Introduction
Diabetic nephropathy (DN) is the leading cause of
end-stage renal disease in the US, which is characterized by damage
to podocytes and increased extracellular matrix accumulation
(1,2), which leads to the progression of
nodular glomerulosclerosis, micro-albuminuria and eventual renal
failure. The molecular and cellular mechanisms responsible for this
disease remain to be fully elucidated. Previous clinical and
experimental studies have demonstrated that the local growth
factor, angiotensin II (AngII) requires consideration as a vital
mediator in the pathogenesis in DN (3). The inhibition of the
renin-angiotensin system by angiotensin-converting enzyme
inhibitors ameliorates proteinuria and the progression of
nephropathy, which suggest that this inhibition may have beneficial
effects on various types of renal cell (4–6).
Podocyte injury is the hallmark characteristic of DN
(7). The local production of AngII
in podocytes contributes to the development of kidney disease
through the generation of reactive oxygen species (ROS) and
oxidative stress, resulting in podocyte apoptosis, matrix
accumulation and aberrant proliferation (8–10).
Several studies have reported that, during the progression of DN,
the suppression of superoxide generation in response to AngII
attenuates renal function, although results following the
application of antioxidants remain inconsistent (11,12).
Several potentially beneficial actions of antioxidant
administration on DN have been reported in human and experimental
diabetes (12). The present study
aimed to examine whether the antioxidant melatonin can ameliorate
pathophysiological injury in podocytes, including severe oxidative
stress and apoptosis, in an in vitro AngII-induced DN
model.
Melatonin is the predominant hormone secreted by the
pineal gland and is involved in sustaining circadian rhythms in
various biological processes. Several biological effects of
melatonin are mediated through the activation of melatonin
receptors, whereas others are a result of its powerful
anti-oxidative function. Several studies have found that melatonin
prevents renal damage in Type I and Type II diabetes via reducing
free radical attack (13–16), however, the cell-specific mechanism
remains to be fully elucidated.
The present study demonstrated for the first time to
the best our knowledge, that melatonin exerts an anti-apoptotic
effect in AngII-mediated podocyte injury, and indicates a possible
mechanism to explain the protective effect of melatonin in DN.
Materials and methods
Reagents and cell line
Angiotensin II (A9290-10) was purchased from
Solarbio Bioscience & Technology, Inc. (Shanghai, China). The
chemical reagents used for detecting cell proliferation and
apoptosis comprised a Cell Counting kit-8 (CCK-8; cat. no. CK04;
Dojindo Molecular Technologies, Inc. Kumamoto, Japan) and an
Annexin V-Fluorescein isothiocyanate (FITC) apoptosis detection kit
(BD Pharmingen, San Diego, CA, USA; cat. no. 556547). A reactive
oxygen species (ROS) assay kit (Vigorous Biotech, Beijing, China)
and MitoPT™ JC-1 assay kit (ImmunoChemistry Technologies, LLC,
Bloomington, MN, USA; cat. no. 924) were used to investigate
mitochondrial function.
Cell culture and treatment
Mouse podocytes (5×104 cells/ml) were
acquired from JRDun Biotechnology Co., Ltd. (Shanghai, China) and
propagated at 33°C in RPMI-1640 culture media supplemented with 10%
fetal bovine serum (FBS; GE Healthcare Life Sciences HyClone
Laboratories, Logan, UT, USE) and 1,000 U/ml recombinant interferon
(BioVision, Inc., Milpitas, CA, USA) to enhance the expression of a
thermo-sensitive T antigen, as previously described (17). To establish an injury model, the
podocytes were seeded at 80% confluence in complete medium
containing 10% FBS. After 24 h, the cell culture medium was
replaced with serum-free medium containing 5 μmol Ang II for
24 h at 37°C. Subsequently, different concentrations of melatonin
(Shanghai Amquar Biological Technology Co., Ltd., Shanghai, China)
were added for various periods of time, as indicated. The cells
were collected at different time points for further
characterization.
Apoptosis assay
The cells were washed, trypsinized and suspended in
binding buffer, and the density was adjusted to
5–10×105/ml. Apoptosis was measured by staining with
Annexin V- FITC and propidium iodide (PI). The podocytes in
suspension (195 μl) was added to 5 μl Annexin V-FITC
and 10 μl PI. The stained cells were analyzed by
fluorescence-activated cell-sorting (FACS; FACScalibur; Becton
Dickinson, Mountain View, CA, USA). The apoptotic cells were
calculated as the percentage of IV-gated cells.
Cell viability assay
The podocytes (1×103 per well) were
plated in 96-well plates. Following treatment with Ang II for 24 h,
a series of melatonin concentrations (0.1, 0.5 and 1.0 mmol) were
added for different durations (48 and 72 h). Subsequently, 10%
CCK-8 diluted in serum-free RPMI-1640 (Invitrogen, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was added to each well for 1 h
at 37°C. The absorption of each sample was measured at a wavelength
of 450 nm using a Labsystems MK3 microplate reader (Thermo Fisher
Scientific, Inc.) to detect cell viability, according to the
manufacturer's protocol. Cells without Ang II treatment served as a
control group. Triplicate experiments were performed throughout all
the procedures.
Measurement of intracellular ROS
levels
The levels of intracellular ROS were measured using
a ROS assay kit, according to the manufacturer's protocol. Briefly,
the treated podocytes were washed three times with pre-warmed
phosphate-buffered saline (PBS) and then harvested for further
staining. Dihydroethidium (DHE; 50 μM) was added to the
cells for incubation at room temperature for 30 min, following
which the cells were analyzed using FACS. The fluorescence
intensity was monitored at an excitation wavelength of 488 nm and
an emission wavelength of 605 nm.
Detection of mitochondrial membrane
potential (MMP)
The treated podocytes were washed with pre-warmed
PBS, trypsinized, centrifuged (400 × g at room temperature) for 10
min and adjusted to 1×106 cell/ml. Tetramethylrhodamine,
methyl ester (TMRM) was used as a trans-membrane dye to indicate
the function of the mitochondria. The cells were incubated with 100
nM TMRM medium at 37°C for 15–20 min in the dark, and washed again
with PBS to remove any unbound probe. Analysis was performed using
FACS at an excitation wavelength of 543 nm and an emission
wavelength of 589 nm.
Western blot analysis
The podocytes were washed with PBS several times and
then added to lysis buffer premixed with proteinase and phosphatase
inhibitors. The concentration of protein was quantified using a
bicinchoninic acid assay method (Thermo Fisher Scientific, Inc.).
Subsequently, 35 μg protein for each sample was loaded onto
12% SDS-PAGE gels for separation, and further transferred onto a
polyvinylidene fluoride membrane. The blots were blocked with 5%
non-fat milk and incubated overnight with the following primary
antibodies: Rabbit anti-nephrine (Abcam, Cambridge, UK; cat. no.
Ab58968; 1:1,000 dilution); rabbit anti-B cell lymphoma-2 (Bcl-2;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; cat. no.
SC-492; 1:150 dilution); rabbit anti-Bcl-2-associated X protein
(Bax; Santa Cruz Biotechnology, Inc.; cat. no. sc-493; 1:100
dilution); rabbit anti-caspase-3 (Abcam; cat. no. Ab32351; 1:2,000
dilution); rabbit anti-phosphorylated (phospho)-Janus kinase (JAK)2
(Cell Signaling Technology, Inc., Danvers, MA, USA; cat. no. 3776;
1:800 dilution); rabbit anti-JAK2 (Cell Signaling Technology, Inc.;
cat. no. 3230; 1:1,000 dilution); rabbit anti-phospho-Signal
transducer and activator of transcription 3 (STAT3; Abcam; cat. no.
Ab76315; 1:800 dilution); mouse anti-STAT3 (Abcam; cat. no.
Ab119352; 1:1,000 dilution); rabbit anti-GAPDH (Cell Signaling
Technology, Inc.; cat. no. 5174; 1:1,500 dilution). GAPDH was used
for the normalization of each protein to ensure the loading of
equal quantities of protein. Following washing three times with
Tris-buffered saline-tween buffer, the blots were incubated for 1 h
at room temperature with goat anti-rabbit and goat anti-mouse
horseradish peroxidase-conjugated secondary antibodies (cat. nos.
A0208 and A0216, respectively; 1:1,000; Beyotime Institute of
Biotechnology, Haimen, China). Following another round of washing,
the signals were revealed using enhanced chemiluminescence (EMD
Millipore, Billerica, MA, USA).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA).
Statistical analysis was performed using one-way analysis of
variance tests. P<0.05 was considered to indicate a
statistically significant difference. Data are expressed as the
mean ± standard deviation of triplicate samples. All results were
confirmed in at least three independent experiments.
Results
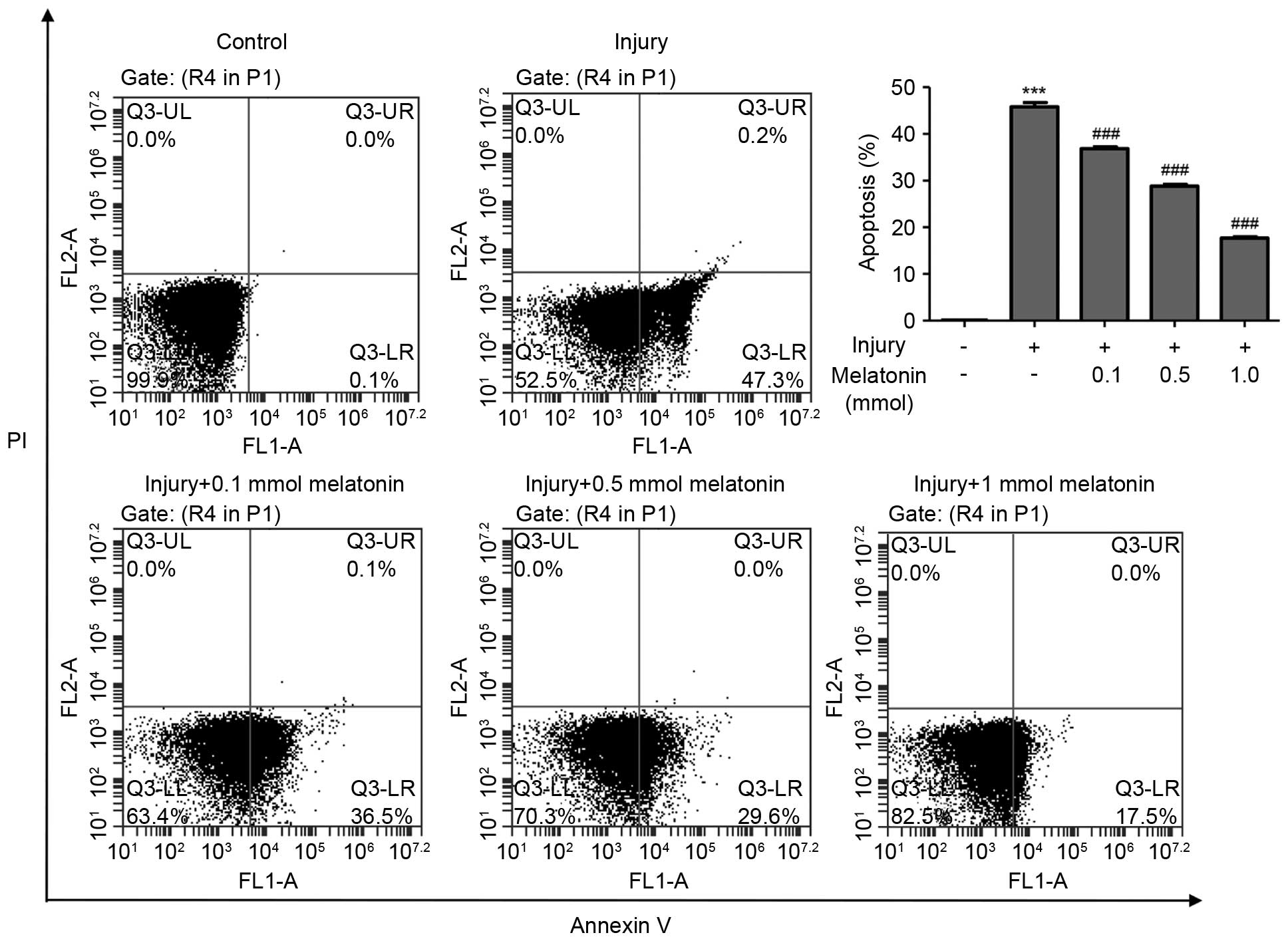
Melatonin protects podocytes from Ang
II-induced apoptosis
In order to investigate the function of melatonin in
DN, the present study established an in vitro model, in
which Ang II was administered to podocytes. Following treatment
with Ang II for 24 h, the podocytes were stained with Annexin V for
further analysis. The FACS results showed the injured podocytes
exhibited high levels of apoptosis (45.800±0.929%), compared with
the control group (0.103±0.003%; Fig.
1). Following exposure to different dosages of melatonin for
another 48 h, the apoptotic rates of the podocytes were
significantly decreased, between 36.87% (0.1 mM melatonin) and
17.70% (1 mM melatonin). These results indicated that melatonin
protected the podocytes from Ang II-induced apoptosis in a
dose-dependent manner.
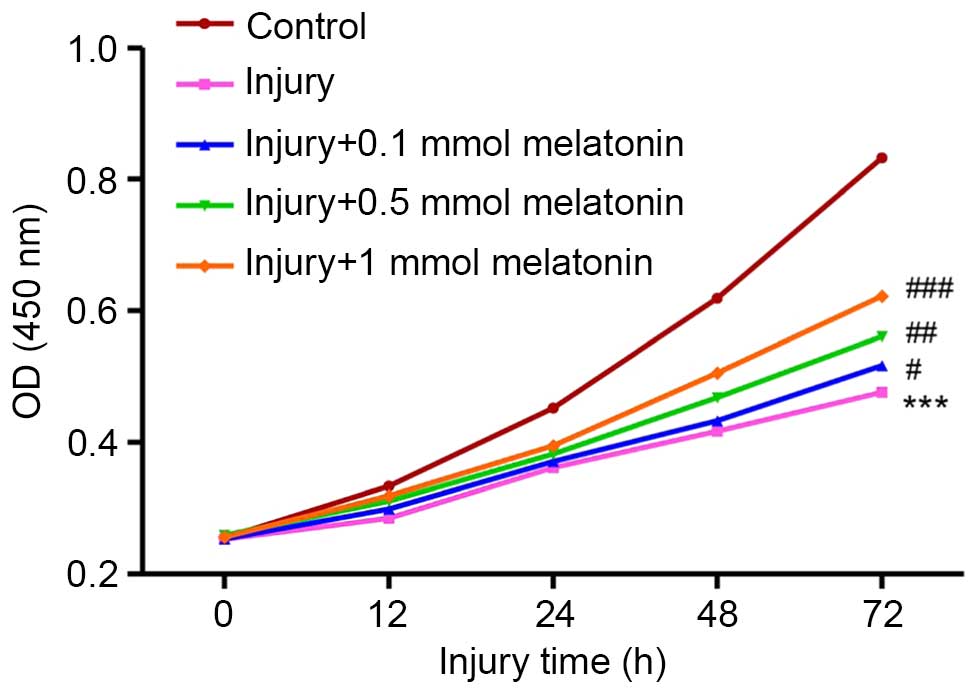
Melatonin restores cell viability in an
Ang II-induced podocytes injury model
The results of the CCK-8 assay demonstrated that,
following 12 h Ang II treatment, the viability of the podocytes was
reduced between 0.334±0.005 and 0.285±0.005, and continued to
decrease significantly, between 0.832±0.005 and 0.476±0.006, until
72 h (Fig. 2). However, melatonin
ameliorated this damage and restored cell viability in a
concentration-dependent manner (Fig.
2). Following incubation for 12 h, the high concentration of
melatonin (1 mmol) rescued almost all the podocytes from injury (1
mmol melatonin, 0.319±0.002; control group, 0.334±0.005). As the
duration of incubation increased, this protective effect was
reduced, with >50% of injured podocytes rescued post-injury
following 72 h in high-concentration melatonin (1 mmol melatonin,
0.622±0.006; control group, 0.832±0.005).
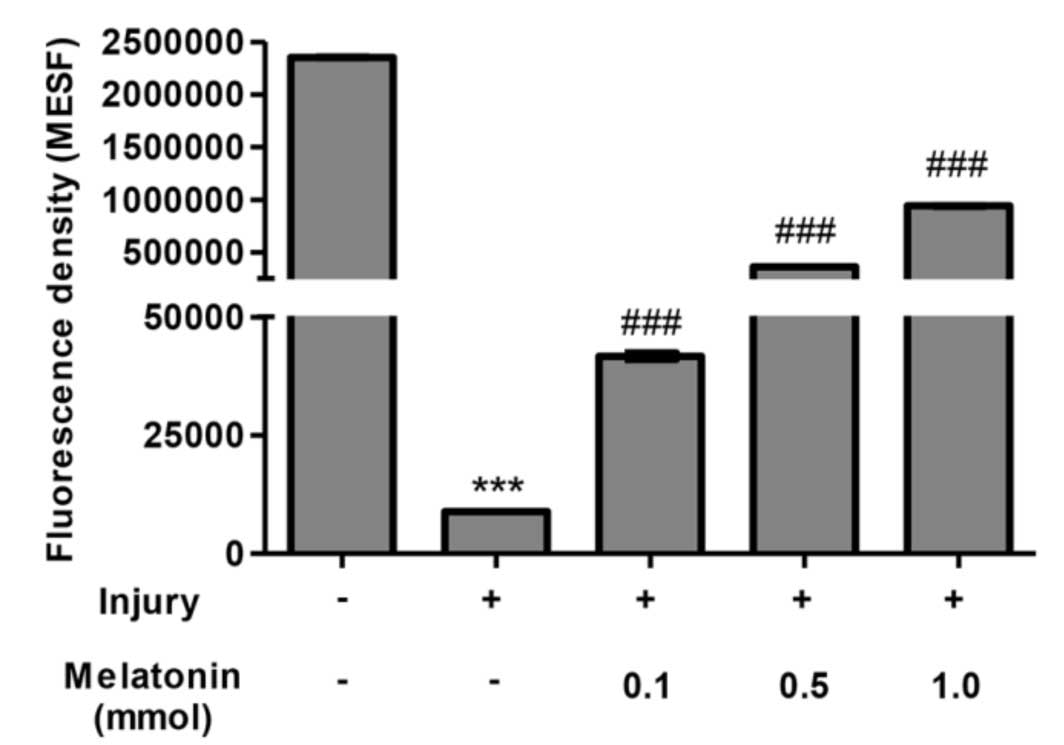
Effect of melatonin on mitochondrial
function and intracellular ROS in injured podocytes
Melatonin has been confirmed as a powerful
anti-oxidant. In order to address the underlying mechanism in the
present study, the mitochondrial function and production of ROS in
the injured podocytes were measured. The use of fluorescent dye for
the assessment of MMP has become a useful tool for monitoring
changes in physiological parameters, as it associated with the
capacity of the cell to generate ATP and maintain normal
mitochondria function. Treatment with Ang II for 24 h markedly
decreased MMP, compared with the control group (0.38% of the
control group (Fig. 3) and reduced
mitochondrial function. Incubation for 48 h with a low
concentration of melatonin (0.1 mmol) was able to partially repair
MMP, and a high concentration melatonin restored almost 40% of the
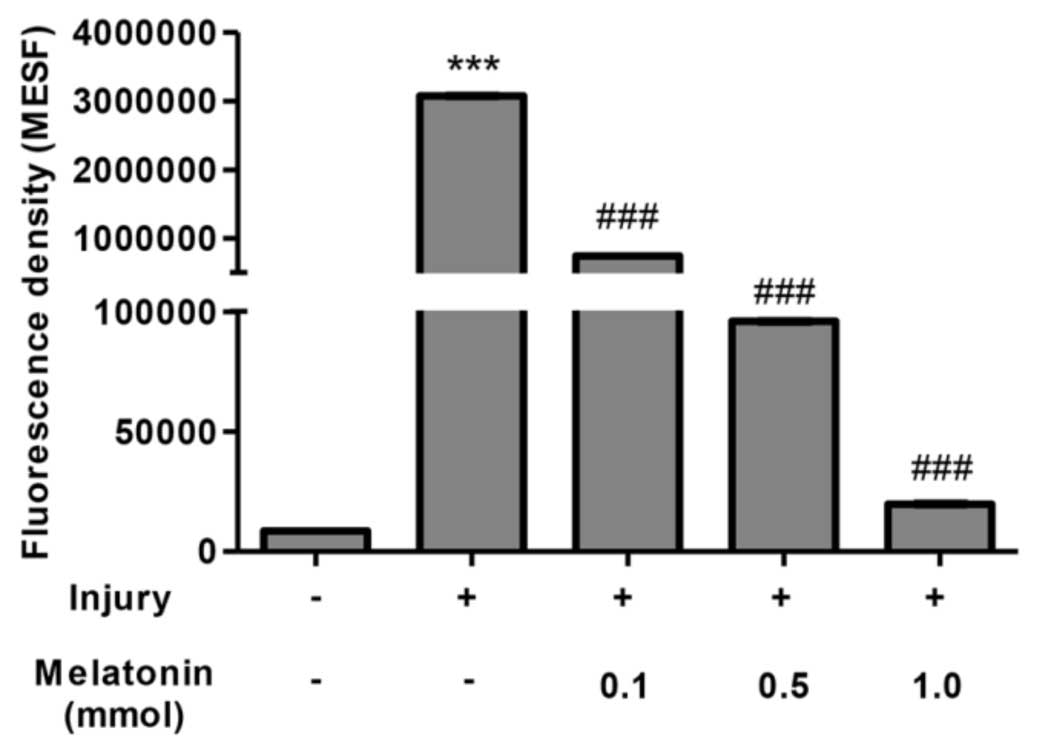
mitochondrial function, compared withe the control group (Fig. 3). Concomitantly, intracellular ROS
was produced due to the damage of MMP, which further reduced
mitochondrial function. As shown in Fig. 4, the results confirmed that Ang II
induced the production of ROS, with an increase of up to 360-fold,
compared with the normal podocytes. Treatment with melatonin
exhibited its potent anti-oxidant effect and eliminated ROS in a
dose-dependent manner. In the cells incubated with a high
concentration of melatonin, ROS were cleared to almost the same
level as in the normal group. Taken together, these results
confirmed melatonin as a potent anti-oxidative reagent, and
demonstrated that melatonin attenuated damage to the podocytes via
the clearance of ROS.
Effects of melatonin on apoptosis and the
JAK/STAT signaling pathway
As described above, melatonin exerted a protective
effect on the podocytes against apoptosis and increased cell
viability. In addition, certain molecules associated with apoptosis
and cell proliferation signaling pathways were detected. Nephrin is
a major cell membrane component connecting the cytoskeleton in
podocytes and is lost during podocyte injury. As shown in Fig. 5, Ang II decreased the expression of
Nephrin (lane B) and melatonin restored its expression, which
increased in a concentration-dependent manner (Lanes C-E). The
Bax/Bcl-2 families are known to be important in the regulation of
apoptosis, and the increased ratio of Bax/Bcl-2 can trigger Caspase
activation and downstream apoptosis. In the present study, Ang II
increased the expression of Bax and repressed the expression of
Bcl-2 level, this change in the Bax/Bcl-2 ratio was corrected by
melatonin. Dose-dependent changes in the expression levels of Bax
and Bcl-2 were observed in the melatonin-treated podocytes.
Caspase-3 activation was also suppressed by melatonin.
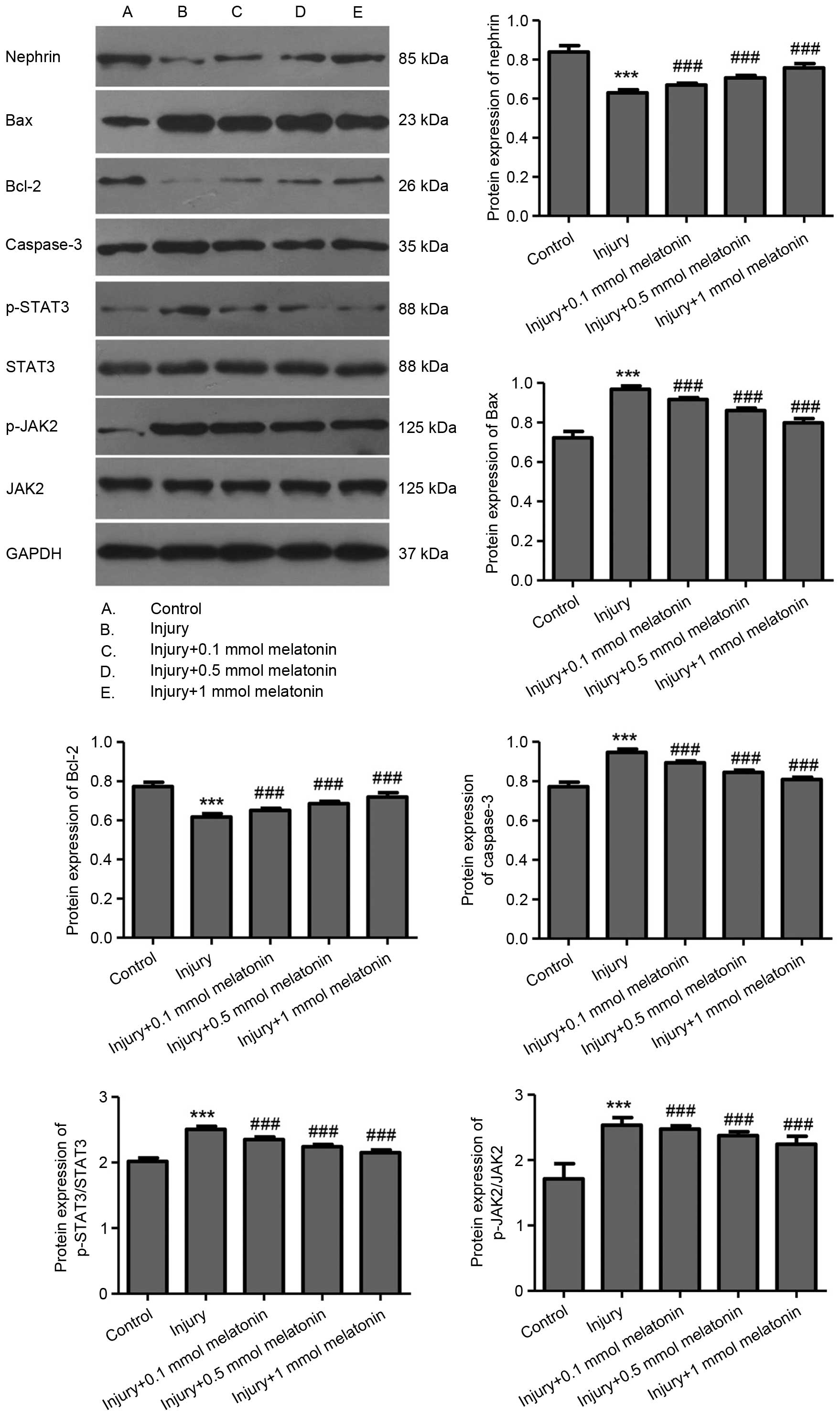 | Figure 5Western blot analysis of the relative
protein levels of Nephrin, Bax, Bcl-2, Caspase-3, p-STAT3/STAT3 and
p-JAK2/JAK2. Podocytes were injured by treatment with angiotensin
II for 24 h, and treated with melatonin at different
concentrations. GAPDH was detected as a loading control. The
molecular weight for each protein is labeled respectively. Data are
expressed as the mean ± standard deviation.
***P<0.001, injury group, vs. control group;
###P<0.001, melatonin group, vs. injury group. Bcl-2,
B cell lymphoma-2; Bax, Bcl-2-associated X protein; STAT3, signal
transducer and activator of transcription 3; JAK2, Janus kinase 2;
p-, phosphorylated. |
The STAT family of transcription factors can control
the activation of several signaling pathways, including
inflammation, proliferation and apoptosis. STAT transcription
factors are activated by tyrosine phosphorylation, predominantly
JAK. In the present study, activation of JAK2/STAT3 phosphorylation
was observed in response to AngII-mediated oxidative stress and
inflammatory disorder. Again, melatonin exerted its anti-oxidative
and anti-inflammatory effects, and the activation of the JAK/STAT
pathway was reduced by melatonin in a dose-dependent manner.
Discussion
DN is one of the predominant causes of end-stage
renal disease. It affects ~30% of patients with Type I diabetes,
and 20% of patients with diabetes <40 years of age succumb to
DN-associated mortality (18). The
glomerulus is the most commonly affected structure in DN, and is
characterized by disordered synthesis of the mesangial matrix
(19), loss of functional
cytoskeleton connections (20) and
enhanced apoptotic rate in podocytes during the progression of
diabetes (21,22), which is partly mediated by Ang II.
Earlier studies have indicated that, as a potent anti-oxidant,
chronic administration of melatonin showed potent beneficial
effects in ameliorating DN in the rat renal tubule and glomerulus
(13,23), and this may have been via a
reduction in oxidative stress. However, few investigations have
focused on elucidating the intracellular mechanisms of the
protective function of melatonin on specific cell types in the
kidney. In the present study, experiments aimed to elucidate the
possible mechanism in podocytes by which melatonin protects the
kidney from oxidative stress and renal damage.
Ang II is considered to be a vital mediator in the
progression of DN (24), as
demonstrated in the results of the present study, in which 24 h
incubation with Ang IIl led to marked podocyte damage. As shown in
Fig. 1, the podocytes were
sensitive to Ang II-induced apoptosis. A study by Gumustekin et
al (25) demonstrated the
presence of TUNEL-positive cells in the glomerular mesangium and
tubular epithelial cells in diabetic rat, and observed that
treatment with melatonin effectively reduced its expression.
Similarly, melatonin-treated podocytes were protected from Ang
II-induced apoptosis in the present study, as shown in Fig. 1. In addition to offering protection
from apoptosis, melatonin increased the number of living cells and
rescued the cells from Ang II-induced cell death (Fig. 2). At a high concentration, almost
50% of the podocytes were rescued. Taken together, these results
confirmed the beneficial effect of melatonin in podocytes.
MMP is an important physiological parameter as it is
associated with the capacity of the cell to generate ATP and
control energy homeostasis (26,27).
During cellular stress, MMP may be adversely altered by the
dysfunction of intracellular ionic charges, consequently leading to
the failure of ATP production and the generation of ROS (27). Therefore, MMP is a key indicator of
cell death and injury. As demonstrated in the present study, Ang II
caused a marked decrease in the MMP of the podocytes (Fig. 3), indicating the possibility of
mitochondrial injury. Several previous studies have reported that,
during the progression of DN in patients and animal models,
oxidative stress is considered a crucial risk factor contributing
to the pathogenesis of renal disease (28–30).
The occurrence of high glucose levels (31) and exposure to mechanical stress
(22) in DN may induce the local
expression of Ang II, acting through Angiotensin II receptors,
which aggravate podocyte injury. As shown in Fig. 4, Ang II not only damaged MMP, it
also produced life-threatening levels of ROS simultaneously.
Melatonin has been confirmed as a potent anti-oxidant. As early as
1999, Ha et al demonstrated that water supplemented with
melatonin reduced the accumulation of plasma lipid peroxides in
streptozotocin-induced DN in rats (13). In subsequent investigations, it was
suggested that melatonin may exert its renal protective effects in
several aspects, for example the clearance of superoxide species in
plasma or urine, including lipid peroxides (13); and increasing the activities of
superoxide dismutase, xanthine oxidase, glutathione peroxidase and
ceruloplasmin (14,16,23).
Although several studies have shown discrepancy in the function of
melatonin in the activity of catalase and superoxide dismutase,
there is substantial evidence supporting the anti-oxidative
protection offered by melatonin and in podocytes, the results of
the present study demonstrated that different concentrations of
melatonin cleared the Ang II-induced production of ROS and rescued
damaged MMP. Taken together, the present study found that melatonin
provided protection in DN through preventing podocyte exposure to
oxidative stress-induced damage.
Podocytes form slit pores, through which the
glomerular filtrate passes, and alterations in this structure are
responsible for the leakage of proteins and blood cells observed in
DN (32). Nephrin is the
predominant component in the family of podocyte membrane proteins,
and is present with other members connected to the actin
cytoskeleton to maintain the normal structure of the glomerulus
(33). In the present study,
although Ang II decreased the expression of nephrin, the
application of melatonin prevented its suppression in a
dose-dependent manner, indicating recovery of the glomerular
cytoskeleton and general structure.
Mitochondrial outer membrane permeabilization is
associated with mitochondrial dysfunction or MMP, and is
responsible for the release of soluble proteins, which are closely
associated with cell death (34).
Once homeostasis is impaired, a mitochondrial-associated cell death
pathway is immediately activated. The release of cytochrome
c into the cytoplasm mediates the binding of apoptotic
protease activating factor-1 to pro-caspase-9 (34), resulting in the activation of
caspase-9 and initiation of the caspase cascade, including caspase
3, and DNA damage. The immediate cellular response to DNA damage
includes cleavage of poly ADP ribose polymerase by caspase-3.
However, the release of cytochrome c is tightly controlled
by members of the Bcl-2 family, known to be associated with the
regulation of apoptosis (34,35).
An increase in the level of Bax or decrease in the level of Bcl-2
can shift the ratio and trigger the apoptotic signal. In the
podocytes examined in the present study, incubation with AngII
activated the expression of caspase-3 and altered the ratio of
Bax/Bcl-2 (lane B, vs. A; Fig. 5),
which led to apoptosis in the Ang II group (Fig. 1). However, in the present study,
dose-dependent downregulation in the expression of caspase 3 and
changes in the Bax/Bcl-2 ratio were observed in the
melatonin-treated podocytes, confirming its protective role as an
anti-oxidant.
STAT proteins belong to a superfamily controlling
transcription-regulating signaling and the response of cells to
environmental stimuli in the absence of protein synthesis through
the STAT pathway (36). In
mammals, there are seven STAT genes, and STAT3 has been widely
investigated due to its numerous functions, including animal cell
growth regulation and inflammation. STATs are activated by tyrosine
phosphorylation, predominantly JAK, leading to their
phosphorylation on activating tyrosine residues, dimerization and
nuclear translocation. JAKs bind specifically to intracellular
domains of cytokine receptor signaling chains and catalyze the
ligand-induced phosphorylation of themselves for activation
(36). DN is possibly the most
well-described renal disorder in which JAK/STAT activation is
important. Ang II-mediated activation of JAK/STAT pathway in
glomerular mesangial cells has been shown to enhance the production
of transforming growth factor (TGF)α, collagen IV and fibronectin,
which is considered to contribute to the accumulation of
extracellular matrix and glomerulosclerosis in DN (37). It has been reported that melatonin
reduces renal inflammation and the accumulation of extracellular
matrix (collagen, α-smooth muscle actin and TGFα) in chronic renal
disease (38). The present study
also demonstrated that, in podocytes, the long-term administration
of Ang II activated the JAK/STAT pathway through promoting the
secretion of certain unknown cytokines, as reported by Abkhezr and
Dryer (39). This activation of
the JAK/STAT signaling pathway was inhibited by different
concentrations of melatonin, possibly via interference with
pro-inflammatory cytokines induced by Ang II, however, the
underlying mechanism requires further investigation.
In conclusion, as a multi-functional hormone
secreted by the pineal gland, the ability of melatonin to
ameliorate the progression of DN was demonstrated in an Ang
II-mediated podocyte-injury model in vitro. Melatonin
provided protection from oxidative stress due to mitochondrial
dysfunction, and inhibited apoptosis and pro-inflammatory signaling
in the podocytes via repression of caspase/Bax/Bcl-2 signaling and
inactivation of the JAK/STAT pathway. However, the mechanism by
which melatonin is involved in this process remains to be fully
elucidated, and investigations concerning the biostructure of
melatonin or specific signaling pathways are required for further
clinical application in DN and other renal disorders.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81170769).
References
|
1
|
Adler S: Structure-function relationships
associated with extracellular matrix alterations in diabetic
glomerulopathy. J Am Soc Nephrol. 5:1165–1172. 1994.PubMed/NCBI
|
|
2
|
Mogensen CE, Christensen CK and Vittinghus
E: The stages in diabetic renal disease. With emphasis on the stage
of incipient diabetic nephropathy. Diabetes. 32(Suppl 2): S64–S78.
1983. View Article : Google Scholar
|
|
3
|
Leehey DJ, Singh AK, Alavi N and Singh R:
Role of angiotensin II in diabetic nephropathy. Kidney Int Suppl.
77:S93–S98. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis EJ, Hunsicker LG, Bain RP and Rohde
RD: The effect of angiotensin-converting-enzyme inhibition on
diabetic nephropathy. The Collaborative Study Group. N Engl J Med.
329:1456–1462. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brenner BM, Cooper ME, de Zeeuw D, Keane
WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z and
Shahinfar S; RENAAL Study Investigators: Effects of losartan on
renal and cardiovascular outcomes in patients with type 2 diabetes
and nephropathy. N Engl J Med. 345:861–869. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gross ML, El-Shakmak A, Szábó A, Koch A,
Kuhlmann A, Münter K, Ritz E and Amann K: ACE-inhibitors but not
endothelin receptor blockers prevent podocyte loss in early
diabetic nephropathy. Diabetologia. 46:856–868. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Somlo S and Mundel P: Getting a foothold
in nephrotic syndrome. Nat Genet. 24:333–335. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma L and Fogo AB: Role of angiotensin II
in glomerular injury. Semin Nephrol. 21:544–553. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fogo AB: Renal fibrosis and the
renin-angiotensin system. Adv Nephrol Necker Hosp. 31:69–87.
2001.PubMed/NCBI
|
|
10
|
Griendling KK and Ushio-Fukai M: Reactive
oxygen species as mediators of angiotensin II signaling. Regul
Pept. 91:21–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Swaminathan S and Shah SV: Novel
approaches targeted toward oxidative stress for the treatment of
chronic kidney disease. Curr Opin Nephrol Hypertens. 17:143–148.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hakim FA and Pflueger A: Role of oxidative
stress in diabetic kidney disease. Med Sci Monit. 16:RA37–RA48.
2010.PubMed/NCBI
|
|
13
|
Ha H, Yu MR and Kim KH: Melatonin and
taurine reduce early glomerulopathy in diabetic rats. Free Radic
Biol Med. 26:944–950. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oktem F, Ozguner F, Yilmaz HR, Uz E and
Dündar B: Melatonin reduces urinary excretion of
N-acetyl-beta-D-glucosaminidase, albumin and renal oxidative
markers in diabetic rats. Clin Exp Pharmacol Physiol. 33:95–101.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Zhang HM, Wu LP, Tan DX, Kamat A,
Li YQ, Katz MS, Abboud HE, Reiter RJ and Zhang BX: Impaired
mitochondrial complex III and melatonin responsive reactive oxygen
species generation in kidney mitochondria of db/db mice. J Pineal
Res. 51:338–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elbe H, Vardi N, Esrefoglu M, Ates B,
Yologlu S and Taskapan C: Amelioration of streptozotocin-induced
diabetic nephropathy by melatonin, quercetin, and resveratrol in
rats. Hum Exp Toxicol. 34:100–113. 2015. View Article : Google Scholar
|
|
17
|
Kim EY, Alvarez-Baron CP and Dryer SE:
Canonical transient receptor potential channel (TRPC)3 and TRPC6
associate with large-conductance Ca2+-activated K+ (BKCa) channels:
Role in BKCa trafficking to the surface of cultured podocytes. Mol
Pharmacol. 75:466–477. 2009. View Article : Google Scholar
|
|
18
|
Lim AKh: Diabetic
nephropathy-complications and treatment. Int J Nephrol Renovasc
Dis. 7:361–381. 2014. View Article : Google Scholar :
|
|
19
|
Mundel P and Kriz W: Structure and
function of podocytes: An update. Anat Embryol (Berl). 192:385–397.
1995. View Article : Google Scholar
|
|
20
|
Faul C, Asanuma K, Yanagida-Asanuma E, Kim
K and Mundel P: Actin up: Regulation of podocyte structure and
function by components of the actin cytoskeleton. Trends Cell Biol.
17:428–437. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Endlich N, Kress KR, Reiser J, Uttenweiler
D, Kriz W, Mundel P and Endlich K: Podocytes respond to mechanical
stress in vitro. J Am Soc Nephrol. 12:413–422. 2001.PubMed/NCBI
|
|
22
|
Durvasula RV, Petermann AT, Hiromura K,
Blonski M, Pippin J, Mundel P, Pichler R, Griffin S, Couser WG and
Shankland SJ: Activation of a local tissue angiotensin system in
podocytes by mechanical strain. Kidney Int. 65:30–39. 2004.
View Article : Google Scholar
|
|
23
|
Anwar MM and Meki AR: Oxidative stress in
streptozotocin-induced diabetic rats: Effects of garlic oil and
melatonin. Comp Biochem Physiol A Mol Integr Physiol. 135:539–547.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lenz O, Elliot SJ and Stetler-Stevenson
WG: Matrix metalloproteinases in renal development and disease. J
Am Soc Nephrol. 11:574–581. 2000.PubMed/NCBI
|
|
25
|
Gumustekin M, Tekmen I, Guneli E, Tugyan
K, Topcu A, Ergonen AT, Ozdemir MH, Uysal N and Bediz CS:
Short-term melatonin treatment improved diabetic nephropathy but
did not affect hemorheological changes in diabetic rats. Pharmazie.
62:693–698. 2007.PubMed/NCBI
|
|
26
|
Ehrenberg B, Montana V, Wei MD, Wuskell JP
and Loew LM: Membrane potential can be determined in individual
cells from the nernstian distribution of cationic dyes. Biophys J.
53:785–794. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nicholls DG and Ward MW: Mitochondrial
membrane potential and neuronal glutamate excitotoxicity: Mortality
and millivolts. Trends Neurosci. 23:166–174. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanwar YS, Wada J, Sun L, Xie P, Wallner
EI, Chen S, Chugh S and Danesh FR: Diabetic nephropathy: Mechanisms
of renal disease progression. Exp Biol Med (Maywood). 233:4–11.
2008. View Article : Google Scholar
|
|
29
|
Scott JA and King GL: Oxidative stress and
antioxidant treatment in diabetes. Ann N Y Acad Sci. 1031:204–213.
2004. View Article : Google Scholar
|
|
30
|
Brezniceanu ML, Liu F, Wei CC, Tran S,
Sachetelli S, Zhang SL, Guo DF, Filep JG, Ingelfinger JR and Chan
JS: Catalase overexpression attenuates angiotensinogen expression
and apoptosis in diabetic mice. Kidney Int. 71:912–923. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Durvasula RV and Shankland SJ: Activation
of a local renin angiotensin system in podocytes by glucose. Am J
Physiol Renal Physiol. 294:F830–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stitt-Cavanagh E, MacLeod L and Kennedy C:
The podocyte in diabetic kidney disease. Scientific World Journal.
9:1127–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Welsh GI and Saleem MA: Nephrin-signature
molecule of the glomerular podocyte? J Pathol. 220:328–337.
2010.
|
|
34
|
Adams JM and Cory S: Life-or-death
decisions by the Bcl-2 protein family. Trends Biochem Sci.
26:61–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aaronson DS and Horvath CM: A road map for
those who don't know JAK-STAT. Science. 296:1653–1655. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chuang PY and He JC: JAK/STAT signaling in
renal diseases. Kidney Int. 78:231–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Quiroz Y, Ferrebuz A, Romero F, Vaziri ND
and Rodriguez-Iturbe B: Melatonin ameliorates oxidative stress,
inflammation, proteinuria and progression of renal damage in rats
with renal mass reduction. Am J Physiol Renal Physiol.
294:F336–F344. 2008. View Article : Google Scholar
|
|
39
|
Abkhezr M and Dryer SE: Angiotensin II and
canonical transient receptor potential-6 activation stimulate
release of a signal transducer and activator of transcription
3-activating factor from mouse podocytes. Mol Pharmacol.
86:150–158. 2014. View Article : Google Scholar : PubMed/NCBI
|