Introduction
Osteoporosis is a serious public health issue that
is characterized by a reduction of bone mass, which is caused by an
imbalance between bone resorption and bone formation (1). Osteoporosis affects numerous
individuals throughout the world (2,3);
however, currently there are no effective and economical
therapeutic strategies to cure osteoporosis (4). Therefore, establishing a novel
therapeutic agent for the prevention and treatment of osteoporosis
is considered to be critical. Traditional Chinese medicine has
become the focus of basic research and clinical studies due to
reduced side-effects when compared with cytokine and hormone
therapy (5).
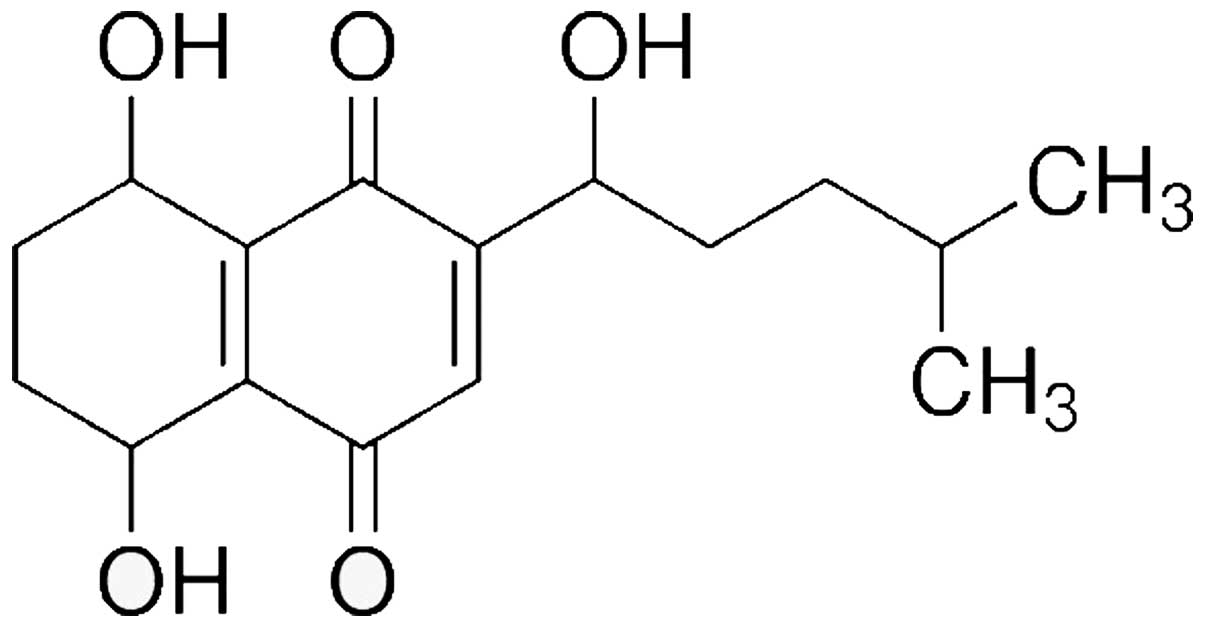
Shikonin (5,8-dihydroxy-2-[(1S)-1-hydroxy-4-methyl
pent-3-en-1-yl] naphthalene-1,4-dione), is a predominant type of
naphthoquinone pigment that is extracted from the Chinese plant,
Lithospermum erythrorhizon (6), with a molecular weight of 288
(Fig. 1). Shikonin performs many
biological activities; it is an antioxidant, anti-inflammatory,
antithrombotic, antiviral, and antimicrobial, it has anticancer
properties and is associated with accelerated wound healing
(7,8). Previous studies have demonstrated
that shikonin inhibits cell growth, mediates cell apoptosis and
alters the cell cycle in various types of tumor cell (9,10).
However, whether shikonin exerts an effect on bone formation
remains unknown. Therefore, the aim of the present study was to
investigate the possible influence and associated mechanisms of
shikonin on MC3T3-E1 cell proliferation and differentiation.
Many studies have demonstrated that bone
morphogenetic proteins (BMPs) and transforming growth factor-β
(TGF-β) are the most important cytokines affecting the
proliferation, differentiation and function of osteoblasts
(11,12). BMP-2, a member of the TGF-β
superfamily, is a key signaling component in osteoblast
proliferation and differentiation (13,14).
SMAD family member 5 (Smad5) is a downstream transcription factor
that is phosphorylated and activated by the receptors of BMP-2.
Phosphorylated Smad5 forms a complex with Smad4 (co-Smad), and
translocates into the nucleus to activate the transcription factor,
runt related transcription factor 2 (Runx2) (15,16).
The BMP-2/Smad5 signal transduction pathway is important in
osteoblast proliferation and differentiation. In the present study,
the function of shikonin on biological behaviors of MC3T3-E1 cells,
such as cell growth, cell division and ALP activity were assessed.
In addition, the potent mechanism of shikonin-enhanced bone
formation was investigated by examining the expression levels of
BMP-2, Smad5, Runx2, alkaline phosphatase (ALP) and osteocalcin
(OC) in the MC3T3-E1 cell line.
Materials and methods
Materials and reagents
Purified shikonin (>98%) was purchased from the
National Institute for the Control Pharmaceutical and Biological
Products (Beijing, China). Shikonin was dissolved in dimethyl
sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) and stored at
−20°C. The final concentrations of shikonin were 0 (control), 10,
50 and 100 ng/ml, and the final concentration of DMSO in the
culture was <0.01%. α-Minimum Essential Medium (α-MEM), fetal
bovine serum (FBS) and trypsin-EDTA were obtained from GE
Healthcare Life Sciences (Hyclone; Logan, UT, USA).
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was purchased from Sigma-Aldrich. Rabbit anti-Smad5 (cat. no.
ab13724), mouse anti-Runx2 (cat. no. ab76956) and mouse anti-GAPDH
(cat. no. ab8245) monoclonal antibodies of were purchased from
Abcam (Cambridge, MA, USA). Invitrogen TRIzol reagent was obtained
from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Primers
were designed and synthesized by Sangon Biotech Co., Ltd.
(Shanghai, China).
Cell culture
The MC3T3-E1 cells were purchased from the Cell
Center of the Chinese Academy of Medical Sciences (Shanghai, China)
and cultured in α-MEM containing 10% FBS and 100 U/ml penicillin
and 100 µg/ml streptomycin in 5% CO2 at 37°C. The
medium was replaced every 3 days, and the cells were subcultured
using 0.25% trypsin with 0.01% EDTA.
Cell proliferation assay
The effect of shikonin on cell proliferation was
evaluated using the MTT assay. The cells were seeded in 96-well
plates at a density of 1.0×103 cells/well. Following
incubation for 24 h at 37°C, the cells were treated with various
final concentrations (0, 10, 50 and 100 ng/ml) of shikonin. Cells
were treated with 20 µl MTT (5 mg/ml) during the final 4 h
of the culture and the optical density of the wells was measured at
490 nm using a microplate reader.
Cell cycle assay
MC3T3-E1 cells (1×105 cells/ml) were
plated in four tissue culture flasks. After 24 h, cells were
treated with various concentrations of shikonin (0, 10, 50 and 100
ng/ml) for 48 h. Then, cells were harvested, fixed in 70% ethanol
for 12 h, washed with phosphate-buffered saline (PBS) and stained
in 5 mg/ml propidium iodide in PBS supplemented with RNase A (Roche
Diagnostics, Indianapolis, IN, USA) for 30 min at room temperature.
Data were analyzed using CellQuest v3.3 (BD Biosciences, San Jose,
CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
After 48 h of shikonin treatment, total RNA was
extracted with TRIzol reagent. Then total RNA was used to
synthesize cDNA using SuperScript II reverse transcriptase
(Invitrogen; Thermo Fisher Scientific, Inc.) with 5 µg oligo
(dT) primers per sample. qPCR was performed using SYBR Green PCR
master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) in
a total volume of 20 µl using a 7900HT Fast Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) as
follows: 95°C for 30 sec, and 40 cycles of 95°C for 5 sec and 60°C
for 30 sec. A dissociation step was performed to generate a melting
curve to confirm the specificity of the amplification and GAPDH
served as the reference gene. The relative levels of gene
expression were represented as ΔCq=Cqgene − Cqreference,
and the fold change of gene expression was calculated according to
the 2−ΔΔCq method (17). Experiments were repeated in
triplicate. The primer sequences were as follows: Forward,
5′-GCTGGTCACAGATAAGGCCA-3′ and reverse, 5′-TTTCTCGTTTGTGGAGCGGA-3′
for BMP-2; forward, 5′-GTGAAGCGATTGTTGGGCTG-3′ and reverse
5′-CAGGTGGCATATAGGCAGGG-3′ for Smad5; forward,
5′-GCGCATTCCTCATCCCAGTA-3′ and reverse, 5′-AGTTCTGAAGCACCTGCCTG-3′
for Runx2; forward, 5′-TGACCTTCTCTCCTCCATCC-3′ and reverse,
5′-CTTCCTGGGAGTCTCATC CT-3′ for ALP; forward,
5′-TGCTTGTGACGAGCTATCAG-3′ and reverse, 5′-GAGGACAGGGAGGATCAAGT-3′
for OC; and forward, 5′-GTGAAGCAGGCATCTGAGGG-3′ and reverse,
5′-GCCGTATTCATTGTCATACCAGG-3′ for GAPDH.
Western blot analysis
Total proteins from cell lines were harvested in
lysis buffer (Thermo Fisher Scientific, Inc.) and quantified
according to the Bradford method. Fifty micrograms of protein were
separated by SDS-PAGE (12%) at a constant voltage (110V) for 2 h,
and transferred onto a polyvi-nylidene difluoride membrane. The
membranes were blocked in 5% nonfat dry milk diluted with
Tris-buffered saline Tween-20 [TBST; 20 mmol/l Tris-HCl, 150 mmol/l
NaCl (PH 7.5) and 0.1% Tween 20] at room temperature for 1 h.
Samples were incubated overnight at 4°C with monoclonal antibodies
against Smad5 (1:1,000), Runx2 (1:1,000) and GAPDH (1:1,000)
followed by incubation for 2 h with a goat-anti rabbit
peroxidase-conjugated IgG (cat. no. ab6721; Abcam; 1:1,000) and
anti-mouse horseradish peroxidase-conjugated IgG (cat. no.
ab131368; Abcam; 1:1,000). The bound proteins were visualized using
enhanced chemiluminescence (Thermo Fisher Scientific, Inc.) and
detected using a BioImaging System (UVP Inc., Upland, CA, USA). The
relative protein levels were calculated based on GAPDH as the
loading control.
ALP staining
To observe the influence of shikonin on osteoblast
differentiation, staining of ALP (an early maker of osteoblast
differentiation) was performed. Cells (2×105 cells/well)
were plated and cultured in 6-well plates for 24 h at 37°C, and
treated with 0 (control), 10, 50, 100 ng/ml shikonin. The medium
was replaced every 3 days. A week later, cells were washed three
times with PBS and fixed in 10% paraformaldehyde for 10 min at
25°C. The cells were stained using 300 µg/ml
5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium buffer
(Thermo Fisher Scientific, Inc.) for 20 min at 25°C. ALP-positive
cells were stained blue/purple. The stained cells were visualized
using a digital microscope (DP73; Olympus, Tokyo, Japan).
Statistical analysis
All statistical analysis were performed using
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Data were presented as the mean ± standard error of the mean, and
statistically analyzed using a two-tailed Student's t test
and one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Shikonin stimulates cell
proliferation
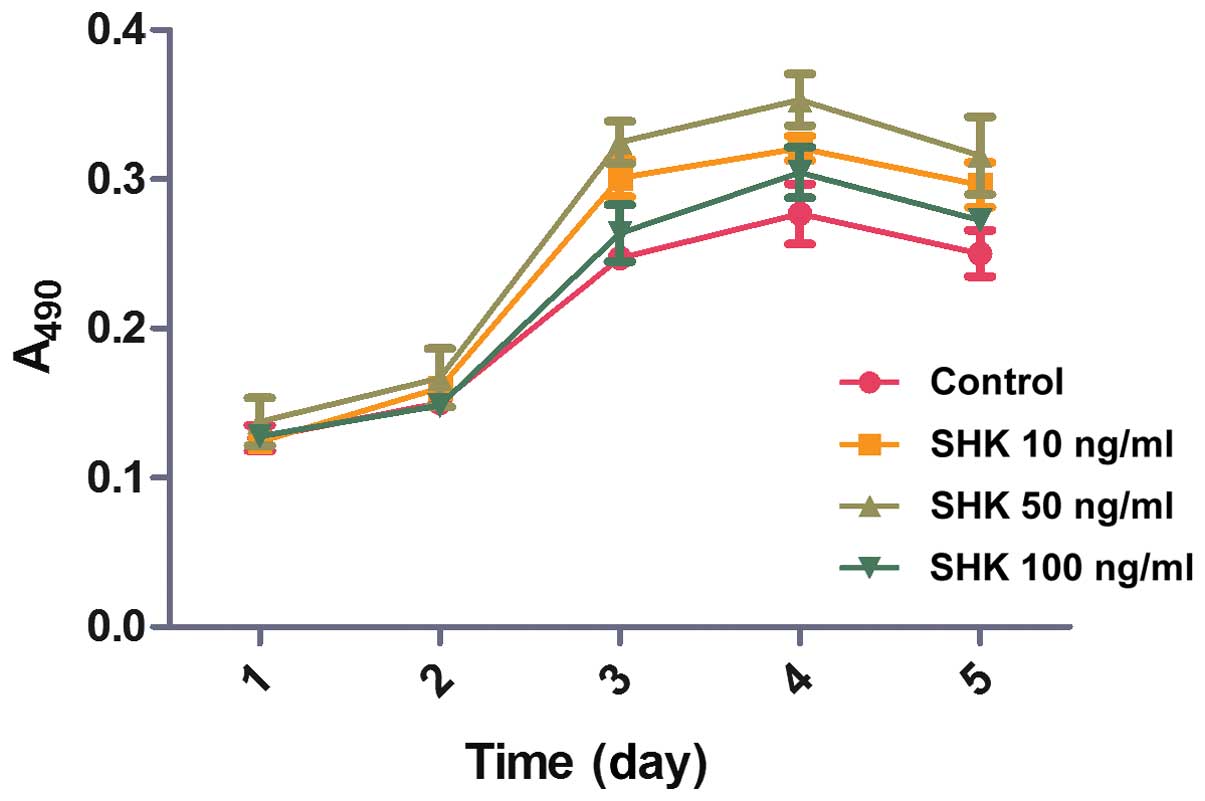
The effects of different concentrations of shikonin
on the proliferation of MC3T3-E1, following 24, 48, 72, 96 and 120
h treatments, were examined by MTT (Fig. 2) to examine whether shikonin
stimulates MC3T3-E1 cell proliferation in vitro. During the
initial 2 days, no statistically significant differences in
MC3T3-E1 cell viability were observed between the groups. However,
compared with the control and the 100 ng/ml shikonin group, a
marginally greater quantity of cells were observed in the 10 and 50
ng/ml shikonin groups on day 3 and 4. On day 4, the speed of cell
proliferation peaked in the 50 ng/ml shikonin group and declined
thereafter. These results demonstrated that shikonin treatment
promotes MC3T3-E1 cell proliferation.
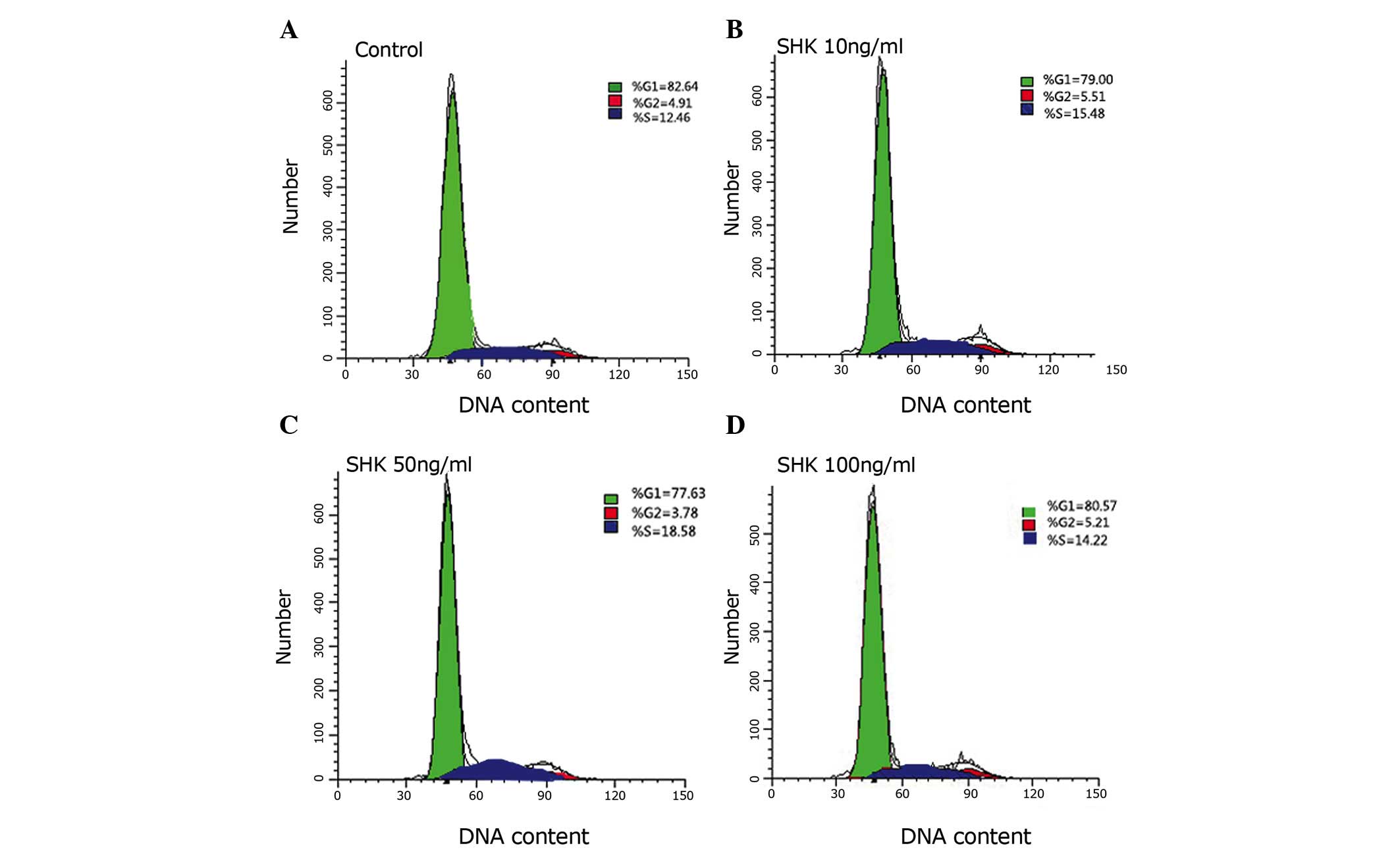
Shikonin stimulates cell division
Subsequently, cell cycle analysis was performed to
assess the effect of shikonin on MC3T3-E1 cell cycle progression.
As shown in Fig. 3, MC3T3-E1 cells
treated with 10, 50 and 100 ng/ml shikonin exhibited increased
percentages of S-phase cells, particularly in the 50 ng/ml shikonin
groups. These data indicate that certain concentrations of shikonin
accelerate cell cycle progression.
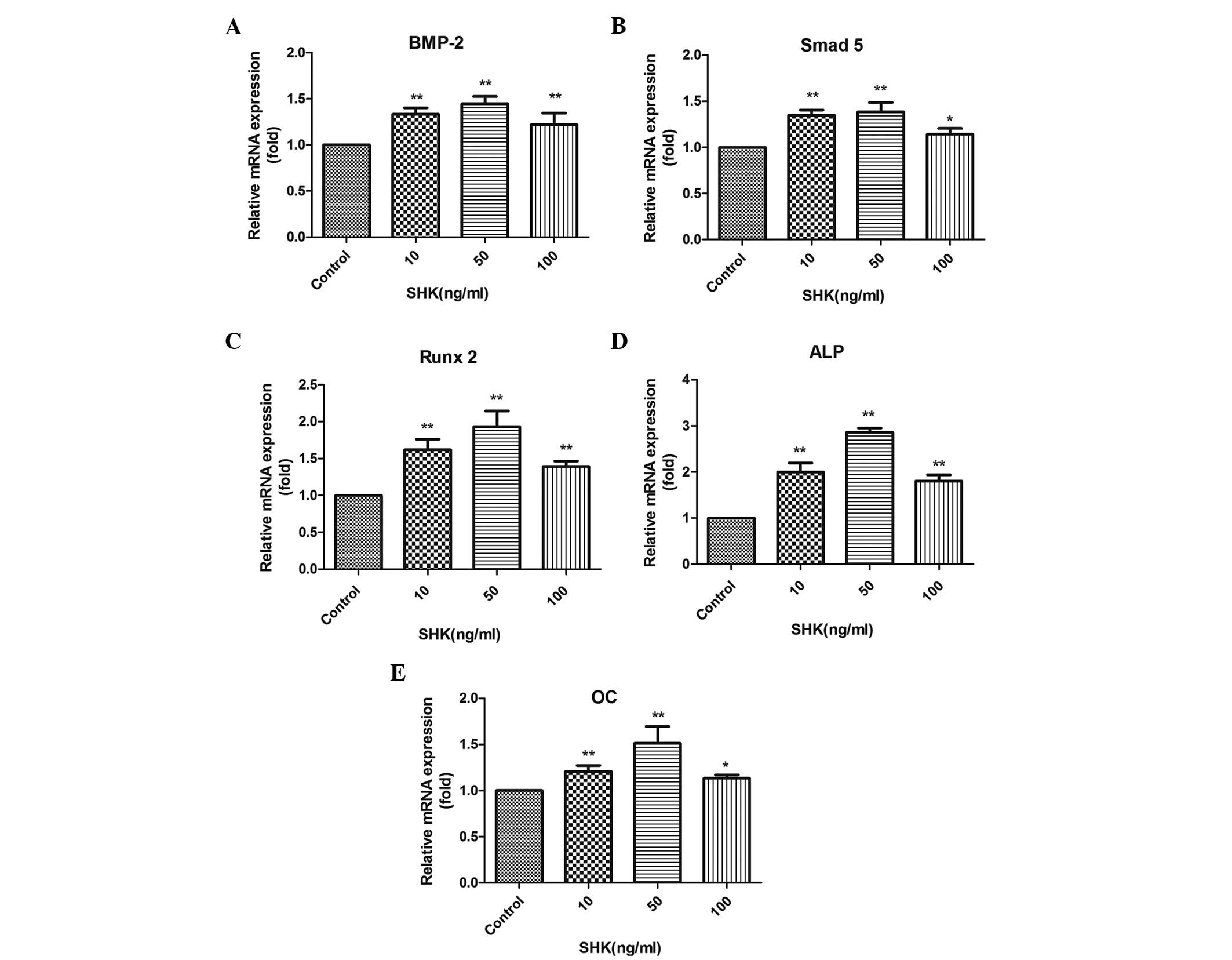
Effects of shikonin on BMP-2, SMAD5,
Runx2, ALP and OC mRNA expression levels
Total mRNA was extracted after MC3T3-E1 cells were
treated with 0, 10, 50 or 100 ng/ml shikonin for 48 h, and the mRNA
expression levels of BMP-2, Smad5, Runx2, ALP and OC were detected
by RT-qPCR. The BMP-2, Smad5, Runx2, ALP and OC expression level in
the cells treated with 10, 50 and 100 ng/ml shikonin increased
significantly compared with the untreated control cells (P<0.01,
P<0.05, P<0.01, P<0.01 and P<0.05, respectively)
(Fig. 4). In addition, the
expression levels of BMP-2, Smad5, Runx2, ALP and OC were increased
to the highest levels in the 50 ng/ml shikonin group. This
demonstrated that shikonin promotes osteoblast differentiation via
its effect on BMP-2, SMAD5, Runx2, ALP and OC expression
levels.
Effects of shikonin on Smad5 and Runx2
protein expression levels
To further investigate the mechanism by which
shikonin stimulates osteoblast differentiation, western blotting
was performed to examine the shikonin-induced changes in Smad5 and
Runx2 protein expression (Fig. 5).
Different concentrations of shikonin (10, 50 and 100 ng/ml)
markedly increased Smad5 and Runx2 protein expression levels in the
MC3T3-E1 cells compared with the control cells, particularly in the
50 ng/ml group. These findings revealed that shikonin regulates the
expression levels of Smad5 and Runx2 proteins, which influences
osteoblastic differentiation.
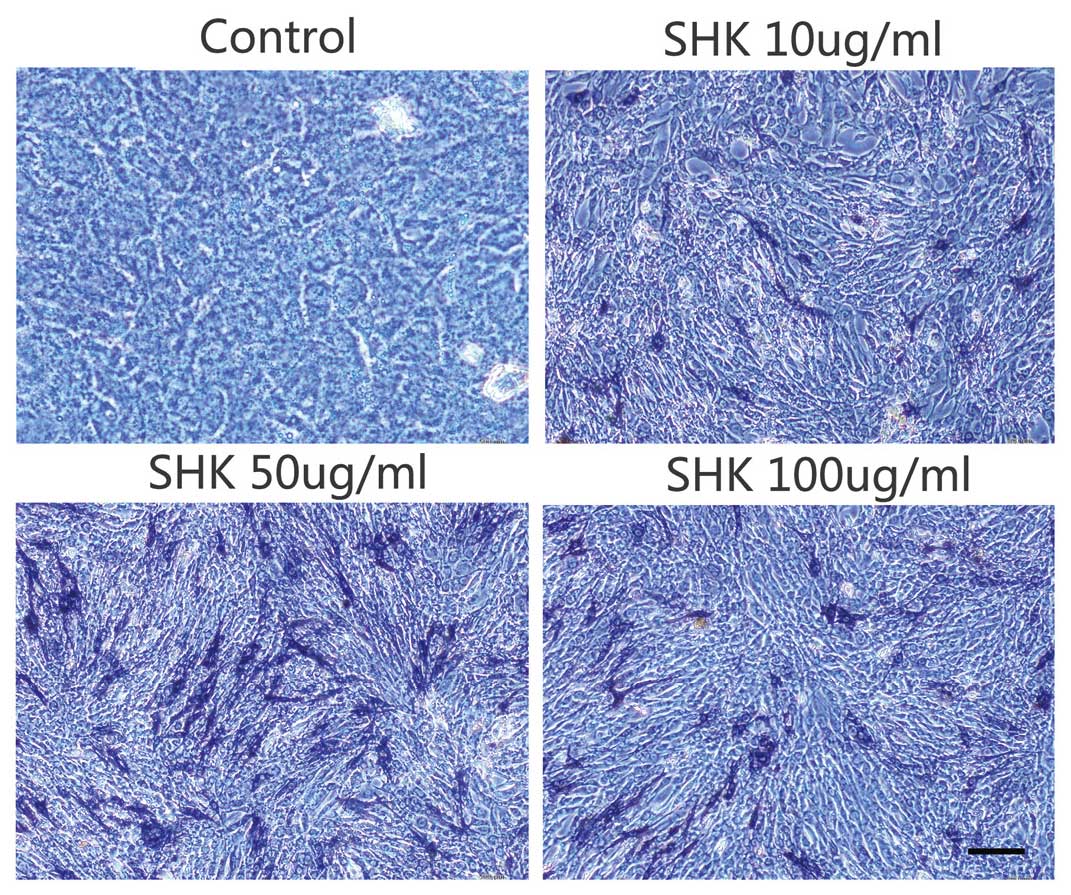
Effects of shikonin on ALP activity
The ALP activity in the MC3T3-E1 cells was examined
by ALP histochemical staining 7 days after treatments with 0, 10,
50 or 100 ng/ml shikonin. The results demonstrate that treatment
with different concentrations of shikonin elicits significantly
greater ALP activity when compared with the control group (Fig. 6), particularly in the 50 ng/ml
group. These results indicate that shikonin enhanced the activity
of ALP in MC3T3-E1 cells.
Discussion
In the present study, the osteoprotective effects of
shikonin and its potential mechanism in MCET3-E1 cells were
examined. The results clearly demonstrated that treatment with 10,
50 and 100 ng/ml of shikonin, particularly 50 ug/ml shikonin,
enhances cell viability, stimulates cell cycle progression,
resulting in a greater number of cells in the S-phase, and promotes
ALP activity in MC3T3-E1 cells. Additionally, shikonin upregulated
the expression levels of BMP-2, Smad5, Runx2, ALP and OC,
indicating that the BMP-2/Smad5 signal transduction pathway may be
involved in shikonin-induced cell proliferation and
differentiation.
Osteoporosis, a progressive disorder of aging bones,
is widely recognized as a major public health issue (18). Bone is a dynamic tissue, which is
mediated by the balance between osteoblastic bone formation and
osteoclastic bone resorption (19). Osteoblasts, osteoclasts, and
osteocytes are important in bone generation, maintenance and
remodeling (20). Multiple
factors, which cause the imbalance of osteoblasts and osteoclasts
at the bone remodeling process, result in the loss of bone mass
(21). Hence, therapeutic agents
that increase the activity of osteoblasts may be administered to
treat osteoporosis.
Due to fewer associated side-effects, Chinese herbs
require investigation to identify more effective therapeutic agents
that promote osteoblast proliferation and differentiation. Shikonin
has attracted increasing attention, as it exhibits numerous
biological activities, such as anti-inflammatory, antiviral and
anti-cancer actions (22–24). Hence, the effects of shikonin on
MC3T3-E1 cells were evaluated in the current study.
The results of the study confirm that shikonin
promotes the proliferation of MC3T3-E1 cells in a dose- and
time-dependent manner. The rate of cell proliferation peaked in
response to 50 ng/ml shikonin on day 4 and decreased thereafter.
Furthermore, the percentage of S-phase cells in the 50 ng/ml
shikonin group was the greatest, which suggests active DNA
synthesis and cell proliferation. Therefore, shikonin may lead to
osteogenesis by stimulating osteoblast proliferation.
Numerous studies have demonstrated that TGF-β and
BMPs are the most important cytokines affecting the proliferation,
differentiation and function of osteoblasts (11,12).
BMP-2 is a member of the TGF-β superfamily. Various studies have
demonstrated that BMP-2 is a key signaling component in the
regulation of bone induction, repair and maintenance (25–27).
Smad5 is the intracellular mediator of BMP-2 and may be
phosphorylated by heterotetrameric serine/threonine kinase
receptors of BMP-2 (28). After
forming a complex with Smad4, phosphorylated Smad5 entered into the
nucleus, activating the transcription factors of Runx2 (15,16).
Our results indicated that the levels BMP-2, Smad5, Runx2, ALP and
OC expression increased in shikonin-treated MC3T3-E1 cells,
particularly in the 50 ng/ml group.
ALP is an early maker of osteoblast differentiation,
thus, the effects of shikonin on ALP activity were detect by ALP
staining. The results demonstrated that treatment with shikonin
enhanced ALP activity, particularly in the 50 ng/ml group,
suggesting that shikonin promotes osteoblast differentiation.
Shikonin, an active ingredient isolated from the
Chinese plant, Lithospermum erythrorhizon, is widely
administered as a traditional Chinese medicine to treat certain
diseases, such as wet typhus, purpura, eczema and erysipelas. The
present study demonstrates that shikonin stimulates MC3T3-E1 cell
proliferation and differentiation via the BMP-2/Smad5 signaling
pathway.
In conclusion, in addition to the anti-inflammatory,
antiviral and anti-cancer effects of shikonin, the present study is
the first, to the best of our knowledge, to demonstrate that
shikonin stimulates osteoblast proliferation and differentiation.
Therefore, shikonin may present as a novel and potent candidate for
the management of osteoporosis. However, further investigations are
required to reveal the mechanism by which shikonin acts to promote
bone formation.
Acknowledgments
This study was supported by grants from the National
Nature Science Foundation of China (grant nos. 81370981 and
31201053) and the Outstanding Scientific Fund of Shengjing
Hospital.
References
|
1
|
Bone H: Future directions in osteoporosis
therapeutics. Endocrinol Metab Clin North Am. 41:655–661. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma L, Kapoor D and Issa S:
Epidemiology of osteoarthritis: An update. Curr Opin Rheumatol.
18:147–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lane NE: Epidemiology, etiology, and
diagnosis of osteoporosis. Am J Obstet Gynecol. 194(2 Suppl):
S3–S11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobayashi Y, Uehara S, Koide M and
Takahashi N: The regulation of osteoclast differentiation by Wnt
signals. Bonekey Rep. 4:7132015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou H, Wang S, Xue Y and Shi N:
Regulation of the levels of Smad1 and Smad5 in MC3T3-E1 cells by
Icariine in vitro. Mol Med Rep. 9:590–594. 2014.
|
|
6
|
Andújar I, Rios JL, Giner RM and Recio MC:
Pharmacological properties of shikonin-a review of literature since
2002. Planta Med. 79:1685–1697. 2013. View Article : Google Scholar
|
|
7
|
Chen X, Yang L, Oppenheim JJ and Howard
MZ: Cellular pharmacology studies of shikonin derivatives.
Phytother Res. 16:199–209. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Zhou Y, Jia G, Han B, Liu J, Teng
Y, Lv J, Song Z, Li Y, Ji L, et al: Shikonin suppresses tumor
growth and synergizes with gemcitabine in a pancreatic cancer
xenograft model: Involvement of NF-kB signaling pathway. Biochem
Pharmacol. 88:322–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han W, Li L, Qiu S, Lu Q, Pan Q, Gu Y, Luo
J and Hu X: Shikonin circumvents cancer drug resistance by
induction of a necroptotic death. Mol Cancer Ther. 6:1641–1649.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang IC, Huang YJ, Chiang TI, Yeh CW and
Hsu LS: Shikonin induces apoptosis through reactive oxygen
species/extracellular signal-regulated kinase pathway in
osteosarcoma cells. Biol Pharm Bull. 33:816–824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knoll BI, McCarthy TL, Centrella M and
Shin J: Strain-dependent control of transforming growth factor-beta
function in osteoblasts in an in vitro model: Biochemical events
associated with distraction osteogenesis. Plast Reconstr Surg.
116:224–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fagenholz PJ, Warren SM, Greenwald JA,
Bouletreau PJ, Spector JA, Crisera FE and Longaker MT: Osteoblast
gene expression is differentially regulated by TGF-beta isoforms. J
Craniofac Surg. 12:183–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao H, Ke Y, Zhang Y, Zhang CJ, Qian W and
Zhang GL: Icariin stimulates MC3T3-E1 cell proliferation and
differentiation through up-regulation of bone morphogenetic
protein-2. Int J Mol Med. 29:435–439. 2012.
|
|
14
|
Liang W, Lin M, Li X, Li C, Gao B, Gan H,
Yang Z, Lin X, Liao L and Yang M: Icariin promotes bone formation
via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19
human osteoblastic cell line. Int J Mol Med. 30:889–895.
2012.PubMed/NCBI
|
|
15
|
Cheng H, Jiang W, Phillips FM, Haydon RC,
Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, et al:
Osteogenic activity of the fourteen types of human bone
morphogenetic proteins (BMPs). J Bone Joint Surg Am.
85-A:1544–1552. 2003.PubMed/NCBI
|
|
16
|
Ghosh-Choudhury N, Singha PK, Woodruff K,
St Clair P, Bsoul S, Werner SL and Choudhury GG: Concerted action
of Smad and CREB-binding protein regulates bone morphogenetic
protein-2-stimulated osteoblastic colony-stimulating factor-1
expression. J Biol Chem. 281:20160–20170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
18
|
Hiligsmann M, Vanoverberghe M, Neuprez A,
Bruyère O and Reginster JY: Cost-effectiveness of strontium
ranelate for the prevention and treatment of osteoporosis. Expert
Rev Pharmacoecon Outcomes Res. 10:359–366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Compston J: Osteoporosis: Social and
economic impact. Radiol Clin North Am. 48:477–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swarnkar G, Sharan K, Siddiqui JA,
Chakravarti B, Rawat P, Kumar M, Arya KR, Maurya R and
Chattopadhyay N: A novel flavonoid isolated from the steam-bark of
Ulmus Wallichiana Planchon stimulates osteoblast function and
inhibits osteoclast and adipocyte differentiation. Eur J Pharmacol.
658:65–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tabuchi M, Miyazawa K, Kimura M, Maeda H,
Kawai T, Kameyama Y and Goto S: Enhancement of crude bone
morphogenetic protein-induced new bone formation and normalization
of endochondral ossification by bisphosphonate treatment in
osteoprotegerin-deficient mice. Calcif Tissue Int. 77:239–249.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen X, Yang L, Zhang N, Turpin JA,
Buckheit RW, Osterling C, Oppenheim JJ and Howard OM: Shikonin, a
component of Chinese herbal medicine, inhibits chemokine receptor
function and suppresses human immunodeficiency virus type 1.
Antimicrob Agents Chemother. 47:2810–2816. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeh CC, Kuo HM, Li TM, Lin JP, Yu FS, Lu
HF, Chung JG and Yang JS: Shikonin-induced apoptosis involves
caspase-3 activity in a human bladder cancer cell line (T24). In
Vivo. 21:1011–1019. 2007.
|
|
24
|
Wiench B, Eichhorn T, Paulsen M and
Efferth T: Shikonin directly targets mitochondria and causes
mitochondrial dysfunction in cancer cells. Evid Based Complement
Alternat Med. 2012:7260252012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kamiya N, Ye L, Kobayashi T, Mochida Y,
Yamauchi M, Kronenberg HM, Feng JQ and Mishina Y: BMP signaling
negatively regulates bone mass through sclerostin by inhibiting the
canonical Wnt pathway. Development. 135:3801–3811. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshida Y, Tanaka S, Umemori H, Minowa O,
Usui M, Ikematsu N, Hosoda E, Imamura T, Kuno J, Yamashita T, et
al: Negative regulation of BMP/Smad signaling by Tob in
osteoblasts. Cell. 103:1085–1097. 2000. View Article : Google Scholar
|
|
27
|
Harada S and Rodan GA: Control of
osteoblast function and regulation of bone mass. Nature.
423:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang L, Zhang X, Guo Y, Chen X, Li R, Liu
L, Shi C, Guo C and Zhang Y: Involvement of BMPs/Smad signaling
pathway in mechanical response in osteoblasts. Cell Physiol
Biochem. 26:1093–1102. 2010. View Article : Google Scholar
|