Introduction
Neural stem cells (NSCs) are multipotent stem cells,
which are derived from neural tissues, either from the central or
peripheral nervous systems. NSCs are self-renewing and can give
rise to various cell types of the nervous system, including
neurons, astrocytes and oligodendrocytes, through asymmetric cell
division (1). NSC-derived
neurogenesis is critical for various aspects of central nervous
function, including spatial learning and memory, mood regulation
and motor control (2–4). Previous studies have suggested that
NSC transplantation may have beneficial effects in the treatment of
numerous pathological conditions, such as neurodegenerative
diseases, seizures and brain tumors (5–7).
Therefore, NSCs may be considered a promising therapeutic option
for the treatment of ischemic stroke and neurodegenerative diseases
(8). Nevertheless, how to promote
cell survival and proliferation following cell transplantation
remains a significant challenge for stem cell therapy using NSCs to
treat various neurodegenerative disorders and strokes.
Epimedium is a plant of the Epimedium
brevicornum Maxim species of the Berberidaceae family, which
contains various bioactive compounds, including flavonoids and
alkaloids (9). Icariin (ICA) is a
type of flavonoid, and is the most metabolically active component
extracted from Epimedium. ICA possesses various
bioactivities, including antidepressant-like effects and
anti-inflammatory activity (10,11).
In addition, it has previously been reported that ICA improves
memory impairment in a murine model of Alzheimer's disease, and
ameliorates motor dysfunction in spinal cord injury in mice
(12,13). Due to the known actions of ICA, and
the important role NSCs have in neuroregeneration, the present
study hypothesized that ICA may stimulate cell proliferation and
regulate gene expression in NSCs.
Materials and methods
Materials
ICA (purity >98%, high-performance liquid
chromatography grade) with the molecular formula
C33H42O15 and a molecular weight
of 676.65 was purchased from Shaanxi Scidoor Hi-tech Biology Co.,
Ltd. (Xi'an, China). Hank's Balanced Salt Solution (HBSS),
Dulbecco's modified Eagle's medium (DMEM)/F12 medium, fetal bovine
serum (FBS), B27, epidermal growth factor (EGF), basic fibroblast
growth factor (bFGF), Glutmax and poly-L-lysine-coated plates were
purchased from Thermo Fisher Scientific, Inc. (Beijing, China).
Mouse anti-nestin was obtained from BD Pharmingen (cat. no. 560341;
San Jose, CA, USA), mouse anti-β-III-tubulin (cat. no. T8578),
mouse anti-glial fibrillary acidic protein (GFAP; cat. no. G3893)
and rabbit anti-galactocerebroside (GalC; cat. no. G9152) were
obtained from Sigma Aldrich (Shanghai) Trading. Co., Ltd.(Shanghai,
China). Cy2- or Cy3-conjugated secondary antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). RNeasy kit
and AMV reverse transcriptase were obtained from Qiagen, Inc.
(Valencia, CA, USA). Cy3-dCTP and Cy5-dCTP were purchased from
CapitalBio, Inc. (Beijing, China). SYBR Premix Ex Taq for
quantitative polymerase chain reaction (qPCR) was obtained from
Sangon Biotech Co., Ltd. (Shanghai, China).
NSC isolation and characterization
The use of human fetuses obtained following
spontaneous abortion was approved by the Ethic Committee of Xuanwu
Hospital of Capital Medical University (Beijing, China), and
written informed consent was obtained from the study participants.
The corpus striatum of 16–20-week fetuses were obtained from 5
fetuses following spontaneous abortion, and were dissected and
chopped in cooled HBSS using a tissue chopper. The tissue was
further triturated by pipetting up and down 10 times to dissociate
cells. After filtering through a 300 micron stainless steel sieve,
in order to remove the large pieces of tissue, the dissociated
cells were pelleted by centrifugation at 200 × g for 5 min at room
temperature, washed once with HBSS, and were re-suspended at a
density of 2×106 cells/ml in DMEM/F12 medium containing
2% B27, 20 ng/ml EGF, 20 ng/ml bFGF and 1% Glutmax (DMEM/F12
complete medium). Cells were seeded in 100 mm dishes and cultured
at 37°C in a humidified atmosphere containing 5% CO2.
Cell medium was changed every 3 days. After an initial culture for
7–10 days typical NSC clusters appeared, which were pelleted by
centrifugation at 200 × g for 5 min at room temperature and were
treated with accutase. Accutase-digested cells were washed once
with HBSS, re-suspended in DMEM/F12 complete medium and used for
all subsequent assays. For NSC characterization, the cells were
seeded onto poly-L-lysine-coated chamber slides
(1×104/cm2). Following a 12 h culture, the
cells were subjected to immunostaining for the analysis of nestin
expression.
NSC differentiation
Growth factor-free DMEM/F12 medium supplemented with
1% FBS was used to induce cell differentiation. Cell clusters were
digested with accutase as aforementioned, were washed once with
HBSS, re-suspended in differentiation medium, and were seeded onto
poly-L-lysine-coated chamber slides
(1×104/cm2). Cells were cultured for 7 days,
and were subsequently subjected to fixation for immunostaining, in
order to determine the expression of β-III-tubulin, GFAP and
GalC.
Immunostaining
Cells were fixed in ice-cold 4% paraformaldehyde for
15 min, followed by three washes with phosphate-buffered saline
(PBS; pH 7.4). After blocking with 10% goat serum (Jackson
ImmunoResearch, Inc., West Grove, PA, USA) in PBS containing 0.3%
Triton X-100 at 37°C for 1 h, the cells were incubated with various
primary antibodies at 4°C overnight. The antibodies used were as
follows: Mouse anti-nestin (1:1,000), mouse anti-β-III-tubulin
(1:1,000), mouse anti-GFAP (1:500) and rabbit anti-GalC (1:100).
After three washes with PBS, Cy2- or Cy3-conjugated secondary
antibodies (1:500) were added to the cells and were incubated at
37°C for 30 min, followed by three washes with PBS. Cells were then
mounted with VectaShield mounting medium (Vector Laboratories,
Inc., Burlingame, CA, USA) and 4′,6-diamidino-2-phenylindole was
used for nuclear counter-staining. Fluorescent signals were
visualized using a confocal laser microscope system (MRC1024;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell treatment and proliferation
assay
To study the effects of ICA on NSC proliferation,
accutase-dissociated single cells were suspended in DMEM/F12
complete medium at a density of 2×105 cells/ml. A 100
µl cell suspension was added into each well of a 96-well
plate, which had previously been coated with poly-L-lysine.
Following a 24 h culture, the medium was replaced with serum- and
growth factor-free medium containing various concentrations of ICA
(final concentrations, 0.1, 1 and 10 µM). After a further 24
h of culture, the medium was removed and serum- and growth
factor-free medium (without phenol red) containing 10% Cell
Counting kit (CCK)-8 reagent was added (Dojundo Molecular
Technologies, Inc., Kumamoto, Japan). The cells were cultured for 4
h at 37°C, and the optical density (OD) of each well was measured
at 450 nm (OD450) using a microplate reader. Cells cultured in
serum- and growth factor-free medium served as a control. In a
separate experiment, individual NSCs in DMEM/F12 complete medium
were seeded into each well of a 24-well plate (104
cells/cm2) coated with poly-L-lysine. Following a 24 h
culture, the medium was replaced with serum- and growth factor-free
medium containing 10 µM ICA (final concentration). Cells
were maintained in culture for 7 days, and the formation of
neurospheres was observed by microscopy. The diameters of 15
randomly chosen neurospheres from each well were measured using a
micrometer installed in the microscope (IX70; Olympus Corporation,
Tokyo, Japan). The results from three wells of each treatment group
were averaged and compared.
cDNA microarray analysis
cDNA microarray analysis was performed for gene
expression profiling, and the results were compared between the
cells treated with ICA and the untreated control cells. Cell
treatment with 10 µM ICA was conducted, as aforementioned.
NSCs cultured in serum- and growth factor-free medium without ICA
served as a control. RNA was extracted from the cells using the
RNeasy kit, according to manufacturer's protocol, and was
quantified using the spectrophotometric method. Total RNA (2
µg) was used for first-strand cDNA synthesis using AMV
reverse transcriptase, according to the manufacturer's protocol.
Cy3-dCTP was added to the cDNA of the control cells, whereas
Cy5-dCTP was added to the cDNA of ICA-treated cells. Microarray
analysis was performed using the 22 K Human Genome Array
(CapitalBio, Inc.). The slide contained gene-specific 70-mer
oligonucleotides representing 21,329 human genes, including four
human housekeeping genes as positive controls, and 12 negative
controls, which were designed to have no significant homology with
known human gene sequences. The labeled samples were quantitatively
adjusted based on the efficiency of Cy-dye incorporation and mixed
into 80 µl hybridization solution [3X saline-sodium citrate
(SSC), 0.2% sodium dodecyl sulfate (SDS), 25% formamide and 5X
Denhardt's). cDNA in hybridization solution was denatured at 95°C
for 3 min prior to loading onto the microarray. The array was
hybridized at 42°C overnight, and washed once with washing solution
(0.2% SDS and 2X SSC) at 42°C for 5 min, followed by one wash with
0.2% SSC at room temperature for 5 min. Finally, the array was
scanned using a confocal LuxScan 10 KA scanner (CapitalBio, Inc.).
The data of the obtained images were extracted using LuxScan 3.0
software (CapitalBio, Inc.). Genes with a signal intensity >800
(Cy3 or Cy5) were considered to be expressed. In every two channel
slides, the intensity ratio of Cy5 to Cy3 of each spot was
calculated following normalization with LOWESS regression.
Statistical data and differential analysis files were generated
using SAM software 3.0 (Stanford University, Stanford, CA, USA).
Significantly altered genes were selected based on a P<0.05 and
>2 fold change. All differentially expressed genes were analyzed
using the free web-based Molecular Annotation System 2.0 (MAS 2.0;
http://bioinfo.capitalbio.com/mas3/).
qPCR
qPCR was performed to verify the array results.
Primers (Table I) were designed
based on published gene sequences using the Oligo 5.0 program
(Molecular Biology Insights, Inc., Colorado Springs, CO, USA), and
were synthesized by Sangon Biotech Co., Ltd. The PCR mix (20
µl) contained 10 µl 2X SYBR Taq Premix buffer, 1
µl cDNA, 200 nM of each primer (final concentration) and 9.0
µl nuclease-free water. The reaction mixture was incubated
at 95°C for 10 sec, followed by 40 amplification cycles at 95°C for
5 sec, 60°C for 10 sec and 72°C for 10 sec. A melting curve
analysis was run to determine the specificity of amplification
following each qPCR analysis. Relative mRNA expression levels were
calculated using the 2−∆∆Cq method (14), following normalization against
glyceraldehyde 3-phosphate dehyrdrogenase levels.
 | Table IQuantitative polymerase chain reaction
primer sequences. |
Table I
Quantitative polymerase chain reaction
primer sequences.
| Genes | Reference number | Sequence | Product size |
|---|
| FZD7 | NM_003507 | F:
5′-GGCGCCTCTGTTCGTCTAC-3′ | 106 bp |
| | R:
5′-GGTCTTGGTGCCGTCGTGT-3′ | |
| CTNNB1 | NM_001904 | F:
5′-CACGTGCAATCCCTGAACTGA-3′ | 121 bp |
| | R:
5′-CGCATGATAGCGTGTCTGGAA-3′ | |
| DVL3 | NM_004423 | F:
5′-GGTACTGCGGGAGATTGTG-3′ | 168 bp |
| | R:
5′-GGAAGGTGCCGGTCAT-3′ | |
| GSK-3β | NM_002093 | F:
5′-GTCCGATTGCGTTATT-3′ | 176 bp |
| | R:
5′-TTCGGAACAGCTGATACAT-3′ | |
| FGFR1 | NM_023105 | F:
5′-AAGGCATCATTTGGTGAACAGAAC-3′ | 133 bp |
| | R:
5′-TCACACAACATTGCTTCAAGGTAGG-3′ | |
| GAPDH | NM_002046 | F:
5′-ATGACATCAAGAAGGTGGTG-3′ | 177 bp |
| | R:
5′-CATACCAGGAAATGAGCTTG-3′ | |
Statistical analysis
All results are expressed as the mean ± standard
error of the mean, and were analyzed using one-way analysis of
variance followed by Tukey's post-hoc test using SPSS 16.0 software
(SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
NSC isolation and characterization
NSCs were isolated from the corpus striatum of
16–20-week human fetuses obtained following spontaneous abortion.
After culturing in DMEM/F12 complete medium for 7–10 days, typical
NSC clusters appeared in the culture. Immunostaining analysis
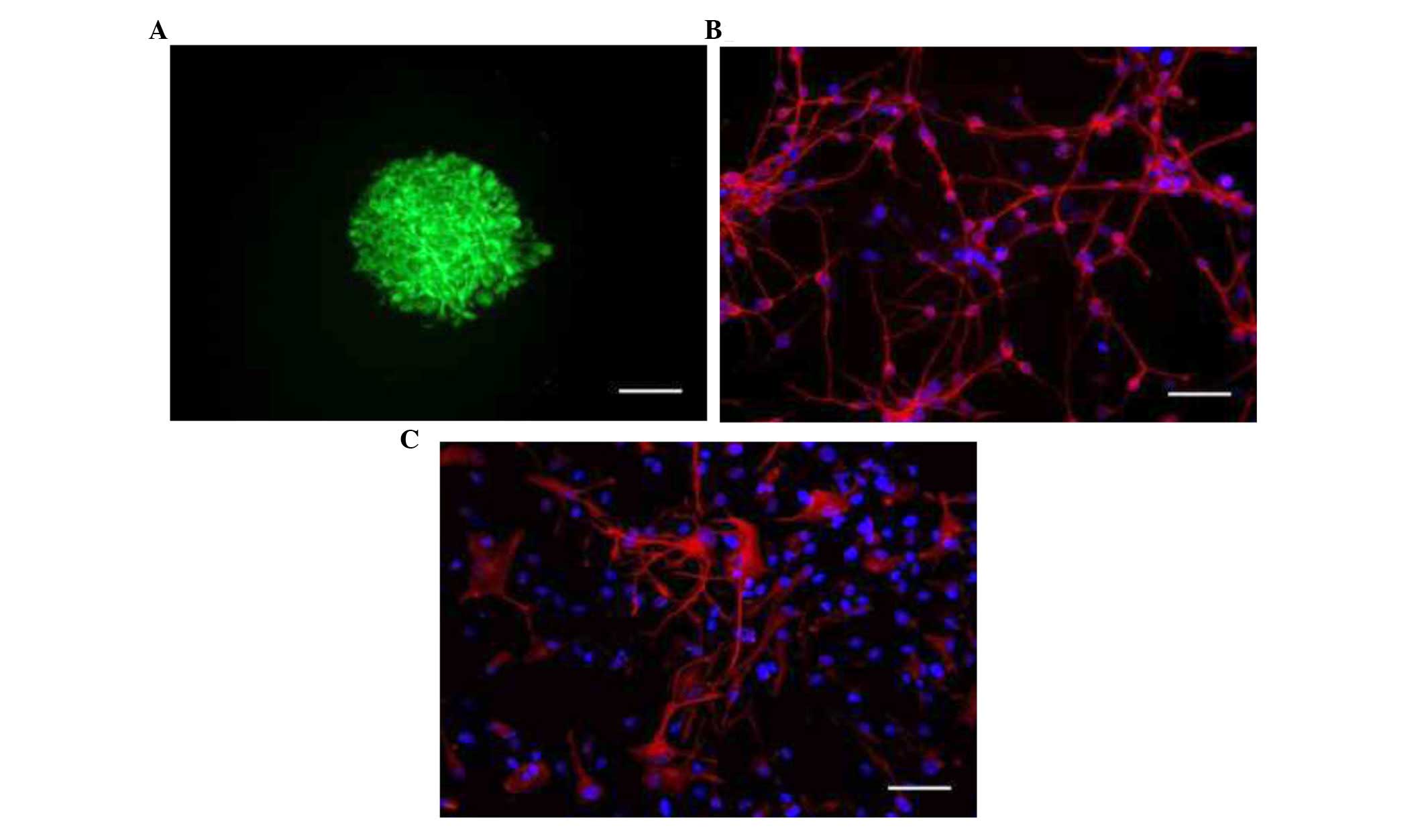
revealed that neurosphere-forming cells expressed nestin, a marker
of NSCs (Fig. 1A). Cell
differentiation assay demonstrated that NSCs were able to
differentiate into different cell types of the nervous system, as
indicated by the expression of β-III-tubulin, a specific neuronal
marker (Fig. 1B); and GFAP, an
astrocyte marker (Fig. 1C). Few
cells expressed GalC, an oligodendrocyte marker (data not
shown).
NSC proliferation is stimulated by
icariin in vitro
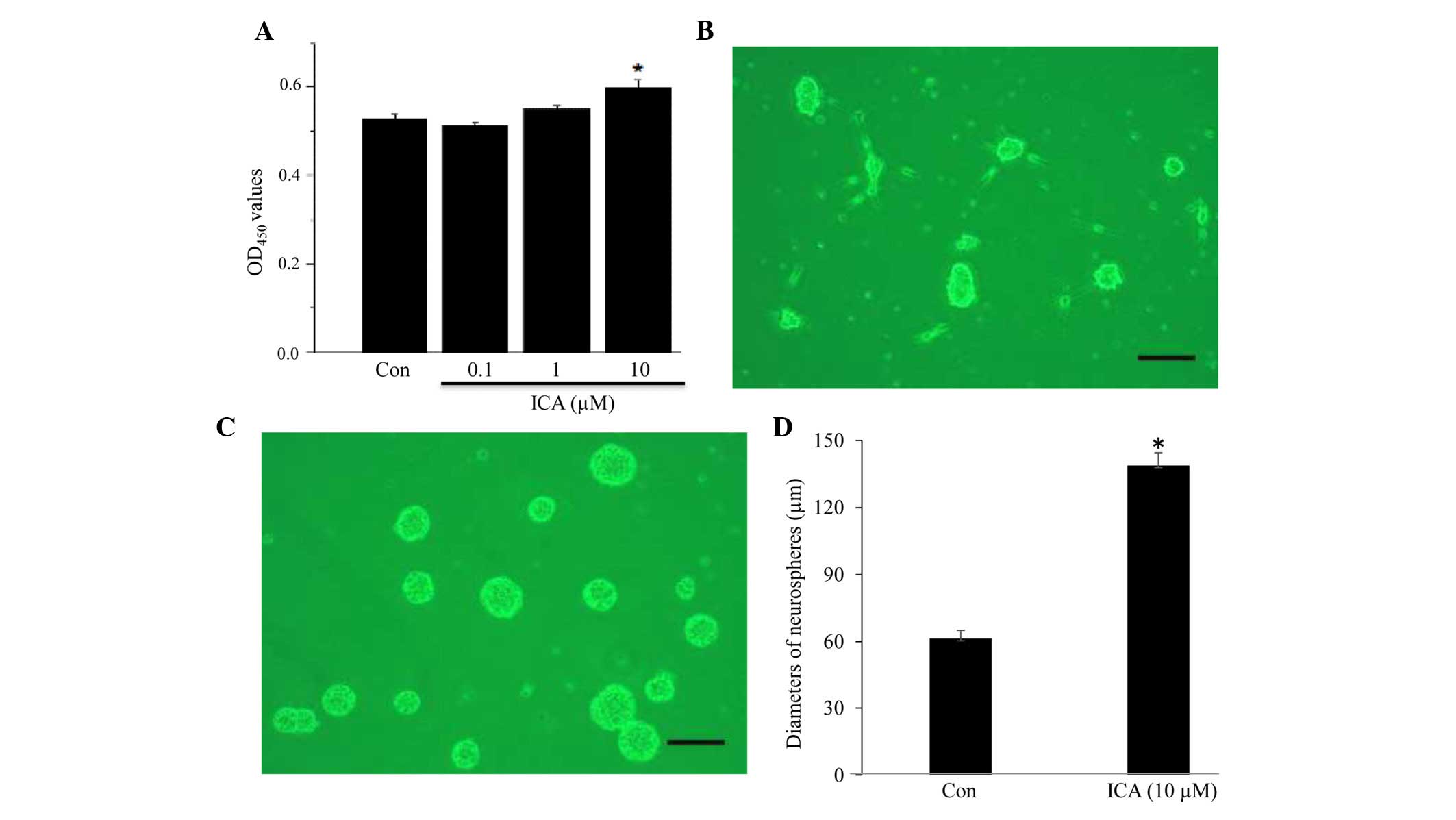
Using a CCK-8 cell proliferation assay kit, the
effects of ICA on NSC proliferation were determined. As shown in
Fig. 2, NSCs were incubated with
various concentrations of ICA. In the presence of 0.1 or 1
µM ICA, cells did not exhibit markedly increased
proliferation compared with the control cells (Fig. 2A). Conversely, cells incubated with
10 µM ICA exhibited a significantly increased proliferation
rate compared with the untreated cells (n=4, P<0.05; Fig. 2A). When ICA concentration increased
to 50 µM, cell toxicity was observed (data not shown). In a
separate assay, the formation of neurospheres under the influence
of ICA was examined. Neurospheres formed in ICA-treated cells
appeared larger compared with those in the control group (Fig. 2B and C). By measuring neurosphere
diameter, it was demonstrated that the diameter of neurospheres
formed in the ICA-treated group was significantly greater than that
of the neurospheres in the control group (n=5, P<0.05; Fig. 2D).
Gene expression profiling by cDNA
microarray
Gene expression in 10 µM ICA-treated cells
and control cells was profiled by cDNA microarray, and the results
were compared. A total of 779 genes were differentially expressed
between the ICA-treated group and the control group, based on
P<0.05 and >2 fold change criteria (data not shown). Of the
altered genes, those involved in the top 10 cell signaling pathways
analyzed using MAS software were listed in Table II. Since the Wnt signaling pathway
and the bFGF pathway have important roles in NSC proliferation and
neurogenesis, several differentially expressed genes important in
the Wnt signaling pathway were selected (Table III). Fibroblast growth factor
receptor 1 was also further analyzed by qPCR to validate the array
results.
 | Table IIDifferences in gene expression in the
top 10 pathways analyzed using Molecular Annotation System
software. |
Table II
Differences in gene expression in the
top 10 pathways analyzed using Molecular Annotation System
software.
| Pathway | Total genes | P-value | Genes |
|---|
| Regulation of actin
cytoskeleton | 19 | 2.0E-8 | ITGA7↑, PDGFRA↑, ACTN1↑, PFN1↑, GSN↑, PDGFRB↓, GNG12↑, FGFR2↑, FGD3↓, PAK3↓, APC2↓, FGFR1↑, PFN2↓, ARHGEF7↓, ITGA2↑, ITGB5↑, TIAM1↓, PIP5K1B↓, FGFR3↓ |
| Focal adhesion | 22 | 2.3E-7 | CAST↑, ITGA7↑, PDGFRA↑, JUN↑, ACTN1↑, LAMC1↑, PDGFRB↓, COL1A2↑, TNC↑, CCND1↑, SHC3↑, THBS4↑, THBS2↓, MAPK10↓, PAK3↓, SPP1↑, ITGA2↑, COL4A1↑, ITGB5↑, PDGFD↓, LAMA4↑, SRC↑ |
| MAPK pathway | 20 | 5.2E-7 | PRKX↓, MEF2C↓,
PDGFRA↑, JUN↑, CACNB3↓, DUSP4↑, NTRK2↓, DUSP6↑, PDGFRB↓, MYC↑, GNG12↑, FGFR2↑, MAPK10↓, CACNG4↓, MKNK1↓,
GADD45A↑, FGFR1↑, TNFRSF1A↑, HSPA5↑, FGFR3↓ |
| ECM-receptor
interaction | 12 | 8.5E-7 | ITGA7↑, SDC3↑, LAMC1↑, COL1A2↑, TNC↑, THBS4↑, THBS 2↓, SPP1↑, ITGA2↑, COL4A1↑, ITGB5↑, LAMA4↑ |
| Axon guidance | 12 | 2.0E-6 | EPHA3↓, NCK2↓,
EPHA2↑, PAK3↓, SEMA6D↓, ROBO2↓,
RGS3↑, DPYSL2↓, SEMA5B↓,
PLXNA2↓, EPHB3↓, EPHB4↓ |
| Cell
communication | 11 | 7.0E-6 | INA↓, LAMC1↑, GJA1↓, COL1A2↑, TNC↑, THBS4↑, THBS2↓, VIM↑, SPP1↑, COL4A1↑, LAMA4↑ |
| TGF-beta
pathway | 9 | 1.2E-5 | ACVR1↓, RPS6KB2↓,
MYC↑, THBS4↑, THBS2↓, AMH↓, INHBB↓, LTBP1↑, BMP7↑ |
| Wnt pathway | 10 | 3.8E-5 | WNT7B↑, FZD7↑, DVL3↑, GSK- 3β↓, MYC↑, CCND1↑, CTNNB1↑, TCF7↑, LEF1↑, JUN↑ |
| Gap junction | 9 | 5.2E-5 | TUBB3↓, PRKX↓,
PDGFRA↑, PDGFRB↓, EDG2↑, GJA1↓, SRC↓, PDGFD↓, CSNK1E↓ |
| Glutathione
metabolism | 6 | 5.9E-5 | GSS↑, GSTA4↓, GSTO1↑, MGST2↑, GSTZ1↑, GPX7↑ |
 | Table IIIDifferentially expressed genes in the
Wnt signaling pathway. |
Table III
Differentially expressed genes in the
Wnt signaling pathway.
| Gene | Fold change
(ICA/control) | Molecular
functions |
|---|
| WNT7B |
2.482 | Wnt ligand |
| FZD7 |
2.850 | Non-G-protein
coupled receptor |
| DVL3 |
2.191 | Catalytic
activity |
| GSK-3β |
0.468 | Kinase and
inactivating agent |
| MYC |
4.390 | Transcription
regulator |
| CCND1 | 11.677 | Mitotic and cell
cycle regulation |
| CTNNB1 |
2.500 | Coactivator of
transcription |
| TCF7 |
2.804 | RNA polymerase II
transcription |
| LEF1 | 10.663 | Transcription
regulator |
| JUN |
2.086 | Transcription
cofactor |
Gene expression validation by qPCR
The mRNA expression levels of fibroblast growth
factor receptor 1 (FGFR1), and four genes of the Wnt signaling
pathway: Frizzled class receptor 7 (FZD7), dishevelled segment
polarity protein 3 (DVL3), catenin beta-1 (CTNNB1) and glycogen
synthase kinase-3β (GSK-3β), were further determined by qPCR. The
results indicated that the mRNA expression levels of FZD7 (Fig. 3A) and DVL3 (Fig. 3B) were significantly elevated in
the ICA-treated group compared with the control group (P<0.01
and P<0.05, respectively); CTNNB1 expression was increased in
ICA-treated cells; however, the increase was not significantly
different compared with the control cells, as determined by
statistical analysis (Fig. 3C).
The mRNA expression levels of GSK-3β were markedly reduced in the
ICA-treated cells (n=6, P<0.01; Fig. 3D). In addition, FGFR1 expression
was significantly upregulated by ICA (P<0.01; Fig. 3E).
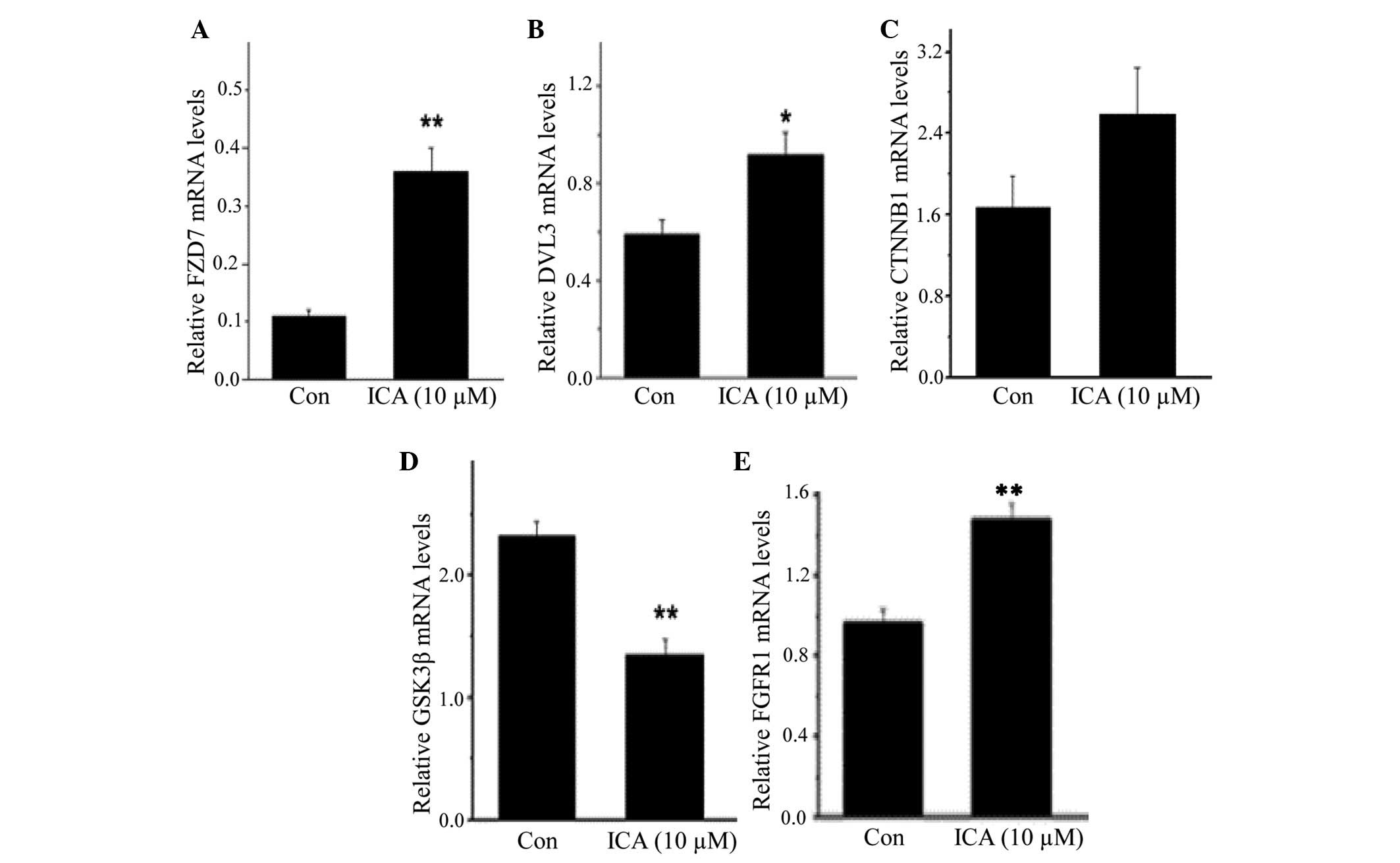 | Figure 3Icariin (ICA) regulated gene
expression in neural stem cells. cDNA microarray analysis
identified genes whose expression was altered following ICA
treatment (Tables II and III). Using quantitative polymerase
chain reaction, the mRNA expression levels of (A) frizzled class
receptor 7 (FZD7), (B) dishevelled segment polarity protein 3
(DVL3), (C) catenin beta-1 (CTNNB1) and (D) glycogen synthase
kinase (GSK)-3β, which are key players in the Wnt signaling
pathway, and (E) fibroblast growth factor receptor 1 (FGFR1), which
is the receptor for fibroblast growth factor, were determined. In
the presence of ICA, the expression levels of FZD7, DVL3 and FGFR1
was significantly increased, whereas GSK-3β was markedly reduced,
verifying the microarray data (n=5 for each gene). Data are
presented as the mean ± standard error of the mean.
*P<0.05, **P<0.01, compared with
control cells. Con, control cells. |
Discussion
NSCs possess the characteristics of self-renewal and
multipotency (1–5). The present study isolated and
cultured NSCs from the corpus striatum of 16–20-week fetuses
obtained following spontaneous abortion, and demonstrated that
these cells expressed nestin, which is an intermediate filament
protein selectively expressed in NSCs and commonly used as a marker
for NSCs (15). Cultured in
differentiation medium, the NSCs expressed β-III-tubulin, a
specific neuronal marker, and GFAP, a marker for astrocytes
(16,17), thus indicating that the NSCs were
multipotent.
In our previous study, Epimedium flavonoids
were revealed to promote the proliferation and differentiation of
NSCs derived from neonatal rats in vitro (9). ICA, which is an important flavonoid
extracted from Epimedium, has not yet been investigated
regarding its role in regulating cell proliferation and gene
expression in NSCs. The present study examined the effects of ICA
on NSC proliferation. The results demonstrated that NSCs treated
with 10 µM ICA exhibited a significantly higher
proliferation rate compared with untreated cells. When NSCs were
incubated with 10 µM ICA for 7 days, larger neurospheres
were formed compared with those in the control group. Measurement
of neurosphere diameter revealed the diameter of neurospheres
formed in the ICA-treated group was significantly greater compared
with in the control group. These data suggested that ICA promoted
NSC proliferation and growth.
In order to provide information regarding the
mechanisms underlying ICA actions, the present study analyzed
ICA-regulated gene expression in NSCs. A cDNA microarray analysis,
which is an important tool used to uncover the molecular basis of
several biological processes (18), was used to profile gene expression
in NSCs. The results indicated that treatment with ICA regulated
gene expression in NSCs, affecting numerous signaling pathways
(Tables II and III). Of these pathways, the Wnt
signaling pathway has been recognized as a key regulator for
self-renewal and maintenance of neural progenitors during
neurogenesis (19–21), and bFGF signaling has been reported
to potently induce NSC proliferation (22–25).
The canonical Wnt pathway cascade is initiated when Wnt signaling
proteins bind to Frizzled protein receptors, such as FZD7, which is
a family of G protein-coupled receptors (26–28).
Subsequently, the receptor interacts with a downstream mediator,
the Dishevelled protein, which is an essential effector for the
accumulation of β-catenin encoded by CTNNB130 (27,29,30).
The accumulated β-catenin then translocates to the nucleus where it
regulates target gene expression to control cell function,
including cell proliferation. Conversely, GSK-3β negatively
regulates Wnt signaling (27,31).
The microarray analysis conducted in the present study demonstrated
that the expression levels of several genes in the Wnt and bFGF
pathways were altered following ICA treatment. The expression
levels of FGFR1, and four important Wnt family members: FZD7, DVL3,
CTNNB1 and GSK-3β were further determined by qPCR. The results
demonstrated that ICA significantly enhanced expression of FGFR1,
FZD7 and DVL3, and significantly reduced the expression of GSK-3β.
It was hypothesized that ICA-regulated expression of these genes
presumably favored the proliferation of human NSCs.
In conclusion, NSCs derived from the corpus striatum
of 16–20-week human fetuses obtained following spontaneous abortion
were successfully isolated. The NSCs expressed nestin, an NSC
marker, and could differentiate into various types of cells of the
nervous system. Treatment with ICA promoted the proliferation and
modulated gene expression in NSCs, thus suggesting that ICA may
exert its neuroprotective effects by regulating NSC activity. Taken
together, the findings suggest that ICA may be potentially applied
for the treatment of neurodegenerative disorders.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81273498 and
81274120), the Beijing Natural Science Foundation (grant nos.
7112061 and 7132110), the Beijing Science and Technology Program
(grant no. Z131102002813066), the Capital Health Development
Scientific Grant (grant no. 2011-1001-05), and the Beijing Health
and Technical High-level Personnel Plan (grant nos. 2011-1-7 and
2009-3-66).
References
|
1
|
Gage FH: Mammalian neural stem cells.
Science. 287:1433–1438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang CL, Zou Y, He W, Gage FH and Evans
RM: A role for adult TLX-positive neural stem cells in learning and
behaviour. Nature. 451:1004–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thomas RM and Peterson DA: Even neural
stem cells get the blues: Evidence for a molecular link between
modulation of adult neurogenesis and depression. Gene Expr.
14:183–193. 2008.PubMed/NCBI
|
|
4
|
McBride JL, Behrstock SP, Chen EY, Jakel
RJ, Siegel I, Svendsen CN and Kordower JH: Human neural stem cell
transplants improve motor function in a rat model of Huntington's
disease. J Comp Neurol. 475:211–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carletti B, Piemonte F and Rossi F:
Neuroprotection: The emerging concept of restorative neural stem
cell biology for the treatment of neurodegenerative diseases. Curr
Neuropharmacol. 9:313–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Waldau B, Hattiangady B, Kuruba R and
Shetty AK: Medial ganglionic eminence-derived neural stem cell
grafts ease spontaneous seizures and restore GDNF expression in a
rat model of chronic temporal lobe epilepsy. Stem Cells.
28:1153–1164. 2010.PubMed/NCBI
|
|
7
|
Achanta P, Sedora Roman NI and
Quiñones-Hinojosa A: Gliomagenesis and the use of neural stem cells
in brain tumor treatment. Anticancer Agents Med Chem. 10:121–130.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hung CW, Liou YJ, Lu SW, Tseng LM, Kao CL,
Chen SJ, Chiou SH and Chang CJ: Stem cell-based neuroprotective and
neurorestorative strategies. Int J Mol Sci. 11:2039–2055. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao R, Zhang L, Li X and Li L: Effects of
Epimedium flavonoids on proliferation and differentiation of neural
stem cells in vitro. Neurol Res. 32:736–742. 2010. View Article : Google Scholar
|
|
10
|
Pan Y, Kong L, Xia X, Zhang W, Xia Z and
Jiang F: Antidepressant-like effect of icariin and its possible
mechanism in mice. Pharmacol Biochem Behav. 82:686–694. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu CQ, Liu BJ, Wu JF, Xu YC, Duan XH, Cao
YX and Dong JC: Icariin attenuates LPS-induced acute inflammatory
responses: Involvement of PI3K/Akt and NF-kappaB signaling pathway.
Eur J Pharmacol. 642:146–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urano T and Tohda C: Icariin improves
memory impairment in Alzheimer's disease model mice (5xFAD) and
attenuates amyloid β-induced neurite atrophy. Phytother Res.
24:1658–1663. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tohda C and Nagata A: Epimedium koreanum
extract and its constituent icariin improve motor dysfunction in
spinal cord injury. Evid Based Complement Alternat Med.
2012:7312082012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
15
|
Messam CA, Hou J and Major EO:
Coexpression of nestin in neural and glial cells in the developing
human CNS defined by a human-specific anti-nestin antibody. Exp
Neurol. 161:585–596. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katsetos CD, Herman MM and Mörk SJ: Class
III beta-tubulin in human development and cancer. Cell Motil
Cytoskeleton. 55:77–96. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Menet V, Giménez y Ribotta M, Chauvet N,
Drian MJ, Lannoy J, Colucci-Guyon E and Privat A: Inactivation of
the glial fibrillary acidic protein gene, but not that of vimentin,
improves neuronal survival and neurite growth by modifying adhesion
molecule expression. J Neurosci. 21:6147–6158. 2001.PubMed/NCBI
|
|
18
|
Schena M, Shalon D, Davis RW and Brown PO:
Quantitative monitoring of gene expression patterns with a
complementary DNA microarray. Science. 270:467–470. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lie DC, Colamarino SA, Song HJ, Désiré L,
Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearle AR
and Gage FH: Wnt signalling regulates adult hippocampal
neurogenesis. Nature. 437:1370–1375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wexler EM, Paucer A, Kornblum HI, Palmer
TD and Geschwind DH: Endogenous Wnt signaling maintains neural
progenitor cell potency. Stem Cells. 27:1130–1141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Machon O, Backman M, Machonova O, Kozmik
Z, Vacik T, Andersen L and Krauss S: A dynamic gradient of Wnt
signaling controls initiation of neurogenesis in the mammalian
cortex and cellular specification in the hippocampus. Dev Biol.
311:223–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reimers D, López-Toledano MA, Mason I,
Cuevas P, Redondo C, Herranz AS, Lobo MV and Bazán E: Developmental
expression of fibroblast growth factor (FGF) receptors in neural
stem cell progeny. Modulation of neuronal and glial lineages by
basic FGF treatment. Neurol Res. 23:612–621. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kelly CM, Tyers P, Borg MT, Svendsen CN,
Dunnett SB and Rosser AE: EGF and FGF-2 responsiveness of rat and
mouse neural precursors derived from the embryonic CNS. Brain Res
Bull. 68:83–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nelson AD and Svendsen CN: Low
concentrations of extracellular FGF-2 are sufficient but not
essential for neurogenesis from human neural progenitor cells. Mol
Cell Neurosci. 33:29–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Furusho M, Kaga Y, Ishii A, Hébert JM and
Bansal R: Fibroblast growth factor signaling is required for the
generation of oligoden-drocyte progenitors from the embryonic
forebrain. J Neurosci. 31:5055–5066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saito-Diaz K, Chen TW, Wang X, Thorne CA,
Wallace HA, Page-McCaw A and Lee E: The way Wnt works: Components
and mechanism. Growth Factors. 31:1–31. 2013. View Article : Google Scholar :
|
|
28
|
Dann CE, Hsieh JC, Rattner A, Sharma D,
Nathans J and Leahy DJ: Insights into Wnt binding and signaling
from the structures of two Frizzled cysteine-rich domains. Nature.
412:86–90. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wallingford JB and Habas R: The
developmental biology of dishevelled: An enigmatic protein
governing cell fate and cell polarity. Development. 132:4421–4436.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gan XQ, Wang JY, Xi Y, Wu ZL, Li YP and Li
L: Nuclear Dvl, c-Jun, beta-catenin, and TCF form a complex leading
to stabilization of beta-catenin-TCF interaction. J Cell Biol.
180:1087–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hart MJ, de los Santos R, Albert IN,
Rubinfeld B and Polakis P: Downregulation of beta-catenin by human
Axin and its association with the APC tumor suppressor,
beta-catenin and GSK3 beta. Curr Biol. 8:573–581. 1998. View Article : Google Scholar : PubMed/NCBI
|