Introduction
Lower back pain (LBP) is the sixth most common risk
factor for disability worldwide (1), and contributes $20–100 billion
annually in total economic burden (2), which has become a severe
socio-economic problem. Effective therapeutic management for LBP
remains complex, since the underlying causes are unclear; however,
intervertebral disc (IVD) degeneration (IVDD) is the most common
diagnosis and target for intervention (3). Among various genetic and/or
environmental factors that may contribute to the development of
IVDD, cell apoptosis appears to be a major cause (4). A previous investigation confirmed
that the number of apoptotic cells in degenerative IVD tissues was
significantly increased compared with in normal tissues (5). However, the signal transduction
pathways involved in the apoptosis of degenerative IVD cells are
not fully understood.
Chemokines are a family of small, soluble
chemoattractive cytokines that regulate the recruitment of
surrounding responsive cells. In addition, they have previously
been reported to affect cell proliferation, apoptosis,
differentiation and other physiological activities (6). Stromal cell-derived factor-1 (SDF-1)
is one of the most extensively investigated chemokines, which was
originally isolated from bone marrow stromal cells and activates
various cells by binding to the G-protein-coupled receptor, C-X-C
motif chemokine receptor 4 (CXCR4). It has previously been reported
that the SDF-1/CXCR4 axis is involved in degradation of the
cartilage matrix (7). Furthermore,
nuclear factor-κB (NF-κB) is a member of a family of transcription
factors that is involved in mediating cellular responses to damage,
stress and inflammation, and increasing evidence has demonstrated
that the NF-κB signaling pathway is associated with cell apoptosis
in nucleus pulposus cells (NPCs) (8,9).
However, to the best of our knowledge, the effect of the
SDF-1/CXCR4 signaling axis on apoptosis of degenerative NPCs, and
whether this effect is mediated through the NF-κB pathway, has not
been previously investigated. On the basis of these observations,
the present study aimed to examine the potential regulatory effect
of SDF-1/CXCR4 on apoptosis of human NPCs.
Materials and methods
Primary NPC isolation and culture
Degenerative NP tissues were obtained from seven
patients with lumbar disc herniation following surgical discectomy.
The control group consisted of three patients with lumbar vertebral
fracture without previously documented medical history of LBP
(Table I). Written informed
consent was obtained from all tissue donors and the study protocol
was approved by the Ethics Committee of Chongqing Medical
University (Chongqing, China). The degree of IVDD was assessed
according to Pfirrmann classification by pre-operative magnetic
resonance imaging scans (10).
Samples from the normal group exhibited Pfirrmann grades I and II,
whereas the IVDD group exhibited Pfirrmann grades III–V.
 | Table IDemographic data of surgical disc
samples. |
Table I
Demographic data of surgical disc
samples.
| Sample | Gender | Age | Level | Pathology | Pfirrmann grade |
|---|
| 1 | F | 58 | L4/5 | Herniation | III |
| 2 | F | 46 | L5/S1 | Herniation | IV |
| 3 | M | 65 | L4/5 | Herniation | V |
| 4 | F | 39 | L3/4 | Herniation | III |
| 5 | F | 31 | L5/S1 | Herniation | III |
| 6 | M | 45 | L2/3 | Herniation | IV |
| 7 | F | 44 | L4/5 | Herniation | V |
| 8 | M | 21 | L1 | Fracture | I |
| 9 | M | 39 | L1 | Fracture | I |
| 10 | F | 45 | L2 | Fracture | II |
NP tissues from six donors (three per group) were
used for immunohistochemistry (IHC) and western blot analysis. NPCs
from the degenerative group were isolated by enzymatic digestion
and expanded in a monolayer culture model containing Dulbecco's
modified Eagle's medium/F 12 (Hyclone; GE Healthcare Life Sciences,
Logan, UT, USA) with 10% (v/v) fetal calf serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 100 μU/ml
penicillin and 100 μg/ml streptomycin, as described
previously (11). Cells were
incubated at 37°C in an atmosphere containing 5% CO2.
Cells at passage III-V were used throughout the in vitro
experiments.
IHC staining
NP tissues were fixed in 4% paraformaldehyde for 24
h, embedded in paraffin and cut serially at 5 μm for IHC
staining. The IHC staining procedure was performed using a
streptavidin-peroxidase immunohistochemical kit (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) according to the
manufacturer's protocol. Briefly, the tissue sections were
incubated with 0.125% trypsin for 30 min at 37°C for antigen
retrieval and then incubated with primary rabbit monoclonal
antibodies against SDF-1 (1:100; Abcam, Cambridge, UK) and CXCR4
(1:100; Abcam; cat. no. ab124824) overnight at 4°C to stain target
protein expression, and immunoglobulin G (Wuhan Boster Biological
Technology, Ltd) was used as a negative control. Finally, sections
were incubated in 3–3′-diaminoben-zidine (Wuhan Boster Biological
Technology, Ltd) to visualize immunoreactivity. Positively stained
cells in three different areas were counted under a light
microscope (Nikon TS100; Nikon Corporation, Tokyo, Japan).
Cell treatments
Cultured cells were transfected with double-stranded
small interfering RNA (siRNA) targeting CXCR4 (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; cat. no. sc-35422) according
to the manufacturer's protocol using PepMute siRNA Transfection
Reagent (SignaGen Laboratories, Rockville, MD, USA). The cultured
cells were transfected with 50 nM siRNA for 72 h, followed by
treatment with or without 10 ng/ml of SDF-1 (Sigma-Aldrich, St.
Louis, MO, USA) for 24 h at 37°C. In addition, cells were treated
with the NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC; 20
μM; Sigma-Aldrich), as described in a previous study
(12), for 24 h to investigate
whether SDF-1 regulates the apoptosis of degenerative NPCs via the
NF-κB pathway.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the collected tissues
and cells, following homogenization with TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.), using RNAiso PLUS kit (Takara
Biotechnology Co., Ltd., Dalian, China). RT was performed using 1
μg total RNA and random hexamers (Promega Corporation,
Madison, WI, USA) to obtain first-strand cDNA. qPCR was performed
in a 20 μl mixture containing 10 μl SYBR Green
Realtime PCR Master Mix (Takara Biotechnology Co., Ltd.), 1.5–3
μl cDNA and 0.4 μl of each primer (10 μM).
cDNA samples were amplified by qPCR on an ABI Prism 7500 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) under the following
conditions: 95°C for 30 sec; 40 cycles of 95°C for 5 sec, 58°C for
5 sec and 72°C for 30 sec. Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) was used as an internal reference gene and relative
expression levels were calculated by the 2−ΔΔCq method
(13). Primer sequences used were
as follows: SDF-1, forward 5′-CGT GCT GGT CCT CGT GCT GAC-3′,
reverse 5′-GCT TTC TCC AGG TAC TCC TG-3′; CXCR4, forward 5′-GTC CAC
GCC ACC AAC AG-3′, reverse 5′-CTG TTG GTG GCG TGG AC-3′; and GAPDH,
forward 5′-GCA CCG TCA AGG CTG AGAAC-3′ and reverse 5′-TGG TGA AGA
CGC CAG TGGA-3′.
Western blot analysis
Tissues were homogenized and cells were lysed on ice
with radioimmunoprecipitation assay lysis buffer in the presence of
phenylmethane sulfonyl fluoride (Beyotime Institute of
Biotechnology, Haimen, China). Protein concentration was determined
using Enhanced BCA Protein assay kit (Beyotime Institute of
Biotechnology). Proteins (50 μg) were separated by 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein
samples were transferred to polyvinylidene difluoride membranes,
which were blocked with 5% nonfat dry milk in Tris-buffered saline
(TBS) for 1 h at room temperature. The membranes were then probed
with antibodies against SDF-1 (1:1,000, CXCR4 (1:1,000), rabbit
monoclonal phosphorylated P65 (p-P65; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA; cat. no. 3033), rabbit
monoclonal P65 (1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA; cat. no. 8242) and mouse monoclonal β-actin (1:8,000;
Beyo-time Institute of Biotechnology; cat. no. AF0003) overnight at
4°C. The membrane was washed with TBS and incubated with
horseradish peroxidase-conjugated secondary antibody at 37°C for 1
h. Following washing with TBS, the membrane was visualized with
Enhanced Chemiluminescence Plus reagent (Beyotime Institute of
Biotechnology) and protein levels were determined using the
ChemiDoc XRS+ Imaging system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Fluorescence immunocytochemistry
Fluorescence immunocytochemistry was used to detect
nuclear translocation of P65. Briefly, cells seeded in 24-well
plates were fixed with 4% paraformaldehyde, washed three times for
10 min with phosphate-buffered saline, blocked with normal goat
serum for 15 min at room temperature and incubated with P65
antibody (1:100) at 4°C overnight. Subsequently, the samples were
washed and incubated with a goat anti-mouse fluorescein
isothiocyanate-labeled fluorescent secondary antibody (1:500;
Beyotime Institute of Biotechnology; cat. no. A0568) at room
temperature for 2 h. Nuclear counterstaining was performed with
4′,6-diamidino-2-phenylindole (Beyotime Institute of
Biotechnology). The cells were imaged at 550 nm using a
fluorescence microscope.
Annexin V/propidium iodide (PI) apoptosis
detection
Apoptosis was detected using an Annexin V/propidium
iodide (PI) double-staining kit (Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China) according to the manufacturer's protocol. Briefly,
1×104 cells were collected and suspended in binding
buffer, then mixed with 5 μl Annexin V and PI. The cells
were gently vortexed and incubated at room temperature in the dark
for 15 min. Subsequently, 1X termination buffer was added and flow
cytometric analysis on a BD FACScan system (BD Biosciences,
Franklin Lakes, NJ, USA) was performed. The apoptotic rate was
calculated using FlowJo 8.5.2 software (BD Biosciences) as the sum
of the percentage of early (Annexin V+/PI−)
and late apoptotic cells (Annexin
V+/PI+).
Statistical analysis
All the data are expressed as the mean ± standard
deviation and were analyzed using Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA). Each experiment was repeated
three times. The differences between groups were analyzed by
Student's t-test for two groups one-way analysis of variance
followed by Tukey's t-test for comparison of multiple groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Basal SDF-1 and CXCR4 expression
levels
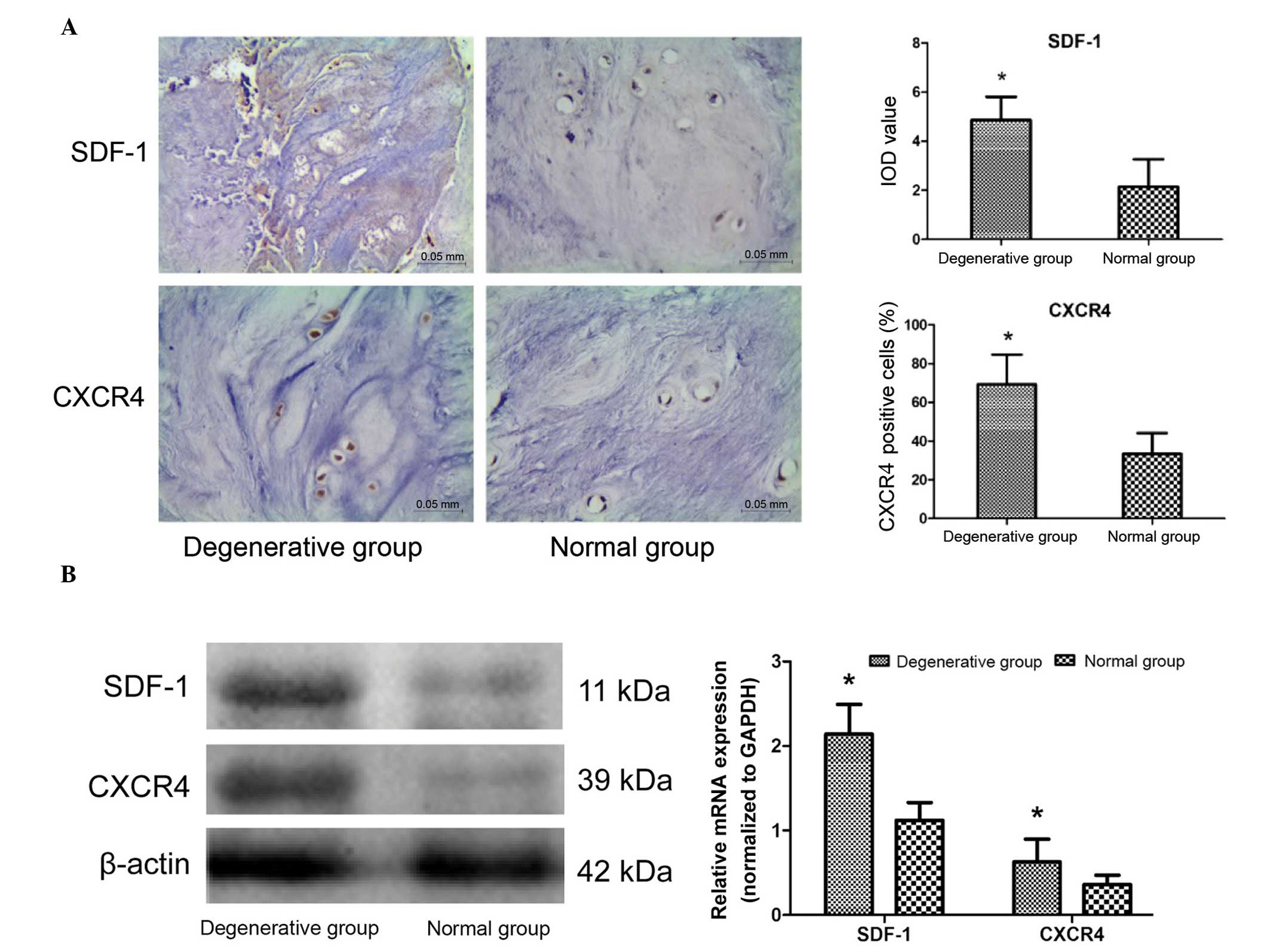
IHC staining demonstrated that SDF-1 and CXCR4 were
expressed in all donor tissues. SDF-1 was predominantly detected in
the extracellular matrix (ECM) and CXCR4 was expressed in the NPCs.
The results demonstrated that the SDF-1 integrated optical density
values and the percentage of CXCR4-positive cells in the
degenerative group was significantly increased compared with the
normal group (P=0.012 and P<0.001, respectively; Fig. 1A). Simultaneously, western blotting
and PCR analysis confirmed that the expression levels of SDF-1 and
CXCR4 were increased in the degenerative group compared with the
normal group (P=0.018 and P<0.001, respectively; Fig. 1B). These results suggest that the
SDF-1/CXCR4 axis is upregulated in degenerative samples, and
therefore may be associated with the IVDD process.
SDF-1/CXCR4 axis regulates NPC
apoptosis
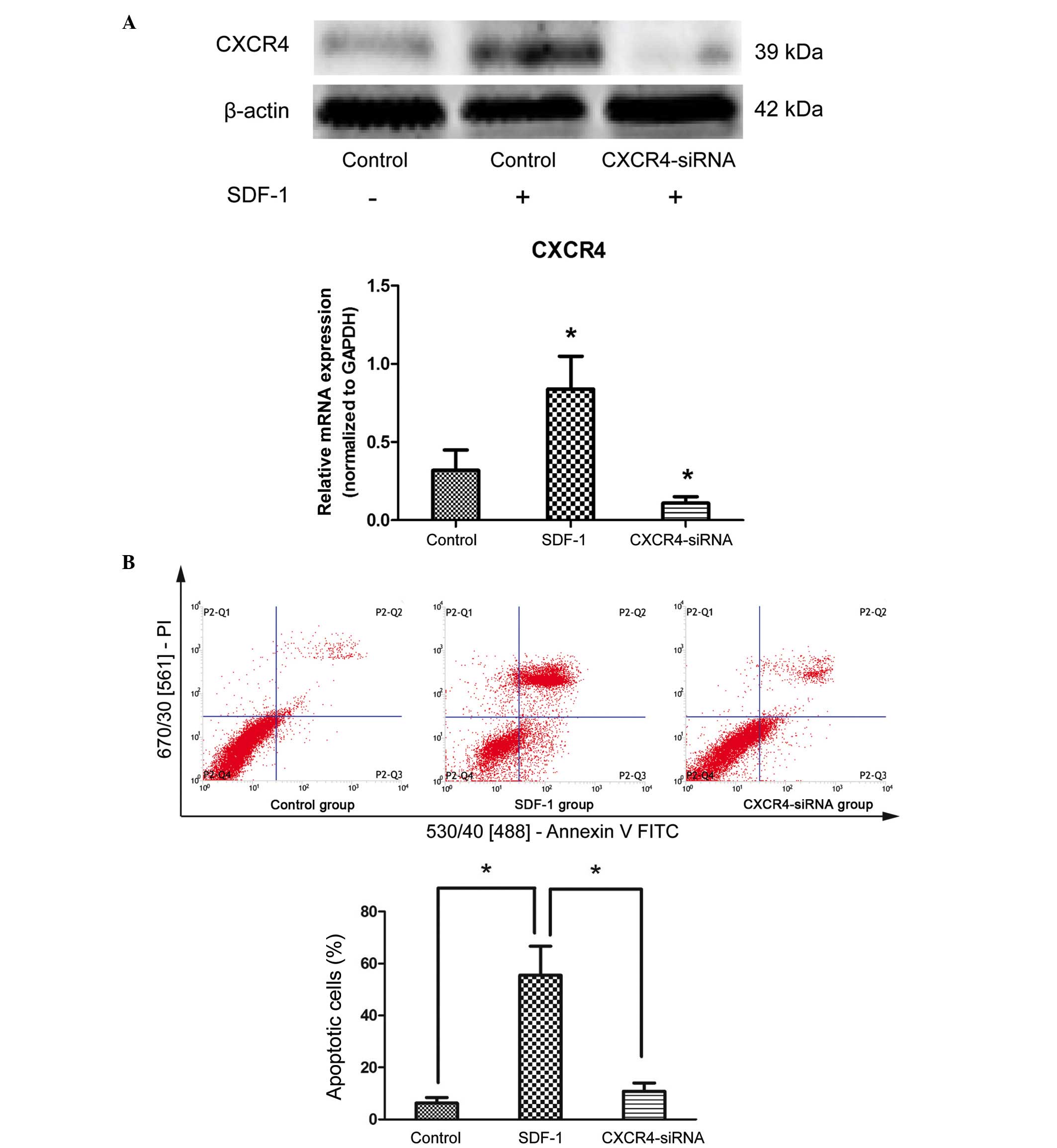
To determine the effects of the SDF-1/CXCR4 axis on
the apoptosis of degenerative NPCs, cells were treated with 10
ng/ml SDF-1 for 24 h. qPCR and western blot analysis demonstrated
that SDF-1 treatment resulted in an increase in CXCR4 expression at
the protein and mRNA levels (P=0.034 and P=0.022, respectively),
compared with untreated cells (Fig.
2A). In addition, the results of Annexin V/PI double-staining
demonstrated that the number of apoptotic NPCs was significantly
increased by SDF-1 treatment compared with untreated cells
(P<0.001; Fig. 2B). To further
confirm that SDF-1 induces cell apoptosis through its receptor,
CXCR4 siRNA was transfected into cells to downregulate the
expression of CXCR4 (P<0.001 compared with the control group;
Fig. 2A). The results demonstrated
that the apoptosis-inducing effects of SDF-1 were reduced following
knockdown of endogenous CXCR4 by siRNA compared with SDF-1
treatment only (P<0.001; Fig.
2B). These results suggest that the SDF-1/CXCR4 axis
participates in the positive regulation of apoptosis in
degenerative NPCs.
Apoptosis-inducing effects of SDF-1/CXCR4
are mediated via the NF-κB pathway
The NF-κB signaling pathway has previously been
demonstrated to regulate cell survival and apoptosis (14). To investigate the association
between NF-κB and SDF-1/CXCR4 signaling, the present study examined
the effect of SDF-1 treatment on the phosphorylation of NF-κB
subunit P65. The levels of p-P65 were markedly increased in NPCs
stimulated with SDF-1 compared with the control cells (P=0.008;
Fig. 3A). Furthermore, treating
NPCs with NF-κB inhibitor, PDTC, or CXCR4 siRNA markedly reduced
the level of p-P65 (Fig. 3A).
Furthermore, the cells were subjected to immunofluorescence to
analyze the nuclear translocation of P65. As demonstrated in
Fig. 3B, PDTC and CXCR4-siRNA
treatment inhibited the nuclear translocation of P65 compared with
SDF-1 treatment. To determine the effect of P65 activation on
SDF-1-induced cell apoptosis, NPCs were treated with 20 μM
PDTC for 24 h, followed by incubation with 10 ng/ml SDF-1 for an
additional 24 h. Annexin V/PI apoptosis detection analysis
demonstrated that, compared with SDF-1 treatment, inhibition of P65
activation by PDTC significantly reduced the SDF-1-induced increase
in NPC apoptosis (P=0.029; Fig.
3C). These results indicate that the apoptosis-inducing effects
of the SDF-1/CXCR4 axis are mediated via NF-κB signaling.
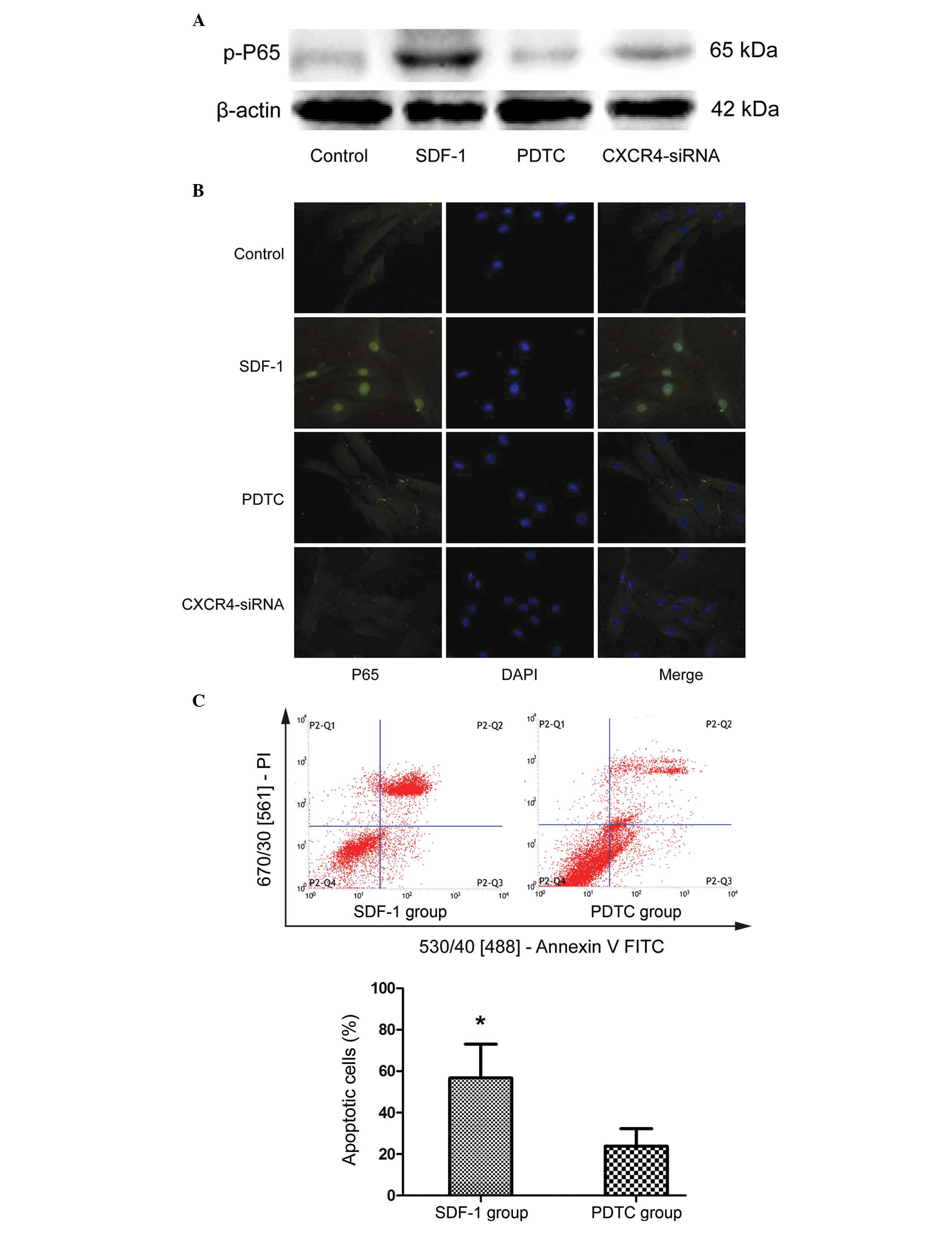 | Figure 3SDF-1/CXCR4 axis induces cell
apoptosis via the NF-κB pathway. (A) NP cells were treated with
SDF-1, NF-κB inhibitor PDTC and CXCR4-siRNA, and the
phosphorylation level of NF-κB subunit P65 was detected by western
blotting. (B) Fluorescence immunocytochemistry was used to detect
nuclear translocation of P65. (C) To observe the effect of NF-κB on
SDF-1-induced cell apoptosis, NP cells were analyzed by flow
cytometry following treatment with PDTC. Data are presented as the
mean ± standard deviation from three independent experiments.
*P<0.05 vs. the PDTC group. NF-κB, nuclear factor-κB;
NP, nucleus pulposus; SDF-1, stromal cell-derived factor-1; PDTC,
pyrrolidine dithiocarbamate; CXCR4, C-X-C motif chemokine receptor
4; siRNA, small interfering RNA; DAPI,
4′,6-diamidino-2-phenylindole; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
Discussion
The present study demonstrated that the expression
levels of SDF-1 and CXCR4 are significantly increased in
degenerative IVD tissues compared with normal NP tissues.
Therefore, it was hypothesized that the SDF-1/CXCR4 signaling axis
may be involved in IVDD. To verify this hypothesis, cultured
degenerative NPCs were treated with SDF-1 following knockdown of
CXCR4 using siRNA. To the best of our knowledge, the present study
is the first to report that the SDF-1/CXCR4 axis accelerates
apoptosis in degenerative NPCs. Furthermore, the number of
apoptotic NPCs was significantly decreased following inhibition of
NF-κB activation using PDTC, which suggests the apoptosis-inducing
effect of the SDF-1/CXCR4 axis in degenerative NPCs is mediated via
NF-κB signaling.
It is well established that NPCs are involved in
resistance against mechanical loading, with the synthesis of ECM
important to maintain spinal stability. Therefore, loss of NPCs has
been demonstrated to be correlated with the pathological process of
IVDD (15). Ahsan et al
(16) analyzed the molecular and
morphological features of 32 herniated disc specimens and four
control disc samples, demonstrating that there was increased
caspase-3 activity and apoptotic-positive stained DNA fragments in
the degenerative disc samples. Furthermore, electron microscopy
findings suggested that there was enhanced programmed cell death in
the degenerative discs (16). In
addition, a previous study indicated that the percentage of
apoptotic cells in degenerative NP specimens was significantly
increased compared with normal controls, as demonstrated by
terminal deoxynucleotidyl transferase dUTP nick end labeling
staining (5). However, the factors
that induce NPC apoptosis during the IVDD process have not been
fully elucidated.
IVDD is characterized by an increase in the
expression levels of proinflammatory cytokines, including tumor
necrosis factor-α and interleukin (IL)-1β, which induce ECM
degradation, chemokine production and changes in cell phenotype
(17). The release of chemokines
promotes the infiltration and activation of immune cells, which
amplifies the inflammatory cascade. SDF-1 is highly expressed in
inflamed tissues, where it attracts activated CXCR4+ T
cells, thus enhancing local inflammatory responses (18). The SDF-1/CXCR4 axis has previously
been associated with the pathogenesis of chronic inflammatory
diseases, including osteoarthritis (19) and rheumatoid arthritis (20). The current study focused on the
differential expression of SDF-1 and CXCR4 in normal and
degenerative IVD tissues. The results demonstrated that the
expression levels of SDF-1 and CXCR4 were increased in degenerative
IVD tissues. The SDF-1/CXCR4 axis has previously been reported to
have various targets, via which it stimulates chondrocyte
proliferation, differentiation and apoptosis (21,22).
Therefore, it was hypothesized that the SDF-1/CXCR4 axis is also
involved in the IVDD process. In support of this, the present study
demonstrated that the number of apoptotic NPCs was significantly
increased following SDF-1 stimulation, and this apoptosis-inducing
effect was inhibited by siRNA-mediated silencing of CXCR4
expression. These results clearly indicated that there is a
positive correlation between SDF-1/CXCR4 expression and cell
apoptosis in IVDD.
NF-κB is a rapidly inducible transcription factor,
which regulates the expression of numerous genes to mediate various
cellular processes, including cell apoptosis, survival and the
immune response (23). It has
previously been demonstrated that the NF-κB signaling pathway is
involved in IL-1β-induced chondrocyte apoptosis (24). Regarding the inflammatory
mechanisms of NF-κB, a previous study reported that the NF-κB
pathway is associated with the release of proinflammatory factors
(25). Furthermore, SDF-1 is
reported to be an important chemokine during the immune response;
however, the molecular interaction between the SDF-1/CXCR4 axis and
NF-κB in degenerative NPCs remains unclear. The current study
demonstrated that treatment with the NF-κB inhibitor, PDTC,
inhibited SDF-1-induced NPC apoptosis in vitro, and
suppressed P65 phosphorylation and nucleus translocation,
indicating that NF-κB-dependent signaling is involved in regulation
of cell apoptosis by SDF-1 in degenerative NPCs.
A limitation of the current study is that in
vitro cultured monolayers of NPCs were used. A previous study
demonstrated that NPCs easily lose their phenotype when cultured
in vitro as a monolayer (26). The use of a 3D alginate culture of
NPCs was considered for the present study; however, the preliminary
testing demonstrated a low siRNA transfection efficiency when using
an alginate scaffold for 3D culture. Therefore, the experiments
were performed using monolayer culture to improve the transfection
efficiency. Furthermore, to elucidate the association between the
SDF-1/CXCR4 axis and NPC apoptosis, further research is required
using in vivo animal models, and the activation state of
upstream and downstream molecules of the NF-κB signaling pathway
should be examined.
In conclusion, the current study demonstrated that
the expression levels of SDF-1 and CXCR4 were increased in
degenerative NP tissues. Knockdown of CXCR4, an SDF-1 receptor,
reduced the number of apoptotic degenerative NPCs. Notably,
activation of NF-κB subunit P65 was associated with the
apoptosis-inducing effect of SDF-1. Therefore, the SDF-1/CXCR4 axis
is considered to induce apoptosis of human degenerative NPCs via
the NF-κB signaling pathway. The results of the present study
suggest that the SDF-1/CXCR4 axis may be a therapeutic target for
the treatment of degenerative disc disease.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81171751 and
81372003).
References
|
1
|
Murray CJ, Vos T, Lozano R, Naghavi M,
Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S,
et al: Disability-adjusted life years (DALYs) for 291 diseases and
injuries in 21 regions, 1990–2010: A systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2197–2223. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parthan A, Evans CJ and Le K: Chronic low
back pain: Epidemiology, economic burden and patient-reported
outcomes in the USA. Expert Rev Pharmacoecon Outcomes Res.
6:359–369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Schepper EI, Damen J, van Meurs JB,
Ginai AZ, Popham M, Hofman A, Koes BW and Bierma-Zeinstra SM: The
association between lumbar disc degeneration and low back pain: The
influence of age, gender, and individual radiographic features.
Spine (Phila Pa 1976). 35:531–536. 2010. View Article : Google Scholar
|
|
4
|
Ding F, Shao ZW and Xiong LM: Cell death
in intervertebral disc degeneration. Apoptosis. 18:777–785. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang D, Hu Z, Hao J, He B, Gan Q, Zhong X,
Zhang X, Shen J, Fang J and Jiang W: SIRT1 inhibits apoptosis of
degenerative human disc nucleus pulposus cells through activation
of Akt pathway. Age (Dordr). 35:1741–1753. 2013. View Article : Google Scholar
|
|
6
|
Pulsatelli L, Dolzani P, Piacentini A,
Silvestri T, Ruggeri R, Gualtieri G, Meliconi R and Facchini A:
Chemokine production by human chondrocytes. J Rheumatol.
26:1992–2001. 1999.PubMed/NCBI
|
|
7
|
Kanbe K, Takagishi K and Chen Q:
Stimulation of matrix metal-loprotease 3 release from human
chondrocytes by the interaction of stromal cell-derived factor 1
and CXC chemokine receptor 4. Arthritis Rheum. 46:130–137. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang XH, Hong X, Zhu L, Wang YT, Bao JP,
Liu L, Wang F and Wu XT: Tumor necrosis factor alpha promotes the
proliferation of human nucleus pulposus cells via nuclear
factor-κB, c-Jun N-terminal kinase, and p38 mitogen-activated
protein kinase. Exp Biol Med (Maywood). 240:411–417. 2015.
View Article : Google Scholar
|
|
9
|
Zhongyi S, Sai Z, Chao L and Jiwei T:
Effects of nuclear factor kappa B signaling pathway in human
intervertebral disc degeneration. Spine (Phila Pa 1976).
40:224–232. 2015. View Article : Google Scholar
|
|
10
|
Pfirrmann CW, Metzdorf A, Zanetti M,
Hodler J and Boos N: Magnetic resonance classification of lumbar
intervertebral disc degeneration. Spine (Phila Pa 1976).
26:1873–1878. 2001. View Article : Google Scholar
|
|
11
|
Zhang Z, Kakutani K, Maeno K, Takada T,
Yurube T, Doita M, Kurosaka M and Nishida K: Expression of silent
mating type information regulator 2 homolog 1 and its role in human
interver-tebral disc cell homeostasis. Arthritis Res Ther.
13:R2002011. View
Article : Google Scholar
|
|
12
|
Zhou H, Sheng L, Wang H, Xie H, Mu Y, Wang
T and Yan J: Anti-β2GPI/β2GPI stimulates activation of THP-1 cells
through TLR4/MD-2/MyD88 and NF-κB signaling pathways. Thromb Res.
132:742–749. 2013. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Shen J, Fang J, Hao J, Zhong X, Wang D,
Ren H and Hu Z: SIRT1 inhibits the catabolic effect of IL-1β
through TLR2/SIRT1/NF-κB pathway in human degenerative nucleus
pulposus cells. Pain Physician. 19:E215–E226. 2016.PubMed/NCBI
|
|
15
|
Wang SZ, Rui YF, Lu J and Wang C: Cell and
molecular biology of intervertebral disc degeneration: Current
understanding and implications for potential therapeutic
strategies. Cell Prolif. 47:381–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahsan R, Tajima N, Chosa E, Sugamata M,
Sumida M and Hamada M: Biochemical and morphological changes in
herniated human intervertebral disc. J Orthop Sci. 6:510–518. 2001.
View Article : Google Scholar
|
|
17
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014. View Article : Google Scholar
|
|
18
|
Palomino DC and Marti LC: Chemokines and
immunity. Einstein (Sao Paulo). 13:469–473. 2015. View Article : Google Scholar
|
|
19
|
Wei F, Moore DC, Wei L, Li Y, Zhang G, Wei
X, Lee JK and Chen Q: Attenuation of osteoarthritis via blockade of
the SDF-1/CXCR4 signaling pathway. Arthritis Res Ther. 14:R1772012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HR, Kim KW, Kim BM, Jung HG, Cho ML
and Lee SH: Reciprocal activation of CD4+ T cells and synovial
fibroblasts by stromal cell-derived factor 1 promotes RANKL
expression and osteoclastogenesis in rheumatoid arthritis.
Arthritis Rheumatol. 66:538–548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sukegawa A, Iwasaki N, Kasahara Y, Onodera
T, Igarashi T and Minami A: Repair of rabbit osteochondral defects
by an acellular technique with an ultrapurified alginate gel
containing stromal cell-derived factor-1. Tissue Eng Part A.
18:934–945. 2012. View Article : Google Scholar
|
|
22
|
Wei L, Sun X, Kanbe K, Wang Z, Sun C,
Terek R and Chen Q: Chondrocyte death induced by pathological
concentration of chemokine stromal cell-derived factor-1. J
Rheumatol. 33:1818–1826. 2006.PubMed/NCBI
|
|
23
|
Lee H, Jeon J, Ryu YS, Jeong JE, Shin S,
Zhang T, Kang SW, Hong JH and Hur GM: Disruption of microtubules
sensitizes the DNA damage-induced apoptosis through inhibiting
nuclear factor κB (NF-κB) DNA-binding activity. J Korean Med Sci.
25:1574–1581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Xu X, Xu T and Qin S:
β-Ecdysterone suppresses interleukin-1β-induced apoptosis and
inflammation in rat chon-drocytes via inhibition of NF-κB signaling
pathway. Drug Dev Res. 75:195–201. 2014.PubMed/NCBI
|
|
25
|
Roman-Blas JA and Jimenez SA: NF-kappaB as
a potential therapeutic target in osteoarthritis and rheumatoid
arthritis. Osteoarthritis Cartilage. 14:839–848. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kluba T, Niemeyer T, Gaissmaier C and
Gründer T: Human anulus fibrosis and nucleus pulposus cells of the
intervertebral disc: Effect of degeneration and culture system on
cell phenotype. Spine (Phila Pa 1976). 30:2743–2748. 2005.
View Article : Google Scholar
|