Introduction
Hypopharyngeal squamous cell carcinoma (HSCC) is
less prevalent, compared with cancer at other major sites of the
head and neck, including the oral cavity, larynx and oropharynx,
with <3,000 cases reported in the United States annually
(1). The majority of patients with
HSCC present with significant comorbidities and advanced stages of
disease. The anatomical proximity of the larynx, advanced stage of
disease at presentation, and higher rates of regional and distant
metastases result in poor rates of prognosis. These factors require
consideration when making treatment decisions. Traditionally,
laryngopharyngectomy with reconstruction of the pharynx has been
the preferred initial treatment for locally advanced operable HSCC.
Efforts to preserve vocal and swallowing functions have resulted in
surgical organ-preserving procedures with improved reconstructive
efforts and/or chemoradiation. Despite advances in surgical
methods, chemotherapy and radiotherapy, the prognosis of patients
with advanced HSCC has not shown satisfactory improvement (2–4).
Therefore, in order to provide more effective treatment to patients
with advanced HSCC, otolaryngologists have increasingly focussed on
novel treatment modalities, including molecular-targeted
therapies.
Livin is a member of the human inhibitor of
apoptosis protein (IAP) family (5). The IAPs comprise a group of
structurally related proteins with anti-apoptotic potential
(6,7). The IAPs may be involved in preventing
tumor cell apoptosis and, therefore, may contribute to
tumorigenesis (8,9). High expression levels of Livin in
neoplasms correlate with more aggressive behavior, leading to
shorter disease-free survival rates, shorter overall survival rates
and chemoresistance (8,9). Furthermore, Livin is expressed at
high levels in various human cancer tissues (8–10).
Therefore, Livin has become the focus of substantial
investigations, however, the role of Livin in human HSCC remains to
be fully elucidated.
Several of the molecular alterations associated with
tumorigenesis occur in cell signaling pathways, which are
responsible for regulating cell proliferation and apoptosis. The
exact mechanism underlying the anti-apoptotic action of Livin
remains to be elucidated. The mitogen-activated protein kinase
(MAPK) pathway is involved in the regulation of cellular functions,
including cell proliferation, differentiation and death (11,12).
Several studies have reported that the activation of MAPK has a
functional role in carcinogenesis (13,14).
However, the impact of Livin on MAPK signaling remains to be
elucidated.
In the present study, the gene expression of Livin
was examined in fresh tissues of advanced cases of HSCC. The
present study also investigated whether Livin affects tumor cell
proliferation, cell cycle progression and oncogenic signaling
pathways, including MAPK and Akt signaling, in human HSCC cell
lines. This study may provide the basis for the application of
Livin as a molecular targeted therapy for HSCC.
Materials and methods
Patients and tumor specimens
Fresh HSCC tissues and paired normal hypopharyngeal
mucosal tissues were collected from four patients (males; aged
50–75 years old) who underwent definitive surgery for HSCC at
Chonnam National University Hwasun Hospital (Jeonnam, Korea). The
protein and mRNA expression levels of Livin were analyzed in these
HSCC tissues and paired normal hypopharyngeal mucosal tissues. The
study was approved by the ethics committee of the Institutional
Review Board of Chonnam National University Hwasun Hospital
(Gwangju, Korea). Written informed consent was obtained from each
patient prior to tissue acquisition.
Cell culture and transfection
The human HSCC cell line, SNU-1041, was provided by
Dr. Sung MW (Seoul National University, Seoul, South Korea). The
cells were cultured in RPMI 1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal
bovine serum (GE Healthcare Life Sciences, Logan, UT, USA), in a
humidified atmosphere of 5% CO2 at 37°C. Small
interfering RNA (siRNA) was used to knock down the endogenous gene
expression of Livin in SNU-1041 cells. SNU-1041 cells were seeded
onto 6-well plates at 2×105 cells/well and transfected
with Livin-specific siRNA (Bioneer, Daejeon, Korea) or negative
control siRNA (Qiagen, Germantown, MD, USA), using Lipofectamine
RNAiMAX (Invitrogen; Thermo Fisher Scientific, Inc.) for 48h at
37°C.
Cell proliferation assay
The cells were seeded into a 96-well plate
(103 cells/well) and, the following day, were
transfected with Livin siRNA and negative control siRNA. Following
incubation for 24 h at 37°C, cell proliferation and viability were
measured using an EZ-CyTox (water-soluble tetrazolium salt; WST-1)
cell viability assay kit (Daeil Lab, Inc., Seoul, South Korea) at
0, 24, 48 and 72 h. Following the addition of WST-1 reagent for 1-2
h at 37°C, the absorbance at 460 nm was determined using a
microplate reader (Infinite M200; Tecan, Austria GmbH, Austria)
with Magellan V6 data analysis software (Tecan). The ratio of the
absorbance of the transfected cells to that of the negative control
cells at 0 h was calculated.
Cell cycle analysis
The cells transfected with Livin siRNA or negative
control siRNA were collected using trypsin, washed with cold
phosphate-buffered saline (PBS) and fixed in ice-cold 70% ethanol
to determine cell cycle distribution. The fixed cells were washed
with PBS and resuspended in 0.1% sodium citrate, 0.1% Triton X-100
and 50 μg/ml propidium iodide for 20 min at room temperature
in the presence of 10 μg/ml ribonuclease A (Sigma-Aldrich,
St. Louis, MO, USA). At least 10,000 events were collected for each
histogram. Cell cycle analysis was performed using a FACSCalibur
flow cytometer (BD Biosciences, San Diego, CA, USA). Data analysis
was performed using WinMDI version 2.9 (The Scipps Research
Institute, San Diego, CA, USA).
Protein isolation and western blot
analysis
The cells were lysed in radioimmunoprecipitation
assay buffer (Biosesang, Inc., Sungnam, Korea) and protein
concentrations were determined using a bicinchoninic acid assay.
Protein lysates (20–30 μg) were separated by SDS-PAGE
(10–12%), then electrophoretically transferred onto polyvinylidene
fluoride membranes. The membranes were incubated for 1 h in 5%
bovine serum albumin (Bioshop Canada Inc., Burlington, ON, Canada)
in Tris-buffered saline (TBS)-Tween 20 at room temperature and
washed 4 times for 15 min with TBS-Tween 20. Specific proteins were
sequentially blotted with primary antibodies against Livin (cat.
no. 5471), phosphorylated (phospho)-Akt (cat. no. 4060),
phospho-extracellular signal-regulated kinase (ERK) 1/2 (cat. no.
4370), phospho-p38 (cat. no. 4511), phospho-c-Jun N-terminal kinase
(JNK; cat. no. 4511), Akt (cat. no. 4691), ERK1/2 (cat. no. 4695),
p38 (cat. no. 9212), JNK (cat. no. 9258), c-myc (cat, no. 9402),
cyclin D1 (cat. no. 2926), cyclin D3 (cat. no. 2936),
cyclin-dependent kinase (CDK)4 (cat. no. 2906), CDK6 (cat. no.
3136), p21 (cat. no. 2946), p27 (cat. no. 2552), β-actin (cat. no.
4970) obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; cat. no.
sc-25778) antibody from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). The primary antibody against Livin detects Livin α (36 kDa)
and Livin β (34 kDa). Primary antibodies were diluted 1:1,000 and
incubated with membranes for 24 h at 4°C. Anti-rabbit (cat. no.
7074; Cell Signaling Technology, Inc.) or anti-mouse horeseradish
peroxidase (HRP) -conjugated secondary antibodies (cat. no. 7076,
Cell Signaling Technology, Inc.) were diluted 1:2,000 and incubated
with membranes at room temperature for 2 h. Immunoreactive proteins
were visualized using an enhanced chemiluminescence detection
system with a HRP (EMD Millipore, Billerica, MA, USA), and were
analyzed using an LAS-4000 luminescent image analyzer (Fujifilm,
Tokyo, Japan).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. RT was performed using 1 μg
total RNA, M-MLV RT (Invitrogen; Thermo Fisher Scientific, Inc.), 1
μl 10 mM dNTP mix (Enzynomics Co., Ltd., Daejeon, Korea), 1
μl oligo dT (500 μg/ml; Promega Corporation, Madison,
WI, USA), 2 μl 0.1 M dithiothreitol (Invitrogen; Thermo
Fisher Scientific, Inc.), 4 μl 5X First-strand buffer
(Invitrogen; Thermo Fisher Scientific, Inc.) and 1 μl RNase
inhibitor (Promega Corporation). The cDNA was then amplified using
specific primers for Livin and GAPDH (Bioneer Corporation, Daejeon,
Korea), as previously described (15). PCR was performed using GoTaq DNA
Polymerase and 5X Green GoTaq reaction buffer (Promega Corporation)
The primer sequences were as follows: Livin α and Livin β, forward
5′-CAC ACA GGC CAT CAG GAC AAG-3′ and reverse 5′-ACG GCA CAA AGA
CGA TGG AC-3′ and GAPDH, forward 5′-ACC ACA GTC CAT GCC ATC AC-3′
and reverse 5′-TCC ACC ACC CTG TTG CTG TA-3′. The PCR was performed
by initial incubation at 94°C for 5 min followed by 32 cycles of
94°C for 30 sec, 58°C for 20 sec and 72°C for 30 sec, with a final
elongation step of 72°C for 7 min. The PCR products were separated
by electrophoresis on a 1% agarose gel containing ethidium bromide.
The signals were quantified by densitometric analysis using
Labworks Image Acquisition software (UVP, Inc., Upland, CA,
USA).
Statistical analysis
The significance of experimental differences were
assessed using Student's t-test. The statistical software
program used was SigmaPlot Software version 6.0 (Systat Software,
San Jose, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Ethical considerations
Local research ethics committee approval was
obtained from the Chonnam National University Hwasun Hospital
Institutional Review Board.
Results
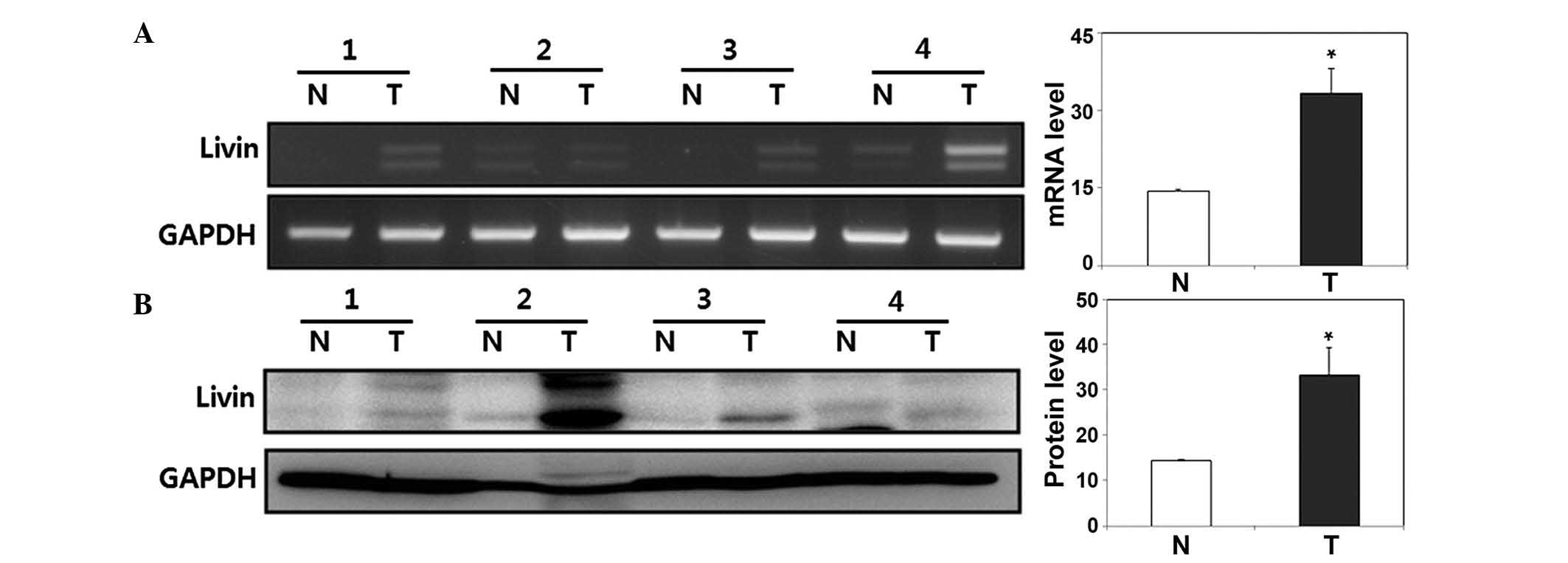
Expression of Livin is higher in human
HSCC tissues, compared with normal hypopharyngeal mucosa
tissues
The present study measured the expression of Livin
at the mRNA and protein levels using RT-qPCR and western blot
analyses in fresh human HSCC tissues and paired normal
hypopharyngeal mucosal tissues. The expression of Livin was
increased in the HSCC tissues. Compared with the paired normal
hypopharyngeal mucosal tissues at the mRNA and protein levels
(P<0.05; Fig. 1).
Knockdown of Livin suppresses tumor cell
growth in the human SNU-1041 HSCC cell line
To examine the role of Livin in tumorigenesis, the
present study used siRNA to inhibit the endogenous expression of
Livin in the human SNU-1041 HSCC cell line. The mRNA and protein
levels of Livin α and Livin β were lower in the Livin siRNA-treated
SNU-1041 cells, compared with those in the negative control
siRNA-treated cells (Fig. 2A).
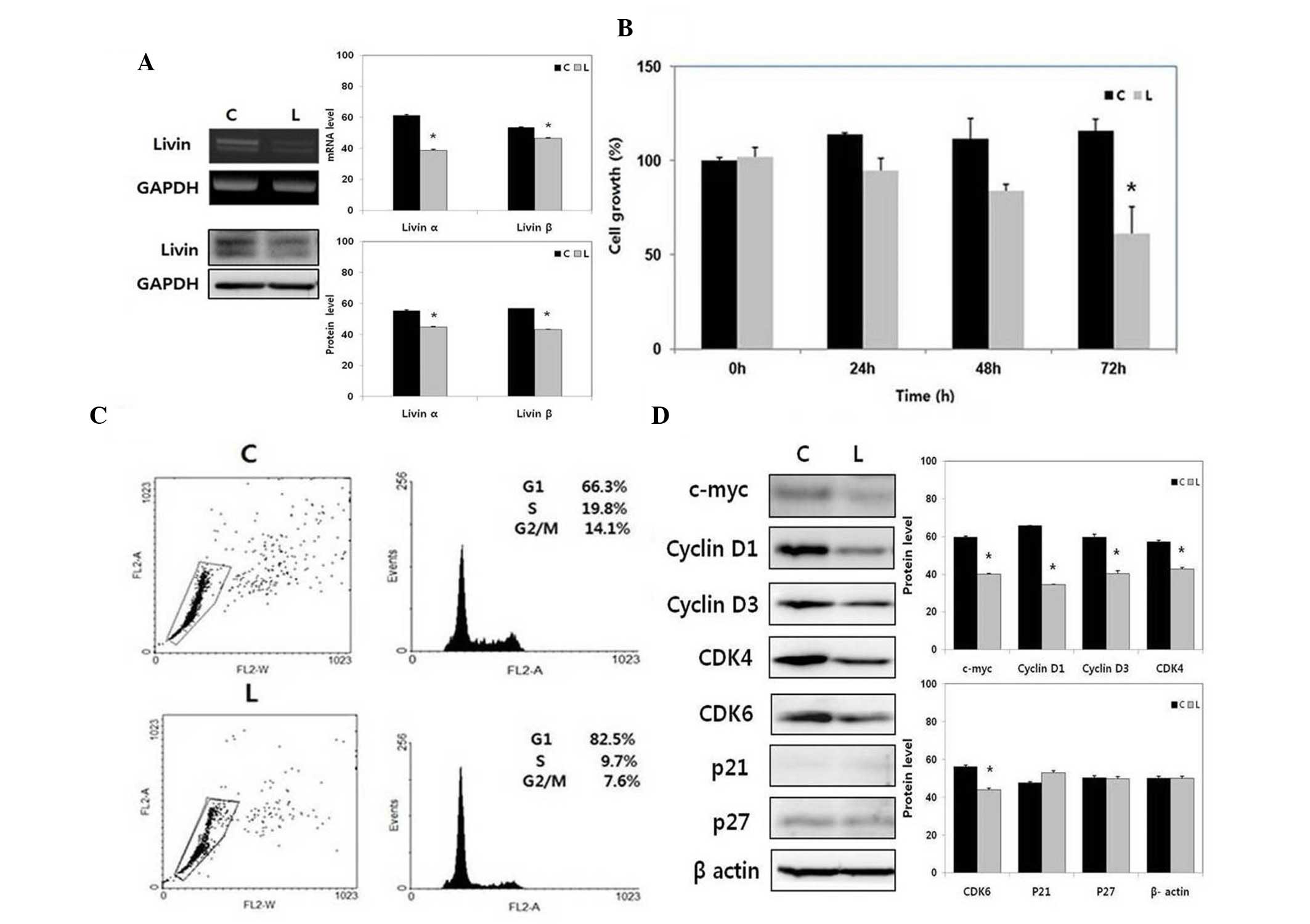 | Figure 2Effect of Livin knockdown on HSCC cell
proliferation and cell cycle. (A) mRNA and protein expression
levels of Livin α and Livin β were decreased by siRNA-mediated
Livin knockdown in the SNU-1041 cells. (B) Livin knockdown
inhibited SNU-1041 proliferation. Values are presented as the mean
± standard error. *P<0.05 vs. C. (C) Livin knockdown
induced cell cycle arrest at the G1 phase in the
SNU-1041 cells. (D) Livin knockdown decreased the expression levels
of cell cycle-positive regulators, c-myc, cyclin D1, cyclin D3,
CDK4, and CDK6 in the SNU-1041 cells. *P<0.01 vs. C.
HSCC, hypopharyngeal squamous cell carcinoma; CDK, cyclin-dependent
kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; siRNA,
small interfering RNA; C, negative control siRNA-transfected
SNU-1041 cells; L, Livin siRNA-transfected SNU-1041 cells. |
Knockdown of Livin significantly
decreases cell proliferation in human HSCC cells
The number of proliferating cells, as determined by
absorbance, was significantly lower in the Livin siRNA-transfected
SNU-1041 cells, compared with the negative control
siRNA-transfected SNU-1041 cells at 72 h post-transfection
(P<0.05; Fig. 2B).
Knockdown of Livin induces cell cycle
arrest at the G1 phase in human HSCC cells
Based on cell cycle analysis, Livin knockdown
resulted in arrest at the G1 phase of the cell cycle in
the SNU-1041 cells (Fig. 2C). The
expression levels of cyclin D1, cyclin D3, CDK4, and CDK6, which
are all key molecules for the G1 phase transition, were
significantly decreased by Livin knockdown in the SNU-1041 cells
(Fig. 2D). The expression of c-myc
was also decreased, however, the CDK inhibitors, p21 and p27, were
not altered by Livin knockdown in the SNU-1041 cells. These results
indicated that Livin knockdown induced cell cycle arrest through
the modulation of cell cycle-positive regulators, including c-myc,
cyclin D1, cyclin D3, CDK4 and CDK6.
Knockdown of Livin decreases the
phosphorylation of MAPK signaling proteins and Akt in human HSCC
cells
To examine the potential mechanisms involved in the
effects of Livin, the present study examined the impact of Livin on
the MAPK/Akt signaling pathways, which are essential for cell
growth and survival. The phosphorylation levels of ERK1/2, p38, JNK
and Akt were decreased by Livin knockdown in the SNU-1041 cells
(Fig. 3). The levels of total
ERK1/2, p38, JNK and Akt were not affected by Livin knockdown in
the SNU-1041 cells.
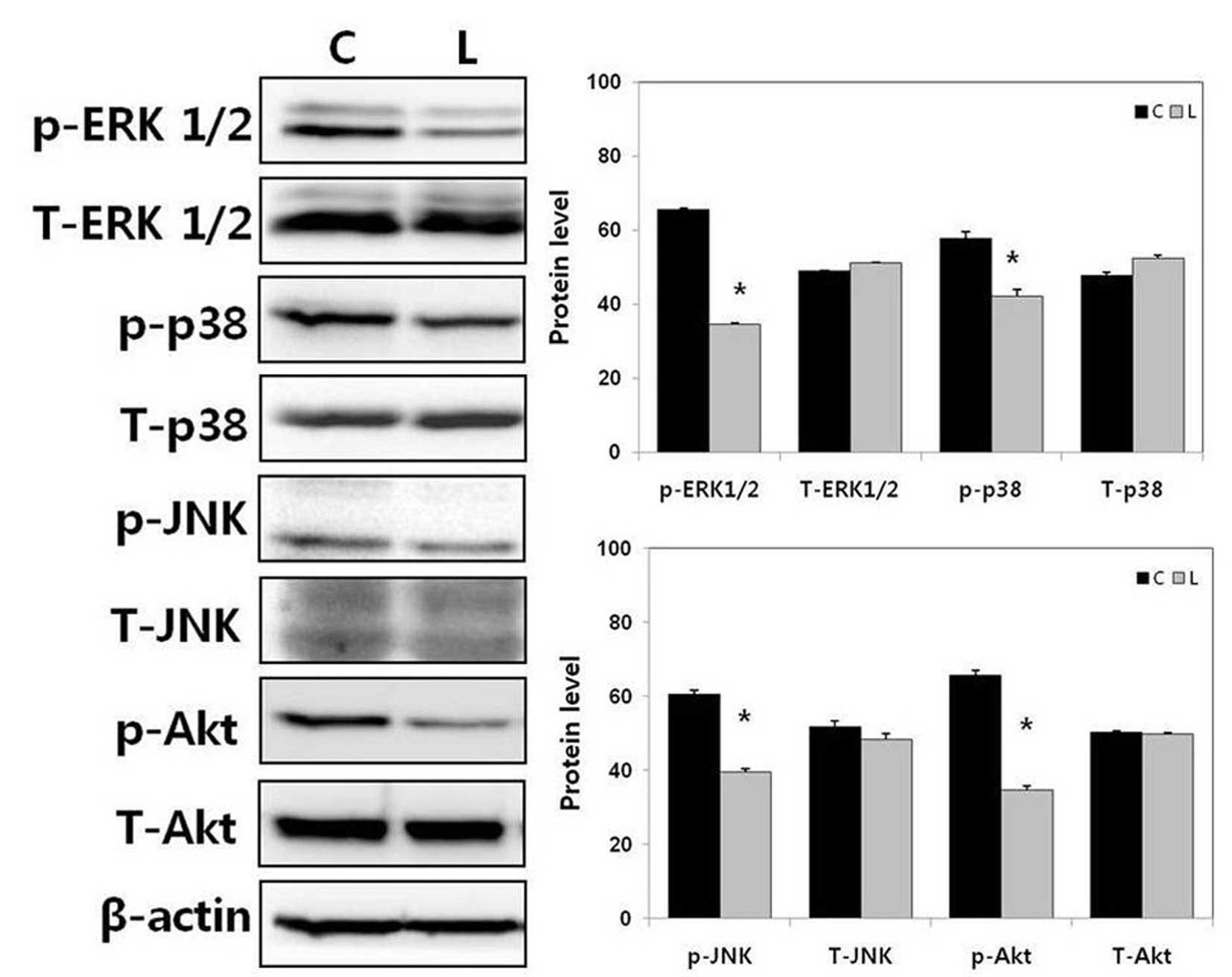 | Figure 3Effect of Livin knockdown on MAPK and
Akt signaling pathways in human HSCC cells. The phosphorylation
levels of ERK1/2, p38, JNK, and Akt were decreased by Livin
knockdown in SNU-1041 cells. *P<0.01 vs. C. HSCC,
hypopharyngeal squamous cell carcinoma; ERK, extracellular
signal-regulated kinase; MAPK, mitogen-activated protein kinase;
JNK, c-Jun N-terminal kinase; siRNA, small interfering RNA; p-,
phosphorylated; C, negative control siRNA-transfected SNU-1041
cells; L, Livin siRNA-transfected SNU-1041 cells. |
Discussion
IAPs have been identified in organisms ranging from
yeast to mammals (6). Human IAP
family members include neuronal apoptosis inhibitory protein,
cellular (c)-IAP1, c-IAP2, X-linked XIAP, Survivin, Apollon,
IAP-like protein 2 and Livin (5–7,16–19).
These proteins contain one or more baculovirus IAP repeat (BIR)
domains and harbor a COOH-terminal RING finger domain (6,7). It
is generally considered that the BIR domain is required for the
suppression of apoptosis by IAPs (20,21).
Livin is a 39-kDa protein consisting of a single BIR domain and a
RING motif, encoded by a gene spanning 4.6 kb on chromosome 20 at
band q13, and composed of six introns and seven exons (5). Livin has been reported to inhibit
tumor cell apoptosis (10,22). Furthermore, Livin has been
implicated in the promotion of cell survival, and in the regulation
of proliferation and the cell cycle (22). However, the impact of Livin on
human HSCC cell behavior remains to be elucidated.
Elevated expression of Livin has been found in
tumors, including melanoma, breast cancer, colon cancer, prostate
cancer and hepatoma (8–10). These findings suggest that Livin
may contribute to tumorigenesis. In the present study, upregulation
in the expression of Livin was observed in fresh HSCC tissues,
compared with paired normal mucosal tissues at the mRNA and protein
levels, although the sample size of patients was small.
Additionally, the present study showed that knockdown of Livin
suppressed tumor cell proliferation and led to cell cycle arrest in
human HSCC cells. Cell cycle promotion with apoptosis resistance is
important in tumor development and progression (23,24).
The cell cycle is positively regulated by a family of cyclins and
CDKs, and negatively regulated by CDK inhibitors, including p21 and
p27 (24). Livin knockdown induced
the suppression of c-myc, cyclin D1, cyclin D3, CDK4, and CDK6 in
human HSCC cells. These results indicated that Livin induced
tumorigenic activities, including cell proliferation and cell cycle
promotion in the human HSCC.
Livin is considered to be a genuine apoptosis
inhibitor due to its direct association with caspase-3 and
caspase-7, as well as its ability to counteract cell death by
interfering with caspase-9 processing (25). In our previous study, it was
reported that Livin inhibits cell apoptosis via the modulation of
caspase 3, caspase 7 and poly ADP ribose polymerase in human
laryngohypopharyngeal squamous cell carcinoma cells (15). Although the majority of studies on
Livin have focused on its role as a caspase inhibitor, there is
increasing evidence that it also acts through other mechanisms
(26,27). Chen et al reported that
Livin abrogates apoptosis of lung adenocarcinoma cells by
regulating the JNK signaling pathway (26). It has also been reported that Livin
enhances JNK phosphorylation, and that activated JNK antagonizes
tumor necrosis factor α and interleukin-converting enzyme-induced
apoptosis (27). MAPK/Akt
signaling is involved in several cellular programs, including cell
proliferation, migration, survival and differentiation (11,12).
The present study showed that Livin knockdown inhibited the
phosphorylation of ERK1/2, p38, JNK and Akt in the human HSCC
cells. To the best of our knowledge, the present study is the first
investigation to describe the effects of Livin knockdown on ERK1/2,
p38 and Akt in cancer cells. These findings suggested that Livin
may promote tumor cell invasiveness by activating the MAPK/Akt
signaling pathways in human HSCC.
In conclusion, Livin knockdown suppressed cell
proliferation and induced cell cycle arrest via the modulation of
cell cycle regulatory proteins in human HSCC cells. Livin knockdown
also reduced the activation of the MAPK/Akt signaling proteins,
including ERK1/2, p38, JNK and Akt in human HSCC cells. Although
further investigations are required to support these findings, the
results of the present study suggested that Livin may enhance
tumorigenesis by modulating the MAPK/Akt signaling pathways in
human HSCC.
Acknowledgments
This study was supported by a grant (grant no. CRI
13013-1) from the Chonnam National University Hospital Biomedical
Research Institute. The authors would like to thank Dr. Sung (Seoul
National University) for providing the SNU-1041 cell line.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sewnaik A, Hoorweg JJ, Kneqt PP, Wieringa
MH, van der Beek JM and Kerrebijn JD: Treatment of hypopharyngeal
carcinoma: Analysis of nationwide study in the Netherlands over a
10-year period. Clin Otolaryngol. 30:52–57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta T, Chopra S, Aqarwal JP, Laskar SG,
D'cruz AK, Shrivastava SK and Dinshaw KA: Squamous cell carcinoma
of the hypopharynx: Single-institution outcome analysis of a large
cohort of patients treated with primary non-surgical approaches.
Acta Oncol. 48:541–548. 2009. View Article : Google Scholar
|
|
4
|
Semrau R, Mueller RP, Stuetzer H, Staar S,
Schroeder U, Guntinas-Lichius O, Kocher M, Eich HT, Dietz A,
Flentje M, et al: Efficacy of intensified hyperfractionated and
accelerated radiotherapy and concurrent chemotherapy with
carboplatin and 5-fluorouraceil: Updated results of a randomized
multicentric trial in advanced head-and-neck cancer. Int J Radiat
Oncol Biol Phys. 64:1308–1316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ashhab Y, Alian A, Polliack A, Panet A and
Ben Yehuda D: Two splicing variants of a new inhibitor of apoptosis
gene with different biological properties and tissue distribution
pattern. FEBS Lett. 495:56–60. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deveraux QL and Reed JC: IAP family
proteins-suppressors of apoptosis. Genes Dev. 13:239–252. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deveraux QL, Stennicke HR, Salvesen GS and
Reed JC: Endogenous inhibitors of caspases. J Clin Immunol.
19:388–398. 1999. View Article : Google Scholar
|
|
8
|
Liu B, Han M, Wen JK and Wang L:
Livin/ML-IAP as a new target for cancer treatment. Cancer Lett.
250:168–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Zhang Q, Liu B, Han M and Shan B:
Challenge and promise: Roles for Livin in progression and therapy
of cancer. Mol Cancer Ther. 7:3661–3669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vucic D, Stennicke HR, Pisabarro MT,
Salvesen GS and Dixit VM: ML-IAP, a novel inhibitor of apoptosis
that is preferentially expressed in human melanomas. Curr Biol.
10:1359–1366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keshet Y and Seger R: The MAP kinase
signaling cascades: A system of hundreds of components regulates a
diverse array of physiological functions. Methods Mol Biol.
661:3–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pratilas CA and Solit DB: Targeting the
mitogen-activated protein kinase pathway: Physiological feedback
and drug response. Clin Cancer Res. 16:3329–3334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bermudez O, Pagès G and Gimond C: The
dual-specificity MAP kinase phosphatases: Critical roles in
development and cancer. Am J Physiol Cell Physiol. 299:C189–C202.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haagenson KK and Wu GS: Mitogen activated
protein kinase phosphatases and cancer. Cancer Biol Ther.
9:337–340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee DH, Yoon TM, Kim SA, Park YL, Lee KH,
Lim SC, Lee JK and Joο YE: Relationship between expression of Livin
and the biological behavior of human oral squamous cell carcinoma.
Oncol Rep. 32:2453–2460. 2014.PubMed/NCBI
|
|
16
|
Badran A, Yoshida A, Ishikawa K, Goi T,
Yamaguchi A, Ueda T and Inuzuka M: Identification of a novel splice
variant of the human anti-apoptopsis gene survivin. Biochem Biophys
Res Commun. 314:902–907. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eckelman BP, Salvesen GS and Scott FL:
Human inhibitor of apoptosis proteins: Why XIAP is the black sheep
of the family. EMBO Rep. 7:988–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roy N, Deveraux QL, Takahashi R, Salvesen
GS and Reed JC: The c-IAP-1 and c-IAP-2 proteins are direct
inhibitors of specific caspases. EMBO J. 16:6914–6925. 1997.
View Article : Google Scholar
|
|
19
|
Vaux DL and Silke J: Mammalian
mitochondrial IAP binding proteins. Biochem Biophys Res Commun.
304:499–504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deveraux QL, Roy N, Stennicke HR, Van
Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS and Reed
JC: IAPs block apoptotic events induced by caspase-8 and cytochrome
c by direct inhibition of distinct caspases. EMBO J. 17:2215–2223.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deveraux QL, Takahashi R, Salvesen GS and
Reed JC: X-linked IAP is a direct inhibitor of cell-death
proteases. Nature. 388:300–304. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho SB, Lee WS, Park YL, Kim N, Oh HH, Kim
MY, Oak CY, Chung CY, Park HC, Kim JS, et al: Livin is associated
with the invasive and oncogenic phenotypes of human hepatocellular
carcinoma cells. Hepatol Res. 45:448–457. 2015. View Article : Google Scholar
|
|
23
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: Role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
25
|
Kasof GM and Gomes BC: Livin, a novel
inhibitor of apoptosis protein family member. J Biol Chem.
276:3238–3246. 2001. View Article : Google Scholar
|
|
26
|
Chen YS, Li HR, Lin M, Chen G, Xie BS, Xu
NL and Lin LF: Livin abrogates apoptosis of SPC-A1 cell by
regulating JNKI signaling pathway. Mol Biol Rep. 37:2241–2247.
2010. View Article : Google Scholar
|
|
27
|
Sanna MG, da Silva Correia J, Ducrey O,
Lee J, Nomoto K, Schrantz N, Deveraux QL and Ulevitch RJ: IAP
suppression of apoptosis involves distinct mechanisms: The
TAK1/JNK1 signaling cascade and caspase inhibition. Mol Cell Biol.
22:1754–1766. 2002. View Article : Google Scholar : PubMed/NCBI
|