Introduction
Infertility is a common disorder affecting some one
in seven couples, and subfertility has become a markedly increasing
problem in affluent countries, with the most commonly identified
cause attributed to 'male factor' (1–3).
Several scientific studies have suggested that a decrease in male
fertility is frequently associated with smoking, which may cause a
decrease in semen quality (4). The
inhalation of cigarette smoke leads to absorption of nicotine,
carbon monoxide and heavy metals throughout the body, which can end
up in the seminal plasma of smokers via various modes of diffusion
and active transport (1,5,6).
Reports have shown that there is a continuous and substantial
number of cell divisions in the sperm cell differentiation and
maturation process (7–9). Simultaneously, cigarette smoking
affects semen quality, particularly among heavy smokers or those
who have smoked for several years (10).
Studies have demonstrated that Chk1 is a Ser/Thr
protein kinase, which controls the G2/M phase transition in
response to DNA damage (11–13).
Following DNA damage, it is released from chromatin and localizes
to the cytoplasm, where a portion localizes to interphase
centrosomes (14). In turn,
activated Chk phosphorylates a number of downstream effectors to
trigger pleiotropic cellular responses, including transcription
regulation, alteration of energy consumption, cell-cycle arrest or
delay, and DNA repair or cell death if the damage is too severe for
repair (14). The harmful
substances in tobacco inhaled by smokers cause DNA damage, which
may elevate DNA fragmentation rates (15,16).
Increased sperm DNA fragmentation rates have been positively
correlated with impaired fertility (17). Thus, the present study aimed to
investigate the expression of Chk1 in sperm cells of smoking and
non-smoking men, and to further examine the correlation between DNA
fragmentation rates and the expression levels of Chk1 with
smoking.
Materials and methods
Study population
The study population consisted of men, who were
referred to the Reproductive Medicine Center of Shanxi Women and
Infants Hospital (Taiyuan, China) between January 2013 and January
2015. All subjects were of the Han population from Shanxi Province
in north China. The study was approved by the ethics committee of
the Shanxi Women and Infants Hospital (Taiyuan, China) and the
individuals in the relationships provided consent.
The inclusion criteria were as follows: Being the
male partner of an infertile couple for a duration of at least 1
year, having regular intercourse, and seeking infertility treatment
at the Reproductive Medicine Center, Shanxi Women and Infants
Hospital over the study period. A careful history was obtained from
each subject to exclude systemic diseases and assess alcohol
assumption; careful physical examination was performed, with
measurement of testicular size to exclude abnormalities of the
external genitalia and cryptorchidism; ultrasonographic examination
was performed to exclude varicoceles; microbiological examination
and spermioculture were performed to exclude infections; an
immunobead binding test was performed to exclude the presence of
anti-sperm antibodies; karyotyping was used to exclude any
chromosomal abnormality; and genetic examination was performed to
exclude Y chromosome microdeletions and cystic fibrosis gene
mutations.
A brief medical history was obtained, primarily by
informal interview with the patient, or from the patient's clinical
notes or a self-reported questionnaire. According to the
standardization of the World Health Organization (WHO) on smoking
and associated literature (18–20),
1,218 men were divided into a smoking group (920 cases) and
non-smoking group (298 cases). The smoking group was grouped into
three groups, according to daily cigarette consumption, as follows:
Mild smoking group (256 cases; ≤9 cigarettes/day), moderate smoking
group (365 cases; 10–19 cigarettes/day), heavy smoking group (299
cases; ≥20 cigarettes/day). The smoking group was grouped into
another three groups, according to the number of years of smoking,
as follows: Short-term smoker group (268 cases; ≤5 years),
medium-term smoker group (282 cases; 5–10 years), long-term smoker
group (370 cases; ≥10 years).
Semen collection and analysis
A semen sample was obtained from all subjects via
masturbation following 2–7 days of abstinence for routine sperm
counts, according to the WHO (2010) criteria (sperm concentration,
motility, morphology and viability) (21). Briefly, the ejaculate volumes were
estimated by specimen weight, assuming a semen density of 1.0 g/ml.
Sperm concentration, motility and viability were detected using a
sperm class analyzer (CASA system; Microoptic S.L., Barcelona,
Spain). Sperm motility was also analyzed using the WHO (2010)
criteria of progressive motility, non-progressive motility and
immotility. In this analysis, the percentage of motile sperm refers
to the percentage of sperm with any flagellar movement, whether
twitching or progressive. A single technician assessed sperm
morphology using the strict methods recommended by the WHO (2010).
In addition to the primary measurements of semen quality (sperm
concentration, volume and percentage of motile sperm), the CASA
system was used to analyze the total sperm count and total motile
sperm count.
Sperm viability analysis
Sperm viability was assessed within 30 min of
ejaculation. Analysis was performed using eosin Y staining (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China), for which 1 g of eosin
was dissolved with 1 g fresh sperm. The percentage of viable sperm,
indicated by an unstained sperm head, and non-viable sperm,
indicated by staining of the sperm head, was assessed by counting a
minimum of 200 spermatozoa. Replicate counts of 200 sperm on each
of two slides were performed using a using a CX31 microscope
(Olympus Corporation, Tokyo, Japan), which were then repeated if
>5% difference was found (4).
Ultraviolet spectrophotometric assay for
measurement of spermatozoa acrosin activity
The spermatozoa in each group were analyzed for
acrosin activity using a Human Spermatozoa Acrosin Activity
Quantitative Assay kit (Huakang Biotech, Shenzhen, China),
according to manufacturer's protocol. At 24°C, the quantity of
substrate hydrolyzing 1.0 µmol BAPNA/min was defined as 1IU
acrosin activity, and determined based on optical density (OD)
values: Acrosin activity (µIU/106 spermatozoa) =
{[(sample tube OD value-control tube OD value) × 2]/(495 × 7.5)} ×
106. OD values were assessed on a DNM-9602 ultraviolet
spectrophotometer (Beijing Perlong New Technology Co., Ltd.,
Beijing, China).
Colorimetric assay for measurement of
seminal plasma zinc
The seminal fluid in each group was analyzed for
zinc using a Seminal Plasma Zinc Quantitative Assay kit (Huakang
Biotech), according to the manufacturer's protocol. The seminal
fluid (1 ml) was centrifuged for 5 min at 1,000 × g at 5 min. The
supernatant was transferred into a test tube for use in seminal
plasma analysis. The sediment was washed with 1 ml physiological
saline solution, mixed on an XH-B vortex-type mixer (Jiangsu
Kangjian Medical Apparatus Co., Ltd., Taizhou, China) for 30 sec,
and centrifuged again, as previously. The supernatant was discarded
and the sediment was used for zinc determination, rather than using
200 µl of liquid sample. The absorbance of the solutions was
measured at 490 nm on aHR801 microplate reader (Shenzhen
Highcreation Technology Co., Ltd., Shenzhen, China). The
concentration in the sample was determined using the following
formula: Seminal plasma zinc (µmol) = zinc concentration
(mmol/l) × semen volume (ml).
Sperm DNA fragmentation analysis
The analysis of DNA fragmentation was performed in
fresh semen using fluorescence staining with a kit supplied by
(Huakang Biotech), based on the fluorescence emission from
individual sperm, which were stained with acridine orange (AO). AO
molecules are intercalated into double-stranded DNA, and green
fluorescence is emitted from the sperm nuclei. The DNA in sperm
with immature nuclei are readily denatured into single strands and,
following AO molecule aggregation in the nuclei, the color of the
fluorescence becomes orange-red. The cell suspension was pipetted
onto a glass slide and observed under a BX51 fluorescence
microscope (Olympus Corporation) with a 480–490 nm filter. The
percentages of green (normal DNA integrity) and orange-red
(abnormal DNA integrity) spermatozoa in each sample of 200
spermatozoa were calculated by the same examiner. The integrity of
sperm nuclear DNA was considered abnormal when the percentage of
denaturation (orange-red spermatozoa on AO staining) was
>34%.
Reverse
transcription-quantitative-polymerase chain reaction (RT-qPCR)
analysis
RT-qPCR analysis was used to assess the
transcriptional expression of tumor-associated genes, including
Chk1. Total RNAs were extracted from the spermatozoa using a Total
RNA Purification kit (Nanjing KeyGen Biotech Co., Ltd.), the
concentration of the RNA was determined using a NanoDrop 1000
(NanoDrop; Thermo Fisher Scientific, Inc., Wilmington, DE, USA) and
reverse transcribed using an Transcriptor First Strand cDNA
synthesis kit (Nanjing KeyGen Biotech Co., Ltd.). The reaction was
performed in the following conditions: 30°C for 10 min; 42°C for 30
min; 99°C for 5 min; and 5°C for 5 min. qPCR was conducted in a
CFX-96 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using a One
Step SYBR PrimeScript RT-PCR kit (Takara Bio., Inc.), as described.
The specific primers (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) were as follows: Chk1, forward
5′-ATATGAAGCGTGCCGTAGACT-3′ and reverse
5′-TGCCTATGTCTGGCTCTATTCTG-3′; GAPDH, forward
5′-ACCACAGTCCATGCCATCAC-3′ and reverse 5′-TCCACCACCCTGTTGCTGTA-3′.
qPCR was performed as follows: Initial denaturation at 98°C for 5
min; 40 cycles of 95°C for 10 sec, and 72°C for 15 sec. The
2−ΔΔCq method was used for quantification, calculated
according to the manufacturer's protocol, with results expressed as
the mean ± standard deviation.
Western blot analysis
Spermatozoa were prepared from each group for
western blot analysis to determine the expression level of the
tumor-associated protein, Chk1. The seminal fluid (1 ml) was
centrifuged for 5 min at 1,000 × g. The precipitate was washed with
1 ml physiological saline solution, mixed on a vortex-type mixer
for 30 sec, and centrifuged again, as above. For analysis of
cellular protein levels, spermatozoa cells were rinsed twice with
ice-cold phosphate-buffered saline and then lysed in ice-cold lysis
buffer [containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM ETDA, 1
mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM
β-glycerophosphate, 1 mM Na3VO4, 1 mM PMSF, and 10 µg/ml
each of leupeptin, aprotinin, and pepstatin) for 30 min. Cell
lysates were centrifuged at 13,000 × g for 10 min at 4℃ and the
protein concentration was determined by the Bradford assay.
Proteins (20 µg) were loaded onto 8% SDS-PAGE gels for
electrophoresis and then transferred onto nitrocellulose membranes.
The transferred nitrocellulose membranes were blocked with 5% dried
skim milk for 1 h at room temperature, then incubated with mouse
anti-Chk1 monoclonal antibody (1:200; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA; cat. no. sc-377231) at 4°C for 12 h. The
membranes were then exposed to goat anti-mouse or rabbit secondary
antibody (1:200; Zhongshan Bioengineering, Beijing, China; cat. no.
PV9005) in blocking buffer for 1 h at room temperature. The bands
were demonstrated by enhanced chemiluminescence reagents (Santa
Cruz Biotechnology, Inc.) for 1 min and analyzed using Image-Pro
Plus 5.1 software (Media Cybernetics, Inc., Rockville, MD,
USA).
Statistical analysis
All data were analyzed using SPSS 17.0 (SPSS, Inc.
Chicago, IL, USA). Normally distributed data are expressed as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference. To assess the normality of
the distribution, a Shapiro-Wilk test was performed. One-way
analysis of variance was used for comparison among multiple groups
if the variance was homogeneous, whereas non-normally distributed
variables were analyzed using a Mann-Whitney U test or
Kruskal-Wallis variance analysis, as appropriate.
Results
Comparison of semen parameters between
smokers and non-smokers
As shown in Tables
I and II, routine semen
parameters in the smoking groups were compared with those of the
non-smoking group. No significant differences in semen volume,
sperm concentration or sperm count were found between the smoking
and non-smoking groups (P>0.05). However, progressive motility
in the smoking group was significantly decreased (P<0.05),
compared with the non-smoking group. No significant changes in
routine semen parameters were observed in the mild smoking group
(P>0.05), whereas the moderate and heavy smoking groups had
significantly decreased progressive motility (P<0.05). No
significant differences were found in the routine semen parameters
in the short-term smoking group, compared with the non-smoking
group (P>0.05), whereas the medium-term smoking group had
significantly decreased progressive motility (P<0.05), and the
long-term smoking group had decreased semen concentration, sperm
count and progressive motility (P<0.05).
 | Table IComparison of routine semen parameters
between non-smokers and smokers grouped according to daily
cigarette consumption. |
Table I
Comparison of routine semen parameters
between non-smokers and smokers grouped according to daily
cigarette consumption.
| Parameter | Non-smoking | Smoking | Daily cigarette
consumption
|
|---|
| Mild | Moderate | Heavy |
|---|
| Cases (n) | 298 | 920 | 256 | 365 | 299 |
| Semen volume
(ml) | 3.63±1.48 | 3.44±1.23 | 3.53±1.18 | 3.40±1.21 | 3.47±1.23 |
| Sperm concentration
(×106/ml) | 45.38±24.83 | 41.57±21.93 | 44.41±21.42 | 42.62±25.26 | 40.13±24.73 |
| Sperm count
(×106) | 49.28±31.29 | 44.62±31.94 | 47.17±29.96 | 45.84±31.24 | 43.18±32.61 |
| Progressive motility
(%) | 27.97±10.66 | 18.26±11.48 | 26.42±12.63 | 19.58±11.24 | 15.21±9.17 |
 | Table IIComparison of routine semen parameters
between non-smokers and smokers grouped according to the duration
of smoking. |
Table II
Comparison of routine semen parameters
between non-smokers and smokers grouped according to the duration
of smoking.
| Parameter | Non-smoking | Short-term | Duration of smoking
|
|---|
| Medium-term | Long-term |
|---|
| Cases (n) | 298 | 268 | 282 | 370 |
| Semen volume
(ml) | 3.63±1.48 | 3.57±1.24 | 3.43±1.42 | 2.12±1.11 |
| Sperm concentration
(×106/ml) | 45.38±24.83 | 43.65±20.39 | 41.59±21.11 | 28.85±22.22 |
| Sperm count
(×106) | 49.28±31.29 | 48.47±21.56 | 44.54±22.39 | 37.67±16.22 |
| Progressive motility
(%) | 27.97±10.66 | 25.67±11.18 | 18.69±12.24 | 13.28±11.43 |
Comparison of sperm morphology between
smokers and non-smokers
As shown in Tables
III and IV, the sperm
morphology in the smoking groups was compared with the non-smoking
group. No significant differences were found in the sperm
morphology in the normal sperm count and abnormal head, body and
tail counts between the smoking and non-smoking groups (P>0.05).
Compared with the non-smoking group, no significant changes were
found in the sperm morphology between the mild smoking group and
moderate smoking group (P>0.05), nor were there significant
differences between the short-term smoking group and medium-term
smoking group (P>0.05). However, the abnormal head rate in the
heavy smoking group and long-term smoking group were significantly
higher, compared with those in the non-smoking group
(P<0.05).
 | Table IIIComparison of sperm morphology
between non-smokers and smokers grouped according to daily
cigarette consumption. |
Table III
Comparison of sperm morphology
between non-smokers and smokers grouped according to daily
cigarette consumption.
| Parameter | Non-smoking | Smoking | Daily cigarette
consumption
|
|---|
| Mild | Moderate | Heavy |
|---|
| Cases | 298 | 920 | 256 | 365 | 299 |
| Normal sperm | 7.22±1.49 | 6.43±1.33 | 7.13±1.38 | 6.97±1.55 | 6.13±1.07 |
| Abnormal head | 82.51±11.66 | 88.38±15.11 | 86.36±13.10 | 86.02±10.16 | 98.22±18.54 |
| Abnormal body | 41.38±8.58 | 49.32±14.43 | 47.73±12.89 | 46.52±14.81 | 52.37±14.23 |
| Abnormal tail | 6.23±7.19 | 11.64±12.77 | 9.38±8.64 | 10.57±10.32 | 12.39±14.71 |
 | Table IVComparison of sperm morphology
between non-smokers and smokers grouped according to duration of
smoking. |
Table IV
Comparison of sperm morphology
between non-smokers and smokers grouped according to duration of
smoking.
| Parameter | Non-smoking | Duration of smoking
|
|---|
| Short-term | Medium-term | Long-term |
|---|
| Cases (n) | 298 | 268 | 282 | 370 |
| Normal sperm | 7.22±1.38 | 7.42±1.23 | 6.29±1.26 | 5.29±1.27 |
| Abnormal head | 82.51±10.62 | 80.91±14.25 | 88.27±12.77 | 99.43±16.28 |
| Abnormal body | 41.38±9.48 | 47.88±8.76 | 51.93±10.36 | 52.50±9.71 |
| Abnormal tail | 6.23±7.44 | 11.59±8.61 | 9.37±11.27 | 11.31±12.19 |
Comparison of sperm viability between
smokers and non-smokers
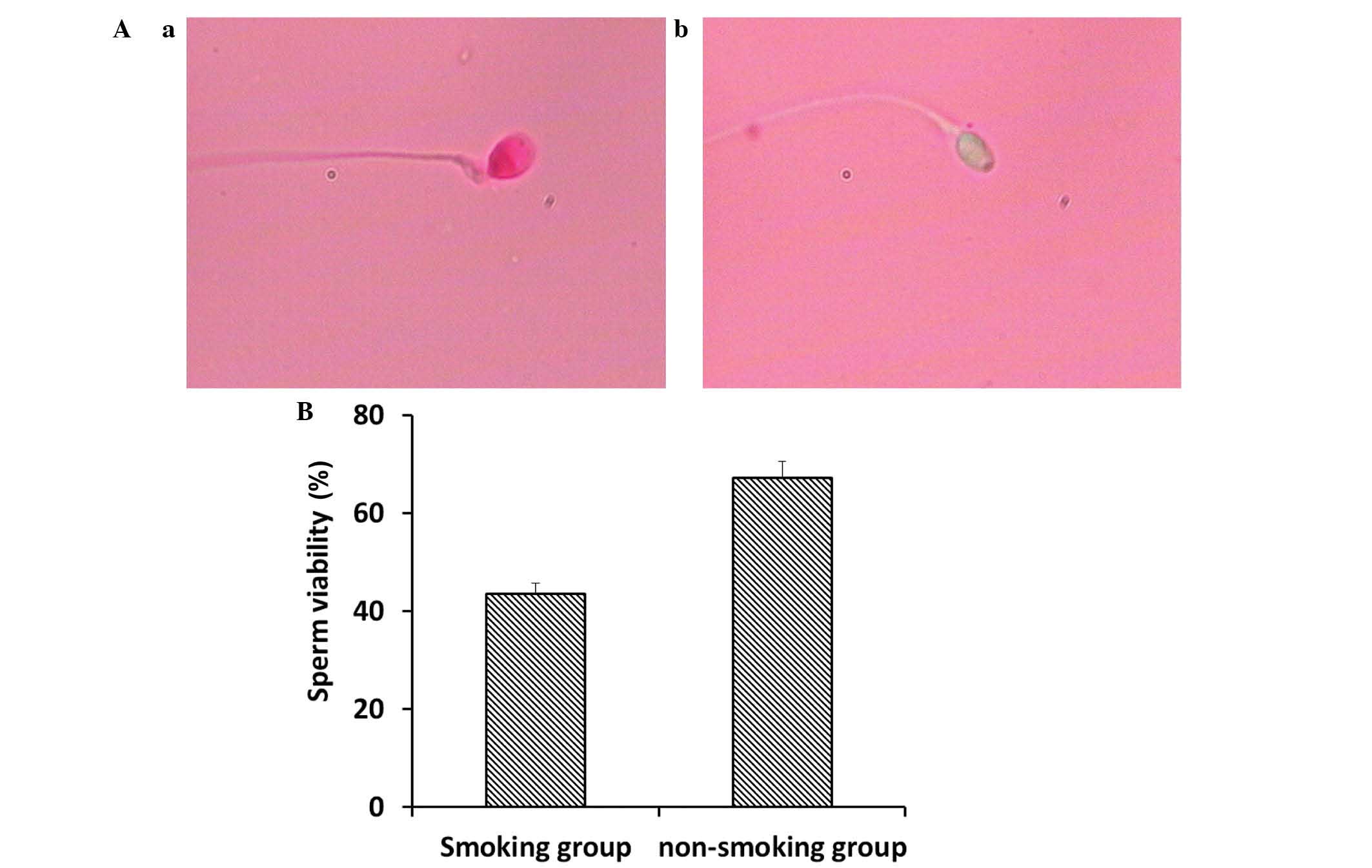
Sperm viability was analyzed using eosin Y staining.
As shown in Fig. 1A, the viable
sperm, in which sperm heads remained unstained, and non-viable
sperm, in which sperm heads were stained, were examined using a
CX31 microscope (Olympus Corporation). Compared with the
non-smoking group, the smoking group had significantly decreased
sperm viability (Fig. 1B;
P<0.05).
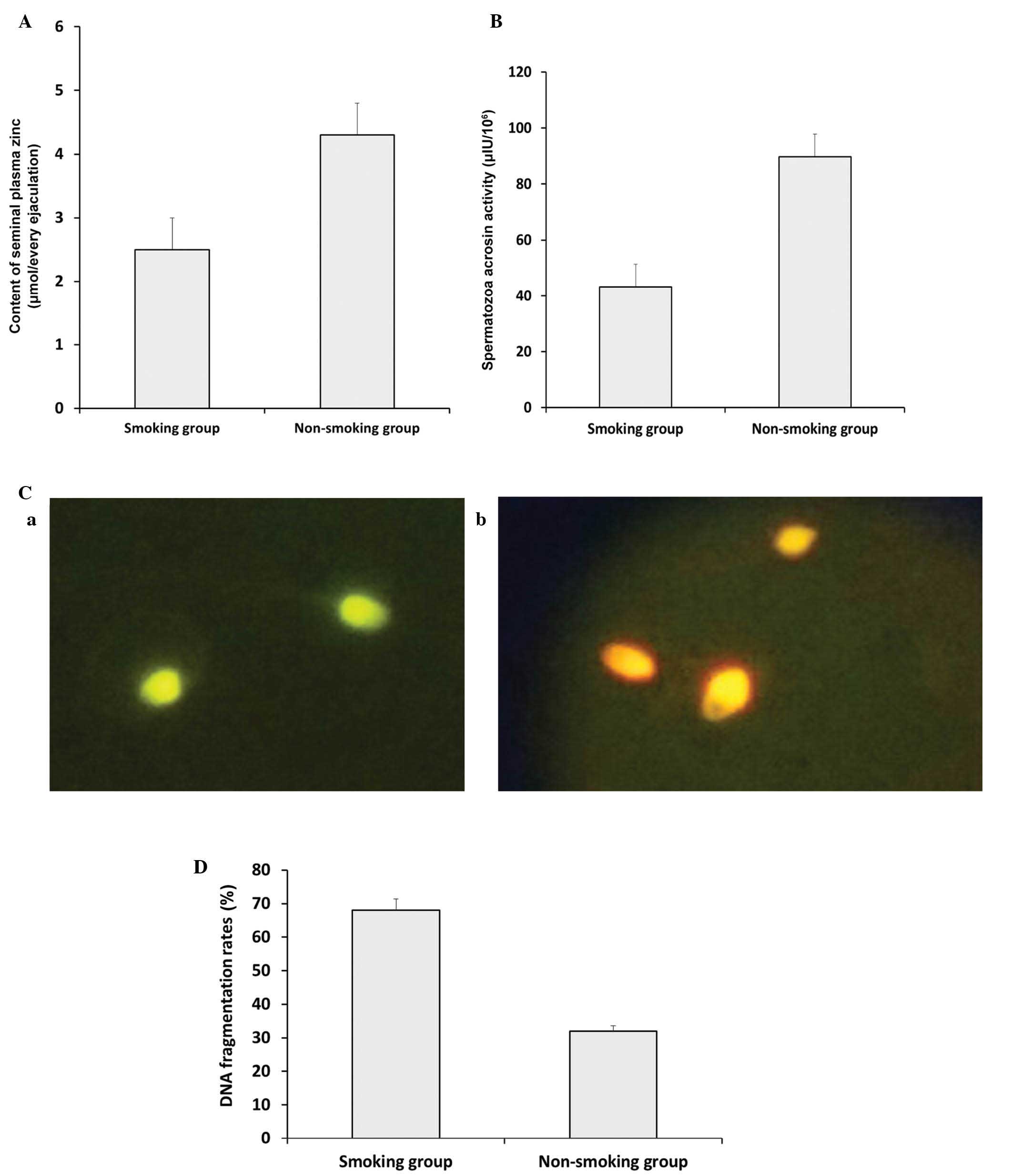
Comparison of plasma zinc, spermatozoa
acrosin activity and DNA fragmentation rates between smokers and
non-smokers
As shown in Fig.
2A, the seminal plasma zinc concentration decreased
significantly in the smoking group, compared with the non-smoking
group (P<0.05). Similar results were obtained for spermatozoa
acrosin activity between the smoking group and non-smoking group
(Fig. 2B; P<0.05). DNA
fragmentation rates were analyzed using AO staining. As shown in
Fig. 2C, the green (normal DNA
integrity) and orange-red (abnormal DNA integrity) spermatozoa were
examined using a BX51 fluorescence microscope (Olympus Corporation)
with a 480–490 nm filter. Compared with the non-smoking group, the
smoking group exhibited a significantly higher DNA fragmentation
rate (Fig. 2D; P<0.05).
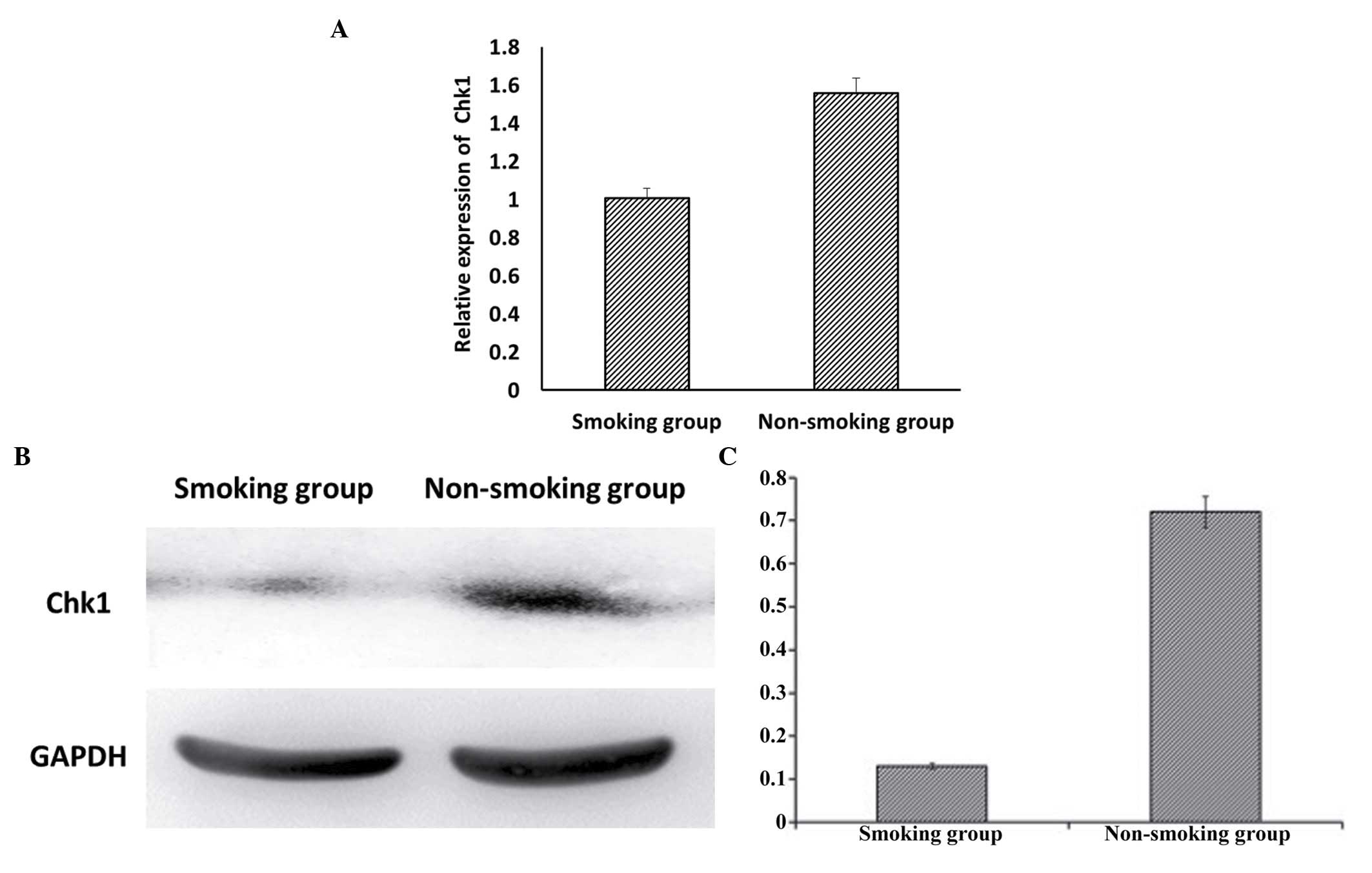
Expression levels of Chk1 in the sperm of
smokers and non-smokers
Analyses using RT-qPCR (Fig. 3A) and western blot analyses
(Fig. 3B) demonstrated that the
expression of Chk1 was significantly decreased in the smoking
group, compared with the non-smoking group (P<0.05).
Association between DNA fragmentation
rates and Chk1 with semen parameters
The present study found a non-linear association
between the relative mRNA expression of Chk1, and the progressive
motility and sperm concentration (P<0.05). However, DNA
fragmentation rates were inversely association (P<0.05) with
progressive motility and sperm concentration (Table V).
 | Table VAssociation between DNA fragmentation
rates and the expression of Chk1 with semen parameters in all
participants. |
Table V
Association between DNA fragmentation
rates and the expression of Chk1 with semen parameters in all
participants.
| Parameter | Mean ± SD | Relative expression
of Chk1
| DNA fragmentation
rate
|
|---|
| r-value | P-value | r-value | P-value |
|---|
| Relative expression
of Chk1 | 1.43±0.28 | – | – | – | – |
| DNA fragmentation
rate | 0.58±0.01 | – | – | – | – |
| Progressive
motility (%) | 22.16±9.32 | 0.042 | 0.027a | 0.042 | 0.027a |
| Sperm concentration
(×106/ml) | 39.27±15.22 | 0.047 | 0.026a | 0.037 | 0.012a |
Discussion
Cigarette smoking is a recognized health hazard, and
the highest prevalence of smokers is in young men of reproductive
age (22). There is considerable
evidence that cigarette smoking has a major role in the etiology of
male infertility (23). Smokers
inhale several toxins, including nicotine, carbon monoxide and
other mutagenic compounds (24).
Cigarette smoking has been associated with detrimental effects on
sperm morphology, density and motility (5). The mechanisms through which smoking
by men may be linked to detrimental effects on reproduction and
sperm parameters remain to be fully elucidated. Direct biological
and toxic effects are possible on male sperm cells a. The
inhalation of cigarette smoke leads to the absorption of nicotine,
carbon monoxide, cadmium and other mutagenic compounds, which may
reach the male reproductive system and cause alterations, including
altered antioxidant concentrations, reactive oxygen species
generation, aneuploidy rates, and DNA damage in spermatozoa and
semen (5,10,22).
In addition, it has been found that heavy smoking-induced DNA
damaged is associated with abnormal spermatozoa and male
infertility (25,26). Thus, it is necessary to evaluate
the effects of cigarette smoking on DNA damage and repair
mechanisms in sperm.
In the present study, it was observed that the
progressive motility of the sperm in the moderate and heavy smoking
groups were significantly decreased, compared with the non-smokers,
whereas no significant changes were observed in the mild smoking
group. No significant differences were observed in the routine
semen parameters of the short-term smoking group, compared with the
non-smoking group. However, the sperm in the medium-term smoking
group had significantly decreased progressive motility, and the
long-term smoking group had decreased semen concentration, sperm
count and progressive motility, compared with the non-smoking
group. Compared with the non-smoking group, no significant changes
were found in the sperm morphology in the mild smoking group or
moderate smoking group. This was also the case for the short-term
smoking group and medium-term smoking group. However, the abnormal
head rates in the heavy smoking group and long-term smoking group
showed significant increases, compared with the non-smoking group.
Compared with the non-smoking group, the smoking group exhibited a
significant increase in sperm viability. The seminal plasma zinc
concentration decreased significantly in the smoking group,
compared with non-smoking group, and the smoking group had
significantly increased DNA fragmentation rates, compared with the
non-smoking group. Similar results were obtained for spermatozoa
acrosin activity between the smoking group and non-smoking group.
These data are consistent with the results of previous studies
(25,26).
It has been reported that, in response to DNA
damage, the activation of the Chk1 facilitates S and G2 checkpoint
arrest (27–29), and it may promote the survival of
cells in the presence of DNA damage-inducing agents. Activated Chk1
phosphorylates a number of downstream effectors to trigger a
pleiotropic cellular response, which includes transcription
regulation, alterations in energy consumption, cell-cycle arrest or
delay and DNA repair or cell death if the damage is too severe for
repair (30–32).
In the present study, the expression of Chk1 was
significantly decreased in the smoking group, compared with the
non-smoking group. There was a nonlinear association between the
relative mRNA expression of Chk1 and the progressive motility and
sperm concentration. However, an inverse association was found
between DNA fragmentation rates and the progressive motility and
sperm concentration. These data suggested that the decrease of
semen quality caused by cigarette smoking was not only correlated
with sperm DNA fragmentation indices, but was also correlated with
a decline in the expression of Chk1. The expression of Chk1 was
correlated with sperm DNA damage and apoptosis, and its reduction
may lead to decreased sperm repair and increased sperm apoptosis,
with a subsequent effect on semen quality.
In conclusion, the results obtained in the present
study provide useful information regarding the expression of Chk1
in sperm cells of smoking and non-smoking men, and the association
between DNA fragmentation rates and the expression levels of Chk1
with smoking. They may also offer information for the prevention
and treatment of male infertility as a result of smoking.
Acknowledgments
The present study was supported by the Research Fund
of National Health and Family Planning Commission of China (grant
no. RFNHFPCC, 201402004).
References
|
1
|
Jo J, Lee SH, Lee JM and Jerng UM: Semen
quality improvement in a man with idiopathic infertility treated
with traditional Korean medicine: A case report. Explore (NY).
11:320–323. 2015. View Article : Google Scholar
|
|
2
|
Agarwal A, Mulgund A, Hamada A and Chyatte
MR: A unique view on male infertility around the globe. Reprod Biol
Endocrinol. 13:372015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilany K, Minai-Tehrani A, Savadi-Shiraz
E, Rezadoost H and Lakpour N: Exploring the human seminal plasma
proteome: An unexplored gold mine of biomarker for male infertility
and male reproduction disorder. J Reprod Infertil. 16:61–71.
2015.PubMed/NCBI
|
|
4
|
Samplaski MK, Dimitromanolakis A, Lo KC,
Grober ED, Mullen B, Garbens A and Jarvi KA: The relationship
between sperm viability and DNA fragmentation rates. Reprod Biol
Endocrinol. 13:422015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdul-Ghani R, Qazzaz M, Dabdoub N,
Muhammad R and Abdul-Ghani AS: Studies on cigarette smoke induced
oxidative DNA damage and reduced spermatogenesis in rats. J Environ
Biol. 35:943–947. 2014.PubMed/NCBI
|
|
6
|
Hamad MF, Shelko N, Kartarius S, Montenarh
M and Hammadeh ME: Impact of cigarette smoking on histone (H2B) to
protamine ratio in human spermatozoa and its relation to sperm
parameters. Andrology. 2:666–677. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahmadnia H, Ghanbari M, Moradi MR and
Khaje-Dalouee M: Effect of cigarette smoke on spermatogenesis in
rats. Urol J. 4:159–163. 2007.PubMed/NCBI
|
|
8
|
Shukla KK, Mahdi AA and Rajender S:
Apoptosis, spermatogenesis and male infertility. Front Biosci
(Elite Ed). 4:746–754. 2012. View
Article : Google Scholar
|
|
9
|
Sun QY, Breitbart H and Schatten H: Role
of the MAPK cascade in mammalian germ cells. Reprod Fertil Dev.
11:443–450. 1999. View
Article : Google Scholar
|
|
10
|
Tian M, Bao H, Martin FL, Zhang J, Liu L,
Huang Q and Shen H: Association of DNA methylation and
mitochondrial DNA copy number with human semen quality. Biol
Reprod. 91:1012014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Albiges L, Goubar A, Scott V, Vicier C,
Lefèbvre C, Alsafadi S, Commo F, Saghatchian M, Lazar V, Dessen P,
et al: Chk1 as a new therapeutic target in triple-negative breast
cancer. Breast. 23:250–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al-Kaabi MM, Alshareeda AT, Jerjees DA,
Muftah AA, Green AR, Alsubhi NH, Nolan CC, Chan S, Cornford E,
Madhusudan S, et al: Checkpoint kinase1 (CHK1) is an important
biomarker in breast cancer having a role in chemotherapy response.
Br J Cancer. 112:901–911. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma YC, Su N, Shi XJ, Zhao W, Ke Y, Zi X,
Zhao NM, Qin YH, Zhao HW and Liu HM: Jaridonin-induced G2/M phase
arrest in human esophageal cancer cells is caused by reactive
oxygen species-dependent Cdc2-tyr15 phosphorylation via
ATM-Chk1/2-Cdc25C pathway. Toxicol Appl Pharmacol. 282:227–236.
2015. View Article : Google Scholar
|
|
14
|
Zuazua-Villar P, Rodriguez R, Gagou ME,
Eyers PA and Meuth M: DNA replication stress in CHK1-depleted
tumour cells triggers premature (S-phase) mitosis through
inappropriate activation of Aurora kinase B. Cell Death Dis.
5:e12532014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sepaniak S, Forges T, Fontaine B, Gerard
H, Foliguet B, Guillet-May F, Zaccabri A and Monnier-Barbarino P:
Negative impact of cigarette smoking on male fertility: From
spermatozoa to the offspring. J Gynecol Obstet Biol Reprod (Paris).
33:384–390. 2004.In French. View Article : Google Scholar
|
|
16
|
Silver EW, Eskenazi B, Evenson DP, Block
G, Young S and Wyrobek AJ: Effect of antioxidant intake on sperm
chromatin stability in healthy nonsmoking men. J Androl.
26:550–556. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozkosem B, Feinstein SI, Fisher AB and
O'Flaherty C: Advancing age increases sperm chromatin damage and
impairs fertility in peroxiredoxin 6 null mice. Redox Biol.
5:15–23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pasqualotto FF, Sobreiro BP, Hallak J,
Pasqualotto EB and Lucon AM: Cigarette smoking is related to a
decrease in semen volume in a population of fertile men. BJU Int.
97:324–326. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou YL, Chen K, Yu BL and Gao XC:
Cigarette smoking decreases sperm nucleoprotein transition in
infertile males. Zhonghua Nan Ke Xue. 19:794–797. 2013.In
Chinese.
|
|
20
|
Trummer H, Habermann H, Haas J and Pummer
K: The impact of cigarette smoking on human semen parameters and
hormones. Hum Reprod. 17:1554–1559. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu JC, Huang YF and Lü NQ: WHO laboratory
manual for the examination and processing of human serum: Its
applicability to andrology laboratories in China. Zhonghua Nan Ke
Xue. 16:867–871. 2010.
|
|
22
|
Sobinoff AP, Sutherland JM, Beckett EL,
Stanger SJ, Johnson R, Jarnicki AG, McCluskey A, St John JC,
Hansbro PM and McLaughlin EA: Damaging legacy: Maternal cigarette
smoking has long-term consequences for male offspring fertility.
Hum Reprod. 29:2719–2735. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davar R, Sekhavat L and Naserzadeh N:
Semen parameters of non-infertile smoker and non-smoker men. J Med
Life. 5:465–468. 2012.
|
|
24
|
Fariello RM, Pariz JR, Spaine DM, Gozzo
FC, Pilau EJ, Fraietta R, Bertolla RP, Andreoni C and Cedenho AP:
Effect of smoking on the functional aspects of sperm and seminal
plasma protein profiles in patients with varicocele. Hum Reprod.
27:3140–3149. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dai JB, Wang ZX and Qiao ZD: The hazardous
effects of tobacco smoking on male fertility. Asian J Androl.
17:954–960. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
La Maestra S, De Flora S and Micale RT:
Effect of cigarette smoke on DNA damage, oxidative stress, and
morphological alterations in mouse testis and spermatozoa. Int J
Hyg Environ Health. 218:117–122. 2015. View Article : Google Scholar
|
|
27
|
Bryant C, Rawlinson R and Massey AJ: Chk1
inhibition as a novel therapeutic strategy for treating
triple-negative breast and ovarian cancers. BMC Cancer. 14:5702014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bryant C, Scriven K and Massey AJ:
Inhibition of the checkpoint kinase Chk1 induces DNA damage and
cell death in human Leukemia and Lymphoma cells. Mol Cancer.
13:1472014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han X, Aslanian A, Fu K, Tsuji T and Zhang
Y: The interaction between checkpoint kinase 1 (Chk1) and the
minichromosome maintenance (MCM) complex is required for DNA
damage-induced Chk1 phosphorylation. J Biol Chem. 289:24716–24723.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
González Besteiro MA and Gottifredi V: The
fork and the kinase: A DNA replication tale from a CHK1
perspective. Mutat Res Rev Mutat Res. 763:168–180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goto H, Kasahara K and Inagaki M: Novel
insights into Chk1 regulation by phosphorylation. Cell Struct
Funct. 40:43–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grabocka E, Commisso C and Bar-Sagi D:
Molecular pathways: Targeting the dependence of mutant RAS cancers
on the DNA damage response. Clin Cancer Res. 21:1243–1247. 2015.
View Article : Google Scholar :
|