Introduction
Pre-eclampsia (PE) is a complication of pregnancy
characterized by the onset of hypertension and proteinuria after 20
weeks of gestation. It affects 5–8% of pregnant women and is one of
the predominant causes of maternal and neonatal mortality and
morbidity worldwide (1,2). Women with chronic hypertension are
3–5 times more likely to develop PE than those with normal blood
pressure (3). Superimposed PE on
chronic hypertension (SPE) is associated with poor pregnancy out
comes (3,4).
Despite the fact that the origin and pathogenesis of
both PE and SPE have been extensively investigated (1,2,5–7),
they remain unclear to date. Recent studies have reported the
implication of microRNAs (miRNAs) in the development of PE
(1,2). miRNAs are a class of 21–25-nucleotide
noncoding single-stranded small RNAs. miRNAs negatively regulate
gene expression by binding to the 3′-untranslated region of the
target mRNAs and thus may possess important control functions in
diverse biological processes (1,2,8).
Aberrant expression of certain miRNAs has been identified in the
placentas and blood from women with PE, suggesting that miRNA
deregulation is involved in PE pathogenesis (1,2).
Chang et al (9) reported
that the top-ranked placental mRNA transcripts differed between
patients with PE and those with SPE, suggesting that the
pathogeneses of these two diseases are driven by different
molecular mechanisms, which may include variations in the miRNA
regulation network. Whether and how the miRNA expression pattern is
changed in the placentas of patients with SPE is yet to be
elucidated. In addition, the severity and frequent occurrence of
SPE substantiates the requirement of the identification of
candidate molecules which may provide novel insights into SPE
pathogenesis.
In the present study, next generation sequencing
(NGS) was performed and the placental miRNA expression profiles
were compared between pregnancies of patients with SPE and normal
pregnancies.
Materials and methods
Study groups and tissue samples
The study was performed on placenta samples
collected from two groups of patients: Patients with SPE (n=5) and
patients with normal pregnancies (control group; n=6). The mean age
in the whole cohort was 31.9 ± 1.4 years. The mean age was 35.0±2.4
years in the SPE group and 29.3±0.6 years in the control group. The
inclusion criterion for patients with SPE was the onset of
proteinuria (≥300 mg of protein in a 24-h specimen) following the
20th week of gestation in women with documented chronic
hypertension and no proteinuria prior to the 20th week of
gestation, which was set by the National High Blood Pressure
Education Program Working Group on High Blood Pressure in Pregnancy
(10). The patients in the control
group had normal pregnancies. Patients with chronic hypertension,
cardiovascular disease, renal disease, hepatitis, diabetes,
intrapartum infection or other pregnancy complications were
excluded from the study.
All placentas were obtained by cesarean section in
the D.O. Ott Research Institute of Obstetrics, Gynecology and
Reproductology (St. Petersburg, Russia). Placental samples were
collected using the previously described systematic sampling
technique to achieve uniformity and adequate sampling (11). Following dissection, tissue
fragments were immediately placed in 0.9% NaCl precooled to 4°C.
Subsequently, placenta villi samples (~30 mg) were selected and
released from blood clots under the Leica M125 stereomicroscope
(Leica Microsystems, Wetzlar, Germany) within 15 min of the
cesarian section. All samples were stabilized in RNAlater (Qiagen,
Inc., Valencia, CA, USA) and stored at −70°C until use.
The study was approved by the Institutional Review
Board of the D.O. Ott Research Institute of Obstetrics Gynecology
and Reproductology (St. Petersburg, Russia). Informed consent was
signed by all patients prior to their inclusion in the study and to
processing of their personal and medical data. The study was
performed in accordance with the Declaration of Helsinki.
Small RNA isolation and library
preparation for sequencing
Small RNA was extracted from placenta samples using
PureLink miRNA Isolation kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol, and
was then stored at −70°C until library preparation.
Small RNA libraries were prepared using the Ion
Total RNA-Seq kit v2 (Thermo Fisher Scientific, Inc.), following
the manufacturer's protocol. Small RNA libraries were prepared
using the Ion Total RNA-Seq kit version 2 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Briefly, for each sample, ~100 ng of small RNA was used as the
starting template. RNA samples (3 µl) were mixed with the
hybridization solution and adaptor mix, incubated at 65°C for 10
min and 16°C for 5 min. Then, ligation reagents were added and the
samples were incubated at 16°C overnight. After ligation, reverse
transcription master mix (RT) was added to synthesize single strand
cDNA. The samples were incubated at 70°C for 5 min, cooled on ice,
then RT enzyme was added and the samples were incubated at 42°C for
30 min. cDNA was size-selected using Purification Module containing
magnetic beads, then eluted with 12 µl nuclease-free water.
The purified cDNA samples were used as templates for subsequent
PCR. Briefly, 6 µl cDNA samples were combined with PCR
primers and Platinum PCR SuperMix High Fidelity reaction mix.
Cycling conditions were as follows: 94°C for 2 min, 2 cycles of
94°C for 30 sec, 50°C for 30 sec, and 68°C for 30 sec, 16 cycles of
94°C for 30 sec, 62°C for 30 sec, and 68°C for 30 sec, then 68°C
for 5 min. PCR products were purified using the Magnetic Bead
Purification Module and eluted with 15 µl nuclease-free
water. For assessment of the yield, size distribution and molar
concentration of the amplified DNA libraries, the samples were run
on 2200 TapeStation Instrument with High Sensitivity D1K ScreenTape
and High Sensitivity D1K Reagents (all purchased from Agilent
Technologies, Santa Clara, CA, USA). The quantity of library
required for template preparation was determined according to the
manufacturer's protocol (Ion Total RNA-Seq Kit v2, Thermo Fisher
Scientific, Inc.).
Ion Torrent sequencing
Each library template was clonally amplified by
emulsion polymerase chain reaction (PCR) on Ion Sphere Particles
(ISPs) using the Ion One Touch 200 Template kit with the Ion One
Touch 2 system (Thermo Fisher Scientific, Inc.). ISPs were prepared
and cleaned according to the manufacturer's protocol.
Quantification of recovered particles was performed using a Qubit
2.0 fluorometer and an Ion Sphere quality control kit (both from
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The optimal percentage of template-positive ISPs was
considered to be 50–150. Relative fluorescent unit values obtained
outside this range were not used in subsequent ISP enrichment. The
ISPs were enriched using Dynabeads MyOne Strepavidin C1 beads
(Thermo Fisher Scientific, Inc.). The ISPs were then loaded into
Ion 318 Chip and sequenced on the Ion Torrent Personal Genome
machine (Thermo Fisher Scientific, Inc.).
Statistical analysis
Statistical analysis was performed using Statistica
10.0 (StatSoft, Inc., Tulsa, OK, USA). The Mann-Whitney test was
selected for the comparison of continuous variables and Fisher's
exact test for the comparison of categorical variables. P<0.05
was considered to indicate a statistically significant
difference.
Computational analysis
Sequencing data were processed using a comprehensive
analysis pipeline for deep miRNA sequencing (CAP-miRSeq) (12). First, according to the CAP-miRSeq
algorithm, pre-processing of read sequences was performed. The
quality of the reads was checked by FastQC (12) and low quality bases were trimmed
from the 3′ end using Cutadapt (13). Reads containing 17 bases or less
were then discarded. All types of RNA were evaluated in the
sequencing libraries. The remaining reads were aligned to the
GENCODE (release 18) transcripts using Bowtie software (14). RNA quantification was performed
using HTseq-count (12). The
sequencing reads were then analyzed to identify miRNAs using Bowtie
and the Human Genome RefSeq Hg19 or miRBase (15) as a reference. Subsequently, the
expression levels of detected miRNAs were estimated using miRDeep2
(16), and differential analysis
of miRNAs was conducted in SPE samples, which were compared with
the control. For each dataset, counts were normalized to the total
number of read sequences, and then the normalized number of counts
for each RNA was compared between the patient groups. The
normalization and differential expression was performed using
Bioconductor edgeR (17). The
false discovery rate (FDR) was calculated according to Benjamini's
method (18). miRNAs were
considered to be differentially expressed at a P<0.01 and
FDR<0.05.
Results
Study groups
The clinical characteristics of the patients from
the two groups were compared (Table
I), and it was found that the patients' age, gestational age at
delivery, fetal weight and length at birth did not differ between
the SPE and control groups. Patient systolic and diastolic blood
pressure prior to and during pregnancy, as well as levels of
proteinuria, were shown to be significantly higher in the SPE group
compared with the control group (P<0.05).
 | Table IClinical characteristics of the
patients with normal (control) and SPE pregnancies. |
Table I
Clinical characteristics of the
patients with normal (control) and SPE pregnancies.
| Characteristic | Control (n=6) | SPE (n=5) |
|---|
| Maternal
characteristic |
| Age, years | 29.3±0.6 | 35.0±2.4 |
| Ethnicity | Russian, 6/6 | Russian, 5/5 |
| Systolic blood
pressure before pregnancy, mmHg | 109.2±4.5 | 131.0±4.0a |
| Diastolic blood
pressure before pregnancy, mmHg | 65.8±3.7 | 86.0±2.4a |
| Systolic blood
pressure at diagnosis, mmHg | 108.3±3.1 | 160.0±8.9a |
| Diastolic blood
pressure at diagnosis, mmHg | 70.8±2.7 | 100.0±5.5a |
| Proteinuria at
diagnosis, g/24 h | 0 | 0.6±0.2a |
| Pregnancy
outcome |
| Mode of
delivery | Cesarian section,
6/6 | Caesarian section,
5/5 |
| Gestational age at
delivery, weeks | 38.8±0.2 | 36.3±1.4 |
| Fetal weight,
g | 3,410.0±124.5 | 2724.0±490.4 |
| Fetal length,
cm | 51.2±0.5 | 47.6±2.6 |
miRNA sequencing
The expression profiles of small RNAs in the
placenta samples were compared between the SPE and control groups.
For this purpose, 5 SPE and 6 control small RNA libraries were
prepared using the Ion Torrent system. Sequencing reads obtained
from 5 SPE and 6 control libraries were used to create two separate
libraries: The SPE and control libraries. The small RNAs from the
two libraries were a similar size of 19–24 nt. The total reads
obtained from the SPE and control libraries were 1,502,484 and
2,241,561, respectively (Table
II). After the read sequences had been pre-processed, 1,207,080
and 1,844,141 clean reads were selected from the SPE and control
libraries, respectively, for further analysis (Table II).
 | Table IICharacteristics of miRNA-sequencing
libraries obtained from normal (control) and SPE placentas. |
Table II
Characteristics of miRNA-sequencing
libraries obtained from normal (control) and SPE placentas.
| Characteristic | Control (n=6) | SPE (n=5) |
|---|
| Total reads, n | 2,241,561 | 1,502,484 |
| Trimmed reads, n
(%) | 100,821 (4.5) | 58,019 (3.9) |
| Too short after
trimming (<17 bps), n (%) | 296,599 (13.2) | 236,655 (15.8) |
| Reads sent to
aligner, n | 1,844,141 | 1,207,080 |
| Aligned reads, n
(%) | 1,184,799
(64.2) | 827,455 (68.6) |
| Precursor miRNA
reads, n | 9,060 | 7,576 |
| Mature miRNA reads,
n | 805,102 | 606,821 |
| Known miRNAs with
≥5X coverage, n | 522 | 466 |
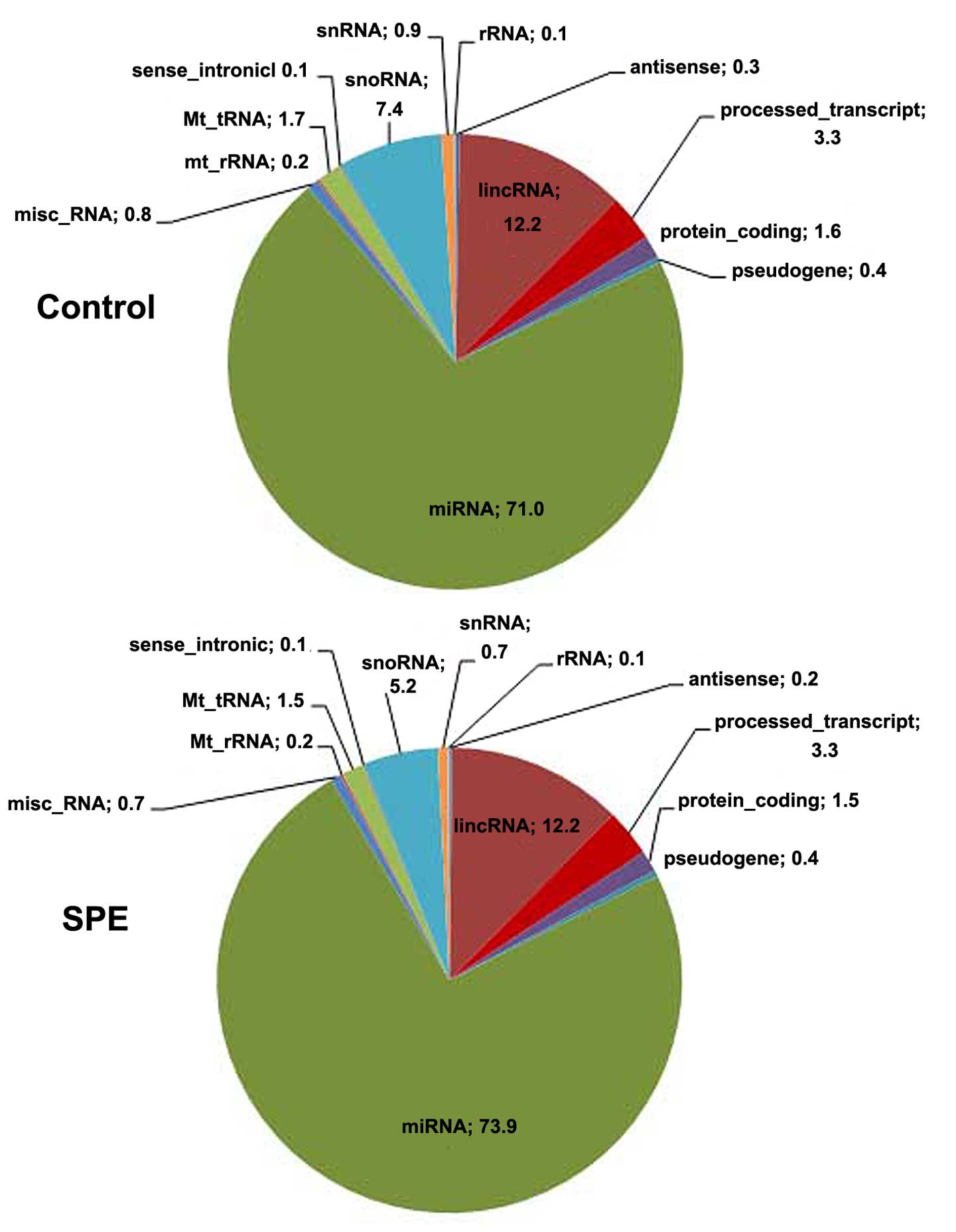
The relative proportions of different RNA categories
were similar in both libraries, with miRNAs represented the largest
fraction of all types of small RNAs (Fig. 1). The number of reads aligned to
the reference genome, and to mature and precursor miRNAs, as well
as the number of miRNAs reads with ≥5x coverage detected are
provided in Table II. In total,
466 known miRNAs were detected in the SPE library and 522 in the
control library (Table II).
miRNA expression
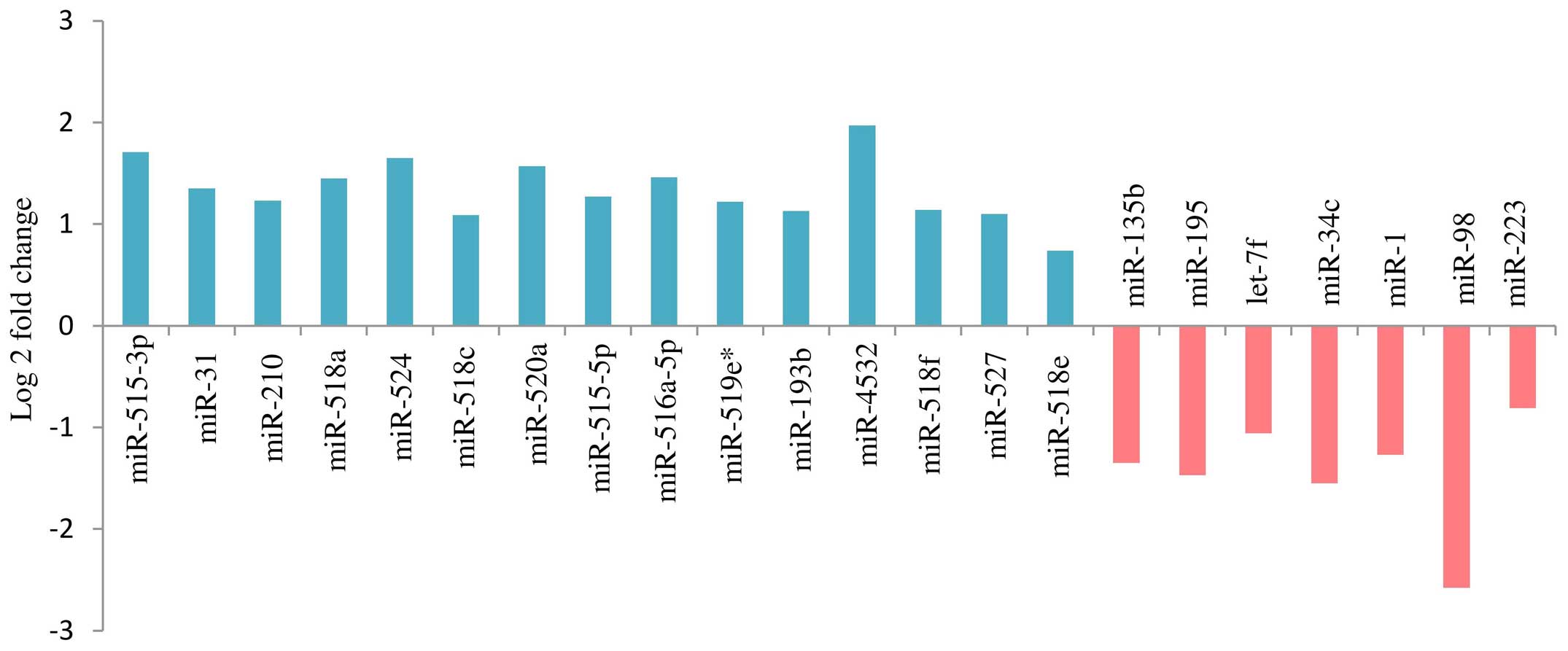
A total of 36 miRNAs (P<0.01) exhibited altered
expression patterns in the SPE placentas, when compared with those
in the control placentas. In 22 of the 36 miRNAs, the difference in
expression was significant (FDR<0.05). Among them, 15 were
upregulated and 7 downregulated in the SPE placentas, as compared
with the control (Fig. 2).
Discussion
The miRNA expression profiles were detected and
compared between the SPE and normal placentas. The experimental
approach followed in the present study was based on NGS using Ion
Torrent Sequencer. In contrast to reverse
transcription-quantitative PCR and microarray hybridization, NGS
does not depend on the design of primers, can detect an unlimited
number of miRNAs in one experiment, is suitable for quantitative
evaluation of low-expressing transcripts and allows for a precise
analysis of small numbers of valuable samples (7).
A total of 22 miRNAs were found to be differentially
expressed in the SPE placentas compared with the control placentas
(Fig. 2). Of these 22 miRNAs, 11
were mapped to the C19MC cluster, the largest human miRNA gene
cluster, whose expression is almost exclusively confined to the
placenta (Table III). The
cluster spans ~100 kb at chromosome 19q13.41 and contains 54
predicted miRNA genes, 43 of which have been cloned and sequenced
(2). The precise biological
function of the C19MC cluster is unknown. These results, in
combination with findings from other miRNA studies of PE (2,19,20),
suggested that the C19MC aberrant expression may comprise a
contributing factor to the development of placental dysfunction. In
the present study, all 11 differentially expressed miRNAs from the
C19MC cluster were found to be overexpressed in SPE placentas.
Eight of these miRNAs (miR-515-3p, miR-515-5p, miR-516a-5p,
miR-518c, miR-518f, miR-519e*, miR-520a and miR-524)
have been reported to be upregulated in placentas from patients
with PE (19–22). The other 3 overexpressed miRNAs
detected in the present study (miR-518a, miR-527 and miR-518e) had
not been previously reported to be associated with PE, and could
therefore be involved in pathogenic biochemical pathways specific
to SPE (Table III).
 | Table IIIDeregulated microRNAs in SPE (present
study) and in PE pregnancies (literature data). |
Table III
Deregulated microRNAs in SPE (present
study) and in PE pregnancies (literature data).
| miRNA | Chromosome | SPE pregnancies
(present study)
| PE pregnancies
(literature data)
|
|---|
| Regulation of
expression | Method | Tissue | Regulation of
expression | Method | Refs. |
|---|
| miR-515-3p | 19, C19MC | Up | NGS | Placenta | – | Mcroarray | (52) |
| Plasma | Pregnancy-specific
miRNA | RT-qPCR | (52) |
| Placenta | Up | RT-qPCR-based
array, not NGS | (20) |
| Placenta | Up, severe PE | Mcroarray (not
validated by RT-qPCR | (22) |
| Plasma | Up, severe and mild
PE | NGS (not checked by
RT-qPCR) | (7) |
| miR-515-5p | 19, C19MC | Up | NGS | Placenta | – | Microarray | (52) |
| Plasma | Pregnancy-specific
miRNA | RT-qPCR | (52) |
| Serum | Pregnancy-specific
miRNA | RT-qPCR | (51) |
| Placenta | Up | RT-qPCR-based
array, not NGS | (20) |
| miR-516a-5p | 19, C19MC | Up | NGS | Placenta | – | Microarray | (52) |
| Placenta | Up, severe PE | Microarray (not
checked by RT-qPCR) | (21) |
| miR-518a | 19, C19MC | Up | NGS | Placenta | – | Microarray | (52) |
| Serum | Pregnancy-specific
miRNA | RT-qPCR | (51) |
| miR-527 | 19, C19MC | Up | NGS | Placenta | – | Microarray | (52) |
| Serum | Pregnancy-specific
miRNA | RT-qPCR | (51) |
| miR-518c | 19, C19MC | Up | NGS | Placenta | – | Microarray | (52) |
| Plasma | Pregnancy-specific
miRNA | RT-qPCR | (52) |
| Placenta | Up | NGS and
RT-qPCR-based array | (20) |
| Plasma | Up, severe and mild
PE | NGS (not checked by
RT-qPCR) | (7) |
| miR-518e | 19, C19MC | Up | NGS | Placenta | – | Microarray | (52) |
| Plasma | Pregnancy-specific
miRNA | RT-qPCR | (52) |
| Serum | Pregnancy-specific
miRNA | RT-qPCR | (51) |
| Placenta | Up | NGS, not
RT-qPCR-based array | (20) |
| Serum | Up | NGS | (53) |
| miR-518f | 19, C19MC | Up | NGS | Placenta | – | Microarray | (52) |
| Placenta | – | NGS | (20) |
|
miR-519e* | 19, C19MC | Up | NGS | Serum | Pregnancy-specific
miRNA | RT-qPCR | (51) |
| Placenta | Up, severe PE | Microarray (not
checked by RT-qPCR) | (19) |
| Placenta | Up, severe PE | Microarray (not
validated by RT-qPCR) | (22) |
| Placenta | Up | NGS and
RT-qPCR-based array | (20) |
| miR-520a | 19, C19MC | Up | NGS | Placenta | – | Microarray | (52) |
| Placenta | Up | NGS and
RT-qPCR-based array | (20) |
| miR-524 | 19, C19MC | Up | NGS | Placenta | – | Microarray | (52) |
| Serum | Pregnancy-specific
miRNA | RT-qPCR | (51) |
| Placenta | Up, severe PE | Microarray (not
validated by RT-qPCR) | (21) |
| Placenta | Up, severe PE | Microarray
(validated by RT-qPCR) | (22) |
| Placenta | Up | RT-qPCR-based
array, not NGS | (20) |
| miR-210 | 11 | Up | NGS | Placenta | – | RT-qPCR-based
array | (48) |
| Placenta | Up | Microarray
(validated by RT-qPCR) | (11) |
| Placenta | Up, severe PE | Microarray
(validated by RT-qPCR) | (19) |
| Placenta | Up | RT-qPCR-based
array | (28) |
| Placenta | Up, severe PE | Microarray
(validated by RT-qPCR) | (20) |
| Placenta | Up | NGS and
RT-qPCR-based array | (20) |
| Placenta | Up, severe PE
(basal plate, not in the chorionic plate) | RT-qPCR | (29) |
| Placenta | Up, late onset
PE | NGS (not checked by
RT-qPCR) | (30) |
| Plasma | Up | RT-qPCR | (8) |
| Plasma | Up, severe PE | RT-qPCR | (22) |
| Plasma | Up, severe and mild
PE | RT-qPCR | (31) |
| miR-195 | 17 | Down | NGS | Placenta | – | NGS | (20) |
| Placenta | – | RT-qPCR-based
array | (48) |
| Placenta | Down, severe
PE | Microarray
(validated by RT-qPCR) | (22) |
| Placenta | Down, severe
PE | RT-qPCR | (33) |
| Placenta | Down, severe
PE | Microarray (not
checked by RT-qPCR) | (19) |
| miR-223 | X | Down | NGS | Placenta | – | RT-qPCR-based
array | (48) |
| Placenta | Down, severe
PE | Microarray (not
checked by RT-qPCR) | (19) |
| Placenta | Down, severe
PE | Microarray
(validated by RT-qPCR) | (22) |
| Placenta
(FFPE) | Down | PNA-based
microarray (not checked by RT-qPCR) | (34) |
| Placenta | Down, early-onset
PE | NGS (validated by
RT-qPCR) | (30) |
| Placenta | Down | NGS, not
RT-qPCR-based array | (20) |
| Plasma | Down, severe and
mild PE | NGS (not checked by
RT-qPCR) | (7) |
| Serum | Down | NGS | (53) |
| miR-34c | 11 | Down | NGS | Placenta | – | RT-qPCR-based
array | (48) |
| Plasma | Pregnancy-specific
miRNA | RT-qPCR-based
array | (48) |
| Placenta | Down | Microarray (not
checked by RT-qPCR) | (11) |
| Placenta | Down, severe
PE | Microarray (not
validated by RT-qPCR) | (21) |
| miR-1 | 18 | Down | NGS | Placenta | – | Microarray | (52) |
| Placenta | Down | Microarray
(validated by RT-qPCR) | (11) |
| Placenta | Down, severe
PE | Microarray (not
checked by RT-qPCR) | (19) |
| miR-193b | 16 | Up | NGS | Placenta | Up | NGS and
RT-qPCR-based array | (20) |
| Placenta | Up, severe PE | Microarray
(validated by RT-qPCR) | (22) |
| let-7f | 9 | Down | NGS | Placenta | – | NGS | (20) |
| Placenta | Up, severe PE | Microarray (not
checked by RT-qPCR) | (54) |
| Serum | Down | NGS | (53) |
| Plasma | Down, severe and
mild PE | NGS (not checked by
RT-qPCR) | (7) |
| miR-31 | 9 | Up | NGS | Placenta | – | RT-qPCR-based
array | (48) |
| Placenta | Up | NGS, not RT-qPCR-
array | (20) |
| Placenta | Up, severe PE | Microarray (not
validated by RT-qPCR) | (22) |
| miR-98 | X | Down | NGS | Placenta | – | RT-qPCR-based
array | (48) |
| Placenta | Up | NGS, not
RT-qPCR-array | (20) |
| miR-135b | 1 | Down | NGS | Placenta | – | Microarray | (52) |
| Placenta | – | NGS | (20) |
| Placenta | – | RT-qPCR-based
array | (48) |
| Plasma | Pregnancy-specific
miRNA | RT-qPCR-based
array | (48) |
| miR-4532 | 20 | Up | NGS | – | – | – | – |
Notably, the C19MC cluster is regulated by genomic
imprinting with only the paternally inherited allele being
expressed in the placenta, while the maternal one displays a
methylation imprint (23,24). The C19MC expression pattern in
human cells, including placental cells, has been shown to be
associated with the methylation status of the distal CpG-rich
region (23). Demethylating agents
induce the upregulation of miRNAs from the C19MC cluster in cancer
cells (23). These data suggest
that the PE/SPE-associated deregulation of the C19MC cluster could
be initiated at the beginning of the pregnancy (during
preimplantation development), or even earlier (during
gametogenesis), when the genome-wide DNA methylation reprogramming
through demethylation and remethylation occurs (25–27).
Of the 22 deregulated miRNAs detected in the present
study, 11 are located outside the C19MC cluster and are mapped to
chromosomes 1, 9, 11, 16, 17, 20 and X. Among these miRNAs, 8 have
been previously reported to be deregulated in the PE placenta
(miR-210, miR-195, miR-223, miR-1, miR-34c, miR-193b, miR-let-7f
and miR-31), while for the remaining 3 (miR-98, miR-135b and
miR-4532), PE-associated aberrant expression has not been
previously reported in the literature (Table III).
The present results on miRNA deregulation (up or
down) in SPE placentas are largely consistent with the data
reported by previous studies focusing on expression in placentas
from patients with PE (11,19–22,28–32),
suggesting that miRNA-mediated molecular mechanisms of SPE and PE
pathogeneses are similar (Table
III). A number of biochemical pathways of miRNA-mediated PE
pathogenesis have been suggested. The overexpression of miR-210,
repeatedly shown in PE placentas (11,19,20,22,28–30),
may be provoked by hypoxia, which in turn can result from impaired
placentation in PE and SPE pregnancies (19). Ectopic expression of miR-210
inhibits the migration and invasion capability of trophoblast cells
by targeting Ephrin-A3 and Homeobox-A9, which are responsible for
cell migration and vascular remodeling (33). Aberrant miR-210 expression may also
contribute to PE and, thus, to SPE by interfering with potassium
channel modulatory factor 1-mediated signaling in the placenta
(29). The expression of another
miR-210 relevant target, hydroxysteroid (17-b) dehydrogenase 1
(HSD17B1) has been shown to be decreased in the placentas
from patients with PE (20).
Notably, another upregulated miRNA in the placentas of the SPE
group detected in the present study, miR-518c, has been
experimentally demonstrated to target HSD17B1 (20), thus suggesting its possible
involvement in PE.
The activin A receptor, type IIA (ACVR2A), a
candidate gene for PE predicted by a genome-wide linkage study
(34), is regulated by miRNA.
miR-195 has been shown to repress ACVR2A and promote the
invasion of trophoblast cells (31). The decrease in miR-195 expression
that was detected in the placenta from patients with SPE in the
present study and in those from patients with PE from other studies
(19,22,31),
results in an increase in ACVR2A. The latter can provoke inhibition
of trophoblast invasion and insufficient remodeling of the spiral
arteries, thus influencing the central pathophysiological features
of PE (31) and SPE. mRNA of
ACVR2A may also be a target for the miR-223 (miRDB database;
http://mirdb.org/), which was reported to be
downregulated in placentas from patients with PE (19,20,22,30,32)
and SPE, as shown in the present study. An important biological
role of ACVR2A, miR-195 and miR-223 in the establishment of
pregnancy renders these molecules a focal point of interest for
further studies.
miR-34c, downregulated in placentas from patients
with PE (11,21) and SPE, normally mediates the
p53-dependent suppression of endometrial cell proliferation. Thus,
it may potentially contribute to PE and SPE development through the
deregulation of the cell cycle (11). The downregulation of miR-1 may
affect the risk of PE development through its effect on calcium
signaling (11) and/or through its
influence on the expression of metallopeptidase inhibitor 3, which
is involved in the regulation of trophoblast invasion (35,36).
The upregulation of miR-193b may decrease the expression of
plasminogen activator, urokinase gene, encoding urokinase-type
plasminogen activator (37), which
results in the reduction of fibrinolytic activity in placenta
vessels, promoting the development of PE (38,39).
The participation of poorly expressed miR-let-7f in the development
of PE has not yet been demonstrated; however, the role of let-7
family members in proliferation, apoptosis and inflammation
(40), suggests their contribution
to the onset of PE through the deregulation of these processes. The
PE-associated miR-31 overexpression (20), which was also observed in SPE
placentas of the present study, can contribute to placenta
dysfunction in several ways. For eample, through targeting
factor-inhibiting hypoxia-inducible factor gene, miR-31 can
activate hypoxia pathways (41),
suggesting an involvement of this particular miRNA in the hypoxic
response. Integrin-β3, which has been found to be significantly
decreased in placentas from patients with PE (42), is known to be another target for
miR-31 in cancer cells (43).
In the present study, 3 deregulated miRNAs (miR-98,
miR-135b and miR-4532), which were located outside the C19MC
cluster and had not been previously reported to be deregulated in
placentas from patients with PE, were identified in placentas from
patients with SPE. These findings suggest that the aforementioned
miRNAs may be of significance in the development of SPE. miR-135b
and miR-98 were downregulated in the placentas from the SPE group,
while miR-4532 was upregulated in the same samples. Several
predicted targets of these miRNAs could be associated with SPE
pathogenesis. Interleukin-6, the validated target of miR-98 in
melanoma cells (44), has been
reported to be significantly increased in maternal and umbilical
serum of PE patients (45). The
target for miR-135b predicted by the miRDB database corresponds to
rho-associated coiled-coil protein kinase 2 (ROCK2) known to
be elevated in PE placentas (46).
ROCK2 has been mapped within the PE linkage peak on
chromosome 2p25 in genome-wide linkage screening (47). miR-135b is expressed in placenta
tissue and found in the maternal circulation (48), confirming its feasible functional
significance in pregnancy. miR-4532 may contribute to SPE by
downregulating collagen, type I, α 1 (COL1A1), a component
of the extracellular matrix (49).
The expression of COL1A1 mRNA has been shown to be lower in
early-onset PE placentas than in the gestational age-matched
control (49). In their candidate
gene study, Goddard et al (50) reported that single nucleotide
polymorphism variants in the COL1A gene are associated with
a risk of PE. These findings are consistent with our hypothesis
that miR-98, miR-135b, and miR-4532 are associated with SPE
pathogenesis. Direct functional studies of these miRNA interactions
with relevant targets could lead to a greater understanding of the
molecular mechanisms underlying this association.
In conclusion, the present results provided novel
insights in to the pathogenesis of SPE. It was found that the
expression pattern of 22 miRNAs was significantly altered in SPE
placentas. Of these 22 miRNAs, 16 had already been described to be
associated with PE, and 6 had not previously been reported. These
findings indicated that miRNA-mediated biochemical pathways of SPE
pathogenesis largely overlap with those of PE; however, the
detection of these 6 SPE-specific deregulated miRNAs suggested the
existence of specific differences in the pathological mechanisms
between PE and SPE. The differentially expressed miRNAs may serve
as targets for the prevention and treatment of SPE. Notably, 12 of
these miRNAs (miR-515-3p, miR-515-5p, miR-518a, miR-518e, miR-527,
miR-518c, miR-519e*, miR-524, miR-210, miR-223, let-7f
and miR-135b) have been previously reported to circulate in the
blood of pregnant women (7,8,22,33,48,51–54),
which makes them potential biomarkers of SPE; however, this
requires further investigation.
Acknowledgments
Equipment from the Biobank and resource centre of
the Development of Molecular and Cellular Technology, St.
Petersburg State University was used in the present study. The
collecting of tissue samples and small RNA isolation were supported
by the Russian Federation President (grant no. 16.120.11.5773 MC).
Library preparation and Ion Torrent sequencing were supported by
the Russian Scientific Foundation (grant no. 14 50 00069).
Computational analysis was supported by the Russian Federation
Government (grant no. 074 U01). Dr Olga A. Efimova and Mr. Andrei
V. Tikhonov are grantees of RF President scholarship (grant nos.
SP-1405.2015.4 and SP-127.2015.4).
Abbreviations:
|
SPE
|
superimposed PE on chronic
hypertension
|
|
PE
|
pre-eclampsia
|
References
|
1
|
Zhao Z, Moley KH and Gronowski AM:
Diagnostic potential for miRNAs as biomarkers for
pregnancy-specific diseases. Clin Biochem. 46:953–960. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen DB and Wang W: Human placental
microRNAs and preeclampsia. Biol Reprod. 88:1302013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perni U, Sison C, Sharma V, Helseth G,
Hawfield A, Suthanthiran M and August P: Angiogenic factors in
superimposed preeclampsia: A longitudinal study of women with
chronic hypertension during pregnancy. Hypertension. 59:740–746.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Genest DS, Falcao S, Michel C, Kajla S,
Germano MF, Lacasse AA, Vaillancourt C, Gutkowska J and Lavoie JL:
Novel role of the renin-angiotensin system in preeclampsia
superimposed on chronic hypertension and the effects of exercise in
a mouse model. Hypertension. 62:1055–1061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Glotov AS, Vashukova YS, Glotov OS,
Nasykhova YuA, Mazur AM, Kurilov RV, Pekhov VM, Khrameyeva YeE,
Ivashchenko TE and Baranov VS: Study of the population frequencies
of gene polymorphisms, associated with preeclampsia. Russian
Journal of Genetics:. Applied Research. 4:388–396. 2014.
|
|
6
|
Glotov AS, Tiys ES, Vashukova ES, Pakin
VS, Demenkov PS, Saik OV, Ivanisenko TV, Arzhanova ON, Mozgovaya
EV, Zainulina MS, et al: Molecular association of pathogenetic
contributors to pre-eclampsia (pre-eclampsia associome). BMC Syst
Biol. 9(Suppl 2): S42015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Ge Q, Guo L and Lu Z: Maternal
plasma miRNAs expression in preeclamptic pregnancies. Biomed Res
Int. 2013:9702652013.PubMed/NCBI
|
|
8
|
Gunel T, Zeybek YG, Akçakaya P, Kalelioğlu
I, Benian A, Ermis H and Aydınlı K: Serum microRNA expression in
pregnancies with preeclampsia. Genet Mol Res. 10:4034–4040. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang SD, Chao AS, Peng HH, Chang YL, Wang
CN, Cheng PJ, Lee YS, Chao A and Wang TH: Analyses of placental
gene expression in pregnancy-related hypertensive disorders. Taiwan
J Obstet Gynecol. 50:283–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
No authors listed. Report of the National
high blood pressure education program working group on high blood
pressure in pregnancy. Am J Obstet Gynecol. 183:S1–S22. 2000.
View Article : Google Scholar
|
|
11
|
Enquobahrie DA, Abetew DF, Sorensen TK,
Willoughby D, Chidambaram K and Williams MA: Placental microRNA
expression in pregnancies complicated by preeclampsia. Am J Obstet
Gynecol. 204:178.e12–178.e21. 2011. View Article : Google Scholar
|
|
12
|
Sun Z, Evans J, Bhagwate A, Middha S,
Bockol M, Yan H and Kocher JP: CAP-miRSeq: A comprehensive analysis
pipeline for microRNA sequencing data. BMC Genomics. 15:4232014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martin M: Cutadapt removes adapter
sequences from high-throughput sequencing reads. EMBnet Journal.
17:102011. View Article : Google Scholar
|
|
14
|
Langmead B, Trapnell C, Pop M and Salzberg
SL: Ultrafast and memory-efficient alignment of short DNA sequences
to the human genome. Genome Biol. 10:R252009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kozomara A and Griffiths-Jones S: MiRBase:
Integrating microRNA annotation and deep-sequencing data. Nucleic
Acids Res. 39(Database Issue): D152–D157. 2011. View Article : Google Scholar :
|
|
16
|
Friedlander MR, Mackowiak SD, Li N, Chen W
and Rajewsky N: MiRDeep2 accurately identifies known and hundreds
of novel microRNA genes in seven animal clades. Nucleic Acids Res.
40:37–52. 2012. View Article : Google Scholar :
|
|
17
|
Robinson MD, McCarthy DJ and Smyth GK:
EdgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar
|
|
18
|
Benjamini Y, Drai D, Elmer G, Kafkafi N
and Golani I: Controlling the false discovery rate in behavior
genetics research. Behav Brain Res. 125:279–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu X, Han T, Sargent IL, Yin GW and Yao
YQ: Differential expression profile of microRNAs in human placentas
from preeclamptic pregnancies vs normal pregnancies. Am J Obstet
Gynecol. 200:661.e1–661.e7. 2009. View Article : Google Scholar
|
|
20
|
Ishibashi O, Ohkuchi A, Ali MM, Kurashina
R, Luo SS, Ishikawa T, Takizawa T, Hirashima C, Takahashi K, Migita
M, et al: Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by
miR-210 and miR-518c that are aberrantly expressed in preeclamptic
placentas: A novel marker for predicting preeclampsia.
Hypertension. 59:265–273. 2012. View Article : Google Scholar
|
|
21
|
Wang W, Feng L, Zhang H, Hachy S, Satohisa
S, Laurent LC, Parast M, Zheng J and Chen DB: Preeclampsia
up-regulates angiogenesis-associated microRNA (i.e., miR-17, -20a
and -20b) that target ephrin-B2 and EPHB4 in human placenta. J Clin
Endocrinol Metab. 97:E1051–E1059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu P, Zhao Y, Liu M, Wang Y, Wang H, Li
YX, Zhu X, Yao Y, Wang H, Qiao J, et al: Variations of microRNAs in
human placentas and plasma from preeclamptic pregnancy.
Hypertension. 63:1276–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai KW, Kao HW, Chen HC, Chen SJ and Lin
WC: Epigenetic control of the expression of a primate-specific
microRNA cluster in human cancer cells. Epigenetics. 4:587–592.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noguer-Dance M, Abu-Amero S, Al-Khtib M,
Lefèvre A, Coullin P, Moore GE and Cavaillé J: The primate-specific
microRNA gene cluster (C19MC) is imprinted in the placenta. Hum Mol
Genet. 19:3566–3582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pendina AA, Efimova OA, Fedorova ID,
Leont'eva OA, Shilnikova EM, Lezhnina JG, Kuznetzova TV and Baranov
VS: DNA methylation patterns of metaphase chromosomes in human
preimplantation embryos. Cytogenet Genome Res. 132:1–7. 2011.
View Article : Google Scholar
|
|
26
|
Efimova OA, Pendina AA, Tikhonov AV,
Fedorova ID, Krapivin MI, Chiryaeva OG, Shilnikova EM, Bogdanova
MA, Kogan IY, Kuznetzova TV, et al: Chromosome hydroxymethylation
patterns in human zygotes and cleavage-stage embryos. Reproduction.
149:223–233. 2015. View Article : Google Scholar
|
|
27
|
Tang W W, Dietmann S, Irie N, Leitch HG,
Floros VI, Bradshaw CR, Hackett JA, Chinnery PF and Surani MA: A
unique gene regulatory network resets the human germline epigenome
for development. Cell. 161:1453–1467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pineles BL, Romero R, Montenegro D, Tarca
AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic J and Mittal
P: Distinct subsets of microRNAs are expressed differentially in
the human placentas of patients with preeclampsia. Am J Obstet
Gynecol. 196:261.e1–261.e6. 2007. View Article : Google Scholar
|
|
29
|
Luo R, Shao X, Xu P, Liu Y, Wang Y, Zhao
Y, Liu M, Ji L, Li YX, Chang C, et al: MicroRNA-210 contributes to
preeclampsia by downregulating potassium channel modulatory factor
1. Hypertension. 64:839–845. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weedon-Fekjær MS, Sheng Y, Sugulle M,
Johnsen GM, Herse F, Redman CW, Lyle R, Dechend R and Staff AC:
Placental miR-1301 is dysregulated in early-onset preeclampsia and
inversely correlated with maternal circulating leptin. Placenta.
35:709–717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai Y, Yang W, Yang HX, Liao Q, Ye G, Fu
G, Ji L, Xu P, Wang H, Li YX and Peng C: Downregulated miR-195
detected in preeclamptic placenta affects trophoblast cell invasion
via modulating ActRIIA expression. PLoS One. 7:e388752012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi SY, Yun J, Lee OJ, Han HS, Yeo MK,
Lee MA and Suh KS: MicroRNA expression profiles in placenta with
severe preeclampsia using a PNA-based microarray. Placenta.
34:799–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Fei M, Xue G, Zhou Q, Jia Y, Li
L, Xin H and Sun S: Elevated levels of hypoxia-inducible
microRNA-210 in pre-eclampsia: New insights into molecular
mechanisms for the disease. J Cell Mol Med. 16:249–259. 2012.
View Article : Google Scholar
|
|
34
|
Moses EK, Fitzpatrick E, Freed KA, Dyer
TD, Forrest S, Elliott K, Johnson MP, Blangero J and Brennecke SP:
Objective prioritization of positional candidate genes at a
quantitative trait locus for pre-eclampsia on 2q22. Mol Hum Reprod.
12:505–512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lim LP, Lau NC, Garrett-Engele P, Grimson
A, Schelter JM, Castle J, Bartel DP, Linsley PS and Johnson JM:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiang Y, Zhang X, Li Q, Xu J, Zhou X, Wang
T, Xing Q, Liu Y, Wang L, He L and Zhao X: Promoter hypomethylation
of TIMP3 is associated with pre-eclampsia in a Chinese population.
Mol Hum Reprod. 19:153–159. 2013. View Article : Google Scholar
|
|
37
|
Ikeda Y, Tanji E, Makino N, Kawata S and
Furukawa T: MicroRNAs associated with mitogen-activated protein
kinase in human pancreatic cancer. Mol Cancer Res. 10:259–269.
2012. View Article : Google Scholar
|
|
38
|
Buchholz T, Lohse P, Rogenhofer N, Kosian
E, Pihusch R and Thaler CJ: Polymorphisms in the ACE and PAI-1
genes are associated with recurrent spontaneous miscarriages. Hum
Reprod. 18:2473–2477. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lam KK, Chiu PC, Chung MK, Lee CL, Lee KF,
Koistinen R, Koistinen H, Seppala M, Ho PC and Yeung WS:
Glycodelin-A as a modulator of trophoblast invasion. Hum Reprod.
24:2093–2103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang XD, Zhang YH, Ling YH, Liu Y, Cao
HG, Yin ZJ, Ding JP and Zhang XR: Characterization and differential
expression of microRNAs in the ovaries of pregnant and non-pregnant
goats (Capra hircus). BMC Genomics. 14:1572013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu CJ, Tsai MM, Hung PS, Kao SY, Liu TY,
Wu KJ, Chiou SH, Lin SC and Chang KW: MiR-31 ablates expression of
the HIF regulatory factor FIH to activate the HIF pathway in head
and neck carcinoma. Cancer Res. 70:1635–1644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou Y, Damsky CH and Fisher SJ:
Preeclampsia is associated with failure of human cytotrophoblasts
to mimic a vascular adhesion phenotype: One cause of defective
endovascular invasion in this syndrome? J Clin Invest.
99:2152–2164. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Augoff K, Das M, Bialkowska K, McCue B,
Plow EF and Sossey-Alaoui K: MiR-31 is a broad regulator of
β1-integrin expression and function in cancer cells. Mol Cancer
Res. 9:1500–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li F, Li XJ, Qiao L, Shi F and Liu W, Li
Y, Dang YP, Gu WJ, Wang XG and Liu W: MiR-98 suppresses melanoma
metastasis through a negative feedback loop with its target gene
IL-6. Exp Mol Med. 46:e1162014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tosun M, Celik H, Avci B, Yavuz E, Alper T
and Malatyalioğlu E: Maternal and umbilical serum levels of
interleukin-6, interleukin-8 and tumor necrosis factor-alpha in
normal pregnancies and in pregnancies complicated by preeclampsia.
J Matern Fetal Neonatal Med. 23:880–886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ark M, Yilmaz N, Yazici G, Kubat H and
Aktaş S: Rho-associated protein kinase II (rock II) expression in
normal and preeclamptic human placentas. Placenta. 26:81–84. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peterson H, Laivuori H, Kerkelä E, Jiao H,
Hiltunen L, Heino S, Tiala I, Knuutila S, Rasi V, Kere J and
Kivinen K: ROCK2 allelic variants are not associated with
pre-eclampsia susceptibility in the Finnish population. Mol Hum
Reprod. 15:443–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chim SS, Shing TK, Hung EC, Leung TY, Lau
TK, Chiu RW and Lo YM: Detection and characterization of placental
microRNAs in maternal plasma. Clin Chem. 54:482–490. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
He P, Shao D, Ye M and Zhang G: Analysis
of gene expression identifies candidate markers and pathways in
pre-eclampsia. J Obstet Gynaecol. 35:578–584. 2015. View Article : Google Scholar
|
|
50
|
Goddard KA, Tromp G, Romero R, Olson JM,
Lu Q, Xu Z, Parimi N, Nien JK, Gomez R, Behnke E, et al:
Candidate-gene association study of mothers with pre-eclampsia and
their infants, analyzing 775 SNPs in 190 genes. Hum Hered. 63:1–16.
2007. View Article : Google Scholar
|
|
51
|
Gilad S, Meiri E, Yogev Y, Benjamin S,
Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H and
Melamed N: Serum microRNAs are promising novel biomarkers. PLoS
One. 3:e31482008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miura K, Miura S, Yamasaki K, Higashijima
A, Kinoshita A, Yoshiura K and Masuzaki H: Identification of
pregnancy-associated microRNAs in maternal plasma. Clin Chem.
56:1767–1771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang Q, Lu J, Wang S, Li H, Ge Q and Lu Z:
Application of next-generation sequencing technology to profile the
circulating microRNAs in the serum of preeclampsia versus normal
pregnant women. Clin Chim Acta. 412:2167–2173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu Y, Li P, Hao S, Liu L, Zhao J and Hou
Y: Differential expression of microRNAs in the placentae of Chinese
patients with severe pre-eclampsia. Clin Chem Lab Med. 47:923–929.
2009. View Article : Google Scholar : PubMed/NCBI
|