Introduction
The vascular endothelium is a physiological barrier
separating the circulating blood from the underlying tissue, and
serves an important role in maintaining physiological homeostasis
of blood vessels (1). Endothelial
cell injury induced by oxidative stress, which is initiated by
excessive generation of reactive oxygen species (ROS) in response
to diverse extracellular detrimental stimuli, is involved in the
pathogenesis of several diseases, including coronary heart disease,
neurodegenerative disorders, diabetes, arthritis, inflammation and
cancer (2–5). Therefore, the protection of
endothelial cells against ROS-induced damage must be considered as
an important strategy for intervention in cardiovascular
diseases.
Apocynum venetum L. (Luo-Bu-Ma) is a
traditional Chinese herbal medicine exhibiting diverse activities,
including inhibition of platelet aggregation and myocardial
ischemia/reperfusion injury, hypotension and an antioxidative
effect, and is widely used for the prevention of cardiovascular
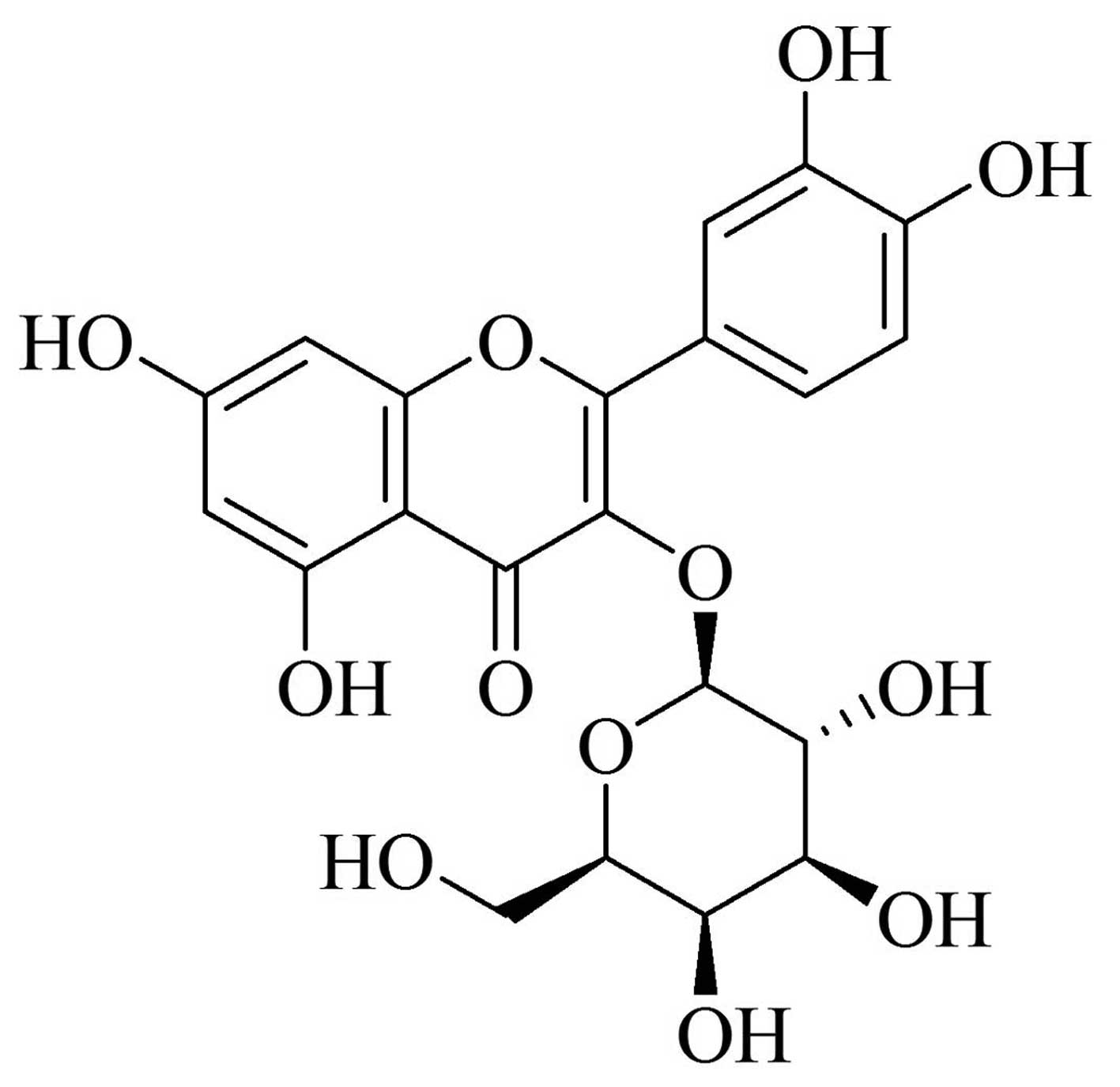
diseases (6–10). Hyperoside (Fig. 1), a flavonoid, is the predominant
active component abundant in A. venetum. Increasing evidence
has demonstrated that hyperoside exhibits anti-inflammatory,
antioxidative and cellular protective effects. Ku et al
(11) reported that hyperoside
exhibited anti-inflammatory effects via the regulation of the
high-mobility group box (HMGB)1-mediated signaling pathway and was
beneficial for the inhibition of vascular inflammation diseases
(11). It was also reported that
hyperoside has a powerful capacity to modulate oxidative
stress-induced melanogenesis through the inhibition of the
formation of peroxynitrite, O2 and NO in B16F10 melanoma
cells (12). Numerous previous
studies also indicated that hyperoside exerted protective effects
on oxidative stress-induced injury of several types of cell
(13–17). The present study demonstrated the
protective effects of hyperoside against
H2O2-induced apoptosis of HUVECs through the
detection of endothelial Ca2+ content, expression levels
of B-cell lymphoma (Bcl)-2, Bcl-2 associated X protein (Bax) and
cleaved caspase-3, which was performed to investigate whether
hyperoside was involved in the prevention of cardiovascular
diseases.
Materials and methods
Materials
Hyperoside was purchased from the Chinese Food and
Drug Inspection Institute (Beijing, China). Dimethyl sulfoxide
(DMSO), 4% paraformaldehyde, H2O2 and
3-(4,5-dimethylthiazol-2-yl)-2,5-dephenyltetrazolium bromide (MTT)
were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Dulbecco's modified Eagle's medium (DMEM), L-glutamine, penicillin
and streptomycin, and TRIzol reagent were purchased from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). The bicinchoninic acid
(BCA) assay kit and cridine orange/ethidium bromide (AO/EB) were
purchased from Beyotime Institute of Biotechnology, Inc. (Nanjing,
China). Antibodies against phosphorylated (p-)p38 (#9211), p38
(#9212), Bcl-2 (#2876), Bax (#2772) and cleaved-caspase-3 (#9661)
were obtained from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit
secondary antibody (#CW0103) and β-actin (#CW0097) were purchased
from CoWin Biotech Co., Ltd. (Beijing, China).
H2O2 was prepared freshly for each experiment
from a 33% (v/v) stock solution. All other chemicals and reagents
were commercially available and of standard biochemical
quality.
Cell culture and treatments
The HUVEC line was purchased from the Cell Bank of
the Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in DMEM supplemented with 10% heat-inactivated fetal
bovine serum (FBS), and 0.1% penicillin/streptomycin at 37°C in 5%
CO2. For all experiments, HUVECs were grown to 70–80%
confluence and were subsequently treated as designed for different
experiments.
Cell viability analysis
MTT assays were used to evaluate cell viability.
HUVECs were seeded into 96-well plates at a density of
1×104 cells/well and cultured at 37°C for 24 h. The
culture medium was then removed and fresh medium for different
treatments was added to each well. Following treatment, 10
µl of 5 mg/ml MTT solution was added to each well and the
cells were incubated for another 4 h. The culture medium was
subsequently replaced with 100 µl DMSO. The optical density
in each well was determined using a Bio-Rad Microplate Reader
(Model 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 570
nm.
Assessment of apoptosis using AO/EB
fluorescent staining
The cells were seeded at 5×105 cells/well
in 6-well plates and cultured for 24 h. The cells were subsequently
treated with hyperoside for 24 h prior to exposure to
H2O2 for 4 h. The cells were fixed with 4%
formaldehyde in phosphate-buffered saline (PBS) for 10 min and were
washed with ice-cold PBS three times. The cells were stained with
AO/EB solution (5 µl) for 30 sec and changes in cell
morphology were observed using a fluorescence microscope (Olympus
IX-71; Olympus, Tokyo, Japan).
Assessment of intercellular
Ca2+ levels
The cells were seeded at a density of
5×105 cells/well into 6-well plates and were cultured
for 24 h. The cells were subsequently treated with hyperoside for
24 h prior to exposure to H2O2 for 4 h. The
Fluo-3/AM fluorescent probe was added to the HUVEC suspension at a
final concentration of 10 µM and incubated at 37°C for 40
min. The intracellular Ca2+ content in the HUVECs was
subsequently measured with laser-scanning confocal microscopy
(Olympus FV1000, Olympus) with an excitation wavelength of 488 nm
and a measured emission at 530 nm.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The total RNA was extracted from the HUVECs using
TRIzol reagent, according to the manufacturer's protocol. The
concentration of purified RNA samples were measured
spectrophotometrically using a Picodrop (Picodrop Ltd., Walden,
UK). The RT reaction was performed using a SuperScript III
First-Strand Synthesis system (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Amplification of the RT
product by PCR was performed using Promega Taq DNA Polymerase
(Promega Co., Madison, WI, USA). All reactions were performed in a
thermal cycler (Model 2400; Perkin-Elmer, Norwalk, CT, USA) with
primers (Sangon Biotech Co., Ltd., Shanghai, China) specific for
Bcl-2, forward: 5′-CTT CGC CGA GAT GTC CAG CCA-3′ and reverse:
5′-CGC TCT CCA CAC ACA TGA CCC-3′; Bax, forward: 5′-TGC TTC AGG GTT
TCA TCC AGGA-3′ and reverse: 5′-ACG GCG GCA ATC ATC ATC CTC TG-3′;
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward: 5′-TCT
CTG CTC CTC CTG TTC GAC-3′ and reverse: 5′-TTA AAA GCA GCC CTG GTG
AC-3′. Thermal cycling conditions involved an initial denaturation
step at 94°C for 10 min, followed by 30 cycles of denaturation at
94°C for 10 sec, and primer annealing at 60°C for 1 min, which was
followed by agarose gel electrophoresis.
Quantitation of protein samples
Following treatment with various concentrations of
hyperoside prior to H2O2 exposure, the HUVECs
were washed with ice-cold PBS and harvested by trypsinization. The
cells were centrifuged (1,000 rpm for 5 min) and washed with
ice-cold PBS three times. The cells were then suspended in 100
µl ice-cold radioimmunoprecipitation lysis buffer (Beyotime
Institute of Biotechnology, Inc.), sonicated 10 times for 5 sec
with 10 sec pauses in an ice-water bath, and the samples were
centrifuged (13,000 rpm for 5 min at 4°C). The supernatants were
stored at −80°C. Quantification of the protein was performed using
a BCA assay.
Western blot analysis
Equal quantities of protein extracts (40 µg)
were separated on 12% sodium dodecyl sulfate-polyacrylamide gels.
The proteins were subsequently transferred onto nitrocellulose
membranes (Pall Gelman Laboratory Corporation, Ann Arbor, MI, USA).
The membranes were blocked with 5% (w/v) non-fat milk powder in
Tris-buffered saline/0.1% Tween-20 (TBST) for 1.5 h at room
temperature. Following blocking, the membranes were incubated
overnight at 4°C with the primary antibody (dilution, 1:1,000).
After three washes with TBST, the membranes were incubated for 1 h
with horseradish peroxidase (HRP)-conjugated anti-rabbit
immunoglobulin G as the secondary antibody at room temperature
(dilution, 1:2,000). After three washes, the proteins were detected
using an enhanced chemiluminescence detection kit (CoWin Biotech
Co., Ltd., Beijing, China).
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical comparisons were performed using a Student's
t-test, and the differences between multiple groups were assessed
by one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of hyperoside on the viability of
HUVECs
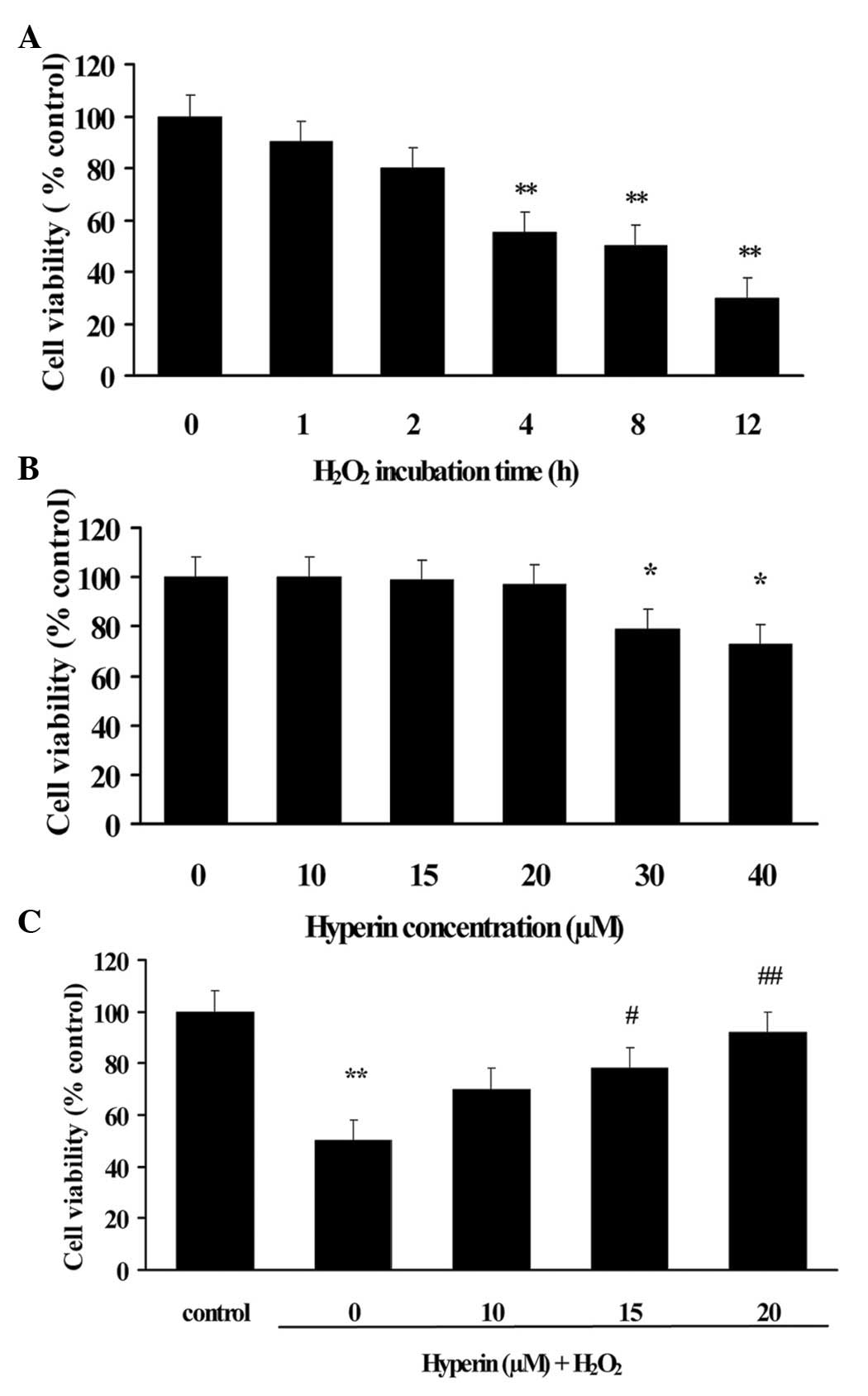
To evaluate the apoptotic induction of
H2O2 on HUVECs, HUVECs were incubated with
200 µM H2O2 for different durations.
As shown in Fig. 2A, the viability
of HUVECs decreased ~50% following exposure to 200 µM
H2O2 for 4 h. Therefore, the exposure of
HUVECs to 200 µM H2O2 for 4 h was
selected to induce apoptosis of HUVECs for the subsequent
experiments.
To ensure the suitable concentrations of hyperoside,
the cytotoxicity of hyperoside was evaluated by MTT assay. HUVECs
were cultured with various concentrations of hyperoside for 24 h
and the cell viability was assessed. The result revealed that
hyperoside at concentrations of <20 µM caused no affect
the viability of HUVECs, as shown in Fig. 2B. Therefore, the concentrations of
hyperoside were confirmed as 10, 15 and 20 µM in the
subsequent experiments.
As shown in Fig.
2C, the H2O2-induced decrease of cell
viability was significantly attenuated by hyperoside treatment in a
dose-dependent manner (P<0.01), which suggested that hyperoside
exhibited protective effect on H2O2-induced
HUVECs injury.
Effects of hyperoside on
H2O2-induced apoptosis in HUVECs
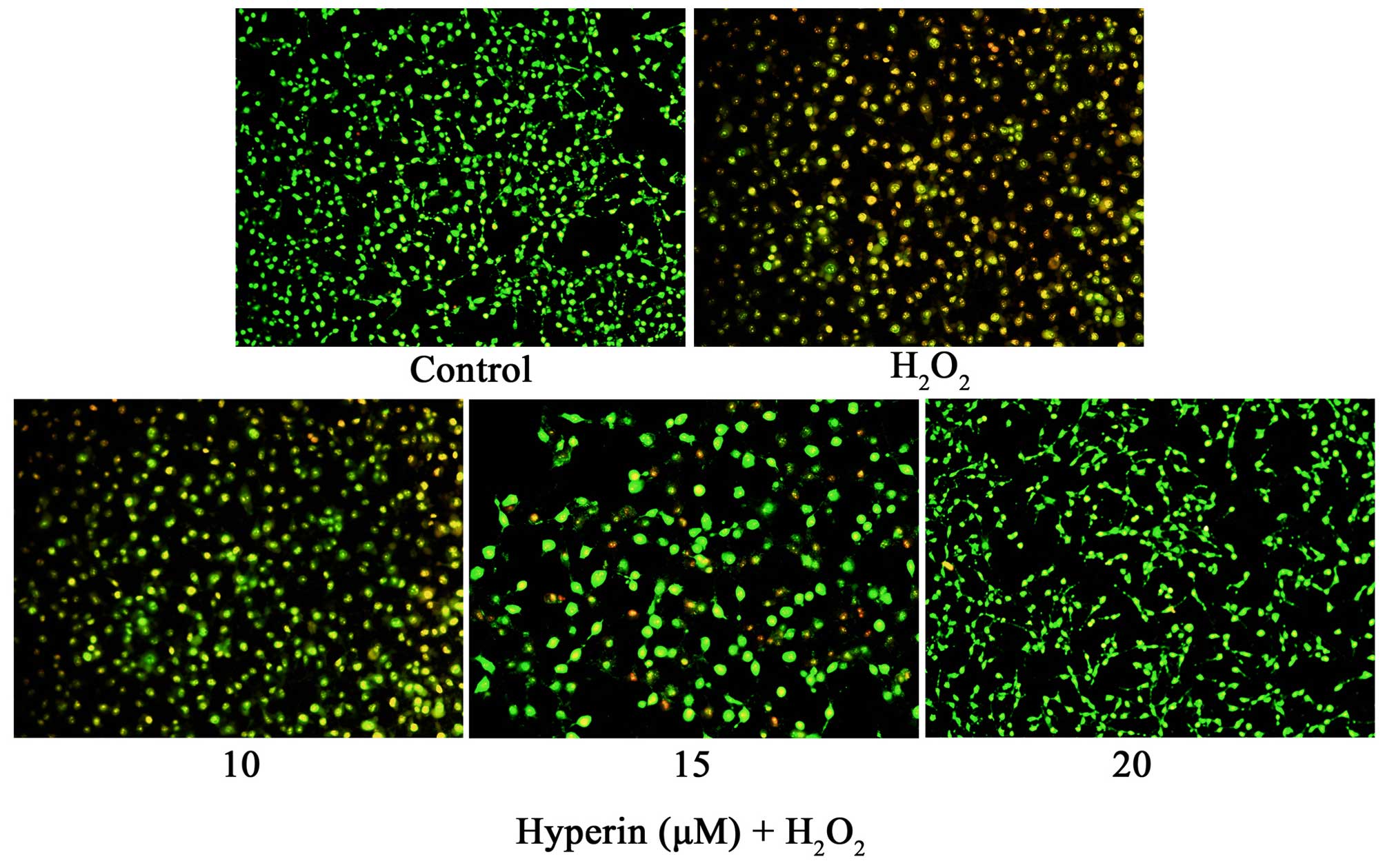
As shown in Fig. 3,
AO/EB staining demonstrated that exposure of HUVECs to 200
µM H2O2 for 4 h induced HUVEC
apoptosis. However, the treatment of HUVECs with 10, 15 and 20
µM hyperoside significantly attenuated the apoptosis of
HUVECs induced by H2O2, which demonstrated
that hyperoside exhibited anti-apoptosis effects in
H2O2-induced HUVECs.
Effects of hyperoside on intercellular
Ca2+ levels in HUVECs
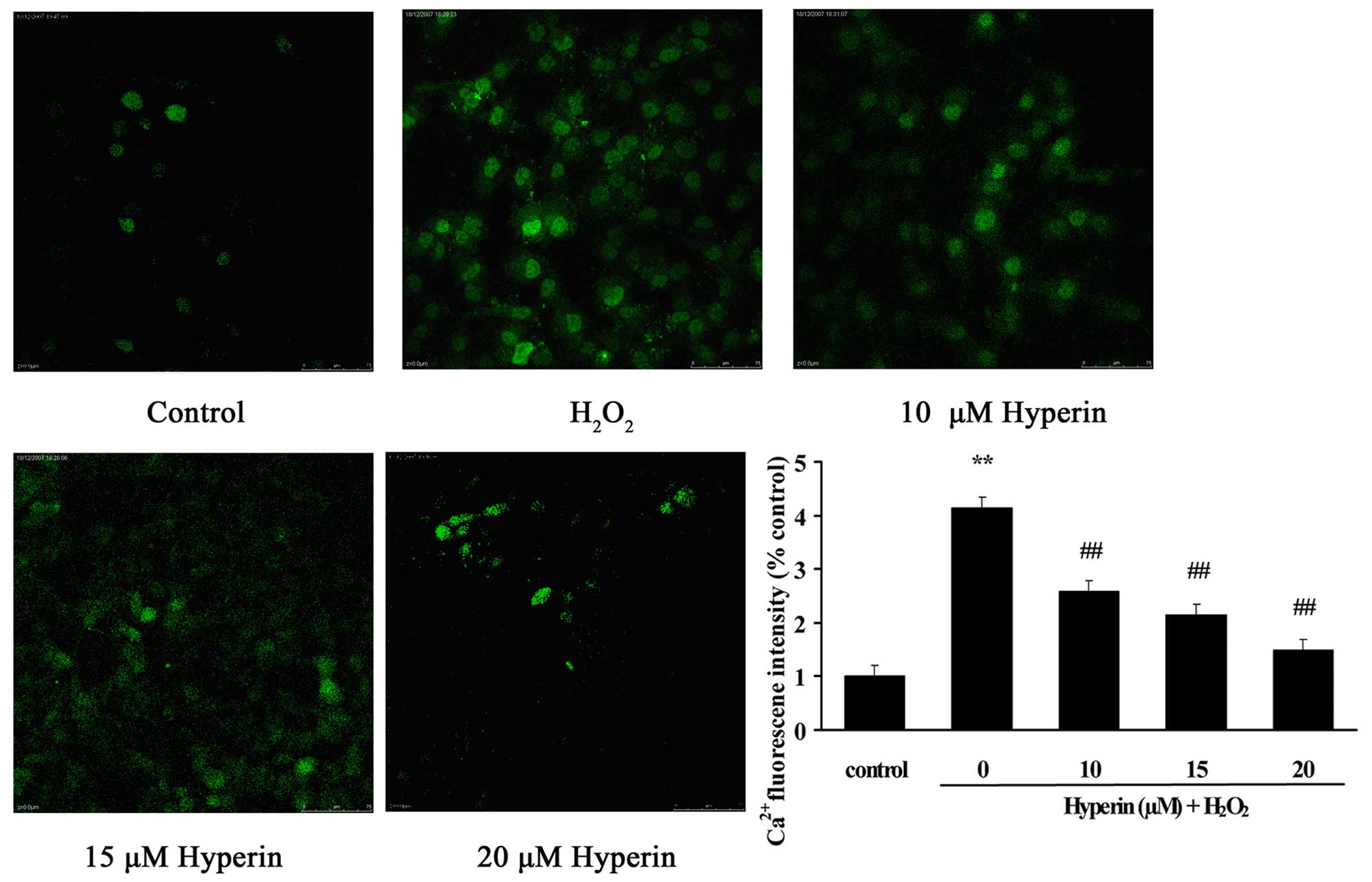
As shown in Fig. 4,
compared with the control group, intercellular Ca2+
content, presented as the fluorescence intensity, was significantly
overloaded in the H2O2-induced HUVECs
(P<0.01). However, the treatment of HUVECs with different
concentrations of hyperoside (10, 15 and 20 µM)
significantly inhibited the increase of fluorescence intensity
induced by H2O2 in a dose-dependent manner
(P<0.01). This indicated that hyperoside exhibited protective
effects on the oxidative stress-induced increase of intercellular
Ca2+ content.
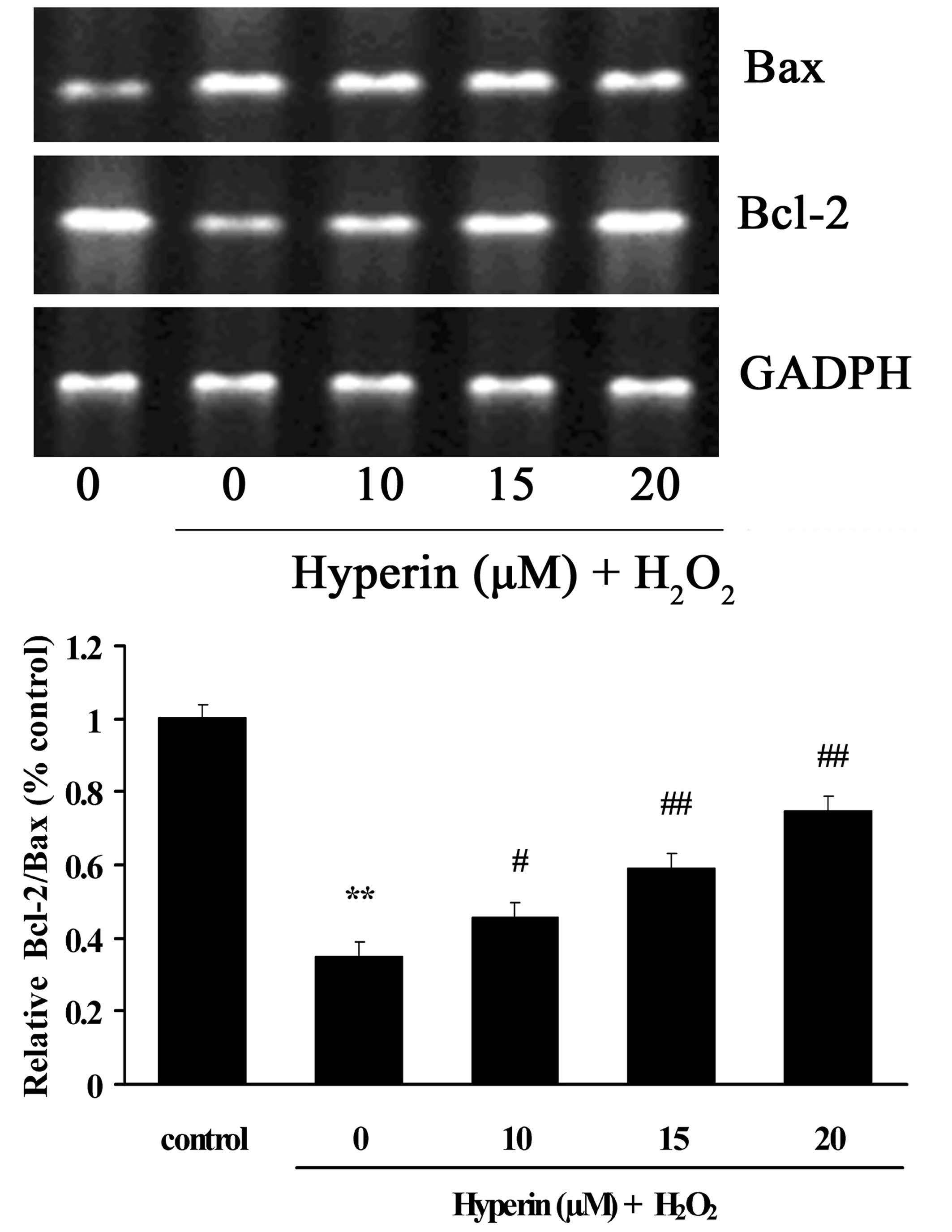
Effects of hyperoside on the mRNA
expression levels of Bcl-2 and Bax
As shown in Fig. 5,
the mRNA expression of Bax was significantly increased in the
H2O2-induced group. By contrast, the mRNA
expression of Bcl-2 was significantly decreased compared with the
control group. However, different concentrations of hyperoside (10,
15 and 20 µM) exhibited significant inhibition on the
H2O2-induced increase of Bax and decrease of
Bcl-2 mRNA in a dose-dependent manner (P<0.01).
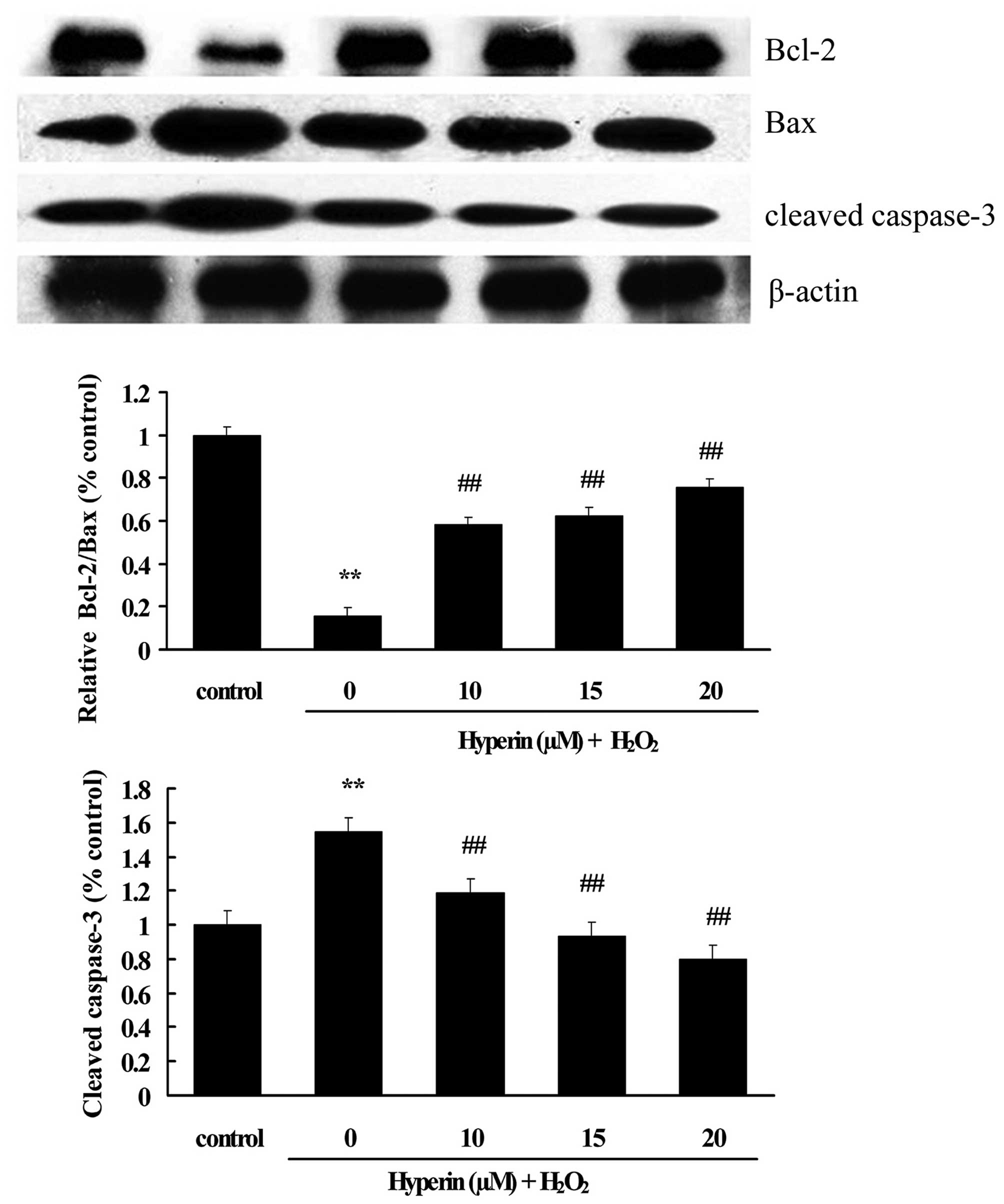
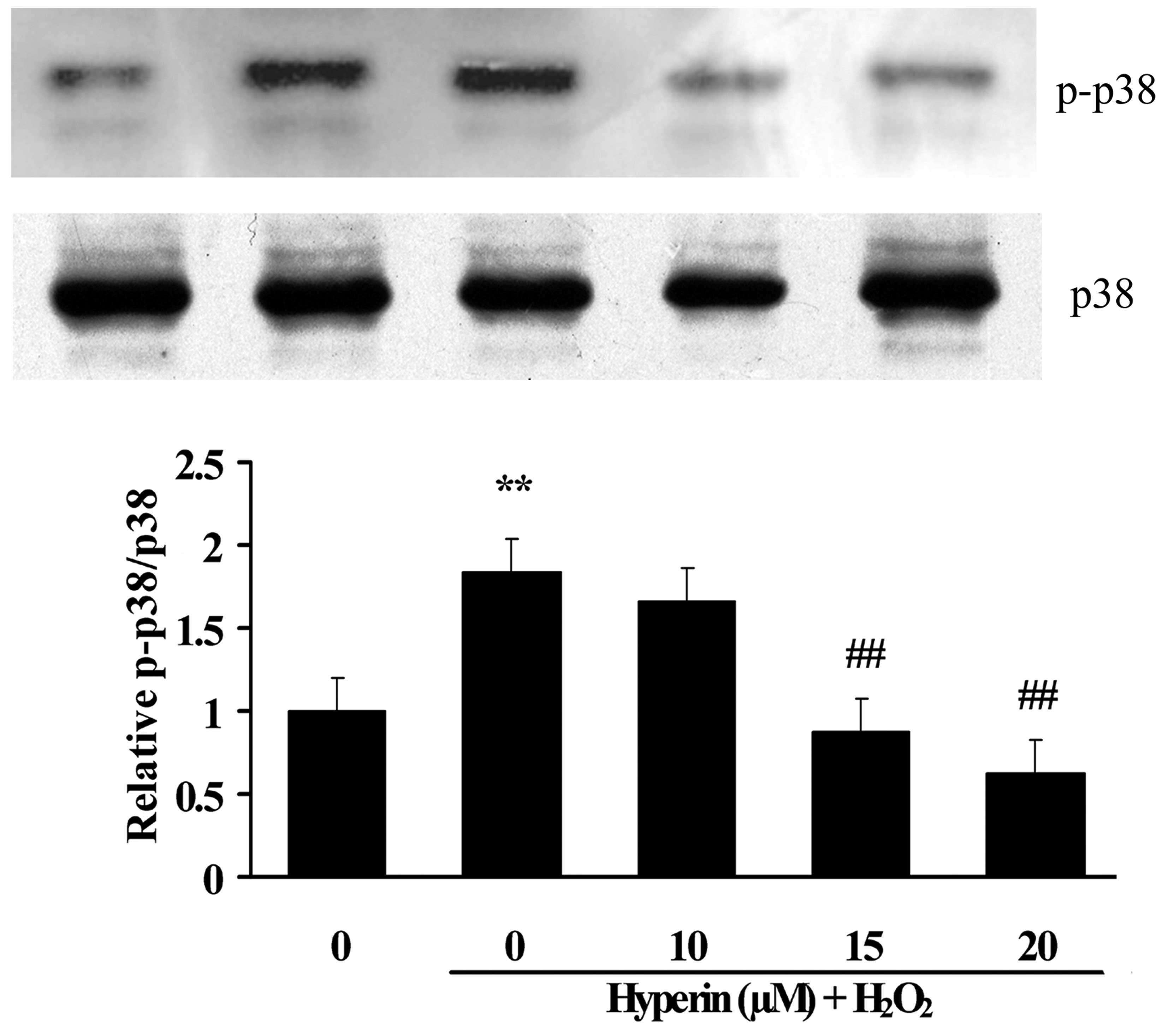
Effects of hyperoside on the expression
of apoptotic-associated proteins
As shown in Figs. 6
and 7, compared with the control
group, the expression of cleaved caspase-3, Bax and p-p38 was
significantly increased, while Bcl-2 was significantly decreased,
in the H2O2-induced group. However, the
treatment with different concentrations of hyperoside (10, 15 and
20 µM) revealed a significant inhibition on the
H2O2-induced increase of cleaved caspase-3,
Bax and p-p38, and the decrease of Bcl-2 in a dose-dependent manner
(P<0.01). This indicated that hyperoside exhibited antiapoptotic
effects on H2O2-induced HUVECs.
Discussion
Apocynum venetum L., known as Luobuma in
China, is a traditional Chinese herb exhibiting diverse activities,
including inhibition of platelet aggregation and myocardial
ischemia/reperfusion injury, hypotension and antioxidative effect,
and is widely used for the prevention of cardiovascular diseases.
Chemical studies have demonstrated that flavonoids were rich in
A. venetum, including quercetin, kaempferol, rutin and
hyperoside (10). Increasing
evidence has demonstrated that hyperoside exhibited
anti-inflammatory, antioxidative and cellular protective effects.
The present study demonstrated the protective effects of farrerol
against oxidative stress-induced apoptosis in HUVECs.
A previous study demonstrated that the elevation of
intracellular Ca2+ concentration was involved in the
induction of mitochondrial dysfunction and leads to
mitochondria-dependent apoptosis (18). Chen et al (19) also reported that hyperoside can
inhibit Ca2+ influx in dissociated neonatal rat brain
cells (19). The present study
found that hyperoside exhibited the inhibition of
H2O2-induced increase of intercellular
Ca2+ concentration in HUVECs, suggesting a protective
effect of hyperoside on H2O2-induced HUVEC
injury.
The Bcl-2 protein family is essential in the
mitochondrial apoptosis pathway. This protein family can be divided
into two categories: i) Anti-apoptotic members, including Bcl-2 and
Bcl-xl; ii) pro-apoptotic members, including Bax and Bak. Numerous
previous studies have demonstrated that the ratio of Bcl-2/Bax
determines the fate of cells to apoptosis or survival. The decrease
of Bcl-2/Bax induced the increase of mitochondrial permeability and
the release of cytochrome c, which resulted in the
activation of caspase-3 and apoptosis (20–24).
The present study revealed that hyperoside increased the mRNA and
protein expression levels of Bcl-2 and decreased the expression of
Bax in the H2O2-induced HUVECs, indicating
the antiapoptotic effects of hyperoside on
H2O2-induced apoptosis of HUVECs.
Caspase components are central in the execution of
apoptosis (25). Caspase-3, which
mediates apoptosis for both extrinsic and intrinsic pathways, is
cleaved and activated in the process of apoptosis. The present
study detected the effect of hyperoside on the expression of
cleaved csspase-3 by western blotting. The result indicated that
hyperoside inhibited the increased expression of cleaved caspase-3
induced by H2O2 in HUVECs, which demonstrated
the antiapoptotic effect of hyperoside on oxidative stress-induced
HUVEC apoptosis.
Increasing evidence has indicated that exposure of
the cells to H2O2 induces the activation of
the members of the mitogen-activated protein kinase (MAPK) pathway,
including extracellular signal-regulated kinases (ERK) 1/2, c-jun
NH2-terminal kinases (JNK) and p38 kinase (26). Among these kinases, the JNK and p38
pathways are commonly considered to be apoptotic, whereas ERK1/2 is
associated with protection from apoptosis (27). The present research indicated that
the hyperoside regulated the activation of p38 MAPK induced by
H2O2 in HUVECs, which demonstrated that the
antiapoptotic effect of hyperoside was likely associated with its
regulation of the p38 MAPK signaling pathway.
In conclusion, the present study demonstrated that
hyperoside protected HUVECs from H2O2-induced
apoptosis, which was likely associated with the regulation of
hyperoside on intracellular Ca2+ content, expression of
apoptosis-associated proteins (Bcl-2, Bax and Cleaved caspase-3)
and activation of the p38 MAPK. The findings suggested that
hyperoside protected cells from oxidative stress-induced injury,
which was likely associated with the prevention of cardiovascular
diseases.
Acknowledgments
The present study was financially supported by the
National Natural Science Foundation of China (nos. 81274132 and
81172938), and the Top Science and Technology Innovation Teams of
Higher Learning Institutions of Shanxi Province.
References
|
1
|
Deanfield JE, Halcox JP and Rabelink TJ:
Endothelial function and dysfunction: Testing and clinical
relevance. Circulation. 115:1285–1295. 2007.PubMed/NCBI
|
|
2
|
Matés JM and Sánchez-Jiménez FM: Role of
reactive oxygen species in apoptosis: Implications for cancer
therapy. Int J Biochem Cell Biol. 32:157–170. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harrison D, Griendling KK, Landrnesser U,
Hornig B and Drexler H: Role of oxidative stress in
atherosclerosis. Am J Cardiol. 91:7A–11A. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choy JC, Granville DJ, Hunt DW and McManus
BM: Endothelial cell apoptosis: Biochemical characteristics and
potential implications for atherosclerosis. J Mol Cell Cardiol.
33:1673–1690. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: The role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kasimu R, Fan Z, Wang X, Hu J, Wang P and
Wang J: Anti-platelet aggregation activities of different fractions
in leaves of Apocynum venetum L. J Ethnopharmacol. 168:116–121.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang W, Liang X, Fu D, Tie R, Xing W, Ji
L, Liu F, Zhang H and Li R: Apocynum venetum leaf attenuates
myocardial ischemia/reperfusion injury by inhibiting oxidative
stress. Am J Chin Med. 43:71–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lau YS, Kwan CY, Ku TC, Hsieh WT, Wang HD,
Nishibe S, Dharmani M and Mustafa MR: Apocynum venetum leaf
extract, an antihypertensive herb, inhibits rat aortic contraction
induced by angiotensin II: A nitric oxide and superoxide
connection. J Ethnopharmacol. 143:565–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HY, Wang JH, Geng M, Wu XQ, Yan L,
Huang K, Shao LM, Yang XB and Huang ZM: Protective effect of
extract of Apocynum venetum on kidneys of streptozotocin-induced
diabetic rats. Yao Xue Xue Bao. 45:26–30. 2010.In Chinese.
|
|
10
|
Xie W, Zhang X, Wang T and Hu J: Botany,
traditional uses, phytochemistry and pharmacology of Apocynum
venetum L. (Luobuma): A review. J Ethnopharmacol. 141:1–8. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ku SK, Zhou W, Lee W, Han MS, Na M and Bae
JS: Anti-inflammatory effects of hyperoside in human endothelial
cells and in mice. Inflammation. 38:784–799. 2015. View Article : Google Scholar
|
|
12
|
Kim YJ: Hyperoside and quercetin modulate
oxidative stress-induced melanogenesis. Biol Pharm Bull.
35:2023–2027. 2012. View Article : Google Scholar
|
|
13
|
Liu Z, Tao X, Zhang C, Lu Y and Wei D:
Protective effects of hyperoside (quercetin-3-o-galactoside) to
PC12 cells against cytotoxicity induced by hydrogen peroxide and
tert-butyl hydroperoxide. Biomed Pharmacother. 59:481–490. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li HB, Yi X, Gao JM, Ying XX, Guan HQ and
Li JC: The mechanism of hyperoside protection of ECV-304 cells
against tert-butyl hydroperoxide-induced injury. Pharmacology.
82:105–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ju HY, Chen SC, Wu KJ, Kuo HC, Hseu YC,
Ching H and Wu CR: Antioxidant phenolic profile from ethyl acetate
fraction of Fructus Ligustri Lucidi with protection against
hydrogen peroxide-induced oxidative damage in SH-SY5Y cells. Food
Chem Toxicol. 50:492–502. 2012. View Article : Google Scholar
|
|
16
|
Liu RL, Xiong QJ, Shu Q, Wu WN, Cheng J,
Fu H, Wang F, Chen JG and Hu ZL: Hyperoside protects cortical
neurons from oxygen-glucose deprivation-reperfusion induced
apoptosis via nitric oxide signal pathway. Brain Res. 1469:164–173.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li ZL, Liu JC, Hu J, Li XQ, Wang SW, Yi DH
and Zhao MG: Protective effects of hyperoside against human
umbilical vein endothelial cell damage induced by hydrogen
peroxide. J Ethnopharmacol. 139:388–394. 2012. View Article : Google Scholar
|
|
18
|
Tornero D, Posadas I and Ceña V: Bcl-x(L)
blocks a mitochondrial inner membrane channel and prevents
Ca2+ overload-mediated cell death. Plos One.
6:e204232011. View Article : Google Scholar
|
|
19
|
Chen ZW and Ma CG: Effects of hyperoside
on free intracellular calcium in dissociated neonatal rat brain
cells. Zhongguo Yao Li Xue Bao. 20:27–30. 1999.PubMed/NCBI
|
|
20
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cory S and Adams JM: The Bcl-2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antonsson B: Mitochondria and the Bcl-2
family proteins in apoptosis signaling pathways. Mol Cell Biochem.
256–257:141–155. 2004. View Article : Google Scholar
|
|
23
|
Hetz C, Bernasconi P, Fisher J, Lee AH,
Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A,
Glimcher LH and Korsmeyer SJ: Pro-apoptotic Bax and Bak modulate
the unfolded protein response by a direct interaction with
IRE1alpha. Science. 312:572–576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Youle RJ and Strasser A: The Bcl-2 protein
family: Opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar
|
|
25
|
Riedl SJ and Shi YG: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Runchel C, Matsuzawa A and Ichijo H:
Mitogen-activated protein kinases in mammalian oxidative stress
responses. Antioxid Redox Signal. 15:205–218. 2011. View Article : Google Scholar
|
|
27
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|