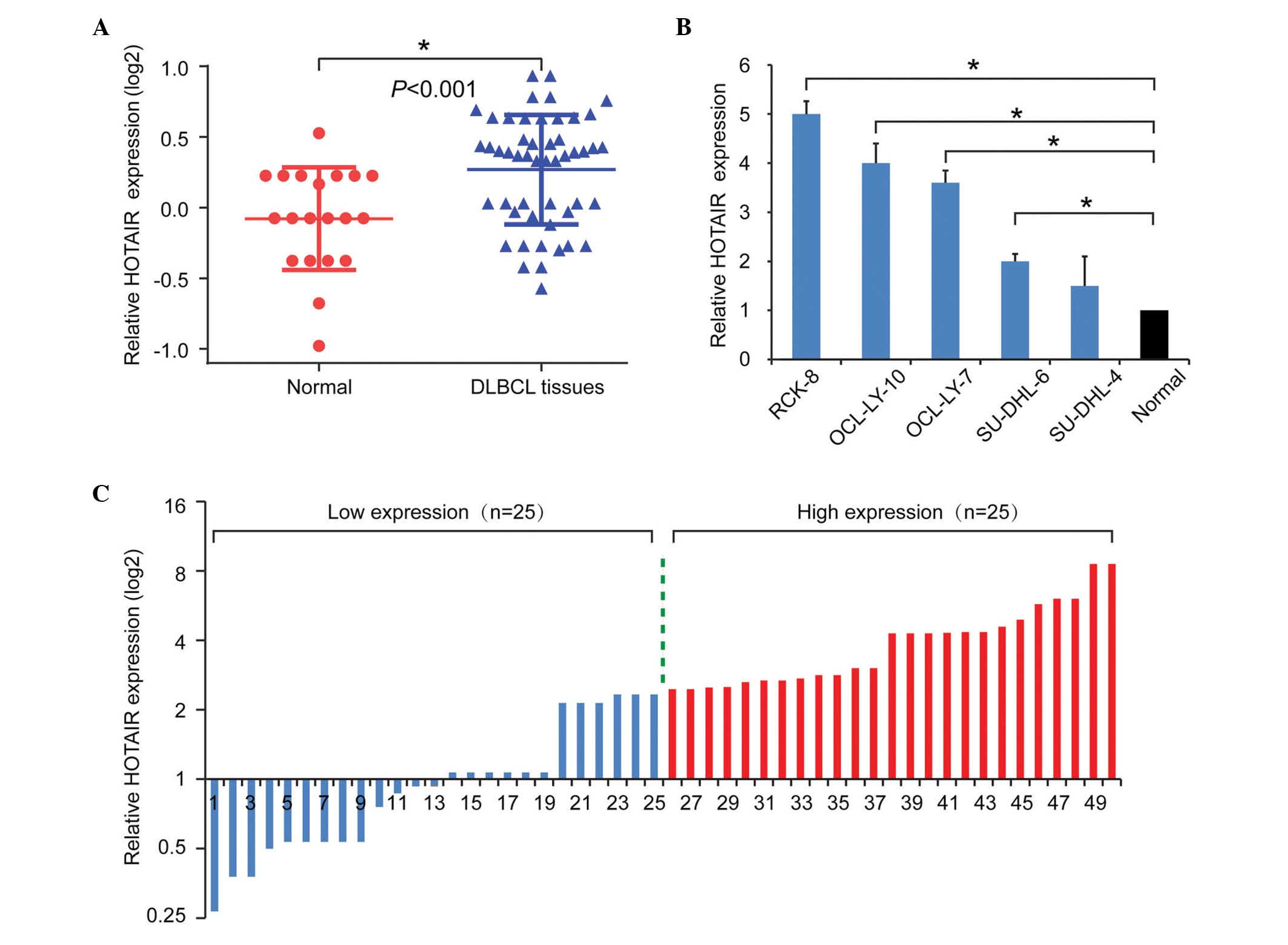
using RT-qPCR. As shown in Fig.
1A, HOTAIR was upregulated in the DLBCL tissues, compared with
the normal control tissues. To further validate the role of HOTAIR
in DLBCL, the expression levels of HOTAIR were also examined in
DLBCL cell lines. Consistently, the expression levels of HOTAIR in
the DLBCL cell lines were significantly upregulated, compared with
the average levels in the normal samples (Fig. 1B). Taken together, these results
suggested tat the upregulation of HOTAIR was a frequent event in
DLBCL.
Overexpression of HOTAIR is correlated
with the clinicopathological features of DLBCL
To further investigate the clinical role of HOTAIR
in DLBCL, the present study analyzed the correlation between the
expression of HOTAIR and patient clinicopathological features. The
median value was used as a cut-off to divide the expression levels
of HOTAIR into a high expression group (n=25) and a low expression
group (n=25; Fig. 1C). As shown in
Table I, elevated expression
levels of HOTAIR were positively associated with advanced clinical
stage (P=0.004), tumor volume (P=0.045), B symptoms (P=0.011) and
International Prognostic Index (IPI) scores (P=0.009).
 | Table ICorrelation between the expression of
HOTAIR and clinicopathological characteristics in diffuse large B
cell lymphoma. |
Table I
Correlation between the expression of
HOTAIR and clinicopathological characteristics in diffuse large B
cell lymphoma.
| Characteristic | n | Expression level of
HOTAIR
| P-value |
|---|
| Low (n) | High (n) |
|---|
| Age (years) | | | | 0.777 |
| <60 | 23 | 11 | 12 | |
| ≥60 | 27 | 14 | 13 | |
| Gender | | | | 0.258 |
| Male | 24 | 10 | 14 | |
| Female | 26 | 15 | 11 | |
| Ann Arbor stage | | | | 0.004a |
| I–II | 20 | 15 | 5 | |
| III–IV | 30 | 10 | 20 | |
| Extra-nodal
status | | | | 0.529 |
| <2 | 14 | 8 | 6 | |
| ≥2 | 36 | 17 | 19 | |
| B symptoms | | | | 0.011a |
| Absent | 27 | 18 | 9 | |
| Present | 23 | 7 | 16 | |
| Bulk (cm) | | | | 0.045a |
| <5 | 21 | 14 | 7 | |
| ≥5 | 29 | 11 | 18 | |
| IPI score | | | | 0.009a |
| 0–2 | 19 | 14 | 5 | |
| 3–5 | 31 | 11 | 20 | |
Survival analysis and prognostic
significance of HOTAIR
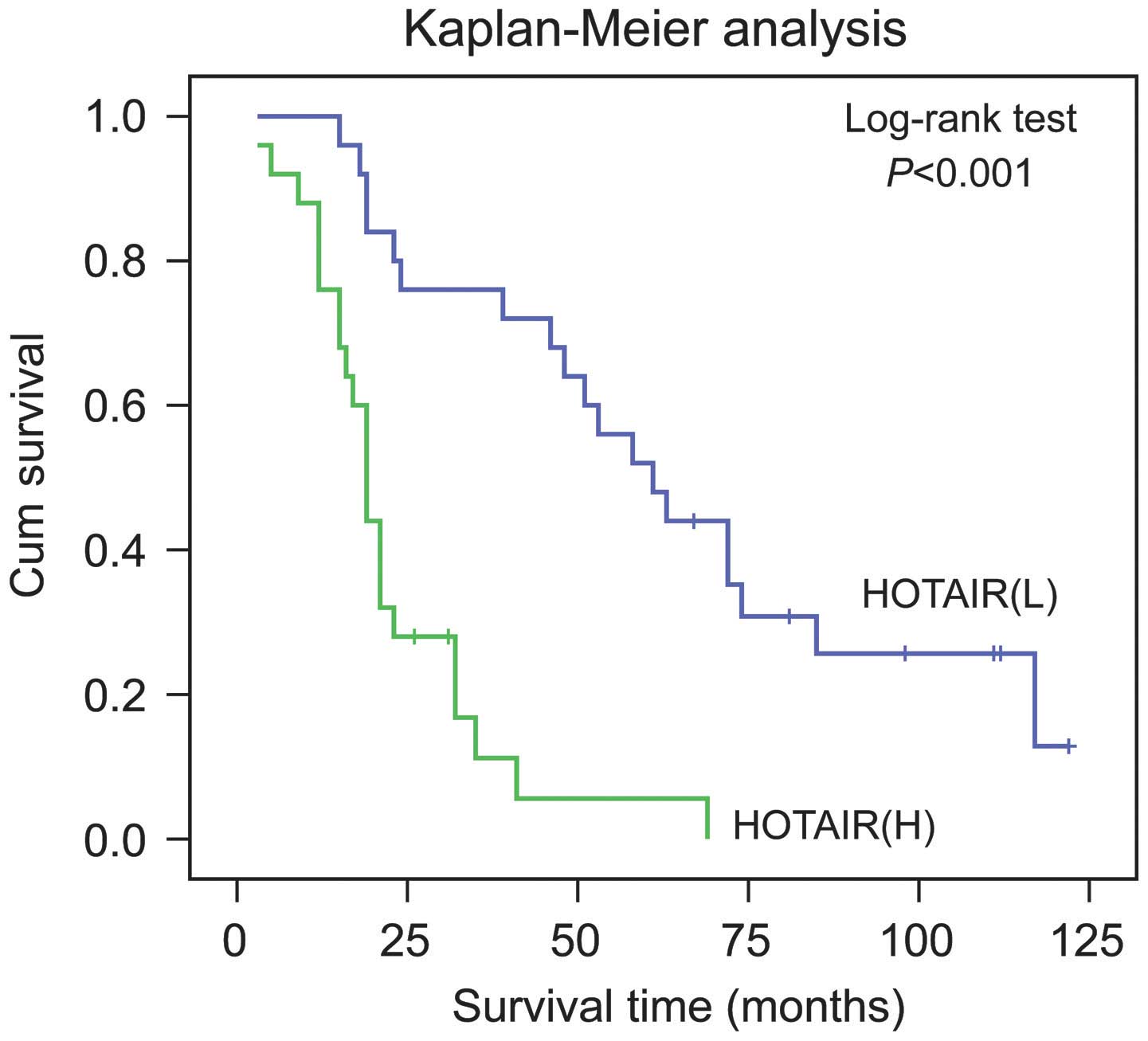
Kaplan-Meier analysis with a log-rank test for OS
was performed to determine the possible association between the
tumor expression levels of HOTAIR and patient survival rates. The
results revealed that patients with higher expression levels of
HOTAIR had a poorer prognosis, whereas the low expression group
possessed a longer OS (P<0.001; Fig. 2).
Using univariate analysis in the Cox proportional
hazards model, decreased OS was associated with the following
characteristics: Expression of HOTAIR, IPI score, clinical stage
and B symptoms (P<0.05; Table
II). Multivariate analysis revealed that the expression of
HOTAIR, in addition to IPI scores, was an independent predictor of
OS (HR, 3.127; 95% CI, 1.217–8.037; P=0.018). These results
suggested that HOTAIR may offer potential as a potent prognostic
factor in patients with DLBCL.
 | Table IIUnivariate and multivariate analyses
of prognostic variables of overall survival in patients with
diffuse large B cell lymphoma. |
Table II
Univariate and multivariate analyses
of prognostic variables of overall survival in patients with
diffuse large B cell lymphoma.
| Variable | Univariate analysis
| Multivariate analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Expression of
HOTAIR | | | | | | 0.018a |
| (high, vs. low) | 5.220 | 2.481–10.984 | <0.001a | 3.127 | 1.217–8.037 | |
| Age (≥60, vs. <60
years) | 0.721 | 0.392–1.327 | 0.293 | | | |
| Gender (female, vs.
male) | 0.778 | 0.424–1.427 | 0.778 | | | |
| Ann Arbor stage | | | | | | |
| (III–IV, vs.
I–II) | 2.780 | 1.437–5.377 | 0.002a | | | |
| Extra-nodal
status | | | | | | |
| (≥2, vs.
<2) | 1.002 | 0.517–1.943 | 0.994 | | | |
| B symptoms | | | | | | |
| (present, vs.
absent) | 2.339 | 1.228–4.454 | 0.010a | | | |
| Bulk (≥5, vs.
<5cm) | 1.491 | 0.793–2.803 | 0.214 | | | |
| IPI score (3–5, vs.
0–2) | 2.948 | 1.512–5.750 | 0.002a | 2.806 | 1.392–5.654 | 0.004a |
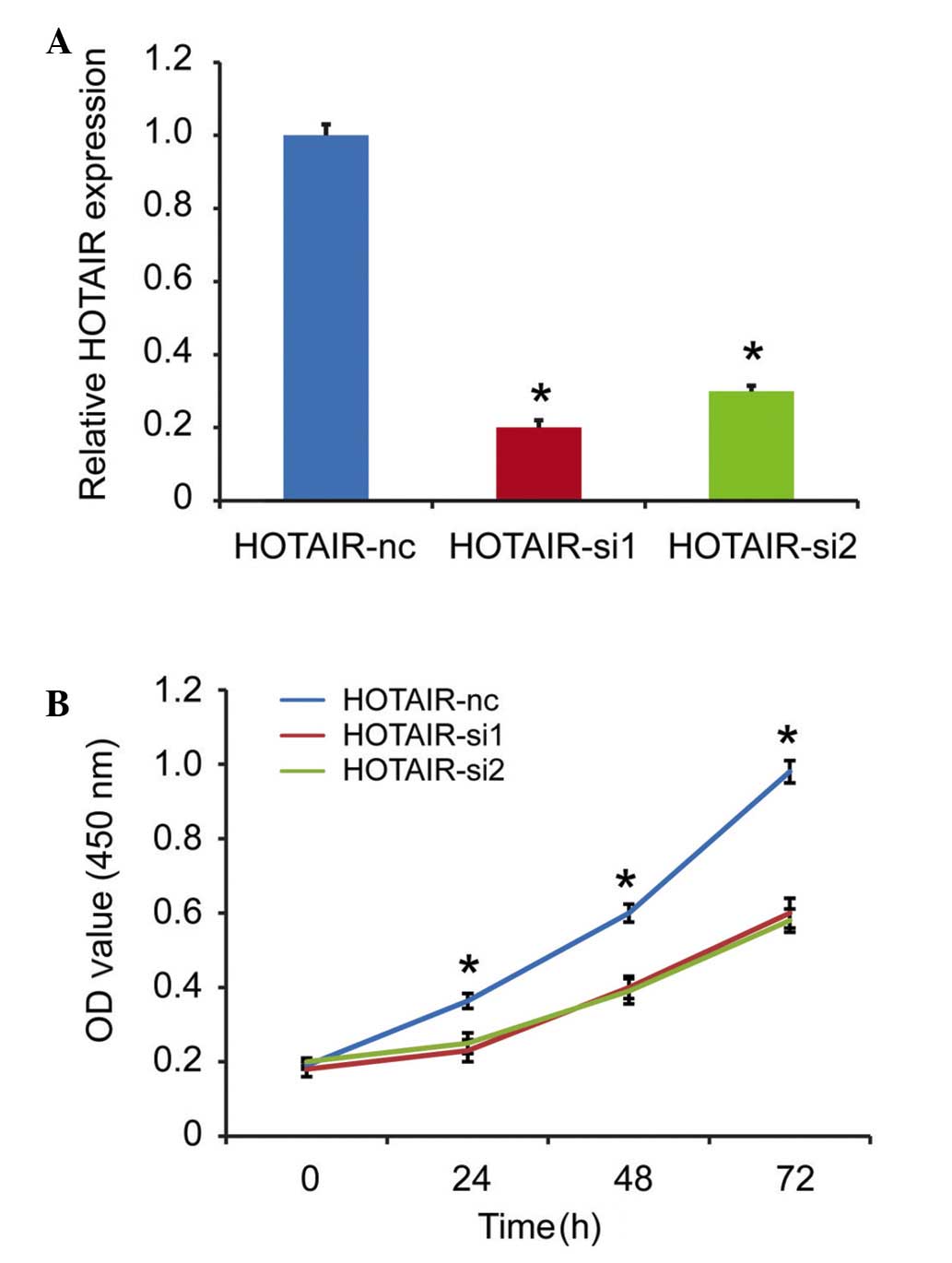
HOTAIR knockdown inhibits cell
proliferation in vitro
As HOTAIR is a prognostic factor for patients with
DLBCL, the present study subsequently investigated the detailed
function of HOTAIR in the DLBCL cells. The RCK-8 cells, which
exhibited the highest expression level of HOTAIR among the cell
lines, was selected as the targeted cell. The expression of HOTAIR
was silenced in the RCK-8 cells, and the silencing efficiency was
confirmed using qPCR. As shown in Fig.
3A, the si1 and si2 fragments effectively decreased the
expression of HOTAIR. A CCK-8 assay was used to detect
proliferation in vitro, and the results showed that HOTAIR
knockdown inhibited cell proliferation, compared with the scramble
group (Fig. 3B).
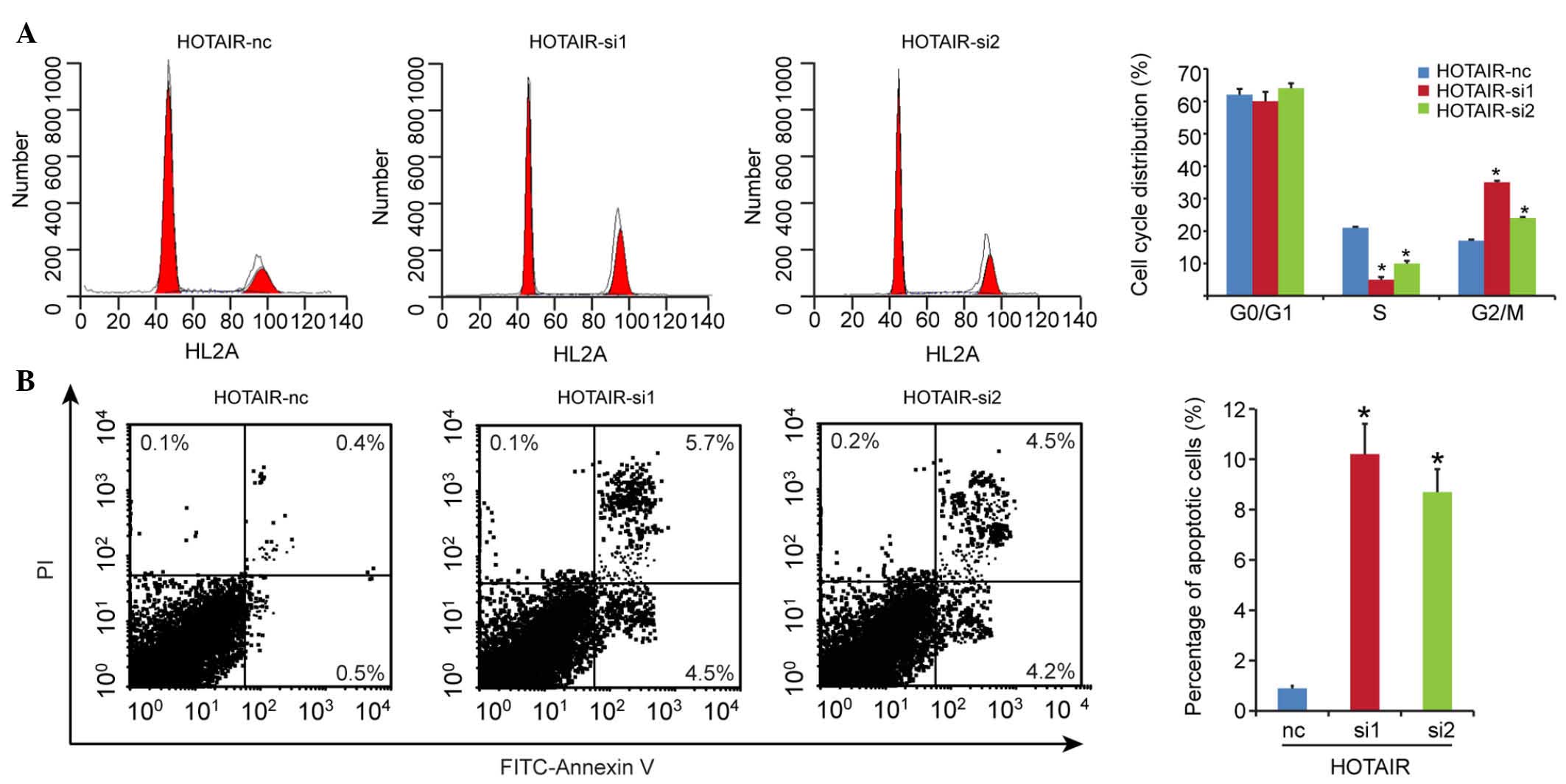
HOTAIR knockdown induces G2/M cell cycle
arrest and promotes cell apoptosis
As the induction of cell cycle arrest is an
important anti-proliferative mechanism, the present study
investigated whether the inhibitory activity of HOTAIR knockdown on
growth involved the control of cell cycle progression. The results
of the cell cycle analysis showed that the G2/M-phase fraction
increased between 17.0±1.5% in the HOTAIR-nc group, and 35.0±0.8%
and 24.0±0.3% in the HOTAIR-si1 and HOTAIR-si2 groups, respectively
(Fig. 4A), which suggested the
induction of G2/M cell cycle arrest following HOTAIR knockdown.
To determine whether apoptosis contributed to the
cell growth inhibition by HOTAIR knockdown, the present study
evaluated the effect of the expression of HOTAIR on cell apoptosis.
Compared with the HOTAIR-nc group (0.9±0.1%), cell apoptosis was
significantly enhanced in the HOTAIR knockdown groups (HOTAIR-si1,
10.2±1.2%; HOTAIR-si2, 8.7±0.9%), as shown in Fig. 4B. These results indicated that cell
cycle arrest and increased apoptosis contributed to the inhibition
of proliferation in DLBCL on silencing HOTAIR.
PI3K/AKT/NF-κB signaling pathway
contributes to cell proliferation mediated by HOTAIR
The serine/threonine kinase, AKT, a downstream
effector of PI3K, is involved in cell survival and anti-apoptotic
signaling. A previous study demonstrated that cell signaling is
mediated by PI3K/AKT via the induction of the NF-κB transcription
factor, which is associated with cell proliferation (12). In the present study, no changes
were found in the protein levels of total PI3K, AKT or NF-κB in
response to HOTAIR knockdown (Fig.
5). However, the levels of PI3K, AKT and NF-κB phosphorylation
were markedly decreased. These results indicated that the
suppression of HOTAIR inhibited the activation of p-Akt and
p-NF-κB, leading to decreased cell proliferation.
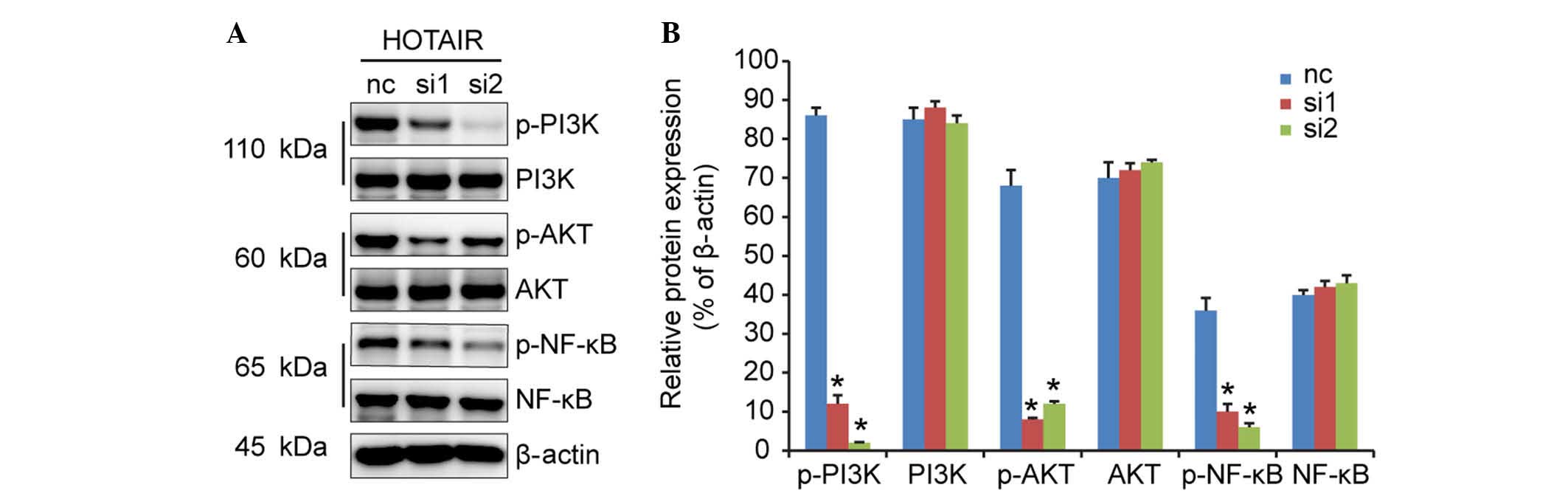 | Figure 5HOTAIR silencing inhibits DLBCL cell
proliferation, possibly through the PI3K/AKT/NF-κB signaling
pathways. (A) Western blot analysis was performed to detect the
expression levels of PI3K, AKT, NF-κB, p-PI3K, p-AKT and p-NF-κB,
and (B) relative protein expression levels were calculated.
*P<0.05 vs. the nc group. HOTAIR, Hox transcript
antisense intergenic RNA; DLBCL, diffuse large B cell lymphoma; nc,
negative control; PI3K, phos-phoinositide 3-kinase; NF-κB, nuclear
factor-κB; p-, phosphorylated; nc, negative control. |
Discussion
DLBCL is the most common subtype of lymphoid
neoplasm, accounting for 30–40% of cases (13), and threatens the health of
individuals worldwide. The pathogenesis and carcinogenesis of DLBCL
are multi-step and heterogeneous processes, and involve different
genetic and epigenetic changes. Despite increasing investigations
focused on elucidating the complex progress, the majority focus on
protein-coding genes rather than another crucial factor, lncRNA,
which have been previously regarded as 'noise' in the process of
the disease. HOTAIR, was first described by Rinn et al
(14) as 2,158 nucleotides and 6
exons in length, and reported to repress transcription in trans
across 40 kilobases of the HOXD locus. A substantial quantity of
data have demonstrated that HOTAIR is involved in several
processes in carcinogenesis and the promotion of malignancy
(15), including those affecting
mobility, invasion, proliferation, apoptosis, aggression and
metastasis (16–18). In response to these findings, the
present study investigated whether HOTAIR is involved in the
malignant processes of DLBCL.
In the present study, it was found that HOTAIR was
upregulated in DLBCL tumor samples, compared with normal tissues.
Consistently, the same trend was observed in the DLBCL cell lines.
The upregulation of HOTAIR was correlated with certain critical
clinicopathological features, including clinical stage, B symptoms,
IPI scores and tumor volumes. Additionally, it was demonstrated
that higher expression levels of HOTAIR in tumor tissues predicted
a poor prognosis in the patients with DLBCL. Univariate and
multivariate analyses confirmed that HOTAIR may be used as a
promising biomarker for patients with DLBCL.
The present study also investigated the biological
function of HOTAIR in DLBCL cell lines. RCK-8 was selected due to
its higher expression level of HOTAIR. HOTAIR knockdown
significantly inhibited cell proliferation. A previously study
showed that inducing cell cycle arrest and promoting cell apoptosis
may be the possible anti-proliferative mechanisms (19). Therefore, the present study
performed cell cycle and apoptosis analyses, and the results
revealed that HOTAIR knockdown arrested the cell cycle in the G2/M
period and induced cell apoptosis. These may have contributed to
the inhibition of proliferation. The present study also examined
the possible pathways involved in this process, and it was found
that the silencing of HOTAIR inhibited the phosphorylation of PI3K,
AKT and NF-κB. Taken together, HOTAIR inhibited cell proliferation,
partly through the PI3K/AKT/NF-κB pathways, which were downstream
of the lncRNA-specific gene.
In conclusion, the present study provided novel
evidence that the overexpression of HOTAIR was a common event
underlying DLBCL, representing a key pro-oncogenic role. It also
functioned as an indicator of poor survival rates in the patients
with DLBCL. In addition, the present study demonstrated that HOTAIR
was imperative for DLBCL carcinogenesis by affecting cell
proliferation, cell cycle and apoptosis. Taken together, the data
obtained in the present study demonstrated the critical role of
HOTAIR in the molecular etiology of DLBCL, and may provide a
therapeutic regimen, dependent on lncRNA, directed against this
life-threatening disease.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shankland KR, Armitage JO and Hancock BW:
Non-Hodgkin lymphoma. Lancet. 380:848–857. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shipp MA, Ross KN, Tamayo P, Weng AP,
Kutok JL, Aguiar RC, Gaasenbeek M, Angelo M, Reich M, Pinkus GS, et
al: Diffuse large B-cell lymphoma outcome prediction by
gene-expression profiling and supervised machine learning. Nat Med.
8:68–74. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kogo R, Shimamura T, Mimori K, Kawahara K,
Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al:
Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu ZH, Wang XL, Tang HM, Jiang T, Chen J,
Lu S, Qiu GQ, Peng ZH and Yan DW: Long non-coding RNA HOTAIR is a
powerful predictor of metastasis and poor prognosis and is
associated with epithelial-mesenchymal transition in colon cancer.
Oncol Rep. 32:395–402. 2014.PubMed/NCBI
|
|
6
|
Zhao W, An Y, Liang Y and Xie XW: Role of
HOTAIR long noncoding RNA in metastatic progression of lung cancer.
Eur Rev Med Pharmacol Sci. 18:1930–1936. 2014.PubMed/NCBI
|
|
7
|
Nakagawa T, Endo H, Yokoyama M, Abe J,
Tamai K, Tanaka N, Sato I, Takahashi S, Kondo T and Satoh K: Large
noncoding RNA HOTAIR enhances aggressive biological behavior and is
associated with short disease-free survival in human non-small cell
lung cancer. Biochem Biophys Res Commun. 436:319–324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu XH, Liu ZL, Sun M, Liu J, Wang ZX and
De W: The long non-coding RNA HOTAIR indicates a poor prognosis and
promotes metastasis in non-small cell lung cancer. BMC Cancer.
13:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013. View Article : Google Scholar
|
|
10
|
Peng W, Wu K and Feng J: Long noncoding
RNA HULC predicts poor clinical outcome and represents
pro-oncogenic activity in diffuse large B-cell lymphoma. Biomed
Pharmacother. 79:188–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou W, Wang G, Zhao X, Xiong F, Zhou S,
Peng J, Cheng Y, Xu S and Xu X: A multiplex qPCR gene dosage assay
for rapid genotyping and large-scale population screening for
deletional α-thalassemia. J Mol Diagn. 15:642–651. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
A clinical evaluation of the international
lymphoma study group classification of non-Hodgkin's lymphoma. The
Non-Hodgkin's lymphoma classification project. Blood. 89:3909–3918.
1997.PubMed/NCBI
|
|
14
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chiyomaru T, Fukuhara S, Saini S, Majid S,
Deng G, Shahryari V, Chang I, Tanaka Y, Enokida H, Nakagawa M, et
al: Long non-coding RNA HOTAIR is targeted and regulated by miR-141
in human cancer cells. J Biol Chem. 289:12550–12565. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hajjari M and Salavaty A: HOTAIR: An
oncogenic long non-coding RNA in different cancers. Cancer Biol
Med. 12:1–9. 2015.PubMed/NCBI
|
|
17
|
Zhou X, Chen J and Tang W: The molecular
mechanism of HOTAIR in tumorigenesis, metastasis, and drug
resistance. Acta Biochim Biophys Sin (Shanghai). 46:1011–1015.
2014. View Article : Google Scholar
|
|
18
|
Wu Y, Zhang L, Wang Y, Li H, Ren X, Wei F,
Yu W, Wang X, Zhang L, Yu J and Hao X: Long noncoding RNA HOTAIR
involvement in cancer. Tumour Biol. 35:9531–9538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiao F, Hu H, Yuan C and Wang L, Jiang W,
Jin Z, Guo Z and Wang L: Elevated expression level of long
noncoding RNA MALAT-1 facilitates cell growth, migration and
invasion in pancreatic cancer. Oncol Rep. 32:2485–2492.
2014.PubMed/NCBI
|