Introduction
Retinal pigment epithelial (RPE) cells are important
for maintaining the function of the visual system. Normal RPE cells
are quiescent and do not proliferate or migrate (1,2).
Epithelial-mesenchymal transition (EMT), proliferation, invasion
and migration of RPE cells are key in the development of
proliferative vitreoretinopathy (PVR) and various other
fibroproliferative eye diseases, which lead to blindness. The
proliferation, directional migration to the vitreous and EMT of
quiescent, differentiated RPE cells contribute to the development
of PVR. During PVR, RPE cells transform into fibroblast-like cells
through EMT (3). EMT may be
triggered by various signaling molecules, including epidermal
growth factor and fibroblast growth factor (FGF); however,
transforming growth factor β-1 (TGF-β1) is considered to be the
primary regulator of EMT (4,5).
TGF-β-induced EMT is known to promote cell migration
and invasion. Lens epithelial cells and corneal epithelial cells
have been shown to undergo TGF-β-mediated EMT (6). TGF-β is a multifunctional cytokine
that is involved in number of biological functions, including cell
growth, differentiation, immunomodulation, oxidative stress and
endoplasmic reticulum (ER) stress (7,8).
TGF-β also contributes to pericellular proteolysis via regulation
of the expression and secretion of plasminogen activators. TGF-β
promotes EMT via the Smad and non-Smad signaling pathways, and
crosstalks between them. TGF-β1 activates Smad-independent
pathways, including ERK, p38 mitogen-activated protein kinase
(MAPK), c-Jun N-terminal kinases (JNK), phosphatidylinositol
3-kinase/Akt and nuclear factor κB (NF-κB) (9).
TGF-β-activated kinase 1 (TAK1) is a
serine/threonine kinase and is a member of the MAPK kinase kinase
family (10). TAK1 is an important
regulator of the cell cycle and apoptosis, and its activity is
regulated by various cytokines, including interleukin-1 and TGF-β
(10). Once activated, TAK1 in
turn activates intracellular kinases, including p38 MAPK, JNK, and
the I-κB kinase complex (11).
Activated TAK1 then transduces signals to several downstream
signaling cascades, including the MKK4/7-JNK, MKK3/6-p38 MAPK, and
NF-κB-inducing kinase-IκB kinase (IKK) (12).
To the best of our knowledge, the present study was
the first to identify TAK1 upregulation in human RPE cells with
TGF-β1-induced EMT. Inhibition of TAK1 activity by LYTAK1
significantly inhibited the proliferation of RPE cells.
Additionally, LYTAK1 significantly prevented TGF-β1-induced EMT via
the regulation of the canonical Smad signaling pathway. The current
study also demonstrated that the NF-κB signaling pathway is
affected by LYTAK1 during EMT. Therefore, the results of the
present study suggest that inhibition of TAK1 activity may be a
novel approach for the treatment and prevention of PVR.
Materials and methods
Cell culture and treatment groups
The ARPE-19 human RPE cell line was provided by
Professor Fu Shang at the Laboratory for Nutrition and Vision
Research, Tufts University (Boston, MA, USA), and cultured in
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.). The cells were grown
to 70% confluence at 37°C in a humidified atmosphere containing 5%
CO2 and were dissociated with a 0.25% trypsin-0.02%
ethylenediaminetetraacetic acid solution (Sigma-Aldrich, St. Louis,
MO, USA). TGF-β1 and LYTAK1 were purchased from Sigma-Aldrich.
The cells were randomly divided into 6 groups:
Control group, TGF-β1 group, TGF-β1 + LYTAK1 (1 µM) group,
TGF-β1 + LYTAK1 (10 µM) group, TGF-β1 + LYTAK1 (25
µM) group and TGF-β1 + LYTAK1 (50 µM) group.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. cDNA was synthesized using a reverse
transcription kit (Takara Biotechnology Co., Ltd., Siga, Japan),
using protocols recommended by the manufacturer. For quantitative
analysis of mRNA expression levels, SYBR PrimeScript RT-PCR kit
(Takara Biotechnology Co., Ltd.) was used to amplify the target
genes with the ABI Prism 7000 sequence detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). PCR primers were as
follows: TAK1, forward 5′-GAATTAGCGCTTTGGGTTGC-3′ and reverse
5′-TTTCTTTCGCAGTGCTGCAT-3′; α-SMA, forward
5′-CTATTCCTTCGTGACTACT-3′ and reverse 5′-ATGCTGTTATAGGTGGTGGTT-3′;
fibronectin, forward 5′-TCTCCTGCCTGGTACAGAATATGTAGTGAG-3′ and
reverse 5′-GGTCGCAGCAACAACTTCCAGGT-3′; and GAPDH, forward
5′-GGCAAATTCAACGGCACAGTC-3′ and reverse
5′-GCTGACAATCTTGAGTGAGTT-3′. The PCR procedure was as follows: 94°C
for 4 min; 94°C for 20 sec, 55°C for 30 sec and 72°C for 20 sec; 2
sec for plate reading for 40 cycles; and melt curve from 65–95°C.
Relative quantification of gene expression was performed using the
2−ΔΔCq method (13).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was used as
an internal control.
Western blot analysis
For total protein extraction, cells were lysed with
100 µl radioimmunoprecipitation assay lysis buffer with
protease inhibitor cocktail (Invitrogen; Thermo Fisher Scientific,
Inc.). The cell lysates were collected following centrifugation
(6,000 × g for 10 min) and mixed with 5X sodium dodecyl sulfate
(SDS) sample buffer (Takara Biotechnology, Inc., Dalian, China),
and a bicinchoninic acid assay was used to measure the protein
levels. The samples (40 µg) were loaded and separated on 10%
SDS-PAGE (Takara Biotechnology, Inc.), and then transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked in 5% non-fat milk and incubated
with the following primary antibodies at 4°C overnight: anti-TAK1
(1:2,000; sc-7967), anti-α-smooth muscle actin (SMA; 1:1,500;
sc-53015), anti-fibronectin (1:2,000; sc-81769), anti-NF-κB p65
(1:2,000; sc-8008), anti-IKKα (1:2,000; sc-7606), anti-p-Smad2
(1:2,000; sc-101801), anti-p-Smad3 (1:2,000; sc-130218) and GAPDH
(1:1,500; sc-166574; all from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). After washing with phosphate-buffered saline with
Tween-20, the membranes were incubated with goat anti-mouse IgG
horseradish peroxidase-conjugated secondary antibodies (1:3,000;
sc-395760; Santa Cruz Biotechnology, Inc.) and goat anti-rabbit
HRP-conjugated secondary antibodies (1:2,000; sc-2030; Santa Cruz
Biotechnology, Inc.) for 1 h at room temperature. The bands on the
membranes were visualized using chemiluminescence detection
reagents (Roche Diagnostics GmbH, Mannheim, Germany). Densitometic
analysis was conducted using Image J version 1.41 (National
Institutes of Health, Bethesda, MD, USA). GAPDH was used as a
control.
Cell proliferation assay
The proliferation of RPE cells was examined using
Cell Counting kit-8 (CCK-8; Sigma-Aldrich) according to the
manufacturer's protocol. Briefly, RPE cells were seeded into
96-well plates at the density of 5×103 cells/well with
100 µl of complete culture medium (Invitrogen; Thermo Fisher
Scientific, Inc.) and cultured for 24 h. Cells were then treated
with 1, 10, 25 and 50 µM LYTAK1 for 24, 48 and 72 h. At the
end of each treatment period, 10 µl CCK-8 solution was added
to each well and incubated for 2 h at 37°C. The absorbance was
determined at 450 nm with a microplate reader (Model 680; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data in the current study are expressed as the mean
± standard deviation. Statistical differences between the means of
two groups was analysed using Student's t-test, and between greater
than two groups using one-way analysis of variance followed by
multiple comparisons performed using a post hoc Bonferroni test in
SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of TAK1 in TGF-β1-induced EMT
of RPE cells
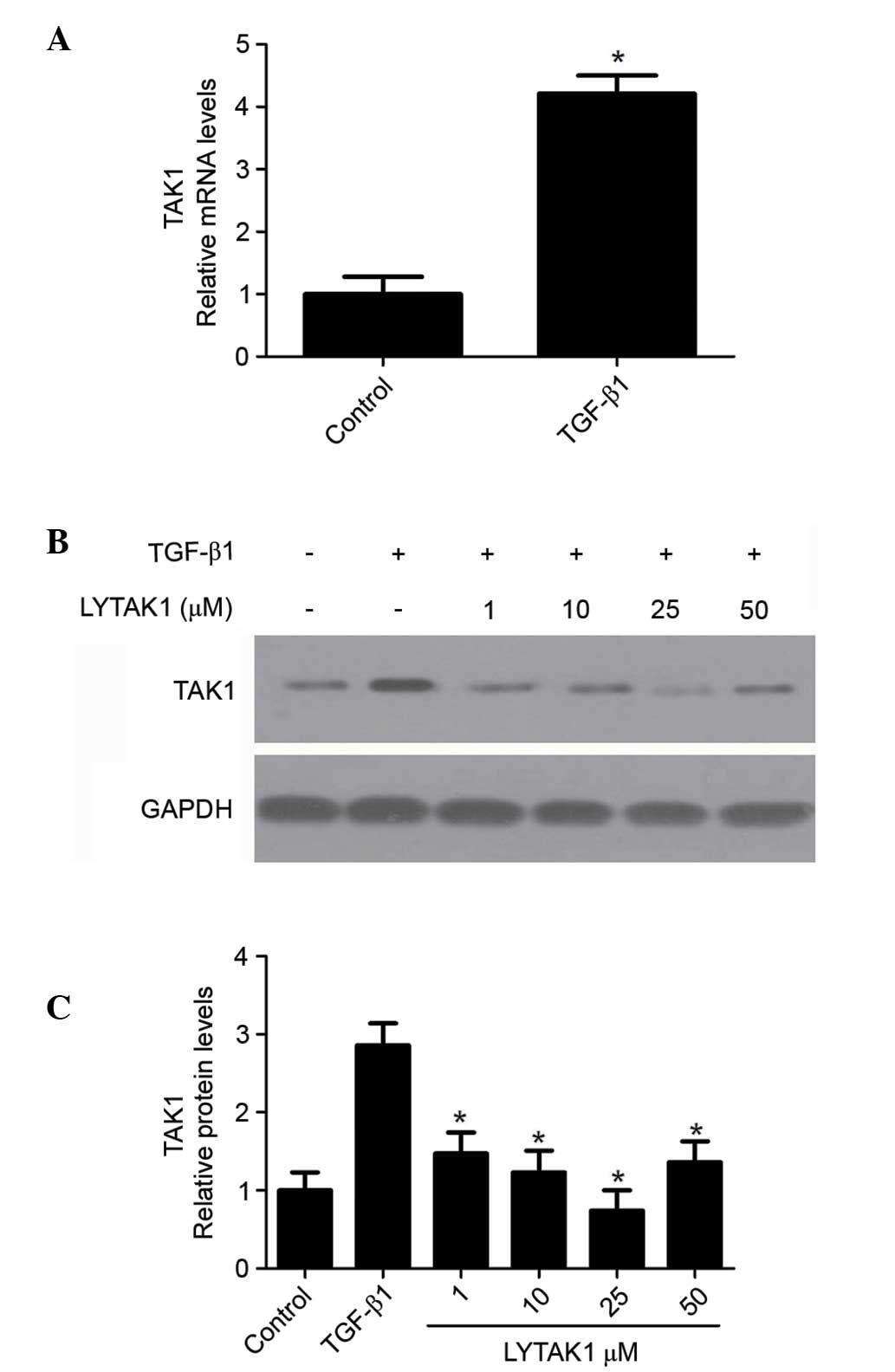
The expression of TAK1 in TGF-β1-induced EMT of
human RPE cells was determined by RT-qPCR. The results indicated
that TGF-β1 significantly increased TAK1 mRNA expression levels
(P<0.05; Fig. 1A).
Additionally, the upregulation of TAK1 expression levels induced by
TGF-β1 were significantly inhibited by LYTAK1 (P<0.05; Fig. 1B and C). These results suggest that
TAK1 is upregulated in TGF-β1-induced EMT in RPE cells.
LYTAK1 prevents TGF-β1-induced EMT in RPE
cells
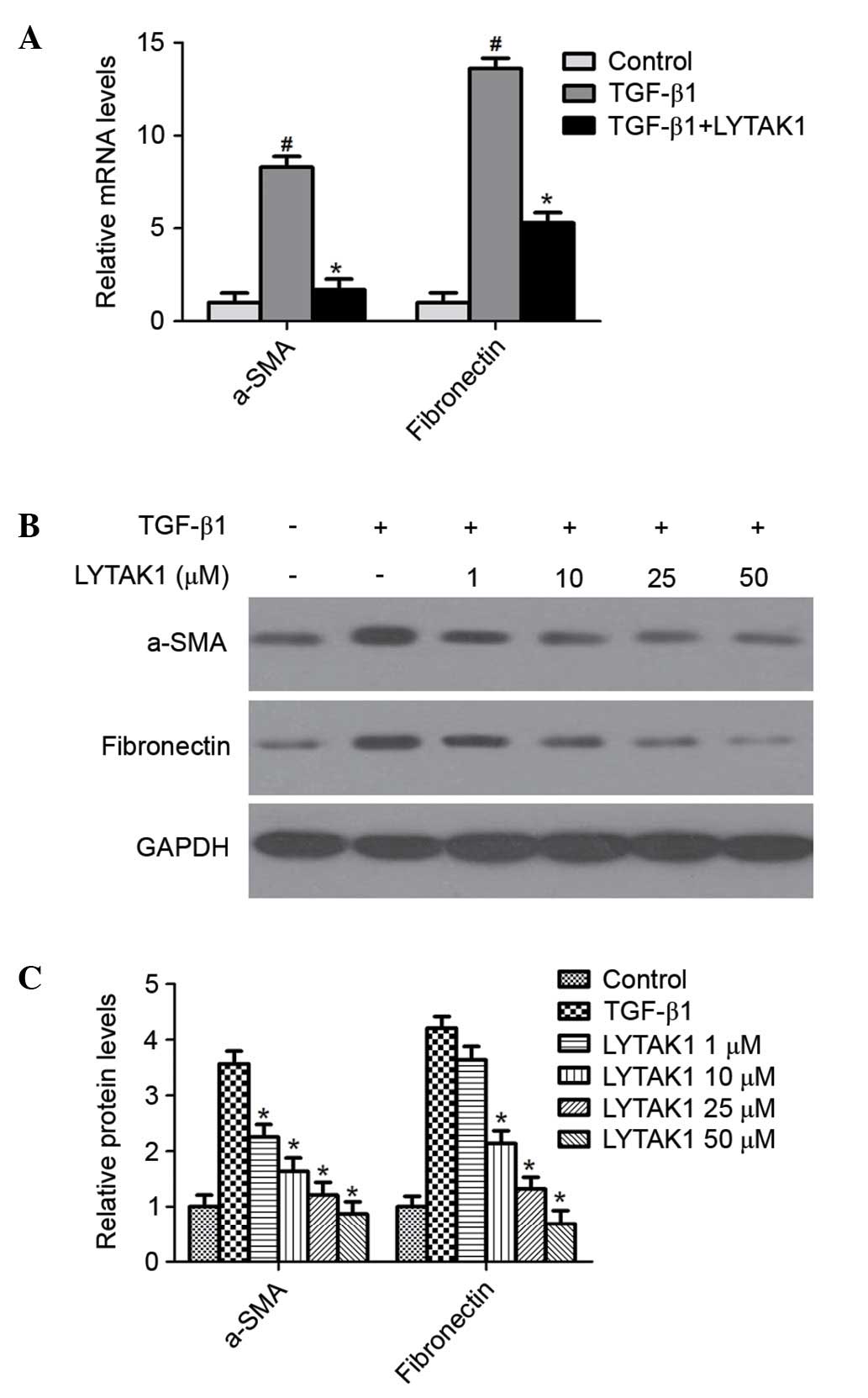
During EMT, the expression levels of the mesenchymal
markers fibronectin and α-smooth muscle actin (α-SMA) are
upregulated. In order to investigate whether TAK1 inhibition
prevents TGF-β1-induced EMT in RPE cells, the mRNA and protein
levels were determined by RT-qPCR and western blotting
respectively. TGF-β1 significantly increased the expression of
α-SMA and fibronectin compared with the control at the mRNA
(P<0.05; Fig. 2A) and protein
levels (P<0.05; Fig. 2B and C).
Notably, LYTAK1 treatment significantly reduced the upregulation of
α-SMA and fibronectin induced by TGF-β1 in a
concentration-dependent manner (P<0.05; Fig. 2). Therefore, these data indicate
that the TAK1 inhibitor, LYTAK1 significantly attenuates
TGF-β1-induced EMT in RPE cells.
LYTAK1 inhibits the proliferation of RPE
cells
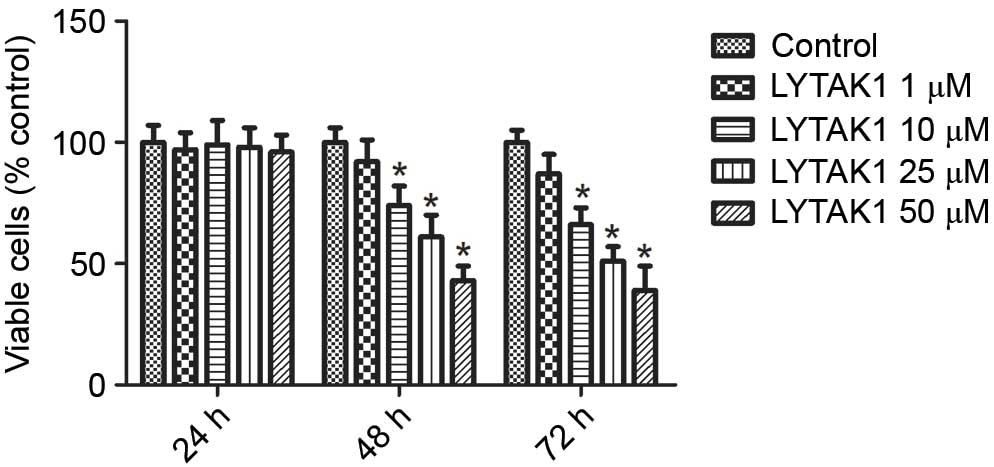
RPE cell proliferation is important in the
progression of PVR. Therefore, the effect of the TAK1 inhibitor,
LYTAK1, on the growth of RPE cells was examined. As indicated in
Fig. 3, LYTAK1 exhibited
significant inhibitory effects on cell growth for 48 and 72 h in a
concentration dependent manner (P<0.05); however, no effect was
observed after 24 h of treatment. These results indicate that
LYTAK1 may successfully inhibit the proliferation of RPE cells.
LYTAK1 inhibits the TGF-β1 signaling
pathway by suppressing the phosphorylation of Smad2
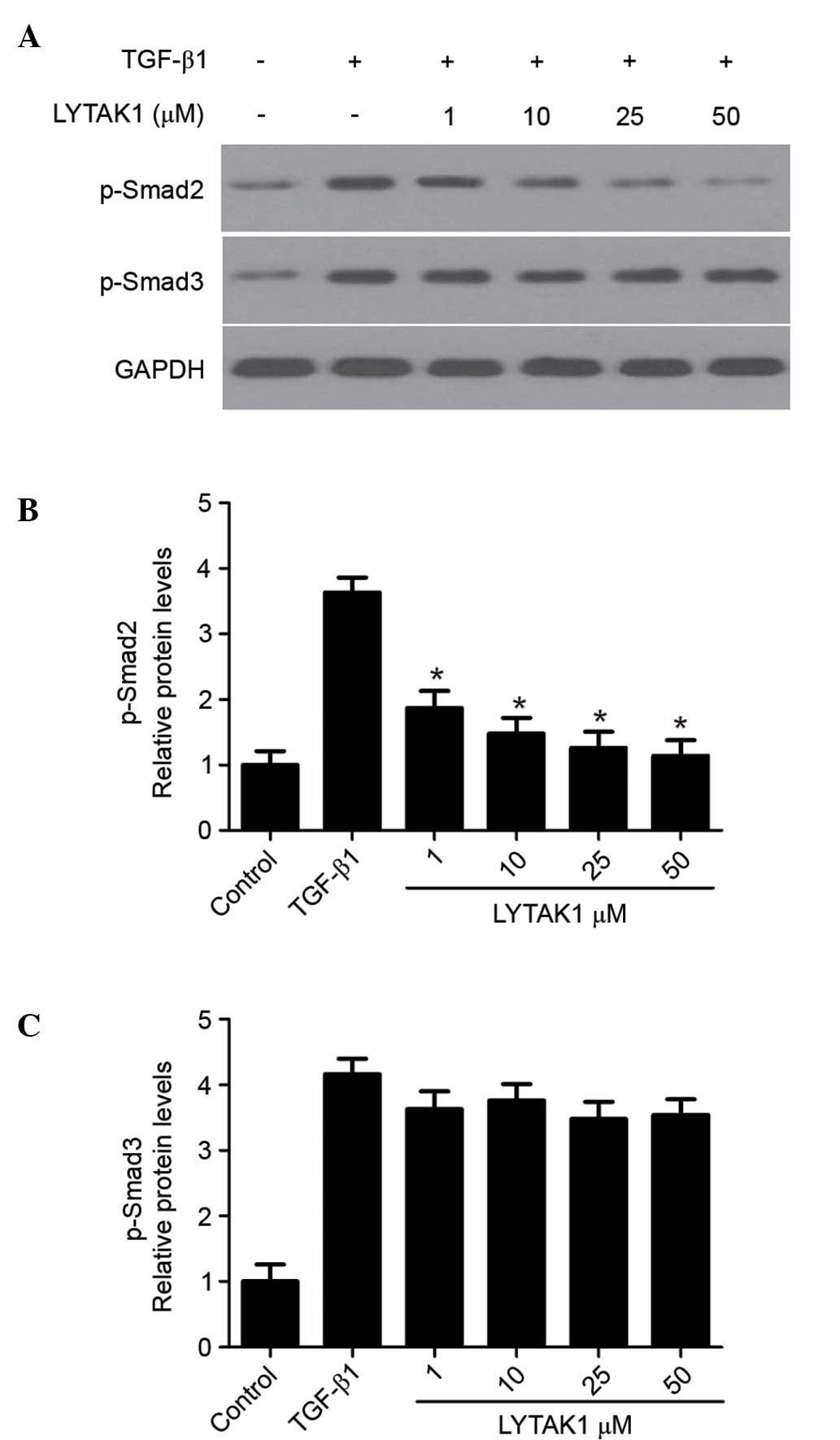
In order to determine the underlying mechanism of
the inhibitory effect of LYTAK1 on TGF-β1 signaling, the effect of
LYTAK1 on the phosphorylation of Smad2 and Smad3 was investigated.
As shown in Fig. 4, TGF-β1
treatment led to the phosphorylation of Smad2 and Smad3 after 60
min. When cells were treated with LYTAK1, the phosphorylation of
Smad2 was significantly inhibited in a concentration-dependent
manner when compared with the TGF-β1 group (Fig. 4B; P<0.05). However, no effect on
the phosphorylation of Smad3 was observed (Fig. 4C). These results suggest that
LYTAK1 suppresses TGF-β1 signaling via the phosphorylation of
Smad2.
LYTAK1 reduces TGF-β1-induced EMT via
downregulation of the NF-κB pathway
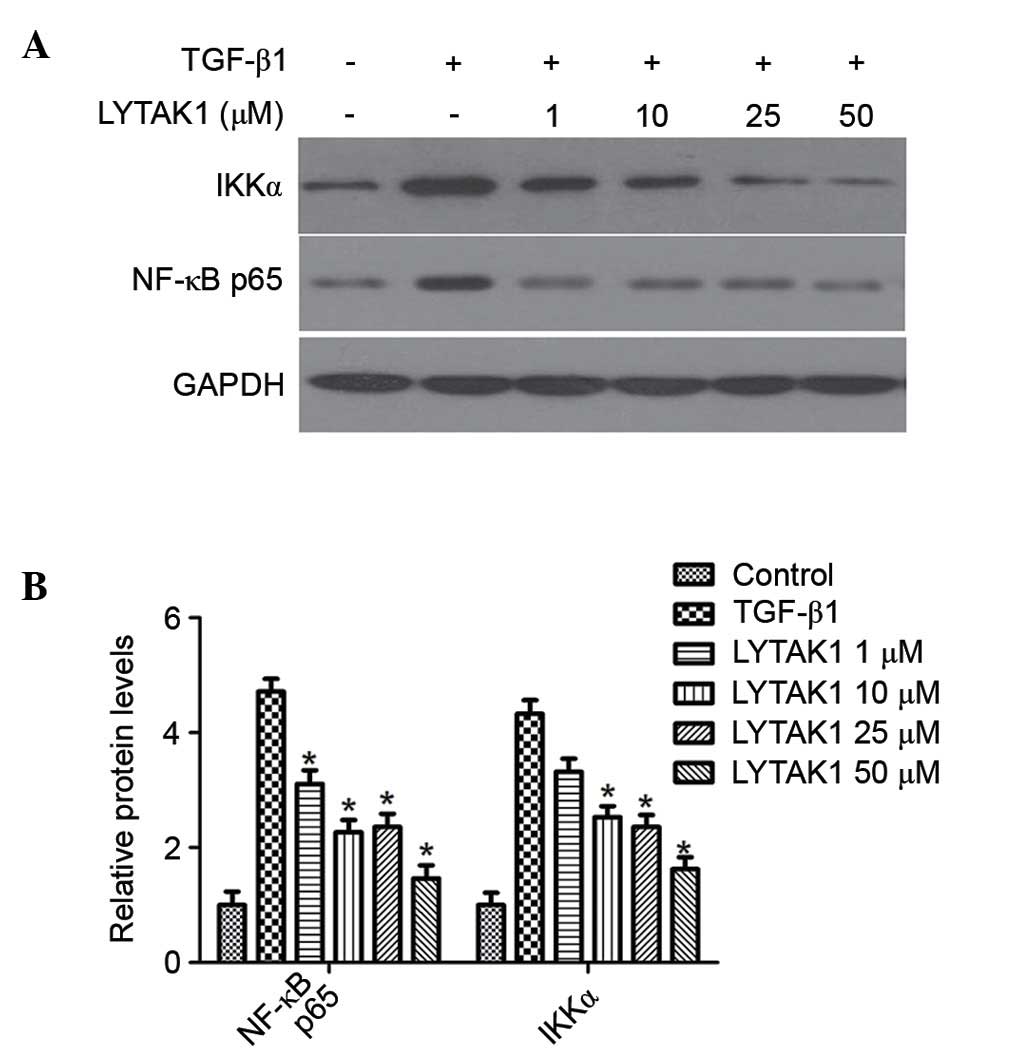
TAK1 is an established upstream kinase required for
NF-κB activation, which is important for cell progression.
Therefore, the effects of LYTAK1 on NF-κB signaling in RPE cells
undergoing TGF-β1-induced EMT was investigated. The results
demonstrated that TGF-β1 treatment alone led to a significant
increase of the phosphorylation of NF-κB p65 and IκB kinases α
(IKKα) (Fig. 5), which are
indicators of NF-κB activation. LYTAK1 treatment was able to
significantly attenuate the TGF-β1-induced phosphorylation of NF-κB
p65 and IKKα in a concentration-dependent manner (P<0.05;
Fig. 5B).
Discussion
Epithelial to mesenchymal transition is an important
morphological step, which occurs during embryonic development. This
process is also observed in adults during wound healing, tumor
progression and organ fibrosis (14). PVR, an ocular fibrotic disease, is
a process that entails fibrocellular proliferation in the vitreous
cavity (15). The gradual
contraction of epiretinal membranes leads to a marked distortion of
the retinal structure and results in retinal detachment and the
loss of vision. RPE cells are the primary cell type involved in the
pathogenesis of PVR, which results from EMT, proliferation, and
migration of transformed RPE cells to the vitreous (16,17).
The importance of EMT in the development of PVR has become
evident.
TAK1 has been identified as a central regulator of
diverse physiological processes, including development, metabolism,
and immune and stress responses, which in turn trigger the
activation of the transcription factors NF-κB and activation
protein 1 (AP-1) (17). A previous
study also described the importance of TAK1 in the progression of
cancer cells (18) and it has been
demonstrated that TAK1 is overexpressed and/or overactivated in
numerous types of cancer (19,20).
Inhibition of TAK1 via pharmacological or genetic methods has been
indicated to induce apoptosis in cancer cells (21). TAK1 has been identified to
contribute to the non-canonical pathway of the TGF-β1 response
(22). Therefore, TAK1 controls
the phosphorylation of downstream proteins, such as p38. The
present study demonstrated that the TAK1 inhibitor, LYTAK1, was
able to suppress the proliferation of RPE cells and decrease the
expression levels of the mesenchymal markers, fibronectin and
α-SMA, indicating that TAK1 is involved in the maintenance of the
mesenchymal properties of transformed RPE cells.
TGF-β1 predominantly acts via the following
pathways: i) The canonical Smad-dependent pathway and ii) the
non-canonical Smad-independent pathway. The canonical TGF-β1/Smad
signaling transmits a signal via binding to two associated
transmembrane type I and type II receptors, which subsequently
phosphorylate receptor-regulated Smad proteins-Smad2 and/or Smad3
(23). The effect of LYTAK1 on the
activation of Smad2 and Smad3 induced by TGF-β1 was examined in the
present study. It was determined that LYTAK1 may inhibit the
phosphorylation of Smad2 induced by TGF-β1; however, it had no
effect on the phosphorylation of Smad3 in RPE cells. These results
suggest that LYTAK1 may mediate the canonical TGF-β1/Smad signaling
via suppression of the phosphorylation of Smad2.
TGF-β1 signaling is vital for the signaling
networks, which control EMT. However, it is not limited to the
canonical Smad signaling pathway, as non-Smad signaling pathways
may also be influenced. TAK1, an MAPK, contributes to the
activation of NF-κB and AP-1, which may suppress proapoptotic
signaling pathways and thus promote resistance to chemotherapeutic
drugs (18). A previous study
indicated that TAK1, via the NF-κB signaling pathway, triggered
increased EMT and led to increased invasiveness of tumors (24). In the present study, LYTAK1 was
identified to inhibit TGF-β1-induced EMT via the NF-κB pathway.
In conclusion, the current study demonstrated that
the inhibition of TAK1 activity with LYTAK1 treatment leads to the
reduction of cell proliferation and TGF-β1-induced EMT in human RPE
cells. The mechanism underlying EMT suppression by LYTAK1 involved
the inactivation of the canonical Smad signaling pathway. In
addition, the NF-κB signaling pathway may also contribute to the
reduction in EMT. The findings of the present study establish a
framework for further research on the importance of EMT in RPE in a
number of proliferative vitreoretinopathies characterized by
EMT.
Acknowledgments
The present study was supported by the Department of
Technology Application and Development of Yunnan Province (grant
no. 2014RA070).
References
|
1
|
Bharti K, Nguyen MT, Skuntz S, Bertuzzi S
and Arnheiter H: The other pigment cell: Specification and
development of the pigmented epithelium of the vertebrate eye.
Pigment Cell Res. 19:380–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strauss O: The retinal pigment epithelium
in visual function. Physiol Rev. 85:845–881. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takahashi E, Nagano O, Ishimoto T, Yae T,
Suzuki Y, Shinoda T, Nakamura S, Niwa S, Ikeda S, Koga H, et al:
Tumor necrosis factor-alpha regulates transforming growth
factor-beta-dependent epithelial-mesenchymal transition by
promoting hyaluronan-CD44-moesin interaction. J Biol Chem.
285:4060–4073. 2010. View Article : Google Scholar
|
|
4
|
Garweg JG, Tappeiner C and Halberstadt M:
Pathophysiology of proliferative vitreoretinopathy in retinal
detachment. Surv Ophthalmol. 58:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamouille S and Derynck R: Cell size and
invasion in TGF-beta-induced epithelial to mesenchymal transition
is regulated by activation of the mTOR pathway. J Cell Biol.
178:437–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saika S: TGFbeta pathobiology in the eye.
Lab Invest. 86:106–115. 2006. View Article : Google Scholar
|
|
7
|
Desmoulière A, Geinoz A, Gabbiani F and
Gabbiani G: Transforming growth factor-beta 1 induces alpha-smooth
muscle actin expression in granulation tissue myofibroblasts and in
quiescent and growing cultured fibroblasts. J Cell Biol.
122:103–111. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoon YS, Lee JH, Hwang SC, Choi KS and
Yoon G: TGF beta1 induces prolonged mitochondrial ROS generation
through decreased complex IV activity with senescent arrest in
Mv1Lu cells. Oncogene. 24:1895–1903. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mu Y, Gudey SK and Landström M: Non-Smad
signaling pathways. Cell Tissue Res. 347:11–20. 2012. View Article : Google Scholar
|
|
10
|
Kim SI, Kwak JH, Na HJ, Kim JK, Ding Y and
Choi ME: Transforming growth factor-beta (TGF-beta1) activates TAK1
via TAB1-mediated autophosphorylation, independent of TGF-beta
receptor kinase activity in mesangial cells. J Biol Chem.
284:22285–22296. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji
J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O and Akira S:
Essential function for the kinase TAK1 in innate and adaptive
immune responses. Nat Immunol. 6:1087–1095. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanada M, Ninomiya-Tsuji J, Komaki K,
Ohnishi M, Katsura K, Kanamaru R, Matsumoto K and Tamura S:
Regulation of the TAK1 signaling pathway by protein phosphatase 2C.
J Biol Chem. 276:5753–5759. 2001. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
García S, López E and López-Colomé AM:
Glutamate accelerates RPE cell proliferation through ERK1/2
activation via distinct receptor-specific mechanisms. J Cell
Biochem. 104:377–390. 2008. View Article : Google Scholar
|
|
16
|
Palma-Nicolás JP, López E and López-Colomé
AM: Thrombin stimulates RPE cell motility by PKC-zeta-and NF-kappa
B-dependent gene expression of MCP-1 and CINC-1/GRO chemokines. J
Cell Biochem. 110:948–967. 2010. View Article : Google Scholar
|
|
17
|
Adhikari A, Xu M and Chen ZJ:
Ubiquitin-mediated activation of TAK1 and IKK. Oncogene.
26:3214–3226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakurai H: Targeting of TAK1 in
inflammatory disorders and cancer. Trends Pharmacol Sci.
33:522–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melisi D, Xia Q, Paradiso G, Ling J,
Moccia T, Carbone C, Budillon A, Abbruzzese JL and Chiao PJ:
Modulation of pancreatic cancer chemoresistance by inhibition of
TAK1. J Natl Cancer Inst. 103:1190–1204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh A, Sweeney MF, Yu M, Burger A,
Greninger P, Benes C, Haber DA and Settleman J: TAK1 inhibition
promotes apoptosis in KRAS-dependent colon cancers. Cell.
148:639–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neil JR, Tian M and Schiemann WP: X-linked
inhibitor of apoptosis protein and its E3 gene ligase activity
promote transforming growth factor-beta-mediated nuclear
factor-kappaB activation during breast cancer progression. J Biol
Chem. 284:21209–21217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Landström M: The TAK1-TRAF6 signalling
pathway. Int J Biochem Cell Biol. 42:585–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akhurst RJ and Hata A: Targeting the TGFβ
signalling pathway in disease. Nat Rev Drug Discov. 11:790–811.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-κappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|