Introduction
Burn injury is one of the most common and severe
trauma morphologies and is typically established from thermal
injury of the skin (1). The
clinical features of burn injuries range from difficult initial
assessment to the tendency of pathological scars (2). According to a previous 33-year
population-based study, adjusted for all-cause mortality, the
mortality rate of burn injury was 1.6 (95% confidence interval,
1.3–2.0), and children with burn injuries had a 1.6-fold higher
mortality rate than those without any injuries (3). The resuscitation of burn trauma
patients remains a challenge, as the healing mechanism remains to
be fully elucidated. When treating burn wounds it is crucial to
restore and close the exposed wound surface promptly, as this will
aid in limiting the loss of water, electrolytes and nutrients, and
preventing the invasion of pathogenic microorganisms (4–6).
Aspects of the wound healing mechanism remain unclear; however, it
has been established that multiple cell types, the extracellular
matrix and soluble mediators are all involved in this process
(7).
The transmembrane 7 superfamily member 2 (Tm7sf2)
gene has been reported to be involved in cholesterol biosynthesis
by encoding the protein 3β-hydroxysterol Δ14-reductase (8). Tm7sf2 participates in the cellular
response to stressful conditions (9) and its loss alters the expression of
proteins that are involved in the differentiation of epidermal
cells by reducing cholesterol sulfate levels (10). The loss of the entire epidermis
following burn injury may induce severe hematopoietic dysfunction,
which will subsequently increase the risk of mortality and
morbidity (11). A previous study
indicated that cholesterol levels of the erythrocyte membrane in
patients with large area burns were significantly increased.
Therefore, it is possible that Tm7sf2 may be involved in the wound
healing process (12).
Electrical burns at 1,000 V may result in extensive
damage to tissues and even renal failure or rhabdomyolysis
(13). The current study performed
electrical burns on rats. The burn serum was then extracted and
used to culture HaCaT human keratinocyte cells were. Tm7sf2 siRNAs
were also produced to determine their effect on these cells. Cell
proliferation, monocyte-endothelial cell adhesion ability and the
expression levels of the autophagy-associated proteins, Beclin1 and
LC3-II, were additionally investigated, in order to determine
whether Tm7sf2 participates in the process of burn wound
healing.
Materials and methods
Burn wound model
Sprague-Dawley rats (age, 5 weeks old; male and
female; n=10) weighing between 250 and 300 g and maintained under
controlled temperature (20±1°C), humidity (55±10%) and illumination
(12 h dark:light), were subjected to limb electrical burns as
described previously (14).
Subsequent to undergoing anesthesia with 3% pentobarbital (10
mg/kg; Sigma-Aldrich, St. Louis, MO, USA), the rats were maintained
in a stationary position on the experimental table in order to
shave the hip and right posterior limb. Next, a null electrode was
placed on the right limb (0.5 cm proximal to ankle), with a live
electrode placed on the ipsilateral gluteus. The electricity
(output voltage: 1,000 V) was administered for 0.1 sec to conduct
the burns using the YLS-5Q Skin Burning Device model (Biowill Co,
Ltd. Shanghai, China). Blood samples were obtained from the tail
vein (using 1 ml syringe) at 6 and 24 and 36 h, subsequent to the
administration of the electric burns, and then centrifuged at 300 ×
g, −4°C for 15 min, to obtain the blood serum.
Cell culture
HaCaT cells (Shanghai Bogoo Biotechnology Co., Ltd.,
Shanghai, China) were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) medium supplemented with 10% (v/v) fetal bovine serum, 100
U/ml streptomycin and 100 U/ml penicillin at 37°C in a 5% (v/v)
CO2 humidified atmosphere. DMEM (Gibco; Thermo Fisher
Scientific, Inc.) containing 0.5 mg/ml collagenase type I was
applied at 37°C for 3 h to separate fibroblasts from rat blood
serum. These fibroblasts were pelleted by centrifugation at 300 ×
g, for 7 min, at room temperature, and then cultured in the
aforementioned conditions. Cells passaged 2–4 times were used for
further experiments.
Tm7sf2 transfection
The full-length wild-type Tm7sf2 coding sequence
(alternate names: D14SR, SR-1; RefSeq accession, NP_003264) was
sub-cloned into pcDNA3.1+ to produce the Tm7sf2
expression vector (pcDNA3.1− Tm7sf2), which was
confirmed by sequencing at Shanghai Majorbio Bio-pharm Technology
Co., Ltd. (Shanghai, China). Tm7sf2-specific siRNA and control
siRNA were synthesized by Shanghai GenePharma Co., Ltd. (Shanghai,
China). Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used for the cell transfection, according to
manufacturer's protocol, and empty construct pcDNA3.1 was also
transfected as a control. Stable Tm7sf2 transfectants were selected
using G418 (Gibco; Thermo Fisher Scientific, Inc.).
Cell interference experiments
HaCaT cells were divided into four groups and
exposed to the following treatments for 20 h: i) Control, 20%
normal serum; ii) burn, 20% electrical burned serum; iii) 20%
normal serum + siTm7sf2; iv) 20% electrical burned serum +
siTm7sf2.
Cell proliferation and invasiveness
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich) colorimetric assay and Matrigel (BD Biosciences,
Franklin Lakes, NJ, USA) invasion chamber assay were used for the
determination of cell proliferative and invasive capacities,
respectively, as described previously (15,16).
Each experiment was performed three times.
Monocyte-endothelial cell adhesion
assay
Calcein-acetomethyl (25 mg; Molecular Probes; Thermo
Fisher Scientific, Inc., in 5 ml dimethyl sulfoxide/ml media;
Sigma-Aldrich) was used for labeling the treated HaCaT cells.
Subsequent to centrifugation at 300 × g, for 7 min, at room
temperature, the cells were washed three times with
phosphate-buffered saline (PBS; Sigma-Aldrich) to allow the
adherence to a compact monolayer of the EA.hy926 human endothelial
cell line (Cell bank of Type Culture Collection of the Chinese
Academy of Sciences, Shanghai, China) at 37°C for 1 h.
Subsequently, the monocytes in the medium were aspirated. A
fluorescence microscope (Olympus CX31; Olympus Corporation, Tokyo,
Japan) was used for the visualization cell adherence at excitation
and emission wavelengths of 488 and 535 nm, respectively. Six
fields were selected per well to calculate the number of adherent
monocytes using Image J software (version 2; National Institutes of
Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was performed as previously described
(17). Total mRNA was isolated
from cells, and complementary DNA (cDNA) was produced using reverse
transcriptase (iScript cDNA Synthesis kit; Bio Rad Laboratories,
Inc., Hercules, CA USA). Tm7sf2 expression levels were measured via
SYBR Green based qPCR (SYBR-Green Master mix; Thermo Fisher
Scientific, Inc.). Reactions were performed with the initial
denaturation at 95°C for 5 min and then 40–50 cycles at 95°C for 30
sec, 58–62°C for 30 sec and 72°C for 30 sec, and a final extension
at 72°C for 10 sec.
Western blot analysis
A 10–12% sodium dodecyl sulfate-polyacrylamide gel
(Thermo Fisher Scientific, Inc.) was used for protein separation at
100 V for 90 min. Next, the proteins were blotted onto
polyvinylidene difluoride membranes, which were blocked with PBST
(0.1% Triton X-100; Sigma-Aldrich in PBS), and probed with primary
antibodies including anti-Tm7sf2 (cat no. sc-162325) Beclin 1 (cat
no. sc-11427) and LC3-II (cat no. sc-28266) all diluted 1:200 in
bovine serum albumin) and horseradish peroxidase-conjugated
secondary antibodies (sc-391122; diluted 1:5,000) and GAPDH
(sc-59540l; diluted 1:1,000). GAPDH was used as a loading control.
All antibodies were purchased from Santa Cruz Biotechnology, Inc.,
(Dallas, TX, USA) unless otherwise stated. Enhanced
chemiluminescence (GE Healthcare Life Sciences; Uppsala, Sweden)
was utilized to develop the immunoreactive protein bands, which
were analyzed by a densitometer (Image Pro Plus, version 1.63;
Media Cybernetics, Rockville, MD, USA).
Statistical analysis
Each sample was processed and measured three times.
The data are presented as the mean ± standard deviation. The
statistical analysis was performed using SPSS software (version
17.0; SPSS, Inc., Chicago, IL, USA) statistical software, one-way
analysis of variance, followed by Fisher's Protected Least
Significant Difference post-hoc analysis used to determine
statistical differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
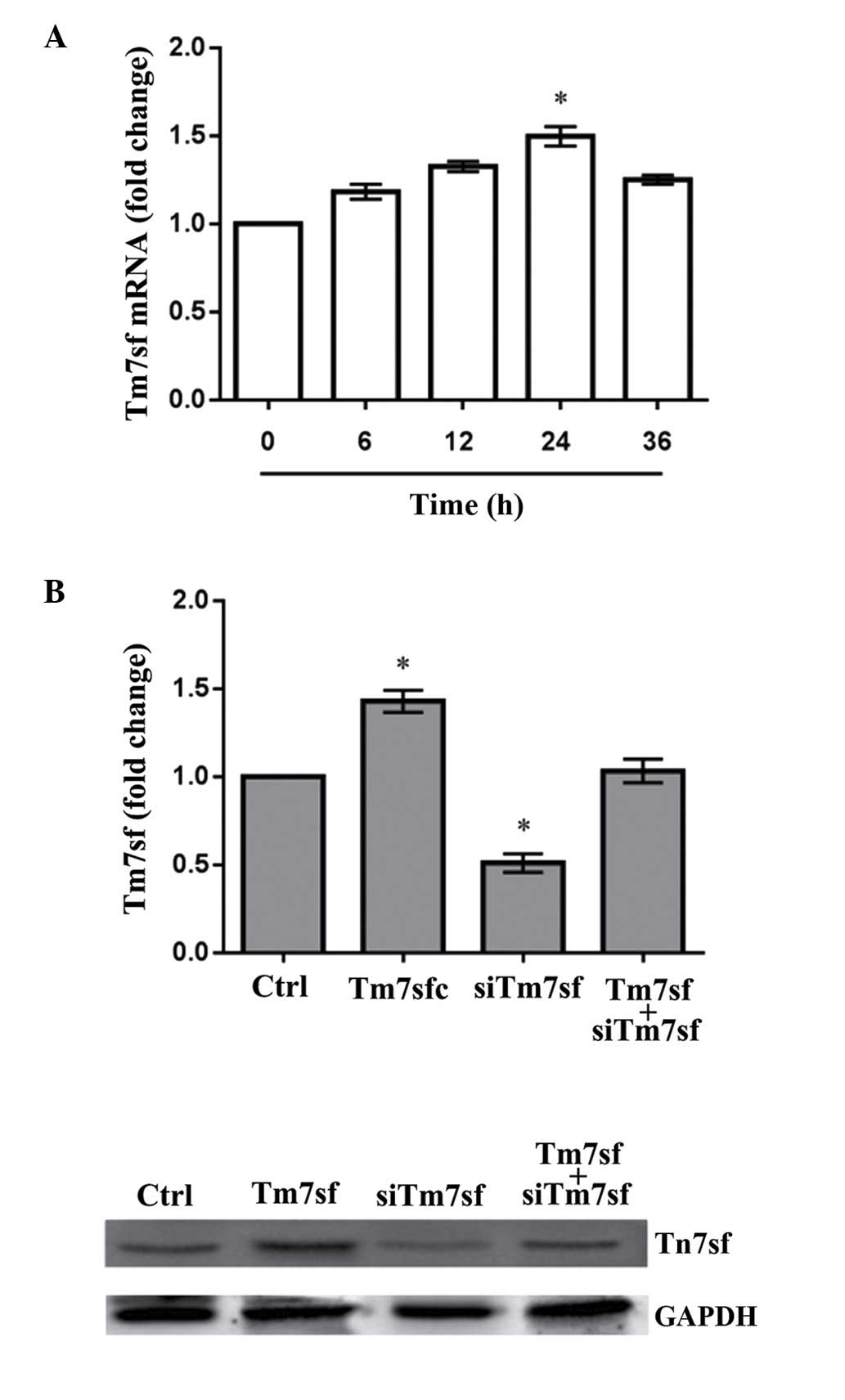
Tm7sf2 level detection
Compared with the control group, Tm7sf2 mRNA levels
in rats with electric burns were significantly higher with the
increasing time following the administration of the burn
(P<0.05). The levels were then reduced after 24 h (Fig. 1A). In the 20% electrical burned
serum group, the protein levels of Tm7sf2 were significantly higher
than the control group (P<0.05). In the siTm7sf2-transfected
cells, Tm7sf2 expression was significantly lower compared with the
control group (P<0.05), however was restored to normal with the
addition of Tm7sf2 (Fig. 1B;
Table I).
 | Table ITm7sf microRNA and protein expression
levels. |
Table I
Tm7sf microRNA and protein expression
levels.
| Time (h)/group | mRNA level | Protein level |
|---|
| 0 | 1.00±0.00 | – |
| 6 | 1.18±0.07 | – |
| 12 | 1.32±0.05 | – |
| 24 | 1.49±0.09 | – |
| 36 | 1.25±0.05 | – |
| Control | – | 1.00±0.00 |
| Tm7sf2 | – | 1.43±0.11 |
| siTm7sf2 | – | 0.51±0.09 |
| Tm7sf2+siTm7sf2 | – | 1.03±0.12 |
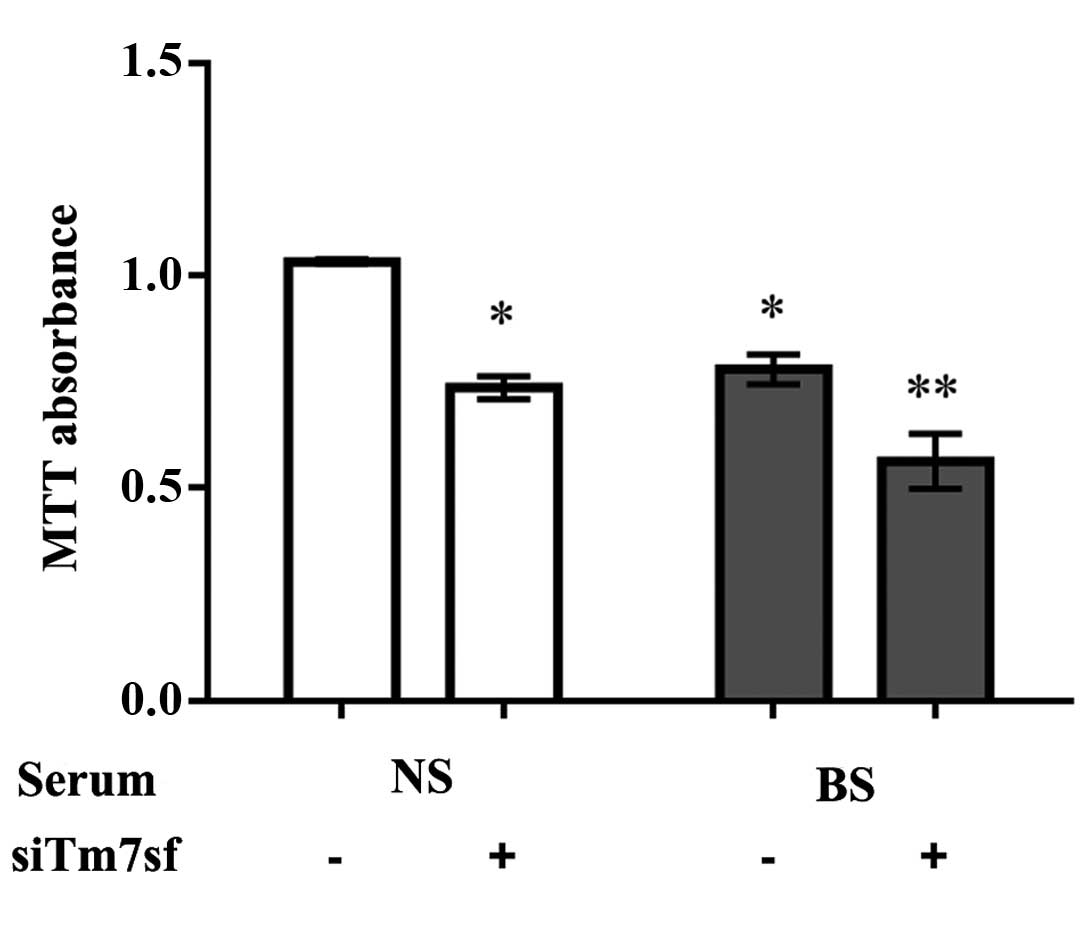
Cell proliferation
The MTT assay demonstrated that the burn serum
significantly reduced cell proliferation when compared with the
normal serum (P<0.05). Furthermore the proliferation of cells
treated with Tm7sf2 siRNA was significantly lower in the burn serum
group compared with the control cells (P<0.05; Fig. 2; Table II).
 | Table IIProliferation of HaCaT cells. |
Table II
Proliferation of HaCaT cells.
| Group | siTm7sf | Absorbance level
(Abs) |
|---|
| Normal serum | − | 1.03±0.01 |
| + | 0.74±0.05 |
| Burn serum | − | 0.78±0.06 |
| + | 0.56±0.11 |
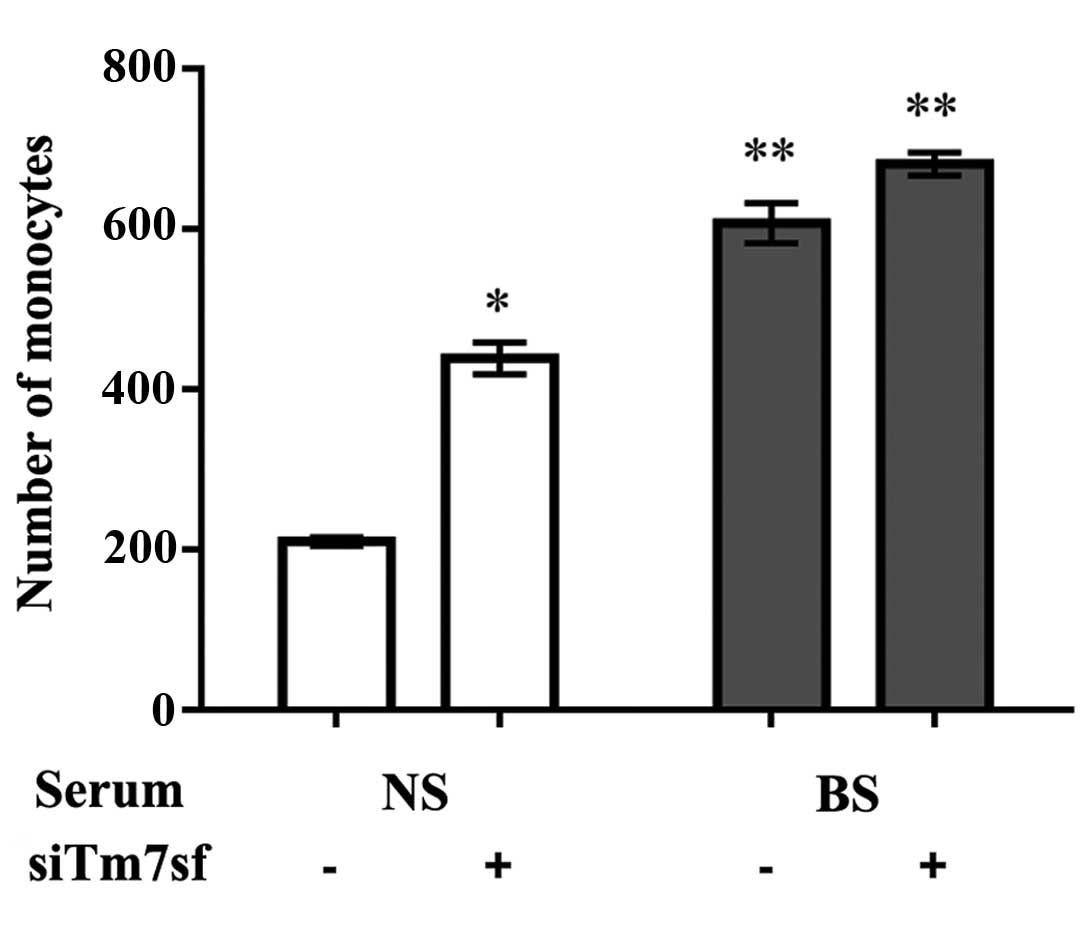
Monocyte-endothelial cell adhesion
The number of monocytes represent the adhesion
strength between the EA.hy926 and HaCaT cells (18). The 24 h treatment with burn serum
induced the highest expression levels of Tm7sf2, therefore cell
adhesion of the HaCaT cells was detected subsequent to 24 h of
treatment. The burn serum treatment significantly enhanced the
capacity of monocyte-endothelial cell adhesion, even if the cells
had been transfected with siTm7sf2, compared with normal serum
treatment (P<0.05; Fig. 3;
Table III).
 | Table IIINumbers of monocytes. |
Table III
Numbers of monocytes.
| Group | siTm7sf | No. |
|---|
| Normal serum | − | 209.67±9.50 |
| + | 438.00±33.65 |
| Burn serum | − | 606.67±43.32 |
| + | 680.67±24.58 |
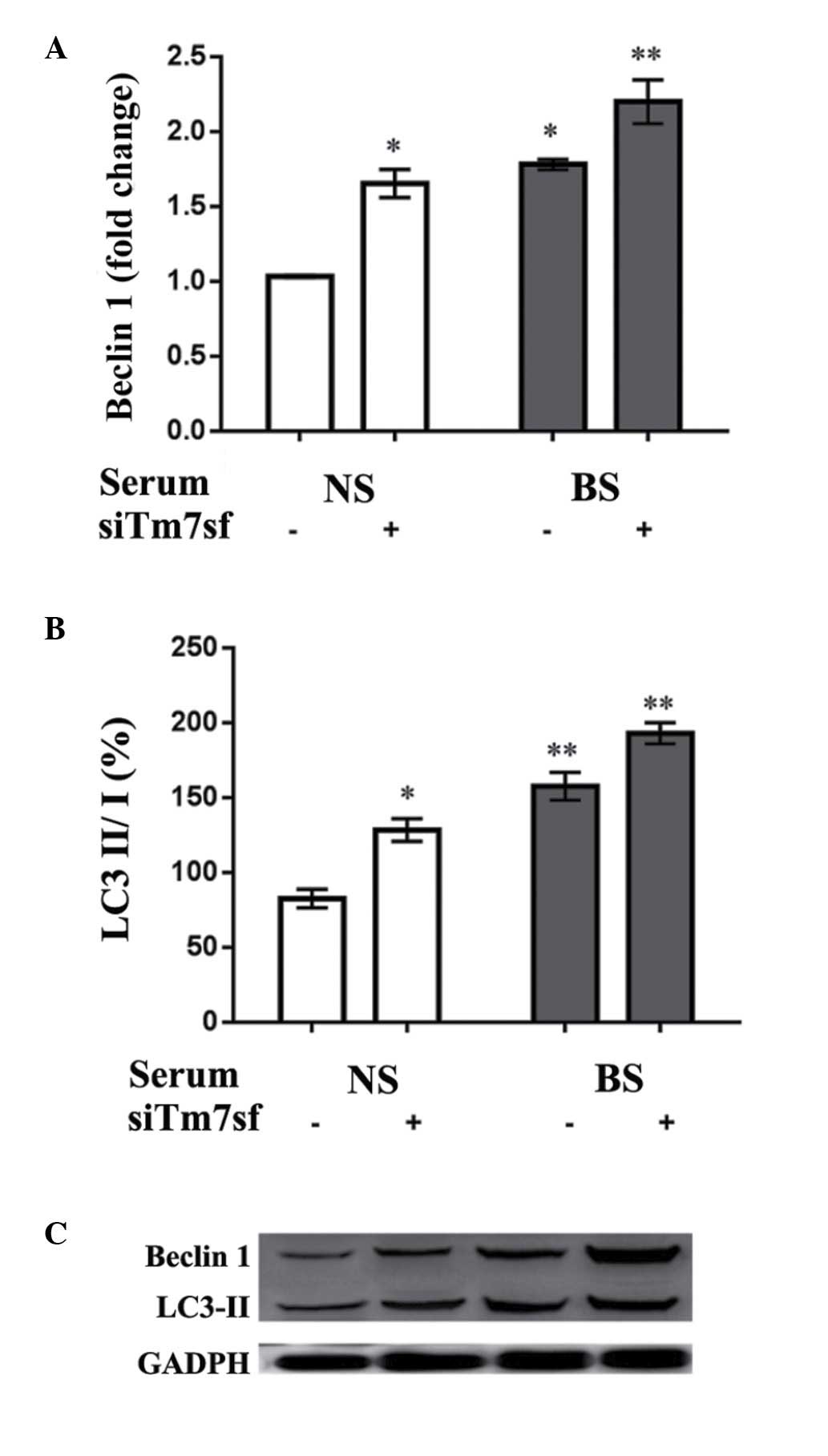
Autophagy-associated protein levels
To investigate the influence of the burn serum and
siTm7sf2 on autophagic-abilities of HaCaT cells, the autophagy
associated Beclin1 and LC3-II protein levels in HaCaT cells were
detected using western blot analysis. As indicated in Fig. 4, burn serum (treated for 24 h)
increased the expression levels of Beclin1 and LC3-II when compared
with control groups (P<0.05). The siTm7sf2 group also presented
increased expression levels, while the effect was more evident in
the burn serum-treated cells (P<0.01; Table IV).
 | Table IVBeclin1 and LC3-II protein expresion
levels in HaCaT cells. |
Table IV
Beclin1 and LC3-II protein expresion
levels in HaCaT cells.
| Group | siTm7sf | Beclin1 | LC3-II |
|---|
| Normal serum | − | 1.03±0.01 | 1.78±0.06 |
| + | 1.65±0.16 | 2.20±0.25 |
| Burn serum | − | 82.43±10.67 | 157.45±16.11 |
| + | 128.32±13.23 | 192.87±12.12 |
Discussion
In the current study, it was identified that burn
serum treatment reduced cell proliferation, increased
monocyte-endothelial cell adhesion, and induced higher expression
levels of Beclin1 and LC3-II in HaCaT cells. Additionally, the
administration of siTm7sf2 had a similar influence on these
properties, suggesting that Tm7sf2 may be important during burn
wound repair.
Thermal injury induces the release of inflammatory
factors (19).
Monocyte-endothelial cellular adhesion, as a key feature in
inflammation, is important in monocyte extravasation (14). The observations of the present
study indicate that silencing of Tm7sf2 and treatment with burn
serum increase cellular adhesion, with the siTm7sf2 + burn serum
treatment resulting in the highest number of monocytes. Therefore,
Tm7sf2 may be involved in inflammation during the wound healing
process.
Autophagy is a catabolic process necessary for the
maintenance of the cellular organelle balance, and is a highly
conserved pathway for the delivery of intracellular macromolecule
waste to lysosomes (20). Severe
burns lead to endoplasmic reticulum stress that triggers autophagy
signaling cascades. However, it has been identified that there is
reduction in autophagy in burn wounds during the initial period of
progression of burn injuries (21). The enhanced level of autophagy deep
in the dermis has been suggested as a potential prosurvival factor
against injury-associated inflammation (22). The Beclin1 protein is associated
with the activation of autophagy and in addition, as a reliable
biomarker of autophagy activity, it forms part of the
phosphatidylinositol 3-kinase complex (23). LC3 is a critical protein for
autophagy, and its cleavage leads to the production of LC3-I, which
is converted to LC3-II subsequent to lipidation via a
ubiquitin-like system that allows LC3 to be involved in autophagic
vesicles (24). The LC3-II/LC3-I
ratio is considered to be an indicator of autophagy (25). Furthermore, the increased
expression levels of Beclin1 and LC3-II, two indicators of
autophagy, observed in the current study suggested that the cells
were undergoing the wound healing processes. Tm7sf2 also appears to
be crucial to these processes.
In conclusion, burn serum extracted from an
electrical burn rat model increased the expression of Tm7sf2. In
addition, Tm7sf2 may participate in the wound healing process by
interacting with LC3-II and Beclin1 and thus, therapeutically
targeting Tm7sf2 may be advantageous to the wound healing process
following burn injuries. However, only rat models were used in the
present study regarding the role of Tm7sf2, and further clinical or
human tissue based studies are required to determine the benefit of
novel therapies targeting Tm7sf2 for burn wound healing.
References
|
1
|
Weber J and McManus A; Nursing Committee
of the International Society for Burn Injuries: Infection control
in burn patients. Burns. 30:16–24. 2004. View Article : Google Scholar
|
|
2
|
Teot L, Otman S, Brancati A and Mittermayr
R: Burn wound healing: Pathophysiology. Handbook of Burns. 2. 1st
edition. Springer; Vienna: pp. 47–54. 2012, View Article : Google Scholar
|
|
3
|
Duke JM, Rea S, Boyd JH, Randall SM and
Wood FM: Mortality after burn injury in children: A 33-year
population based study. Pediatrics. 135:e903–e910. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enoch S and Leaper DJ: Basic science of
wound healing. Surgery. 26:31–37. 2008.
|
|
5
|
Li J, Chen J and Kirsner R:
Pathophysiology of acute wound healing. Clin Dermatol. 25:9–18.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Velnar T, Bailey T and Smrkolj V: The
wound healing process: An overview of the cellular and molecular
mechanisms. J Int Med Res. 37:1528–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gurtner GC, Werner S, Barrandon Y and
Longaker MT: Wound repair and regeneration. Nature. 453:314–321.
2008.Review. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zuleger N, Boyle S, Kelly DA, de las Heras
JI, Lazou V, Korfali N, Batrakou DG, Randles KN, Morris GE,
Harrison DJ, et al: Specific nuclear envelope transmembrane
proteins can promote the location of chromosomes to and from the
nuclear periphery. Genome Biol. 14:R142013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bellezza I, Roberti R, Gatticchi L, Del
Sordo R, Rambotti MG, Marchetti MC, Sidoni A and Minelli A: A novel
role for Tm7sf2 gene in regulating TNFα expression. PLoS One.
8:e680172013. View Article : Google Scholar
|
|
10
|
Bellezza I, Gatticchi L, del Sordo R,
Peirce MJ, Sidoni A, Roberti R and Minelli A: The loss of Tm7sf
gene accelerates skin papilloma formation in mice. Sci Rep.
5:94712015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Loebl EC, Baxter CR and Curreri PW: The
mechanism of erythrocyte destruction in the early post-burn period.
Ann Surg. 178:681–686. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalal R, Sharma CA, Chakravarty BB, Alam
Parwaz CM and Anil Malik C: A study of prognostic factors for
prediction of complications and outcomes in burn patients. Indian J
Burns. 22:56–61. 2014. View Article : Google Scholar
|
|
13
|
Benson A and Dickson WA: Burns. ABC of
wound healing. BMJ. 332:649–652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruan Q, Zhao C, Ye Z, Ruan J, Xie Q and
Xie W: Effect and possible mechanism of monocyte-derived VEGF on
monocyte-endothelial cellular adhesion after electrical burns.
Burns. 41:825–832. 2015. View Article : Google Scholar
|
|
15
|
Zhang T, Liu M, Wang C, Lin C, Sun Y and
Jin D: Down regulation of MiR-206 promotes proliferation and
invasion of laryngeal cancer by regulating VEGF expression.
Anticancer Res. 31:3859–3863. 2011.PubMed/NCBI
|
|
16
|
Yang LH, Xu HT, Han Y, Li QC, Liu Y, Zhao
Y, Yang ZQ, Dong QZ, Miao Y, Dai SD and Wang EH: Axin downregulates
TCF-4 transcription via beta-catenin, but not p53, and inhibits the
proliferation and invasion of lung cancer cells. Mol Cancer.
9:252010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fransson L, Rosengren V, Saha TK,
Grankvist N, Islam T, Honkanen RE, Sjöholm Å and Ortsäter H:
Mitogen-activated protein kinases and protein phosphatase 5 mediate
glucocorticoid-induced cytotoxicity in pancreatic islets and
β-cells. Mol Cell Endocrinol. 383:126–136. 2014. View Article : Google Scholar
|
|
18
|
Edwards AM, Potter U, Meenan NA, Potts JR
and Massey RC: Staphylococcus aureus keratinocyte invasion is
dependent upon multiple high-affinity fibronectin-binding repeats
within FnBPA. PLoS One. 6:e188992011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu H, Ka B and Murad F: Nitric oxide
accelerates the recovery from burn wounds. World J Surg.
31:624–631. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: A double edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song J, de Libero J and Wolf SE: Hepatic
autophagy after severe burn in response to endoplasmic reticulum
stress. J Surg Res. 187:128–133. 2014. View Article : Google Scholar
|
|
22
|
Xiao M, Li L, Li C, Zhang P, Hu Q, Ma L
and Zhang H: Role of autophagy and apoptosis inwound tissue of deep
second degree burn in rats. Acad Emerg Med. 21:383–391. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ichimura Y, Kirisako T, Takao T, Satomi Y,
Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi
M, et al: A ubiquitin like system mediates protein lipidation.
Nature. 408:488–492. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kabeya Y, Mizushima N, Yamamoto A,
Oshitani-Okamoto S, Ohsumi Y and Yoshimori T: LC3, GABARAP and
GATE16 localize to autophagosomal membrane depending on form II
formation. J Cell Sci. 117:2805–2812. 2004. View Article : Google Scholar : PubMed/NCBI
|