Introduction
Coronary heart disease (CHD) is becoming
increasingly prevalent in China. For patients presenting with an
acute ST-segment elevation myocardial infarction (STEMI), timely
myocardial reperfusion treatment has become a primary strategy.
Despite the use of this technique, the morbidity and mortality of
patients with STEMI remains problematical. The process of
myocardial ischemia-reperfusion (I/R) may, in itself, induce
cardiomyocyte death by activating deleterious signaling cascades.
There remains no effective therapy for preventing myocardial
reperfusion injury. Previously published stem cell therapeutic
studies suggested that stem cells may provide a novel approach to
protect against I/R injury (1–3). At
present, bone marrow mesenchymal stem cells (BM-MSCs) are the most
common stem cell for use in transplantation in heart disease.
However, the growth potential of BM-MSCs decreases with donor age
(4,5), potentially limiting their use in
clinical practice. However, age is not a concern with amniotic
fluid-derived mesenchymal stem cells (AFMSCs), which are isolated
in amniotic fluid. AFMSCs represent a promising alternative in
cell-based therapy, given their superior qualities, including ease
of acquisition, pluripotency, self-renewal capability,
comparatively few ethical concerns, and an absence of teratoma
formation (6,7).
However, the survival rate of transplanted cells may
be markedly weakened due to an impaired myocardial
micro-environment, for example, in the cases of reperfusion injury
and inflammation, or in the presence of pro-apoptotic factors
(8–10). A low survival rate is a critical
problem that needs to be addressed in order to improve the
efficiency of stem cell therapy. The activation of Akt, a
serine-threonine kinase, maintains stem cells by promoting
viability and proliferation (11,12).
Furthermore, Akt has been identified as a key molecule for the cell
cycling of cardiac myocytes during differentiation, and for the
survival/proliferation of progenitor cells (13). Recently, MSCs transduced with Akt
have been shown to protect against myocardial injury by enhancing
the efficacy of stem cells, inhibiting apoptosis, reducing infarct
size and promoting angiogenesis (14–16).
In the present study, it was hypothesized that the
genetic modification of AFMSCs with the pro-survival gene, Akt,
would improve the survival of transplanted AFMSCs and enhance their
therapeutic efficacy in a rabbit I/R model.
Materials and methods
Cell culture
AFMSCs were isolated from rabbit amniotic fluid, as
previously described (17). AFMSCs
were cultured on a 100 mm diameter-plate (37°C, 5% CO2)
in a growth medium consisting of low glucose Dulbecco's modified
Eagle's medium (L-DMEM; Beyotime Institute of Biotechnology,
Shanghai, China) supplemented with 20% fetal bovine serum (FBS;
Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin and
streptomycin (Beyotime Institute of Biotechnology), and detached
from the substrate by incubation in 0.25% trypsin/EDTA (Beyotime
Institute of Biotechnology). The cells were subsequently reseeded
at a split ratio of 1:3 when growing to 90% confluence in
culture.
Lentiviral transduction of AFMSCs
Rabbit Akt gene was generated by polymerase chain
reaction (PCR) using 1 µl high-fidelity DNA polymerase (New
England Biolabs, Ipswich, MA, USA) and primers containing
EcoRI and Xmal sites (forward:
5′-CCGGAATTCGCCACCATGAGCGATGTTACCATTGTGAAAGA-3′; reverse:
5′-TCCCCCCGGGTTATTCCCGTCCACTTGCAGA-3′). The gene fragment and
plasmid vector were connected by In-Fusion® technology
(Clontech Laboratories, Mountain View, CA, USA) following enzyme
digestion, and transformed into competent DH5α cells. Positive
clones containing the lentiviral expression vector were obtained
following screening and sequencing. The lentiviral vector was used
to transfect 293T cells and package the virus, which led to the
determination of the virus titers. AFMSCs at cell passage 3 were
transduced with lentivirus vectors in vitro via different
values of multiplicity of infection (MOI). The transduction
efficiency was obtained according to the optimal MOI, and the
expression of the Akt gene was determined using a western blot
assay.
Western blot analyses
Protein extracts were obtained from cell lysates of
AFMSCs and homogenized myocardium tissue samples by treatment with
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Protein concentrations were
determined using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Proteins were separated using sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (10% separating
gel and 5% stacking gel; 100 V, blots run for 100 min) and
transferred on to polyvinylidene difluoride (PVDF) membranes for 90
min at 250 mA in Towbin transfer buffer. The PVDF membranes were
blocked for 2 h at room temperature with TBST blocking buffer
containing 5% dry milk and reacted overnight at 4°C with the
following primary antibodies: Mouse monoclonal anti-Akt antibody
(cat. no. 2920; 1:1,000, Cell Signal Technology), mouse monoclonal
anti-phosphorylated (P)-Akt antibody (cat. no. 12694; 1:1,000, Cell
Signal Technology), mouse monoclonal anti-B-cell lymphoma 2 (Bcl-2)
antibody (cat. no. 692; 1:1,000, Abcam, Cambridge, UK), mouse
monoclonal anti-connexin 43 antibody (cat. no. 11369; 1:1,500,
Abcam), mouse monoclonal anti-caspase-3 antibody (cat. no. 9668;
1:1,000, Cell Signaling Technology) and mouse monoclonal
anti-vascular endothelial growth factor (VEGF) antibody (cat. no.
ab1316; 1:1,000, Abcam). After being washed three times, the
membranes were treated with goat anti-mouse IgG (cat. no. A0216,
Beyotime Institute of Biotechnology; the dilution used was 1:5,000
for the AFMSCs and 1:2,000 for the homogenized myocardium tissue
samples). β-actin and glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) served as an internal control for the AFMSCs and myocardium
tissue sample, respectively. The enhanced chemiluminescence
technique was used for specific protein identification, with
Millipore Immobilon Western Chemiluminescent Horseradish Peroxidase
substrate (Millipore Corp., Billerica, MA, USA).
5-Bromo-2-deoxyuridine (Brdu)
labeling
Once AFMSCs or Akt-AFMSCs had grown to 50%
confluence in culture on a 100 mm diameter-plate (37°C, 5%
CO2), the culture medium was removed and the cells were
incubated with 10 µmol/l BrdU (Sigma-Aldrich, St. Louis, MO,
USA). At 72 h after the culture had been initiated, AFMSCs or
Akt-AFMSCs were detached with 0.25% trypsin/EDTA, centrifuged at
22°C at 1,000 rpm (250 g) for 5 min using a MiniSpin®
centrifuge (Eppendorf, Hamburg, Germnay), resuspended at a density
of 3×1010/l in 1 ml L-DMEM, and immediately prepared for
transplantation.
Myocardial I/R procedure and cell
transplantation
All animals were maintained and used as approved by
the Animal Experimental Ethics Committee of Xin Hua Hospital
Affiliated to Shanghai Jiaotong University School of Medicine. Male
New Zealand White rabbits (aged 4 months; 2.4–2.8 kg) were
anesthetized with an intravenous injection of sodium pentobarbital
(30 mg/kg; Merck & Co., Inc., Darmstadt, Germany). The chest
was opened via mid-sternotomy. A silk thread was passed through the
myocardium around a prominent branch of the left anterior
descending coronary artery (LAD). Ischemia was induced by pulling
the ends of the suture through a segment of a soft tube, which was
firmly attached against the artery with a clamp (18,19).
The successful induction of ischemia was verified by ST-segment
elevation on the electrocardiogram. At 30 min following the
ligation, the rabbits were used as a model of myocardial I/R. Prior
to the initiation of myocardial reperfusion by releasing the clamp,
a suspension of AFMSCs or Akt-AFMSCs (3.0×1010/l in 1 ml
L-DMEM; Sigma-Aldrich) was injected into the left ventricular
anterior wall at five points.
All the rabbits were randomly divided into four
groups (n=8 per group): The sham group (sham), which received the
identical surgical procedures as the other groups, excluding the
LAD-ligation; the control group (control), which received I/R
without cell transplantation; the AFMSC group, which received I/R
with transplantation of AFMSCs; and the Akt-AFMSC group, which
received I/R with Akt-AFMSC transplantation. The control group
without cell therapy received an identical volume of the medium.
Functional and histological evaluations were performed 3 weeks
following I/R. Rabbits were anesthetized with 3% sodium
pentobarbital, and echocardiography was performed. Subsequently,
hearts were quickly excised, cut transversely and processed for
morphological, biochemical and molecular studies.
Detection of donor cells in vivo
For the detection of AFMSCs, specimens were fixed
with 4% paraformaldehyde for 24 h, steeped in 30% sucrose solution
overnight for dehydration, embedded in optimum cutting temperature
compound (Sakura Finetek Japan Co., Ltd., Tokyo, Japan). frozen and
cut into 5 µm sections. Briefly, sections were incubated
with mouse monoclonal anti-Brdu antibody (cat. no. B2531; 1:100,
Sigma-Aldrich) to detect donor cells. Following a washing step, the
sections were incubated with fluorescein isothiocyanate-conjugated
goat anti-mouse antibody (cat. no. 115095003; 1:100, (Jackson
ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Nuclei
were counterstained with 4,6-diamidino-2-phenylindole (DAPI;
Beyotime Institute of Biotechnology) after having been washed with
PBS. Subsequently, the coverslips were coated with a neutral resin
(Ruiqi Biotechnology Co., Ltd., Shanghai, China) for fluorescence
microscopic analysis.
Immunohistochemistry
For immunohistochemical staining, heart tissues were
fixed in 4% paraformaldehyde, embedded in paraffin and cut into 5
µm sections. The sections were subsequently deparaffinized,
rehydrated, and the antigens were retrieved in citrate buffer (pH
6.0) by boiling in a microwave. The sections were then
peroxidase-blocked in 3% hydrogen peroxide
(H2O2) in methanol for 10 min, blocked with
5% bovine serum albumin in PBS for 30 min, and subsequently
incubated with primary antibodies, including mouse monoclonal
anti-cardiac troponin T (cTNT) antibody (cat. no. ab10214; 1:100,
Abcam) and mouse monoclonal anti-von Willebrand factor (vWF)
antibody (cat. no. ab778; 1:100, Abcam) at 4°C overnight. Following
washing in PBS, the sections were incubated with goat anti-mouse
secondary antibodies for 1 h at room temperature. Nuclei were
counterstained with hematoxylin (Sigma-Aldrich). Capillary
endothelial cells were identified on the basis of immunoreactivity
with vWF, and quantified as the number of cells/mm2.
Five fields on the slide were randomly chosen for the counting of
stained capillaries at a ×200 magnification in a blinded manner.
The sections were also stained with hematoxylin-eosin.
Ultrastructural analysis by transmission
electron microscopy
The fixed tissues were washed with phosphate buffer
[0.1 M, (pH 7.4)] and subsequently post-fixed in identical buffer
[0.1 M phosphate buffer (pH 7.4)] containing 1% osmium tetraoxide
(Shunbai Biotechnology Co., Ltd., Shanghai, China) at 4°C for 2 h.
The specimens were subsequently embedded in epoxy-resin
(Araldite® CY212; Agar Scientific Ltd., Stansted, UK) to
produce tissue blocks and ultrathin sections (70–80 nm). The
sections obtained were stained with uranyl acetate and lead acetate
(Shunbai Biotechnology Co., Ltd.), and subsequently examined under
a Philips Technai™ 10 transmission election microscope (Phillips,
Amsterdam, The Netherlands).
Echocardiography
Rabbit heart function was assessed by transthoracic
echocardiography at day 21 following the induction of I/R. All
echocardiographic analyses were performed in a blinded manner by
the same echocardiographer. The left ventricular internal
dimension-diastole (LVIDd) and left ventricular internal
dimension-systole (LVIDs) were assessed in short-axis
two-dimensional views at the level of the mid-papillary muscle with
a GE Vivid 7 system (GE Healthcare Life Sciences, Shanghai, China)
and a standard phased-array. LVIDd and LVIDs were measured from at
least three consecutive cardiac cycles. The ejection fraction was
calculated as (LVIDd − LVIDs) / LVIDd × 100.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) staining
TUNEL assays were performed using an in situ
apoptotic cell death detection kit (Roche/Applied Biosystems,
Foster City, CA, USA) following the manufacturer's instructions.
Sections from each experimental group were examined using a BX53
Olympus microscope (Olympus, Hamburg, Germany). Individual nuclei
were visualized at a magnification of ×200 for quantitative
analyses. The percentages of apoptotic cells were calculated as the
ratio of the number of TUNEL-positive cells to the total number of
cells.
Quantitative reverse transcription PCR
(RT-qPCR)
The total RNA was extracted, and cDNA was
synthesized according to the manufacturer's instructions (Takara
Bio, Inc., Otsu, Japan). RT-qPCR was performed using a real-time
PCR system (Applied Biosystems® ABI 7500; Thermo Fisher
Scientific, Waltham, MA, USA) with the following primers: GATA-4
forward primer, 5′-cagtgagagccttcctcctac-3′ and reverse primer,
5′-catagccttgtggggacag-3′; glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) forward primer, 5′-atggtgaaggtcggagtgaa-3′ and reverse
primer, 5′-tgggtggaatcatactggaac-3′. GAPDH was used as an
endogenous control. Relative changes in expression were calculated
using the 2−ΔΔCq method.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Statistical analyses were performed using an unpaired
t-test. P<0.05 was considered to indicate a statistically
significant value.
Results
Genetic modification of AFMSCs with the
Akt gene
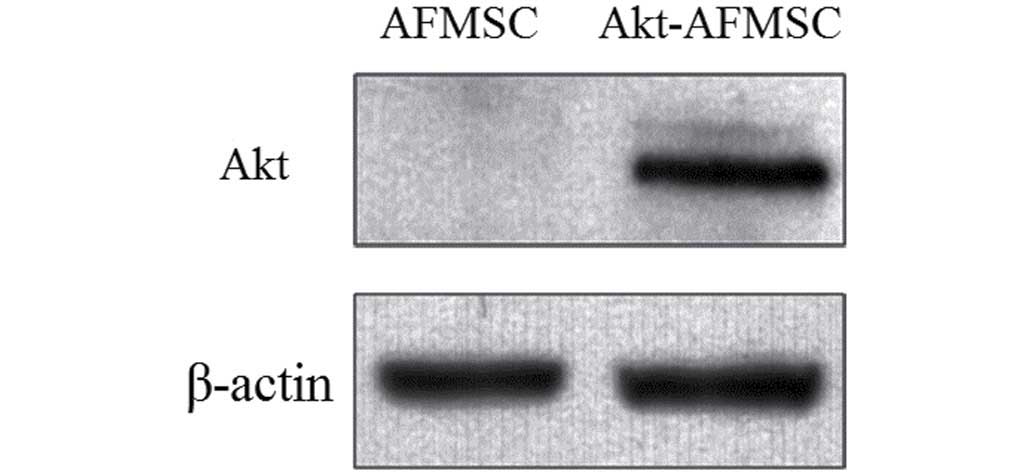
To determine whether stably transfected Akt-AFMSCs
exhibited an increased expression of Akt, western blot analysis was
performed. A marked increase in the level of Akt in the Akt-AFMSCs
compared with the AFMSCs was observed (Fig. 1).
Histological and ultrastructural effects
of Akt-AFMSCs on myocardium in I/R rabbits
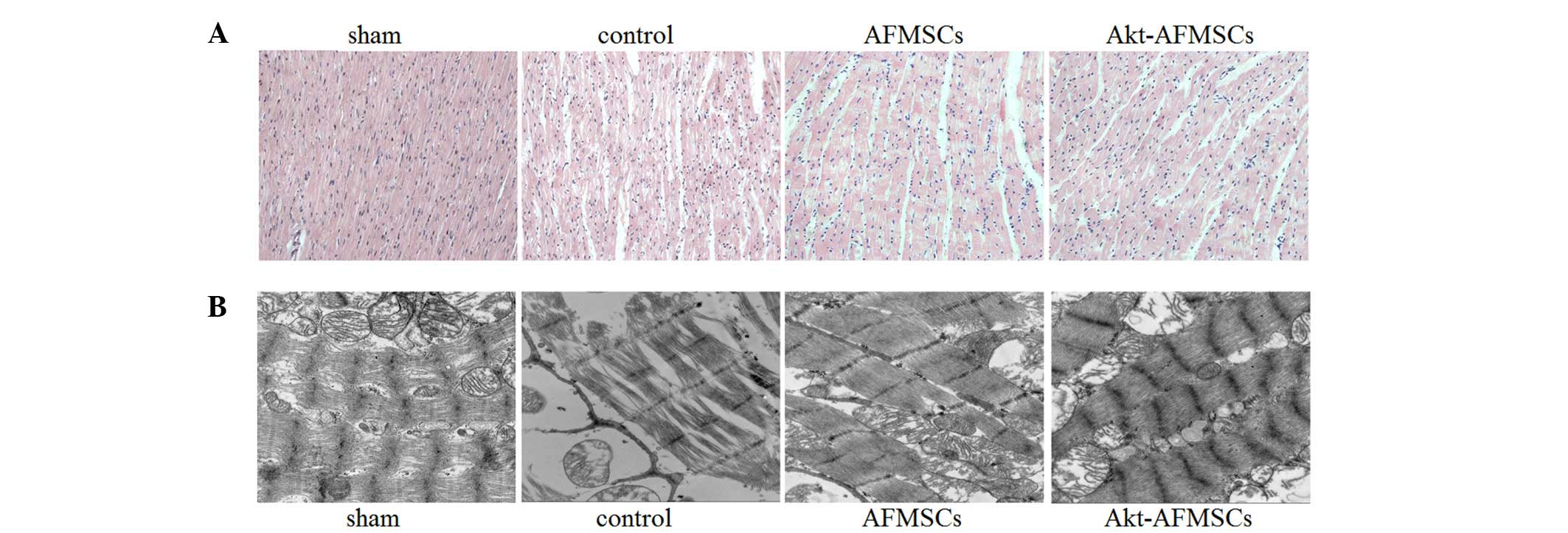
The sham group exhibited normal myocardium
structure, whereas the control group showed significant membrane
damage of cardiomyocytes, with inflammatory cell infiltration and
myonecrosis. The AFMSC and Akt-AFMSC groups revealed significant
structural improvements (Fig. 2A).
Compared with the sham group, observations made under the
transmission electron microscope demonstrated that the control
group were subjected to significant disorganization of chromatin
condensation, swollen and disrupted cristae in the mitochondria and
a loss of cytoplasmic vacuoles and myofibers (Fig. 2B). However, there was a modest
alleviation of ultrastructural damage in hearts transplanted with
AFMSCs or Akt-AFMSCs.
Echocardiography in Akt-AFMSCs
transplanted in an I/R model
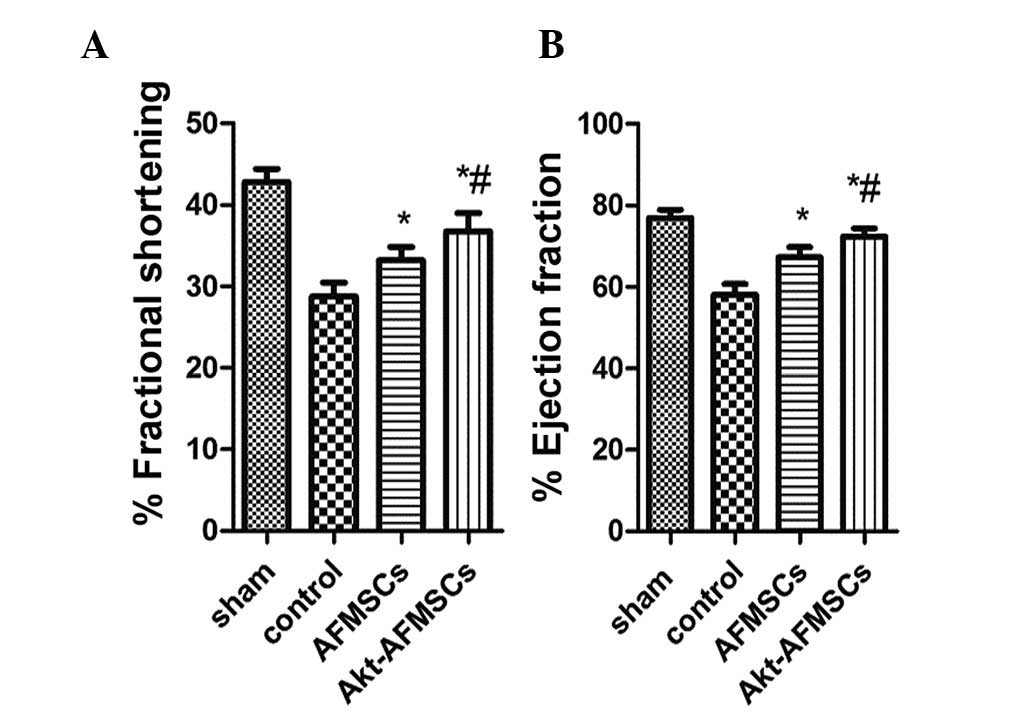
Echocardiography was performed to assess the effects
of transplanted Akt-AFMSCs on cardiac function 3 weeks following
I/R. As shown in Fig. 3A and B,
I/R injury significantly decreased fractional shortening and the
ejection fraction in the rabbit heart (P<0.05). Compared with
the control group, the AFMSC and Akt-AFMSC groups exhibited higher
fractional shortening (29.18±2.36 vs. 33.65±2.81 and 36.89±3.02%,
P<0.05, respectively) and ejection fractions (58.62±3.47 vs.
67.42±3.03 and 72.02±2.89, P<0.05, respectively), suggesting
that Akt-AFMSCs exhibit an improved level of cardiac function
against I/R injury.
Tracking of AFMSCs in vitro and in
vivo
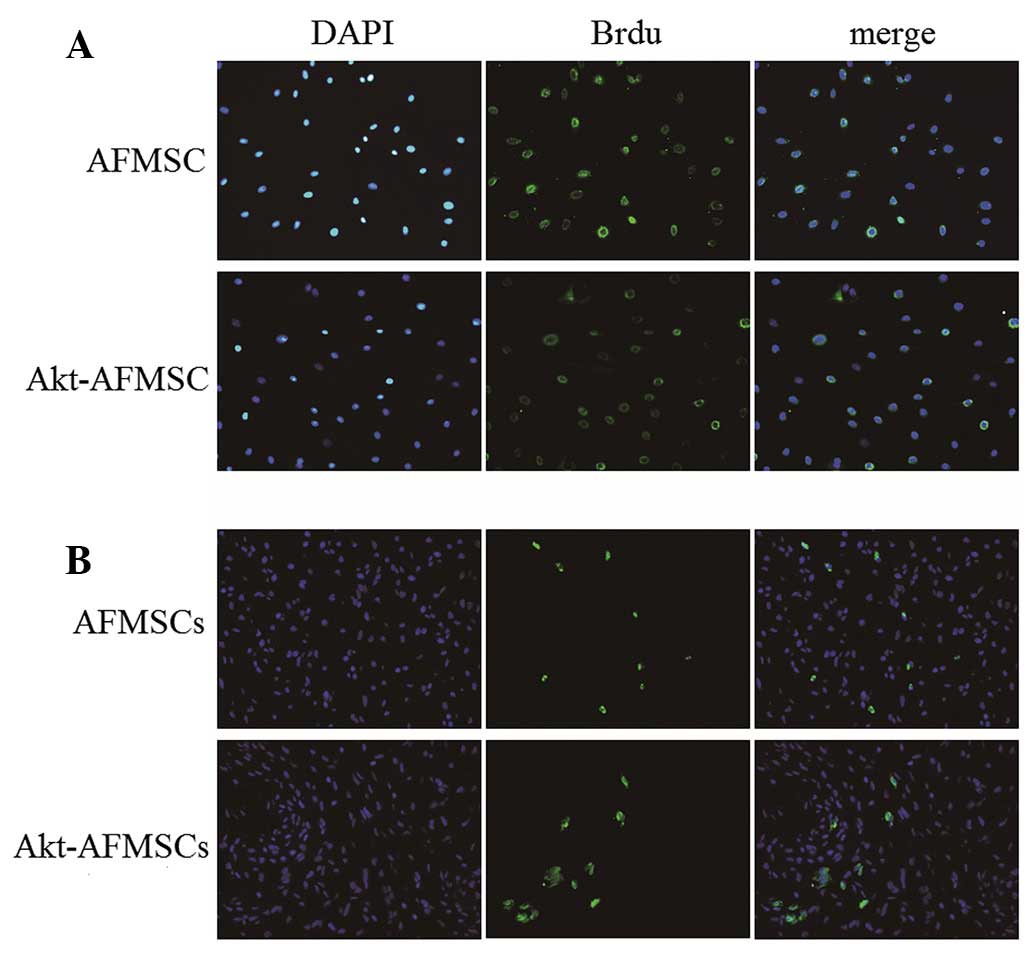
In our cell culture system, Brdu was used to label
AFMSCs for subsequent cell tracking. Following treatment with Brdu
at 10 µM for 72 h, the percentages of AFMSCs and Akt-AFMSCs
labeled with Brdu were 90±2.8 and 89±3.5%, respectively (Fig. 4A). Immunohistochemical staining of
Brdu in tissues was performed in order to track the cells in the
myocardium. As shown in Fig. 4B, a
greater number of cells remained in the Akt-AFMSC group compared
with the AFMSC group. (P<0.05). The average number of
Brdu-labeled Akt-AFMSCs and AFMSCs was 14.8±3.0/high-power field
and 9.3±2.6/high-power field, respectively.
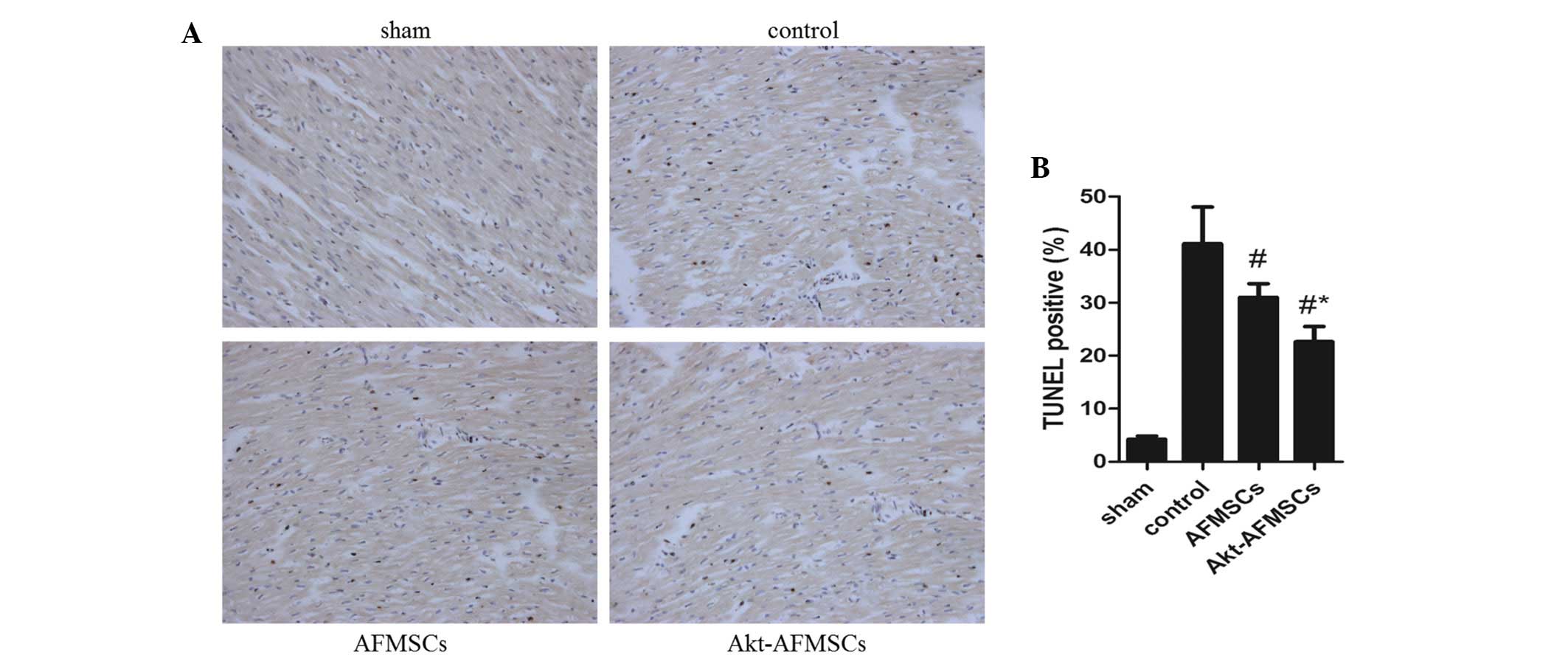
Cardiomyocyte apoptosis following
Akt-AFMSC transplantation
The number of TUNEL-positive cardiomyocytes was
significantly lower (P<0.05) in hearts transplanted with AFMSCs
and Akt-AFMSCs (Fig. 5A). Notably,
the number of apoptotic muclei was markedly reduced in the
Akt-AFMSCs group compared with the AFMSCs group (Fig. 5B), suggesting that Akt is a
potential pro-survival factor that inhibits I/R-induced
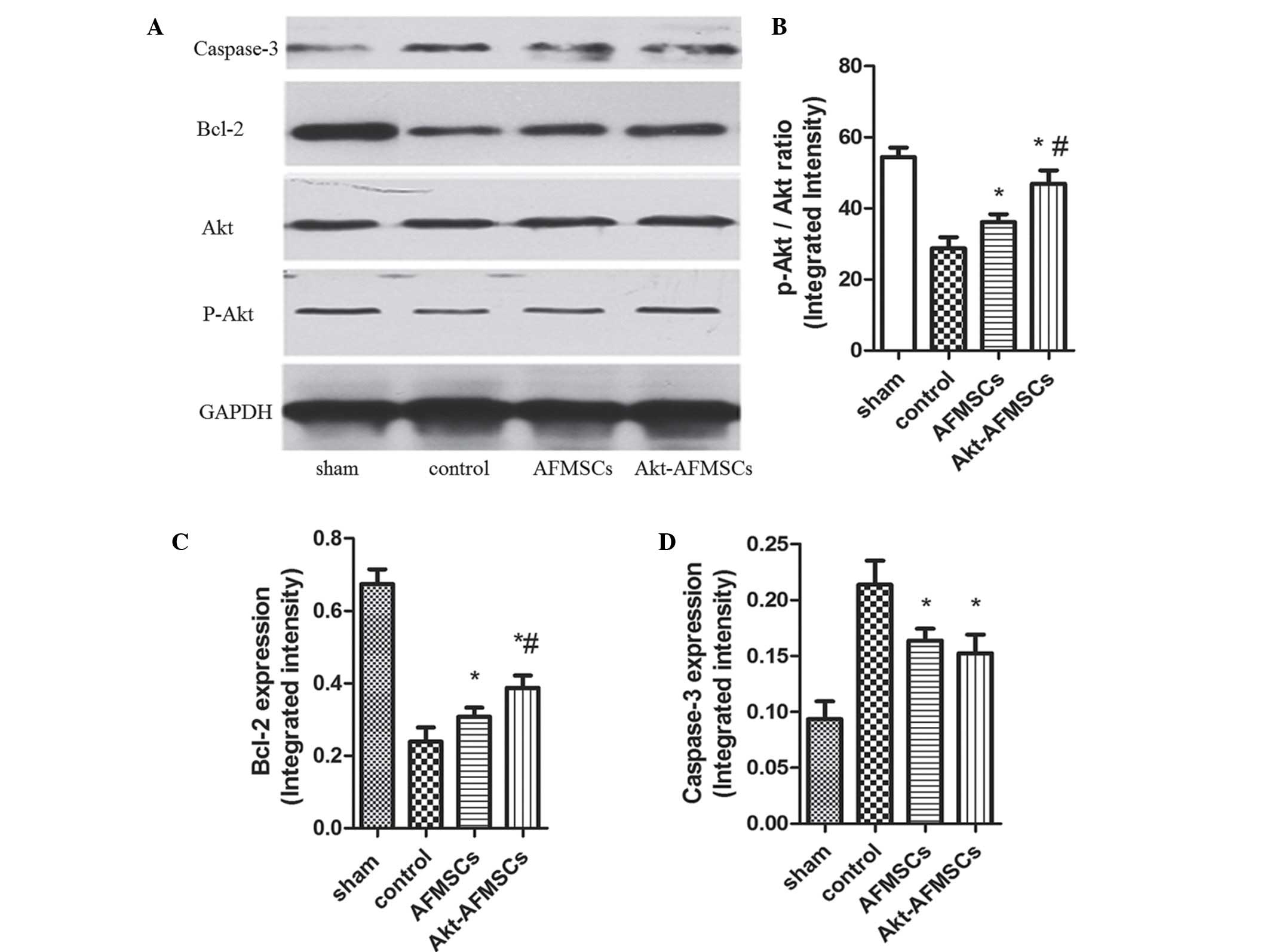
cardiomyocyte apoptosis. To examine whether P-Akt, which can
inhibit protein synthesis in apoptosis, is implicated in the
cardioprotection of Akt-overexpressing AFMSCs in transplantation,
western blot analysis was performed for Akt and P-Akt (Fig. 6A). These data suggest that hearts
transplanted with either Akt-AFMSCs or AFMSCs exhibited a marked
increase in P-Akt expression compared with the controls.
Furthermore, transplantation of the Akt-AFMSCs increased the
expression of P-Akt compared with the AFMSC group (Fig. 6B). However, no statistical
significances attributable to Akt were observed in all the
groups.
Multiple signaling pathways contribute to apoptosis,
including caspase-3 and Bcl-2. Western blot analysis was performed
for Bcl-2 and caspase-3 (Fig. 6A).
Bcl-2 exerts a major role in determining the death or survival of
cells after the application of apoptotic stimuli. Fig. 6C shows how the Bcl-2 level was
decreased in the control group, and this decrease was partly
reversed by the transplantation of Akt-AFMSCs or AFMSCs. Caspase-3
is an important component of the final pathway leading to the cell
apoptosis, and myocardial caspase-3 activity is considered as a
marker of I/R-induced cardiomyocyte apoptosis. As shown in Fig. 6D, myocardial I/R resulted in a
noticeable increase in caspase-3 compared with the sham group. A
significant decrease in caspase-3 was observed in the Akt-AFMSC and
AFMSC groups compared with the control group. However, no
statistically significant differences were observed in the
caspase-3 levels between the Akt-AFMSC and AFMSC groups.
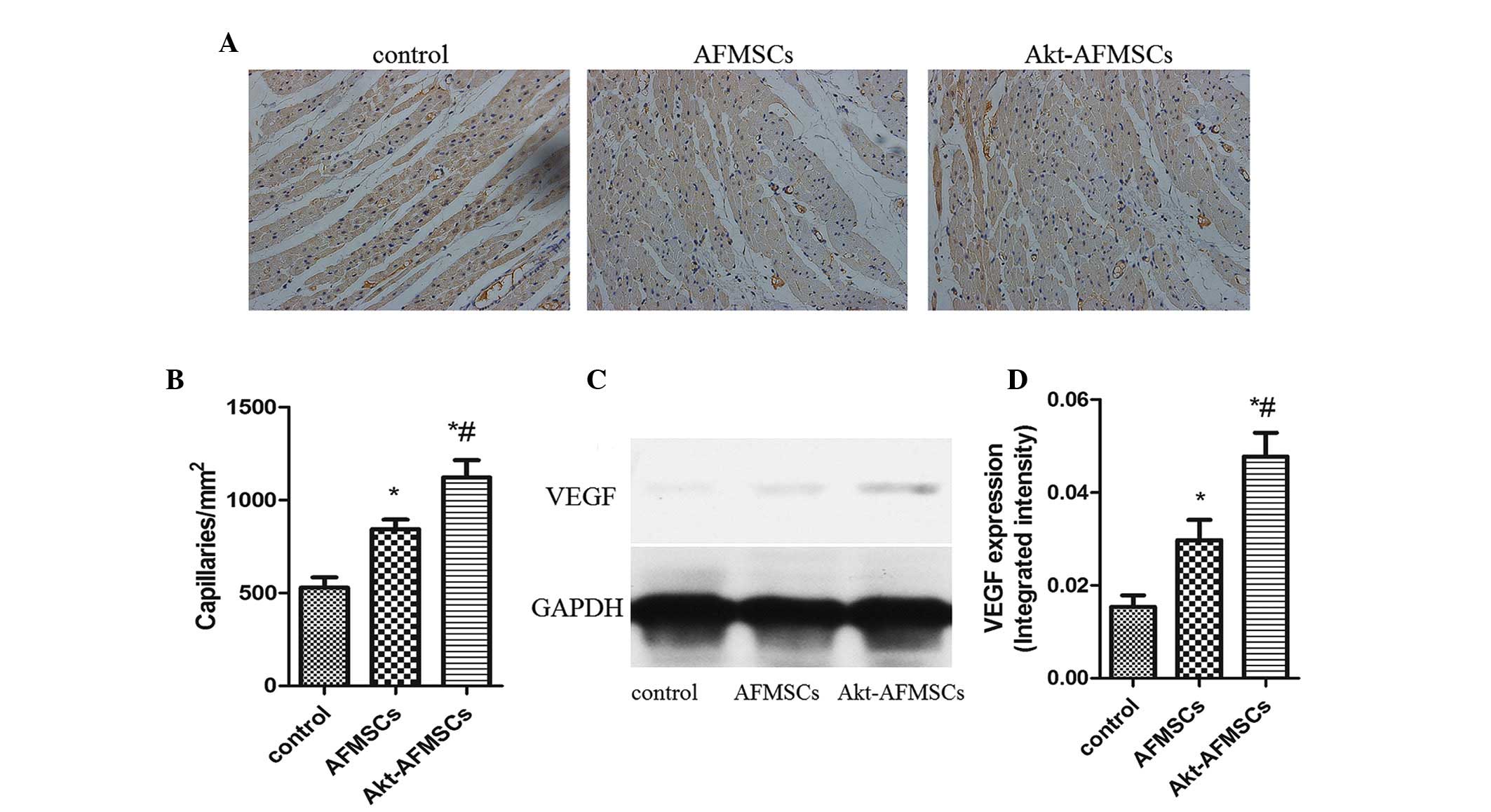
Angiogenesis in A kt-A FM SCs
transplantation
Immunohistological analysis revealed that the
capillary density was significantly higher in the Akt-AFMSC and
AFMSC groups compared with the control group. Notably, in the
Akt-AFMSC group, the increased level of angiogenesis was greater
compared with the AFMSC group (Fig. 7A
and B). To examine whether Akt-AFMSCs exerted any effect on
secretion of VEGF in the heart, western blot analysis was performed
for VEGF in myocardium tissue (Fig.
7C). The expression of VEGF in the zone of I/R injury in the
AFMSC group was significantly higher compared with the control
group, and also higher in the Akt-AFMSC group compared with the
AFMSC group (Fig. 7D), suggesting
that genetic modification of AFMSCs with the Akt gene may further
promote the release of VEGF.
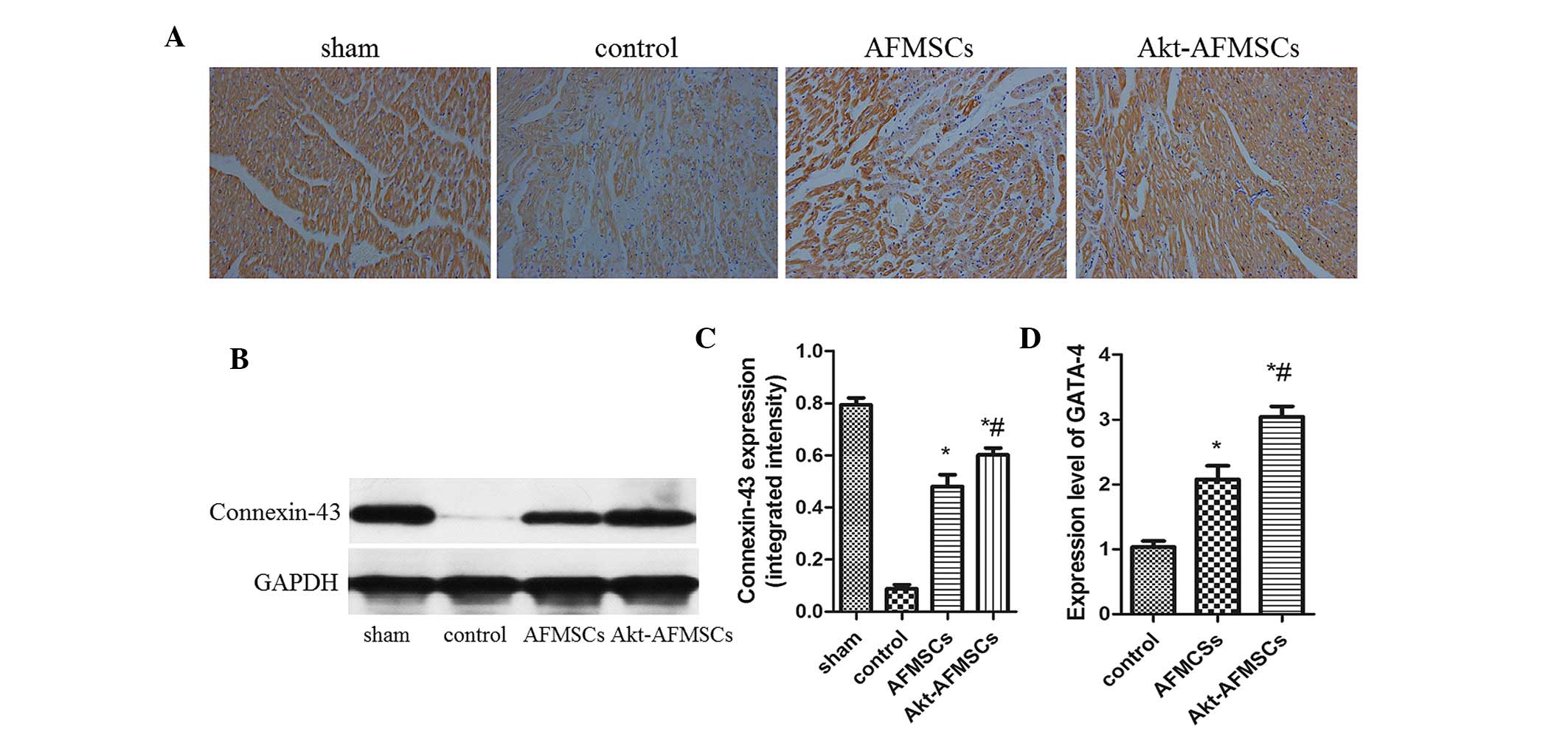
Expression of GATA-4, connexin 43 and
cTNT in the transplanted Akt-AFMSCs
Immunohistological analysis revealed that the
expression of cTNT was markedly higher in the Akt-AFMSC and AFMSC
groups compared with the control group (Fig. 8A). The Akt-AFMSC group also
revealed a greater increase in the expression of cTNT compared with
the AFMSC group. The Akt-AFMSC group also exhibited a higher
expression of the cardiac myocyte-specific markers, GATA-4,
connexin 43 and cTNT, suggesting that the transplantation of
Akt-AFMSCs may promote cardiac regeneration in myocardial I/R
injury. At 21 days following myocardial I/R, the difference in
protein expression of connexin-43, as determined by western
blotting, in the Akt-AFMSC, control and AFMSC groups reached its
peak (Fig. 8B and C). Compared
with the control group, the Akt-AFMSC and AFMSCs groups revealed
higher levels of gene expression of GATA-4 (1.00±0.24 vs. 3.08±0.23
and 2.11±0.19, P<0.05, respectively) (Fig. 8D).
Discussion
For patients with a STEMI, the opportunity to
intervene in acute myocardial ischemia is limited to the time of
myocardial reperfusion. The effects of myocardial reperfusion by
percuta-neous coronary intervention continues to improve with
earlier times of reperfusion. However, the process of myocardial
I/R may itself induce cardiomyocyte death by activating deleterious
signaling cascades. Previously, a number of emerging therapeutic
strategies for preventing lethal myocardial reperfusion injury have
shown promise in small clinical studies. These strategies include
ischemic post-conditioning, remote ischemic preconditioning,
therapeutic hyperoxemia and hypothermia, pharmacological agents,
including adenosine, atrial natriuretic peptide, atorvastatin,
erythropoietin, exenatide, delcasertib, glucose-insulin-potassium
(GIK), cyclosporin A, sodium nitrite and TRO40303. However, certain
interventions have produced disappointing results, whereas the
effects of others are now being investigated through multicenter
and randomized clinical trials (20). There remains no effective
therapeutic agent for preventing myocardial reperfusion injury.
Previous reports suggested that myocardial I/R
injury may be weakened through the transplantation of stem cells,
such as embryonic stem cells (ESCs) and adult stem cells (ASCs)
(21). These reports prompted our
assessment of whether AFMSC myocardial transplantation confers
protection of the heart against I/R injury. Based on the source of
the stem cells, cells are divided into ESCs and ASCs. As a novel
category of stem cell, AFMSCs have several advantages over other
stem cells. For instance, considered to be at an intermediate stage
between ESCs and ASCs, AFMSCs express the two types of stem cell
markers, proliferate rapidly, and differentiate into cells of all
three embryonic germ layers (22,23).
Unlike ESCs, AFMSCs do not form teratomas when injected into
immune-deficient mice, and the isolation of AFMSCs does not have
ethical implications. ASCs are isolated from the most
differentiated adult tissues (24). Compared with ASCs, the isolation of
AFMSCs is a simpler process, and large numbers of AFMSCs may be
isolated from amniotic fluid, proliferate rapidly, and do not
require a supportive feeder layer (17,25).
In addition, ASCs are few in the adult organism, and their number
decreases with age (26).
Furthermore, recently, a technique for acquiring pluripotent cells
from differentiated cells of an adult organism through genetic
modification, which are termed induced pluripotent stem cells (iPS
cells), was established. However, the use of iPS cells for cell
therapy is limited by the tumorigenicity of these cells. As such,
AFMSCs are an attractive cell source for applications in
regenerative medicine due to their high proliferative capacity,
immunomodulatory activity, multipotency and the lack of significant
immunogenicity.
AFMSC-based research on regenerative medicine
applications is increasingly becoming more prevalent. For
differentiation purposes, AFMSCs have been force-induced into a
number of lineages, including those of liver, cartilage, bone,
pancreas, muscle, neuronal and endothelial lineages in vitro
(27). In addition to
multipotency, AFMSCs have the ability to secrete cytokines, many of
which are pro-regenerative, pro-angiogenic or immunomodulatory.
Previous animal experiments have shown that AFMSCs may be useful in
the treatment of renal failure (28), skin wounds (29), fulminant hepatic failure (30), diabetes mellitus (31), and other condtions. Additionally, a
burgeoning number of studies have shown that AFMSCs may be
differentiated into endothelial and cardio-myogenic lineages
(6,32), and implantation of AFMSCs into
cardiac tissue following myocardial infarction can be beneficial
(33,34).
More attention has been paid to I/R following the
re-establishment of the coronary blood flow following myocardial
infarction. Therapeutic strategies on cardiac protection against
I/R injury are urgently needed. Studies of pre- and
post-conditioning have shown that I/R injury is able to initiate
several pro-survival kinase cascades, including the
phosphoinositide 3-kinase/Akt signaling pathway (35). Previous studies, for example, Mangi
et al (15), have also
shown that Akt-overexpressing MSCs present more benefits against
I/R injury (15). Akt, a
serine-threonine kinase, maintains stem cells by promoting
viability and proliferation. In addition, Akt is an important
factor in the regulation of intercellular glycometabolism that
increases energy support during hypoxia. However, its role in the
genetic modification of AFMSCs in protecting against myocardial I/R
injury has yet to be elucidated. Therefore, the present study was
designed to determine whether overexpressing Akt in AFMSCs would
alleviate I/R injury. The results have demonstrated that
transplanted AFMSCs (especially AFMSCs overexpressing Akt)
significantly improve pathological myocardial I/R injury, cardiac
function, cardiomyocyte apoptosis, angiogenesis and the expression
of GATA-4, connexin 43 and cTNT. These results were similar to
those obtained from a majority of stem cell transplantations
against myocardial I/R injury, indicating that AFMSCs may be more
suitable as a cell source for applications in stem cell therapy due
to their advantage over ESCs and ASCs.
The present study has shown that the Akt-AFMSC group
had more stem cell retention compared with the AFMSC group. This
finding is explained, in part, by Akt gene modifications, which
enhanced the viability of stem cells and resistance to apoptosis.
It has also been demonstrated that Akt is an excellent therapeutic
gene for preserving MSC viability in the early post-transplantation
period (15).
To date, it is generally agreed that the mechanism
of cardiovascular regeneration by stem cells is mediated by the
release of a myriad of cytokines and chemokines. In terms of
AFMSCs, a previous study demonstrated that these cells are able to
secrete a number of growth factors at concentrations higher than
those of MSCs (29). In addition,
Akt-modified MSCs are able to excrete additional factors, including
VEGF, fibroblast growth factor-2, hepatocyte growth factor (HGF),
insulin growth factor (IGF)-1 and thymosin β-4 (36). In the present study, the promotion
of angiogenesis by AFMSCs was accompanied by a higher expression of
VEGF in cardiac tissue. A decrease in cardiomyocyte apoptosis was
accompanied by decreased levels of caspase-3 and elevated levels of
P-Akt and Bcl-2, suggesting that the Akt-AFMSC transplantation
group resulted in a greater benefit compared with the other groups.
Taken together, our results are consistent with the hypothesis that
Akt-overexpressing AFMSCs provide a cardioprotective effect via a
paracrine mechanism.
With regard to higher expression levels of GATA-4,
connexin 43 and cTNT following Akt-AFMSC transplantation, the
effects may be a consequence of the above-mentioned decrease in
cardiomyocyte apoptosis in the Akt-AFMSC group, and/or certain
cytokines excreted by Akt-AFMSCs, which are able to enhance the
migration and activation of resident cardiac stem cells, leading to
differentiation into cardiomyocytes. As for the latter mechanism,
it has been well documented that IGF-1/HGF are able to activate
endogenous cardiac stem cells (37). AFMSCs have also been reported to
excrete HGF (29).
In conclusion, the present study suggests that
AFMSCs and Akt-AFMSCs protect the heart against I/R injury in
rabbits. These results were similar to those for stem cell
transplantation against myocardial I/R injury, indicating that
AFMSCs may be more suitable as a stem cell source for application
in stem cell therapies due to their advantages over ESCs and ASCs.
The protective effect may be due to delivery of secreted cytokines,
promotion of neoangiogenesis, prevention of cardiomyocyte
apoptosis, transdifferentiation into cardiomyocytes, or a
combination of these effects. Genetically, modification through Akt
appears to represent an improved therapeutic approach, promoting
the viability of transplanted AFMSCs, and thereby enhancing their
protective effects against cardiac I/R injury.
Acknowledgments
This work was supported by a Nature Science
Foundation of China Grant (no. 81170124/H0203).
References
|
1
|
Angoulvant D, Ivanes F, Ferrera R,
Matthews PG, Nataf S and Ovize M: Mesenchymal stem cell conditioned
media attenuates in vitro and ex vivo myocardial reperfusion
injury. J Heart Lung Transplant. 30:95–102. 2011. View Article : Google Scholar
|
|
2
|
Preda MB, Rønningen T, Burlacu A,
Simionescu M, Moskaug JØ and Valen G: Remote transplantation of
mesenchymal stem cells protects the heart against
ischemia-reperfusion injury. Stem Cells. 32:2123–2134. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Abarbanell AM, Herrmann JL, Weil
BR, Manukyan MC, Poynter JA and Meldrum DR: TLR4 inhibits
mesenchymal stem cell (MSC) STAT3 activation and thereby exerts
deleterious effects on MSC-mediated cardioprotection. PloS One.
5:e142062010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dexheimer V, Mueller S, Braatz F and
Richter W: Reduced reactivation from dormancy but maintained
lineage choice of human mesenchymal stem cells with donor age. PloS
One. 6:e229802011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanawa M, Igarashi A, Ronald VS, Higashi
Y, Kurihara H, Sugiyama M, Saskianti T, Pan H and Kato Y:
Age-dependent decrease in the chondrogenic potential of human bone
marrow mesenchymal stromal cells expanded with fibroblast growth
factor-2. Cytotherapy. 15:1062–1072. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maioli M, Contini G, Santaniello S,
Bandiera P, Pigliaru G, Sanna R, Rinaldi S, Delitala AP, Montella
A, Bagella L and Ventura C: Amniotic fluid stem cells morph into a
cardiovascular lineage: Analysis of a chemically induced cardiac
and vascular commitment. Drug Des Devel Ther. 7:1063–1073.
2013.PubMed/NCBI
|
|
7
|
Rennie K, Gruslin A, Hengstschläger M, Pei
D, Cai J, Nikaido T and Bani-Yaghoub M: Applications of amniotic
membrane and fluid in stem cell biology and regenerative medicine.
Stem Cells Int. 2012:7215382012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng AS and Yau TM: Paracrine effects of
cell transplantation: Strategies to augment the efficacy of cell
therapies. Semin Thorac Cardiovasc Surg. 20:94–101. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang M, Methot D, Poppa V, Fujio Y, Walsh
K and Murry CE: Cardiomyocyte grafting for cardiac repair: Graft
cell death and anti-death strategies. J Mol Cell Cardiol.
33:907–921. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Balsam LB, Wagers AJ, Christensen JL,
Kofidis T, Weissman IL and Robbins RC: Haematopoietic stem cells
adopt mature haematopoietic fates in ischaemic myocardium. Nature.
428:668–673. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hammerman PS, Fox CJ, Birnbaum MJ and
Thompson CB: Pim and Akt oncogenes are independent regulators of
hematopoietic cell growth and survival. Blood. 105:4477–4483. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SJ, Cheon SH, Yoo SJ, Kwon J, Park JH,
Kim CG, Rhee K, You S, Lee JY, Roh SI and Yoon HS: Contribution of
the PI3K/Akt/PKB signal pathway to maintenance of self-renewal in
human embryonic stem cells. FEBS Lett. 579:534–540. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Catalucci D and Condorelli G: Effects of
Akt on cardiac myocytes: Location counts. Circ Res. 99:339–341.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH,
Joo SY, Nam KI, Cho JG, Kang PM and Park JC: The effects of
mesenchymal stem cells transduced with Akt in a porcine myocardial
infarction model. Cardiovasc Res. 70:530–542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mangi AA, Noiseux N, Kong D, He H, Rezvani
M, Ingwall JS and Dzau VJ: Mesenchymal stem cells modified with Akt
prevent remodeling and restore performance of infarcted hearts. Nat
Med. 9:1195–1201. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu YS, Shen ZY, Ye WX, Huang HY, Hua F,
Chen YH, Chen K, Lao WJ and Tao L: AKT-modified autologous
intracoronary mesenchymal stem cells prevent remodeling and repair
in swine infarcted myocardium. Chin Med J (Engl). 123:1702–1708.
2010.
|
|
17
|
Fei X, Jiang S, Zhang S, Li Y, Ge J, He B,
Goldstein S and Ruiz G: Isolation, culture and identification of
amniotic fluid-derived mesenchymal stem cells. Cell Biochem
Biophys. 67:689–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang S, Zhang M, Goldstein S, Li Y, Ge J,
He B and Ruiz G: The effect of c-fos on acute myocardial infarction
and the significance of metoprolol intervention in a rat model.
Cell Biochem Biophys. 65:249–255. 2013. View Article : Google Scholar
|
|
19
|
Zhang S, Liu X, Goldstein S, Li Y, Ge J,
He B, Fei X, Wang Z and Ruiz G: Role of the JAK/STAT signaling
pathway in the pathogenesis of acute myocardial infarction in rats
and its effect on NF-κB expression. Mol Med Rep. 7:93–98. 2013.
|
|
20
|
Chen C, Xu Y and Song Y: IGF-1
gene-modified muscle-derived stem cells are resistant to oxidative
stress via enhanced activation of IGF-1R/PI3K/AKT signaling and
secretion of VEGF. Mol Cell Biochem. 386:167–175. 2014. View Article : Google Scholar
|
|
21
|
Takashima S, Tempel D and Duckers HJ:
Current outlook of cardiac stem cell therapy towards a clinical
application. Heart. 99:1772–1784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kunisaki SM, Fuchs JR, Steigman SA and
Fauza DO: A comparative analysis of cartilage engineered from
different perinatal mesenchymal progenitor cells. Tissue Eng.
13:2633–2644. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kolambkar YM, Peister A, Soker S, Atala A
and Guldberg RE: Chondrogenic differentiation of amniotic
fluid-derived stem cells. J Mol Histol. 38:405–413. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lanfranchi A, Porta F and Chirico G: Stem
cells and the frontiers of neonatology. Early Hum Dev. 85(Suppl
10): S15–S18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davydova DA: Stem cells in human amniotic
fluid. Izv Akad Nauk Ser Biol. 517–526. 2010.In Russian.
|
|
26
|
Sessarego N, Parodi A, Podestà M,
Benvenuto F, Mogni M, Raviolo V, Lituania M, Kunkl A, Ferlazzo G,
Bricarelli FD, et al: Multipotent mesenchymal stromal cells from
amniotic fluid: Solid perspectives for clinical application.
Haematologica. 93:339–346. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Wang D, Liang T, Guo Q and Zhang
G: Amniotic fluid-derived mesenchymal stem cells: Characteristics
and therapeutic applications. Arch Gynecol Obstet. 290:223–231.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baulier E, Favreau F, Le Corf A, Jayle C,
Schneider F, Goujon JM, Feraud O, Bennaceur-Griscelli A, Hauet T
and Turhan AG: Amniotic fluid-derived mesenchymal stem cells
prevent fibrosis and preserve renal function in a preclinical
porcine model of kidney transplantation. Stem Cells Transl Med.
3:809–820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Skardal A, Mack D, Kapetanovic E, Atala A,
Jackson JD, Yoo J and Soker S: Bioprinted amniotic fluid-derived
stem cells accelerate healing of large skin wounds. Stem Cells
Transl Med. 1:792–802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng YB, Zhang XH, Huang ZL, Lin CS, Lai
J, Gu YR, Lin BL, Xie DY, Xie SB, Peng L and Gao ZL:
Amniotic-fluid-derived mesenchymal stem cells overexpressing
interleukin-1 receptor antagonist improve fulminant hepatic
failure. PloS One. 7:e413922012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Villani V, Milanesi A, Sedrakyan S, Da
Sacco S, Angelow S, Conconi MT, Di Liddo R, De Filippo R and Perin
L: Amniotic fluid stem cells prevent β-cell injury. Cytotherapy.
16:41–55. 2014. View Article : Google Scholar
|
|
32
|
Yeh YC, Wei HJ, Lee WY, Yu CL, Chang Y,
Hsu LW, Chung MF, Tsai MS, Hwang SM and Sung HW: Cellular
cardiomyoplasty with human amniotic fluid stem cells: In vitro and
in vivo studies. Tissue Eng Part A. 16:1925–1936. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bollini S, Pozzobon M, Nobles M, Riegler
J, Dong X, Piccoli M, Chiavegato A, Price AN, Ghionzoli M, Cheung
KK, et al: In vitro and in vivo cardiomyogenic differentiation of
amniotic fluid stem cells. Stem Cell Rev. 7:364–380. 2011.
View Article : Google Scholar
|
|
34
|
Lee WY, Wei HJ, Lin WW, Yeh YC, Hwang SM,
Wang JJ, Tsai MS, Chang Y and Sung HW: Enhancement of cell
retention and functional benefits in myocardial infarction using
human amniotic-fluid stem-cell bodies enriched with endogenous ECM.
Biomaterials. 32:5558–5567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murphy E and Steenbergen C: Mechanisms
underlying acute protection from cardiac ischemia-reperfusion
injury. Physiol Rev. 88:581–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gnecchi M, He H, Noiseux N, Liang OD,
Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS and Dzau
VJ: Evidence supporting paracrine hypothesis for Akt-modified
mesenchymal stem cell-mediated cardiac protection and functional
improvement. FASEB J. 20:661–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ellison GM, Torella D, Dellegrottaglie S,
Perez-Martinez C, Perez de Prado A, Vicinanza C, Purushothaman S,
Galuppo V, Iaconetti C, Waring CD, et al: Endogenous cardiac stem
cell activation by insulin-like growth factor-1/hepatocyte growth
factor intracoronary injection fosters survival and regeneration of
the infarcted pig heart. J Am Coll Cardiol. 58:977–986. 2011.
View Article : Google Scholar : PubMed/NCBI
|