Introduction
Menopause is a natural process that occurs in every
woman's life cycle. Due to the increasing average lifespan of
humans, ~40% of women's lives occur after menopause. The marked
decrease in ovarian secretion of estrogens that occurs around
menopause has been hypothesized as a main cause of numerous
diseases, including osteoporosis and stroke (1,2). In
addition, the association between menopause and pathophysiological
alterations in the liver, which is the primary target organ of
estrogen, has recently attracted more attention. Non-alcoholic
fatty liver disease (NAFLD), which is characterized by excessive
hepatic lipid accumulation due to causes other than significant
alcohol consumption, is considered a risk factor for type 2
diabetes and cardiovascular disease (3). Excessive hepatic fat accumulation can
be caused by increased hepatic fat synthesis and delivery, and by
reduced fat oxidation and exportation. It has previously been
reported that the prevalence of NAFLD is lower in premenopausal
women than in men, whereas the prevalence markedly increases in
postmenopausal women, consequently exceeding that in men (4). Basic and clinical studies have
suggested that estrogens protect from the development of NAFLD
(5,6); however, little is currently known
regarding the mechanisms by which low levels of estrogen contribute
to fatty liver disease. Clarifying the underlying mechanisms may
provide useful information regarding the prevention and treatment
of fatty liver disease in postmenopausal women.
Dysregulated hepatic lipid metabolism is considered
a prerequisite for the development of NAFLD. Triglyceride (TG),
which is the product of a condensation reaction between glycerol
and free fatty acids, is the main form of hepatic lipid. An
imbalance between the synthesis and secretion of TG results in its
accumulation in liver cells, which is the main feature of fatty
liver disease (7). Previous
studies have reported an association between aquaporin 7 (AQP7), a
water-glycerol transporter, and adult-onset obesity. In adipocytes,
AQP7 deficiency increased glycerol kinase activity, enhancing TG
synthesis and ultimately leading to obesity (8,9).
Given the close relationship between AQP7 and lipid metabolism, the
present study hypothesized that AQP7 may serve a role in other
tissues with active lipid metabolism, such as the liver. AQP2,
another member of the AQP family, has been identified as a target
of estrogen, which mediates estrogen-enhanced migration and
invasion of Ishikawa cells (10).
Therefore, the present study aimed to determine whether AQP7 may
act as a target gene of estrogen and serve a role in low level
estrogen-induced fatty liver disease.
The present study investigated the role of AQP7 in
low estrogen-induced hepatic steatosis using an ovariectomized
(OVX) mouse model. To gain further insights into the underlying
mechanism of action, the effects of AQP7-specific small interfering
(si)RNA and estrogen were determined in an oleic acid (OA)-induced
cell model of steatosis.
Materials and methods
Experimental design
All experiments were approved by the ethics
committee of the Zhujiang Hospital of Southern Medical University,
(Guangzhou, China). C57/BL6 female mice (age, 6 weeks) were
purchased from the Shanghai Experimental Animal Center (Shanghai,
China), and were maintained under standard conditions in accordance
with the National Institutes of Health Guide for the Care and Use
of Laboratory Animals (11). Mice
were randomly divided into four groups (n=6/group): Group 1, which
underwent a sham operation; group 2, which underwent a sham
operation and were administered a subcutaneous implantation of
tamoxifen [TAM; Innovative Research of America (Sarasota, FL, USA)]
sustained release tablet; group 3, which underwent bilateral
ovariectomy (OVX); and group 4, which underwent OVX and were
administered a subcutaneous implantation of 17β-estradiol sustained
release tablet (E2; 0.25 mg/pellet; 60-day release; Innovative
Research of America, Sarasota, FL, USA) at the time of OVX. All
mice were anesthetized anaesthetized with sodium pentobarbitol
(0.04 mg/g; Propbs Biotechnology, Beijing, China.) In the OVX
group, after an abdominal incision was performed, ligation was
placed around the oviduct and blood vessels, the ovaries were
removed through an incision, and the incision was closed with
suture. For the sham operation, group, abdominal incision was
performed followed by closure with suture. All mice were sacrificed
by cervical dislocation 8 weeks post-treatment. Liver specimens
were subsequently excised, and were stored in liquid nitrogen or
fixed in 4% (w/v) paraformaldehyde.
Histological analysis of tissues
Liver samples were fixed with 4% (w/v)
paraformaldehyde overnight. Sections (5-µm) were prepared
from the paraffin-embedded tissues and were analyzed by
hematoxylin-eosin (H&E) staining. For Oil Red O (ORO) staining,
8-µm sections were prepared from the frozen tissues, and
were stained with ORO (Sigma-Aldrich, St. Louis, MO, USA) and
lightly counterstained with hematoxylin, as described previously
(12) and visualized using a light
microscope (ECLIPSE Ti-S; Nikon Corporation, Tokyo, Japan).
Cell culture and OA-induced
steatosis
HepG2 cells were purchased from the Cell Bank of the
Shanghai Branch of the Chinese Academy of Sciences (Shanghai,
China) and were cultured in Dulbecco's modified Eagle's medium
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (Hyclone; GE Healthcare Life Sciences)
and 100 U/ml penicillin/streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The HepG2 cells were seeded
into 6-well plates and were divided into four groups: siRNA
negative control (siNC)-transfected group, siAQP7-transfected
group, siNC + estradiol (E2)-treated group, and siAQP7 + E2-treated
group.
HepG2 cells were transfected with the indicated
siRNA oligonucleotides (Shanghai GenePharma Co., Ltd., Shanghai,
China; siAQP7, TAG CCA TGA ACT CTG GATTGT) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, siRNA (50 pmol) and 5 µl Lipofectamine®
2000 were incubated separately with Opti-MEM (Invitrogen; Thermo
Fisher Scientific, Inc.) for 5 min, and were then mixed together
for 20 min at room temperature. Subsequently, the mixture was
applied to the cells plated in 2 ml medium. A total of 48 h
post-transfection, the HepG2 cells were treated with 1 mM OA
solution (Sigma-Aldrich), and E2-bovine serum albumin solution (1
µM; Sigma-Aldrich) was added to the E2-treated group. After
24 h, the medium was removed and the cells were fixed with 4%
paraformaldehyde at room temperature for 10 min; the cells were
then stained with ORO solution (Sigma-Aldrich) and lightly
counterstained with hematoxylin. After the cells were dried and
mounted with glycerin, they were examined under a light microscope
(ECLIPSE Ti-S; Nikon Corporation). Red oil droplets in the cells
were considered to indicate OA-induced steatosis.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from the liver tissues or
from HepG2 cells using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) following homogenization with a
tissue grinder in TRIzol®, according to the
manufacturer's protocol. DNase I-treated (Sigma-Aldrich) RNA was
reverse transcribed using the Superscript III enzyme (Invitrogen;
Thermo Fisher Scientific, Inc.). DNase I-treated RNA was reverse
tran scribed using the Superscript III enzyme (Invitrogen; Thermo
Fisher Scientific, Inc.) by incubation at 37°C for 60 min, 85°C for
5 min and 4°C for 5 min. qPCR was performed with 5 µl
SYBRGreen Mix (Thermo Fisher Scientific, Inc.), 0.5 µl
forward primer, 0.5 µl reverse primer, 2 µl cDNA
template and 9.5 µl ddH2O, on an ABI 7300
real-time PCR machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Primers were purchased from Generay (Shanghai,
China) Thermal cycling conditions were as follows: 95°C for 10 min,
followed by 40 cycles at 95°C for 15 sec and 60°C for 45 sec.
Primer sequences are listed in Table
I. Gene expression was determined using the ΔΔCq method
(13). All data represent the
average of three replicates.
 | Table IPrimer sequences for quantitative
polymerase chain reaction. |
Table I
Primer sequences for quantitative
polymerase chain reaction.
| Primer | Primer sequence | Size (bp) |
|---|
| AQP7 (Mus
musculus) | F:
5′-TGTCTCTTCGCCATCACC-3′ | 213 |
| (NM_007473.4) | R:
5′-CCACCACCAGTTGTTTCC-3′ | |
| ACC (Mus
musculus) | F:
5′-TGCTCCAGGCTAAGCGATTC-3′ | 208 |
| (NM_133904.2) | R:
5′-ATGCCCACCTCGTTACAACC-3′ | |
| FAS (Mus
musculus) | F:
5′-GGGTGGATGCAACTTTAATG-3′ | 134 |
| (NM_007988) | R:
5′-AAAGCACCAGTTCACAGATG-3′ | |
| GPAT (Mus
musculus) | F:
5′-GTTCATCCAGTATGGCATTC-3′ | 130 |
| (NM_008149.3) | R:
5′-TTCATCTTCCTCGTCACTTC-3′ | |
| GAPDH (Mus
musculus) | F:
5′-ATCACTGCCACCCAGAAG-3′ | 191 |
| (NM_008084.2) | R:
5′-TCCACGACGGACACATTG-3′ | |
| AQP7 (Homo
sapiens) | F:
5′-ACGGACCAGGAGAACAAC-3′ | 160 |
| (NM_001170.1) | R:
5′-CCCAACCAGCAATGAAGG-3′ | |
| ACC (Homo
sapiens) | F:
5′-GCAGGTATCCCAACTCTTC-3′ | 139 |
| (NM_198834.2) | R:
5′-GTAGCCCATCATCCACATC-3′ | |
| FAS (Homo
sapiens) | F:
5′-TGCCAAGAAGGGAAGGAG-3′ | 238 |
| (NM_004104.4) | R:
5′-TGGTGTTGCTGGTGAGTG-3′ | |
| GPAT(Homo
sapiens) | F:
5′-GCTGCTCACTTTCATTCTC-3′ | 159 |
|
(NM_001244949.1) | R:
5′-ACATCTTTCCGTCCATCTG-3′ | |
| GAPDH (Homo
sapiens) | F:
5′-CACCCACTCCTCCACCTTTG-3′ | 110 |
|
(NM_001256799.1) | R:
5′-CCACCACCCTGTTGCTGTAG-3′ | |
Western blot analysis
Protein was extracted from ~0.2 g of tissue sample
by homogenization with a tissue grinder in
radio-immunoprecipitation assay cell lysis buffer (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China), and the
protein concentration in the lysates was quantified using an
enhanced bicinchoninic acid protein assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). Equal amounts of protein
(50–80 µg/lane) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and were electroblotted
onto nitrocellulose membranes (EMD Millipore, Bedford, MA, USA).
The blots were blocked with 5% nonfat dry milk for 1 h and were
then incubated with primary antibodies [AQP7 (rabbit polyclonal
antibody, 1:500 cat no. ab32826), fatty acid synthase (FAS; rabbit
monoclonal antibody; 1:1,000; cat no. ab128870),
glycerol-3-phosphate acyltransferase (GPAT; rabbit polyclonal
antibody, 1:800; Sigma-Aldrich; cat no. PRS4613), acetyl-CoA
carboxylase (ACC; rabbit polyclonal antibody; 1:800; cat no. 3662),
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; rabbit monoclonal
antibody; 1:1500; cat no. 5174) both Cell Signaling Technology,
Inc. Danvers, MA, USA] overnight at 4°C, followed by appropriate
polycolonal goat anti-rabbit secondary antibody; Beyotime Institute
of Biotechnology, Haimen, China (1:1,000; cat no. A0208) for 1 h at
room temperature. Detection of specific proteins was performed by
enhanced chemiluminescence (EMD Millipore). Band density was
measured using ImageJ software (version 1.49; National Institutes
of Health, Bethesda, MD, USA) and expression levels were normalized
to GAPDH. Antibodies against AQP7, FAS and GPAT were purchased from
Abcam (Cambridge, MA, USA), and antibodies against ACC and GAPDH
were purchased from Cell Signaling Technology, Inc.
Determination of TG contents
Cell lysates were prepared as aforementioned, and TG
contents in the cell lysates were determined using a colorimetric
assay (TG assay kit; Nanjing Jiancheng Bioengineering Institute).
Results were expressed as mol of TG/g of cellular protein, as
described previously (14).
Statistical analysis
Data are presented as the mean ± standard deviation.
Data was analyzed using GraphPad Software (version 6.0; GraphPad
Software, Inc. La Jolla, CA, USA) Differences between the groups
were determined by one-way analysis of variance, followed by
Sidak's test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Estrogen protects mice from OVX-induced
hepatic steatosis
OVX is often used as an experimental animal model to
simulate menopause (15,16), and TAM is an antagonist to estrogen
(17). In order to study the
effects of estrogen on hepatic steatosis, 24 mice were randomly
divided into four groups (n=6/group): Sham operation group, sham
operation + TAM group, OVX group, or OVX + E2 group. After 8 weeks,
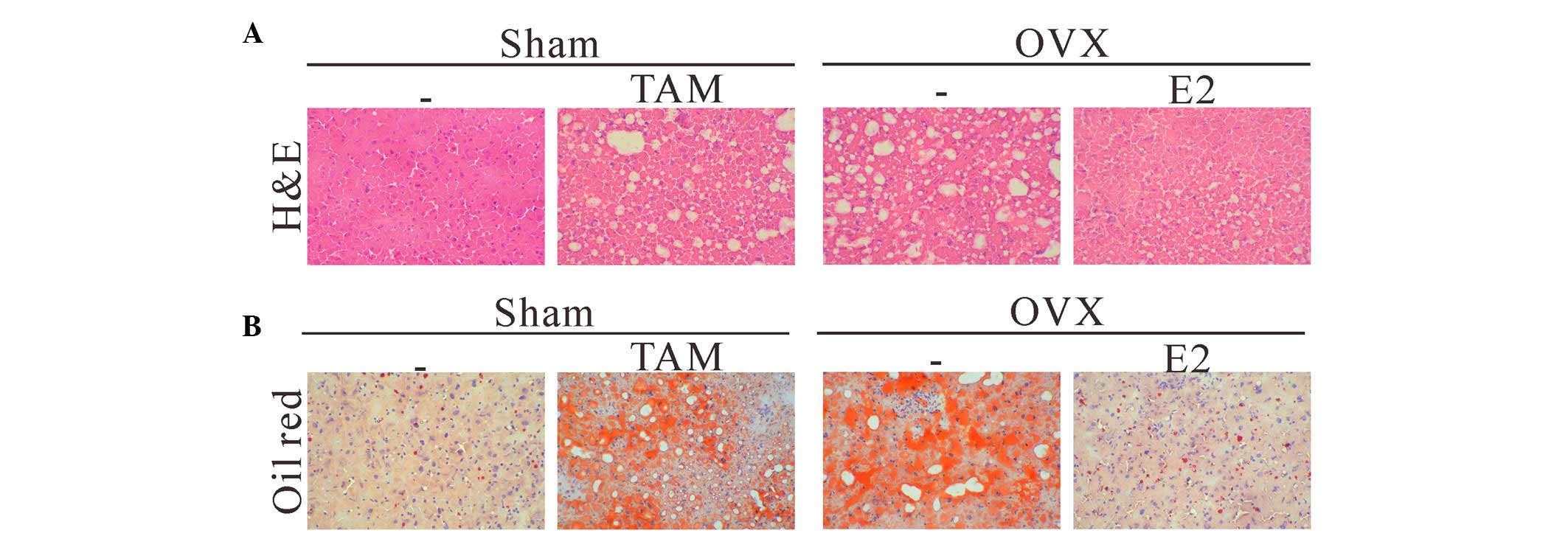
liver specimens were collected. Histological examination of the
H&E-stained liver sections revealed that TAM or OVX led to
marked fat accumulation in the liver specimens, as compared with in
the sham operation group (Fig.
1A). Moderate to marked macrovesicular steatosis without
pattern was present throughout the hepatic lobule, whereas mice in
the sham operation group displayed minimal evidence of hepatic fat
accumulation. Furthermore, treatment with E2 alleviated hepatic
steatosis in the mice following OVX. ORO staining further confirmed
that lipid accumulation was excessive in the livers of the sham
operation + TAM and OVX groups (Fig.
1B), whereas it was normal in the sham operation and OVX + E2
groups. These data indicate that estrogen may exert inhibitory
effects on hepatic steatosis.
Estrogen suppresses the expression of
lipogenic genes
To gain further insight into the mechanisms
underlying the protective effects of estrogen against liver
steatosis, the present study analyzed the hepatic expression levels
of genes associated with lipogenesis and fatty acid oxidation. As
shown in Fig. 2, the mRNA and
protein expression levels of lipogenic genes: ACC, FAS and GPAT,
were significantly increased in the livers of the sham + TAM and
OVX groups, as compared with in the sham operation group. Treatment
with E2 notably decreased the expression levels of these genes.
These data suggest that estrogen may reduce liver lipid
accumulation via inhibiting the expression of lipogenic genes.
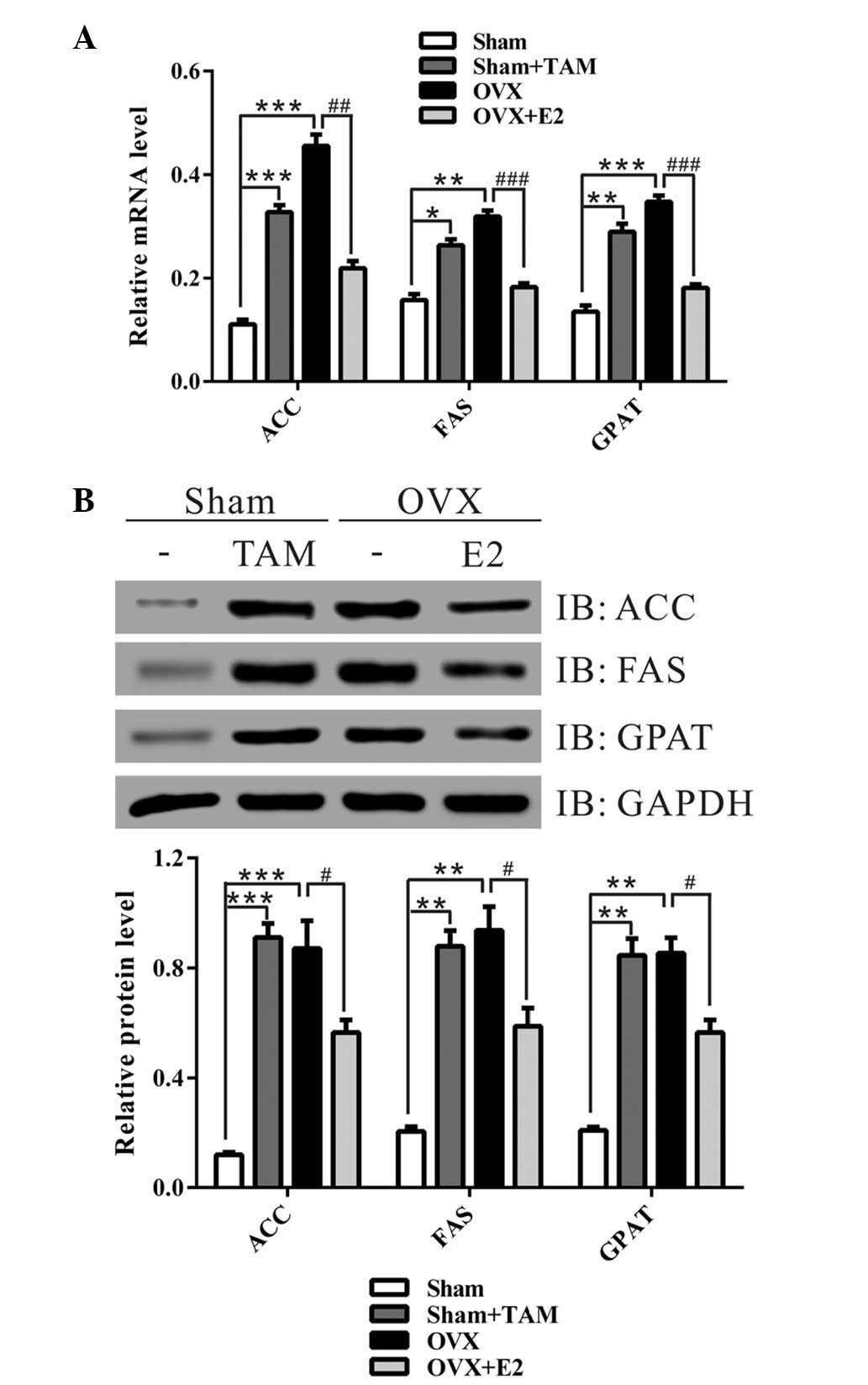 | Figure 2Treatment with estrogen at the time of
ovariectomy (OVX) decreased the expression of lipid-associated
enzymes. (A) Quantitative polymerase chain reaction and (B) western
blot analysis of the liver specimens from the four groups.
Representative western blot images are presented. Data from three
independent experiments are presented as the mean ± standard
deviation. *P<0.05, **P<0.01,
***P<0.001 vs. Sham group; #P<0.05,
##P<0.01, ###P<0.001 vs. OVX group. TAM,
tamoxifen; E2, 17β-estradiol; ACC, acetyl-CoA carboxylase; FAS,
fatty acid synthase; GPAT, glycerol-3-phosphate acyltransferase;
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IB,
immunoblot. |
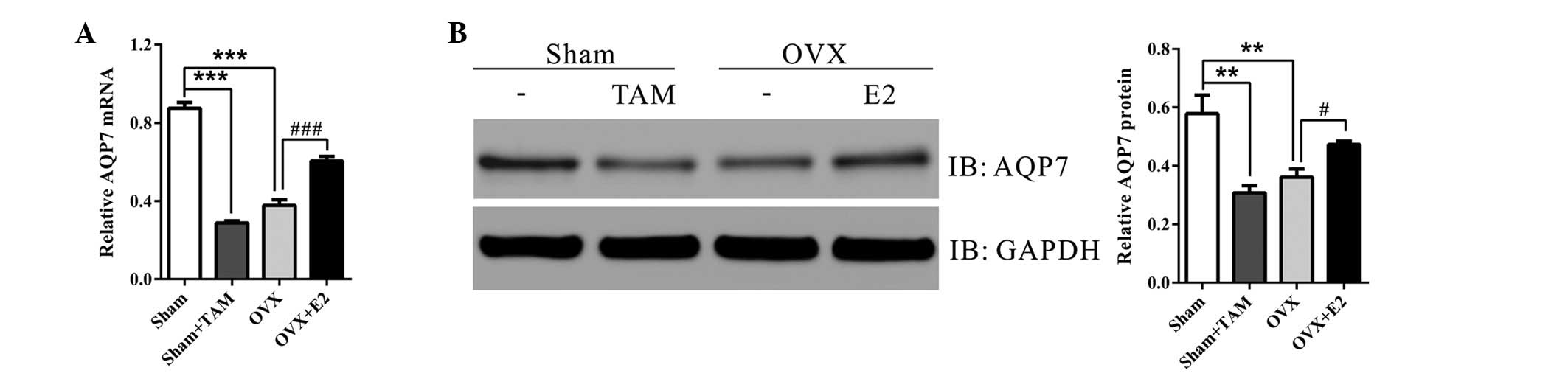
Estrogen increases the expression of
AQP7
To investigate the involvement of AQP7 in
OVX-induced hepatic steatosis, the present study detected the
hepatic mRNA and protein expression levels of AQP7 in the four
groups. As shown in Fig. 3, TAM
treatment and OVX significantly decreased the mRNA and protein
expression levels of AQP7, as compared with in the sham operation
group. Treatment with E2 at the time of OVX markedly increased the
expression levels of AQP7. These data provide evidence of an
association between AQP7 and OVX-induced hepatic steatosis.
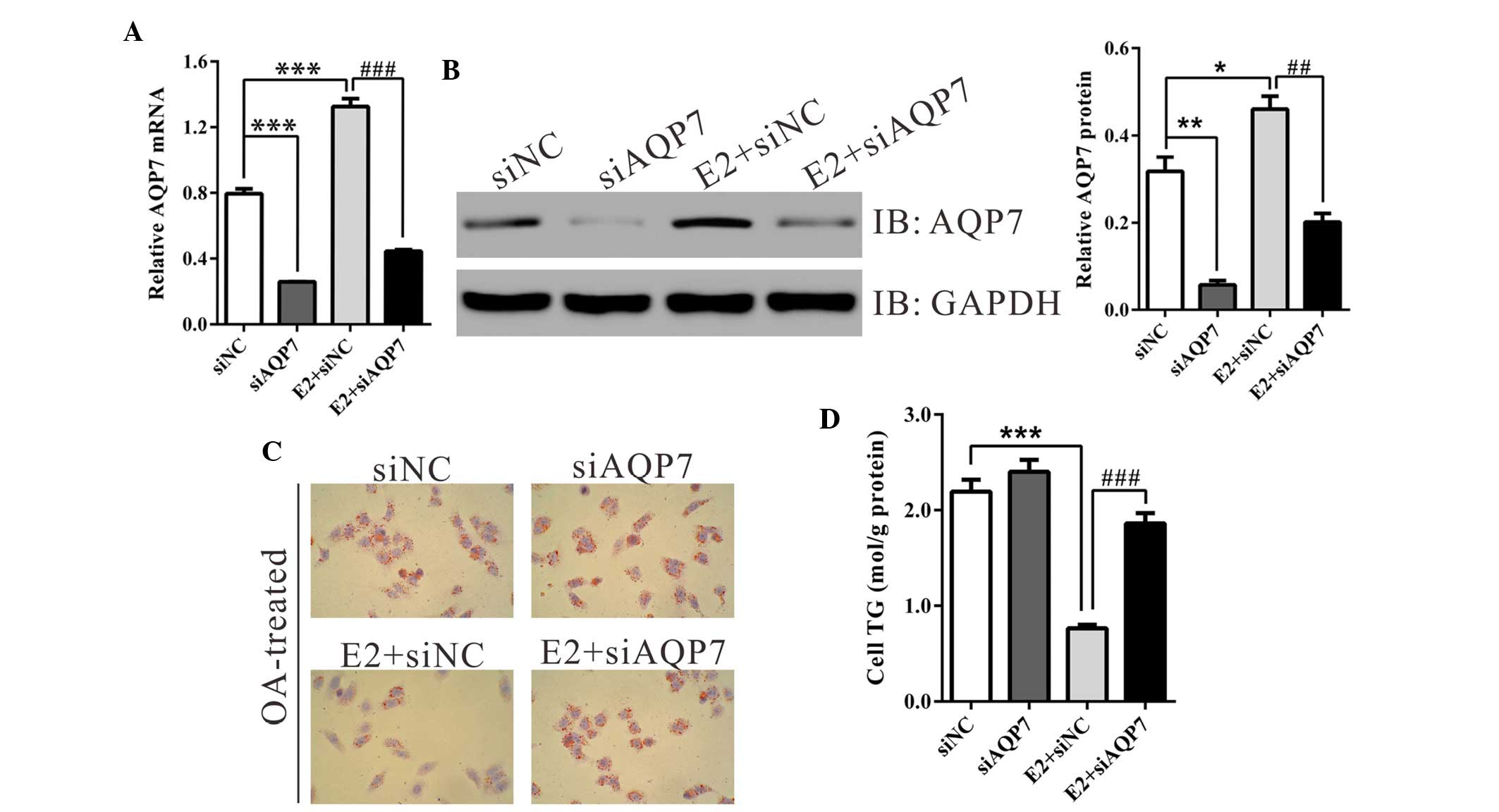
Estrogen improves OA-induced steatosis in
HepG2 cells
In order to determine the function of estrogen and
AQP7 on hepatic steatosis in vitro, an OA-induced steatosis
model was established in HepG2 cells, as described previously
(18). Cells were divided into
four groups: Cells transfected with siNC, cells transfected with
siAQP7, cells transfected with siNC and treated with E2, and cells
transfected with siAQP7 and treated with E2. In line with the in
vivo findings, treatment with E2 in vitro increased the
mRNA and protein expression levels of AQP7 by 69.3 and 66.6%,
respectively. AQP7-specific siRNA efficiently suppressed the
expression of AQP7; the efficiency was >65% (Fig. 4A and B).
Following a 24-h incubation with OA, HepG2 cells in
the siNC-transfected group developed marked steatosis, which
manifested as an accumulation of lipid droplets in the cytoplasm.
Treatment with E2 significantly decreased the number of lipid
droplets (Fig. 4C), whereas
transfection of the HepG2 cells with siAQP7 attenuated the
protective effects of E2 on lipid accumulation.
Consistent with these alterations, a marked
reduction in TG content was observed in the E2-treated group, as
compared with in the corresponding control group (siNC, 2.19±0.13;
E2 + siNC, 0.76±0.04; Fig. 4D).
siAQP7 transfection significantly increased TG content by 144% in
cells treated with E2. These data indicate that estrogen may
protect hepatocytes from steatosis via increasing the expression of
AQP7.
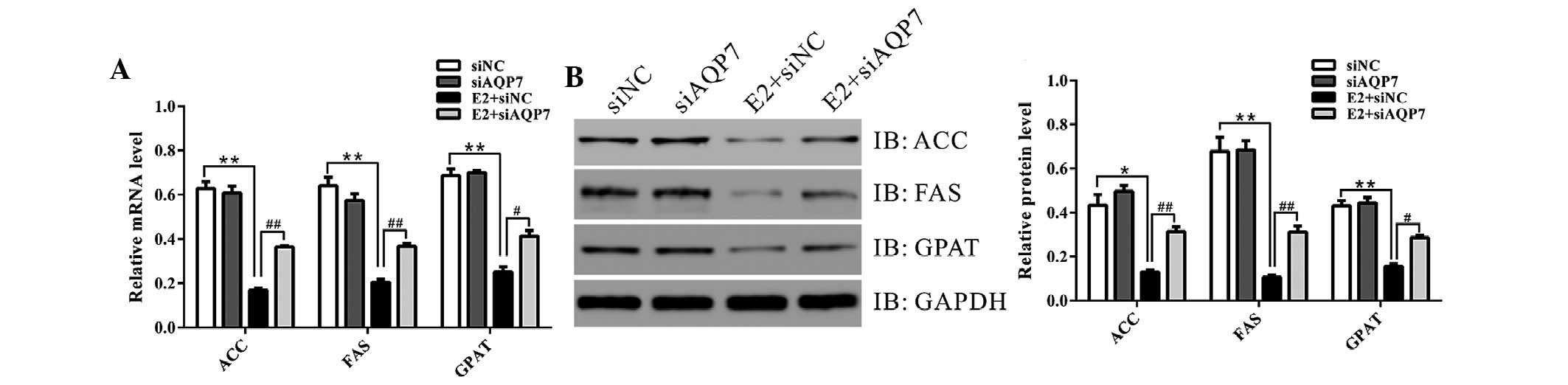
AQP7 siRNA suppresses the expression of
lipid-associated enzymes in vitro
The present study analyzed the expression levels of
genes associated with lipogenesis and fatty acid oxidation in
vitro. Treatment with E2 resulted in a marked decrease in ACC,
FAS and GPAT expression (Fig. 5),
which was partially inhibited by knockdown of AQP7 expression.
These data suggest that estrogen may suppress lipogenesis via
increasing AQP7 expression.
Discussion
Human menopause is associated with an elevated risk
of NAFLD; however, the underlying molecular mechanisms remain
unclear. The present study evaluated the effects of estrogen
depletion on hepatic steatosis using an OVX mouse model (Figs. 1 and 2), which is often used as an experimental
animal model to simulate menopause, and is associated with
increased risk of bone loss (19),
visceral obesity (20) and hepatic
steatosis (21). Liver specimens
from the OVX or TAM (estrogen antagonist)-treated mice displayed
visible steatosis with increased expression of lipogenic genes
(ACC, FAS and GPAT), whereas E2 treatment alleviated OVX-induced
hepatic steatosis and decreased the expression levels of lipogenic
genes. The present study proposed an inhibitory effect of E2 on
lipogenesis and lipid accumulation, which was consistent with
previous studies in human adipose tissue (22) and rat liver (23).
Estrogen regulates human physiology via signaling
through intracellular hormone-specific estrogen receptors (ERs).
Dimeric ERs directly bind to specific DNA sequences of target
genes, known as estrogen response elements (EREs), thus mediating
their expression (24). Functional
EREs have been detected in AQPs, including AQP5 (25) and AQP2 (10). Previous studies have demonstrated
that AQP7 modulates adipocyte glycerol permeability, thereby
controlling TG accumulation and fat cell size (8,9). The
present study hypothesized that AQP7 may act as a target gene of
estrogen and serve a role in OVX-induced hepatic steatosis. The
results demonstrated that OVX and TAM treatment significantly
decreased the hepatic expression levels of AQP7 (Fig. 3), thus suggesting the involvement
of AQP7 in low estrogen-induced lipid accumulation. In order to
further investigate the function of AQP7 during estrogen
depletion-induced lipid accumulation, AQP7-specific siRNA and an
OA-induced HepG2 cell model of steatosis was used. Treatment with
E2 decreased lipid drop formation and TG content in HepG2 cells,
whereas such effects were attenuated by AQP7 siRNA (Fig. 4). Analysis of lipogenic gene
expression (Fig. 5) further
indicated that estrogen was able to decrease lipogenesis by
increasing AQP7 expression; however, the mechanisms underlying the
hormone-induced regulation of AQP7 transcription require further
in-depth investigation.
In conclusion, the present study indicated that
estrogen exposure alleviated hepatic steatosis by regulating the
expression of its target, AQP7, in an OVX mouse model and a
cellular model. In addition to its function in glycerol transport,
AQP7 serves an important role in lipogenesis. The present study
provided potential targets for the prevention and treatment of
fatty liver disease in postmenopausal women.
Acknowledgments
The present study was supported by the Zhejiang
Provincial Natural Science Foundation of China (grant no. LQ
13H040002) and National Natural Science Foundation of China (grant
no. 81200251).
References
|
1
|
Winkler UH: Menopause, hormone replacement
therapy and cardiovascular disease: A review of haemostaseological
findings. Fibrinolysis. 6:5–10. 1992. View Article : Google Scholar
|
|
2
|
Carr MC: The emergence of the metabolic
syndrome with menopause. J Clin Endocrinol Metab. 88:2404–2411.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marchesini G, Brizi M, Bianchi G,
Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S,
Forlani G and Melchionda N: Nonalcoholic fatty liver disease: A
feature of the metabolic syndrome. Diabetes. 50:1844–1850. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki A and Abdelmalek MF: Nonalcoholic
fatty liver disease in women. Womens Health (Lond Engl). 5:191–203.
2009. View Article : Google Scholar
|
|
5
|
Lonardo A, Carani C, Carulli N and Loria
P: 'Endocrine NAFLD' a hormonocentric perspective of nonalcoholic
fatty liver disease pathogenesis. J Hepatol. 44:1196–1207. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu L, Brown WC, Cai Q, Krust A, Chambon
P, McGuinness OP and Stafford JM: Estrogen treatment after
ovariectomy protects against fatty liver and may improve
pathway-selective insulin resistance. Diabetes. 62:424–434. 2013.
View Article : Google Scholar :
|
|
7
|
Farrell GC and Larter CZ: Nonalcoholic
fatty liver disease: From steatosis to cirrhosis. Hepatology. 43(2
Suppl 1): S99–S112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frühbeck G, Catalán V, Gómez-Ambrosi J and
Rodríguez A: Aquaporin-7 and glycerol permeability as novel obesity
drug-target pathways. Trends Pharmacol Sci. 27:345–347. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodríguez A, Catálan V, Gómez-Ambrosi J
and Frühbeck G: Role of aquaporin-7 in the pathophysiological
control of fat accumulation in mice. FEBS Lett. 580:4771–4776.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zou LB, Zhang RJ, Tan YJ, Ding GL, Shi S,
Zhang D, He RH, Liu AX, Wang TT, Leung PC, et al: Identification of
estrogen response element in the aquaporin-2 gene that mediates
estrogen-induced cell migration and invasion in human endometrial
carcinoma. J Clin Endocrinol Metab. 96:E1399–E1408. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Institute of Laboratory Animal Resources
(US) Committee on Care, Use of Laboratory Animals, and National
Institutes of Health (US): Division of Research Resources: Guide
for the care and use of laboratory animals. 8th edition. National
Academies Press; Washington, DC: 2011
|
|
12
|
Yuan H, Zhang H, Wu X, Zhang Z, Du D, Zhou
W, Zhou S, Brakebusch C and Chen Z: Hepatocyte-specific deletion of
Cdc42 results in delayed liver regeneration after partial
hepatectomy in mice. Hepatology. 49:240–249. 2009. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Ricchi M, Odoardi MR, Carulli L, Anzivino
C, Ballestri S, Pinetti A, Fantoni LI, Marra F, Bertolotti M, Banni
S, et al: Differential effect of oleic and palmitic acid on lipid
accumulation and apoptosis in cultured hepatocytes. J Gastroenterol
Hepatol. 24:830–840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mori-Okamoto J, Otawara-Hamamoto Y, Yamato
H and Yoshimura H: Pomegranate extract improves a depressive state
and bone properties in menopausal syndrome model ovariectomized
mice. J Ethnopharmacol. 92:93–101. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bekku N and Yoshimura H: Animal model of
menopausal depressive-like state in female mice: Prolongation of
immobility time in the forced swimming test following ovariectomy.
Psychopharmacology (Berl). 183:300–307. 2005. View Article : Google Scholar
|
|
17
|
Shou J, Massarweh S, Osborne CK, Wakeling
AE, Ali S, Weiss H and Schiff R: Mechanisms of tamoxifen
resistance: Increased estrogen receptor-HER2/neu cross-talk in
ER/HER2-positive breast cancer. J Natl Cancer Inst. 96:926–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okamoto Y, Tanaka S and Haga Y: Enhanced
GLUT2 gene expression in an oleic acid-induced in vitro fatty liver
model. Hepatol Res. 23:138–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pacifici R: Estrogen, cytokines, and
pathogenesis of postmenopausal osteoporosis. J Bone Miner Res.
11:1043–1051. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gloy V, Langhans W, Hillebrand JJ, Geary N
and Asarian L: Ovariectomy and overeating palatable, energy-dense
food increase subcutaneous adipose tissue more than intra-abdominal
adipose tissue in rats. Biol Sex Differ. 2:62011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chung YH, Huang CC, Chiu SC, Lin YH and
Yen TC: Evaluate non-alcoholic fatty liver disease in
ovariectomized mice as a model of postmenopausal women using Tc-99m
Disofenin scintigraphy and ultrasound imaging. J Nucl Med. 55(Suppl
1): p352014.
|
|
22
|
Lundholm L, Zang H, Hirschberg AL,
Gustafsson JA, Arner P and Dahlman-Wright K: Key lipogenic gene
expression can be decreased by estrogen in human adipose tissue.
Fertil Steril. 90:44–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paquette A, Wang D, Jankowski M, Gutkowska
J and Lavoie JM: Effects of ovariectomy on PPAR alpha, SREBP-1c,
and SCD-1 gene expression in the rat liver. Menopause.
15:1169–1175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carroll JS and Brown M: Estrogen receptor
target gene: An evolving concept. Mol Endocrinol. 20:1707–1714.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kobayashi M, Takahashi E, Miyagawa S,
Watanabe H and Iguchi T: Chromatin immunoprecipitation-mediated
target identification proved aquaporin 5 is regulated directly by
estrogen in the uterus. Genes Cells. 11:1133–1143. 2006. View Article : Google Scholar : PubMed/NCBI
|