Introduction
Adolescent idiopathic scoliosis (AIS) is a complex
three-dimensional structural deformity with lateral curvature of
the spine, which presents in late childhood and occurs in up to 3%
of school-aged children worldwide (1,2).
Genetic factors, growth and sex hormone secretion, connective
tissue structure, muscle structure, vestibular dysfunction,
melatonin deficiency, and platelet abnormalities are associated
with the etiology of AIS (3–6).
Studies have previously demonstrated that patients with AIS have
abnormal skeletal growth (7) and
persistent lower bone mineral density (8) compared with sex- and age-matched
controls. Additionally, previous studies have suggested that
relative anterior overgrowth with disproportionate
endochondral-membranous bone growth contributes to the development
of AIS (9). A melatonin receptor
1B (MTNR1B) gene polymorphism (10) and asymmetric expression of
melatonin receptor mRNA in the bilateral paravertebral muscles
(11) have been previously
reported to be associated with AIS. Furthermore, neuroendocrine
research findings implicating melatonin deficiency and melatonin
signaling pathway dysfunction as the source of AIS have been a
focus of great interest (12).
Melatonin signaling pathway dysfunction has
previously been reported in the osteoblasts of patients with severe
AIS (13,14). The abnormal response of osteoblasts
to melatonin and abnormal expression of MTNR1B in osteoblasts of
patients with AIS has been previously reported (15,16).
However, to the best of our knowledge, no results of functional
research on abnormalities in the melatonin signaling pathway
affecting the regulation of osteogenic and chondrogenic
differentiation in AIS have previously been reported.
Mesenchymal stem cells (MSCs) are multipotent
stromal cells that can differentiate into a variety of unique
mesenchymal cell types, including osteoblasts, chondrocytes, and
adipocytes (17). Intramembranous
and endochondral ossification, two important components of bone
formation, begin with MSC proliferation and condensation (18). Previous studies have demonstrated
that melatonin is important for the regulation of osteogenic and
chondrogenic differentiation of MSCs (19–21).
The present study was conducted to provide further
understanding and evidence of the expression of melatonin membrane
receptor 2 (MT2) in human MSCs (hMSCs), and to examine the effects
of melatonin on osteogenic and chondrogenic differentiation of
hMSCs isolated from patients with AIS and normal control subjects.
It was hypothesized that an abnormality of the melatonin signaling
pathway may be involved in abnormal skeletal growth caused by
osteogenic and chondrogenic differentiation in subjects with
AIS.
Materials and methods
Patient data
The Ethics Committee of Sun Yat-sen University
(Guangzhou, China) approved this study, and all participants
provided written informed consent. Patients with severe AIS and
sex-matched control subjects were recruited. The AIS group
comprised patients who met current clinical criteria for severe AIS
and were candidates for surgery. These patients provided detailed
histories and underwent physical examinations, standard
posteroanterior radiography of the whole spine while standing, and
other tests, including magnetic resonance imaging and/or computed
tomography. Patients with congenital scoliosis and scoliosis
secondary to neuromuscular disorders, endocrine disorders, skeletal
dysplasia, connective tissue abnormalities and syndromic disorders
were excluded. Control subjects were surgery candidates recruited
from the traumatology department. Two experienced orthopedic
surgeons preoperatively confirmed the absence of other deformities
of the skeletal system, hereditary diseases, and disorders
affecting bone growth and metabolism in the control subjects.
Isolation and culture of bone
marrow-derived hMSCs
hMSCs were isolated from bone marrow obtained from
patients with AIS and control donors as described previously
(19). The bone marrow samples
were diluted with phosphate-buffered saline (PBS). Cells were then
fractionated using a Lymphoprep (MP Biomedicals, LLC., Santa Ana,
CA, USA) density gradient by resuspension in low-glucose Dulbecco's
modified Eagle medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.), seeded, and incubated at 37°C/5%
CO2. After 48 h, non-adherent cells were removed by
changing the medium. Subsequently, the medium was changed every 3
days. When the cells reached 80–90% confluence, they were
trypsinized, counted, and replated. Cells from passages 3–6 were
used for the experiments. Phenotype analysis of cultured MSCs was
conducted using a flow cytometer (data not shown), as described
previously (18).
Western blotting analysis
For protein extraction, hMSCs were seeded at a
density of 1×106 cells in a 75-cm2 flask and
cultured in DMEM containing 10% FBS. The cells were cultured for an
additional 3 days after reaching confluence. The cells were then
trypsinized and lysed in radioimmunoprecipitation assay buffer
containing 1 mm protease inhibitor cocktail (Sigma-Aldrich, St.
Louis, MO, USA) for 30 min, as described in a previous study
(16). The mixture was centrifuged
at 15,000 × g for 20 min at 4°C. The supernatant was collected for
protein detection. Protein was extracted using a BCA Protein assay
kit (CWbiotech, Beijing, China) and stored at −80°C.
For western blotting, ~16 µl of the prepared
supernatant was mixed with an equal volume of 5X sodium dodecyl
sulfate (SDS) gel loading buffer. The samples were placed in a
boiling water bath for 5 min. Samples were centrifuged at 15,000 ×
g for 5 min. The supernatant (30 µg), in a 25 µl
volume, was subjected to SDS-polyacrylamide gel electrophoresis
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) using 10%
separating gel and 4% stacking gel for 50 min at 180 V. The gel was
removed from the tank and electroblotted onto a methanol-activated
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA) using
the semi-dry method for 60 min at 200 mA. The membrane was washed
three times with Tris-buffered saline Tween-20 (TBST) buffer and
blocked with 5% skim milk for 1 h at 4°C. Subsequently, it was
probed with mouse anti-human melatonin primary antibody for MT1 and
MT2 (1:1,000; Santa Cruz Biotechnology Inc., Dallas, TX, USA; cat.
no. sc-398788) and mouse anti-human β-actin primary antibody
(1:2,000; Santa Cruz Biotechnology, Inc.; cat. no. sc-130300)
overnight at 4°C. The membrane was washed three times with TBST
buffer and incubated with horseradish peroxidase-conjugated goat
anti-mouse secondary antibody (1:4,000; Santa Cruz Biotechnology,
Inc.; cat. no. 395763) for 1 h at room temperature. After washing
three times, the immunocomplex was visualized using
electrochemiluminescence (ECL) western blotting detection reagents
(Thermo Fisher Scientific, Inc.) and an ECL camera (ImageQuant LAS
4000 mini; GE Healthcare Life Sciences, Chalfont, UK).
Following reaction with the first primary antibody,
the same membrane was rinsed with TBST buffer and the antibodies
were stripped with a stripping buffer for 30 min. Then, it was
incubated with anti-β-actin antibody overnight at 4°C as the
reference protein. Following conjugation with the secondary
antibody, the immunocomplex was visualized as described. An
affinity-isolated antigen-specific antibody directed against the
β-actin protein (Santa Cruz Biotechnology, Inc.) was used as a
reference protein for western blots. All results were confirmed in
duplicate.
Osteogenic differentiation
Osteogenic differentiation assays were conducted on
four groups: Control osteogenesis (Control-Os), control
osteogenesis + melatonin (Control-Os/Mel), AIS osteogenesis
(AIS-Os), and AIS osteogenesis + melatonin (AIS-Os/Mel) groups.
hMSCs were plated at a density of 15,000 cells/cm2 in
6-well plates for the reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) assay and 12-well plates for the alkaline
phosphatase (ALP) activity assay in DMEM with 10% FBS, as described
previously (19). When the cells
reached 70–80% confluence, the growth medium was changed to
osteogenic differentiation medium, which consisted of DMEM, 10%
FBS, 0.1 µM dexamethasone, 10 mM β-glycerol phosphate
(Sigma-Aldrich) and 50 µg/ml ascorbic acid (Sigma-Aldrich).
For the ALP activity and RT-qPCR assays, cells were treated for 12
days with osteogenic differentiation medium in the presence of
melatonin at a final concentration of 50 nM.
ALP activity assay
ALP activity was examined using a p-nitrophenyl
phosphate (pNPP) assay (Sigma-Aldrich). Following treatment with
osteogenic differentiation medium, the cells were washed and lysed
in 0.2 ml PBS containing 0.1 M glycine, 1 mM MgCl2 and
0.05% Triton X-100 for 10 min at 4°C. The lysate was incubated with
pNPP solution at 37°C for 30 min, and absorbance at 405 nm was
measured using a microplate reader. The protein content was
determined and ALP activity was expressed as p-NP units/mg
protein/30 min.
Chondrogenic differentiation
Chondrogenic differentiation assays were also
performed on four groups: Control chondrogenesis (Control-CHO),
control chondrogenesis + melatonin (Control-CHO/Mel), AIS
chondrogenesis (AIS-CHO), and AIS chondrogenesis + melatonin
(AIS-CHO/Mel) groups. A high-density micromass culture system was
used as described previously (22). Briefly, culture-expanded MSCs were
trypsinized, washed, and then resuspended at a density of
2×107 cells/ml in a chemically defined chondrogenic
medium consisting of high-glucose DMEM supplemented with 10 ng/ml
recombinant human transforming growth factor-β3 (Peprotech, Inc.,
Rocky Hill, NJ, USA), 100 nM dexamethasone (Sigma-Aldrich), 50
µg/ml ascorbic acid 2-phosphate (Sigma-Aldrich), 1 mM sodium
pyruvate (Sigma-Aldrich), 40 µg/ml proline (Sigma-Aldrich),
and insulin-transferrin-selenium + Universal Culture Supplement
Premix (BD Biosciences, Franklin Lakes, NJ, USA; final
concentrations, 6.25 µg/ml bovine insulin, 6.25 µg/ml
transferrin, 6.25 µg/ml selenous acid, 5.33 µg/ml
linoleic acid, and 1.25 mg/ml bovine serum albumin). Droplets (15
µl) were placed carefully in each interior well of a 24-well
plate. Cells were allowed to adhere at 37°C for 1.5 h, followed by
addition of 500 µl chondrogenic medium containing vehicle or
50 nM melatonin (Sigma-Aldrich). The medium was changed every 3
days, and induced cartilage tissues were harvested on day 14 for
RT-qPCR assay and on day 21 for quantitative analysis of
glycosaminoglycan (GAG).
Quantitative analysis of GAG
Micromasses were washed and digested in PBS
containing 0.03% papain (Merck Millipore, Darmstadt, Germany), 5 mM
cysteine hydrochloride (Sigma-Aldrich), and 10 mM
ethylenediaminetetraacetic acid (Sigma-Aldrich) for 16 h at 65°C.
GAG concentrations were measured using the 1,9-dimethylmethylene
blue (DMMB) dye binding assay (Sigma-Aldrich). Briefly, an aliquot
of the lysate was reacted with DMMB solution for 10 min, and
absorbance at 525 nm was measured using Varioskan Flash (Thermo
Scientific, Inc.). DNA concentrations were calculated using the
fluorescent dye Hoechst 33258 binding assay (Sigma-Aldrich) and a
SpectraMax M5 microplate reader (Molecular Devices, LLC, Sunnyvale,
CA, USA). For comparison, GAG content was normalized to DNA
content.
RT-PCR analysis
Total RNA was extracted from cells or micromasses
using RNAiso Plus reagent (Takara Biotechnology Co., Ltd., Dalian,
China) and then converted to cDNA using PrimeScript RT master mix
(Takara Biotechnology Co., Ltd.), according to the manufacturer's
protocols. qPCR was performed with an iQ5 system (Bio-Rad
Laboratories, Inc.) using SYBR Green I master mix (Toyobo Co.,
Ltd., Osaka, Japan), with the following thermocycling conditions:
Initial denaturation, 95°C for 10 min; 40 cycles of 95°C for 15 sec
and 60°C for 60 sec. The expressions of osteogenic marker genes,
including those for ALP, osteopontin, osteocalcin, and
runt-related transcription factor 2 (RUNX2); and
chondrogenic marker genes, including those for collagen type II
(COL2A1), collagen type X (COL10A1), aggrecan, and
sex-determining region Y-box 9 (SOX9), were examined. The
expression level of the glyceraldehyde-3-phosphate dehydrogenase
gene (GAPDH) served as a reference. The primer sequences
used are listed in Table I. Each
PCR was processed in triplicate. The Cq value of
GAPDH was subtracted from that of the target gene
(ΔCq), and the average of triplicate
ΔCq values was recorded. The relative expression
level of each gene was determined using the 2−ΔΔCq
method (23).
 | Table IPrimers used for reverse
transcription-quantitative polymerase chain reaction assays. |
Table I
Primers used for reverse
transcription-quantitative polymerase chain reaction assays.
| Gene (accession
no.) | Primer
sequence | Product length
(bp) |
|---|
| GAPDH
(NM_002046) |
5′-AGAAAAACCTGCCAAATATGATGAC-3′ | 126 |
|
5′-TGGGTGTCGCTGTTGAAGTC-3′ | |
| ALP
(NM_000478.3) |
5′-AGCACTCCCACTTCATCTGGAA-3′ | 77 |
|
5′-GAGACCCAATAGGTAGTCCACATTG-3′ | |
| Osteopontin
(NM_013227) |
5′-GCGAGGAGTTGAATGGTG-3′ | 140 |
|
5′-CTTGTGGCTGTGGGTTTC-3′ | |
| Osteocalcin
(NM_199173) |
5′-CAGCGAGGTAGTGAAGAGA-3′ | 143 |
|
5′-GACTGGTGTAGCCGAAAG-3′ | |
| RUNX2
(NM_001024630) |
5′-AGAAGGCACAGACAGAAGCTTGA-3′ | 78 |
|
5′-AGGAATGCGCCCTAAATCACT-3′ | |
| COL2A1
(NM_001844) |
5′-GGCAATAGCAGGTTCACGTACA-3′ | 79 |
|
5′-CGATAACAGTCTTGCCCCACTT-3′ | |
| COL10A1
(NM_000493) |
5′-CAAGGCACCATCTCCAGGAA-3′ | 70 |
|
5′-AAAGGGTATTTGTGGCAGCATATT-3′ | |
| Aggrecan
(NM_001135) |
5′-TGCATTCCACGAAGCTAACCTT-3′ | 84 |
|
5′-GACGCCTCGCCTTCTTGAA-3′ | |
| SOX9
(NM_000346) |
5′-AGCGAACGCACATCAAGAC-3′ | 110 |
|
5′-GCTGTAGTGTGGGAGGTTGAA-3′ | |
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation. Differences in responses between hMSCs from AIS
and control subjects were examined by one-way analysis of variance
followed by a Newman-Keuls post-hoc t-test using SPSS
statistical software (version 16.0; SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of MTs in hMSCs
Study participants were 12 patients with severe AIS
(8 females, 4 males; age 14–19 years) and 12 control subjects (8
females, 4 males; age 15–24 years; Tables II and III). hMSCs isolated from bone marrow
were defined morphologically by a fibroblast-like appearance.
Overall, expression levels of MT1 and MT2 were irregular in hMSCs
from patients with AIS. MT1 expression was low in MSCs from 4
subjects with AIS (P2, P3, P6 and P10), and MT2 expression was low
in MSCs from 6 subjects with AIS (P2-P5, P10 and P11; extremely low
in P2 and P10; Fig. 1A). By
contrast, all MSC samples isolated from control subjects exhibited
uniform bands for MT1 and MT2 (Fig.
1B). When the levels from all samples were combined, MT2
demonstrated significantly reduced expression in the AIS group
compared with the control group (P=0.00261; Fig. 1C).
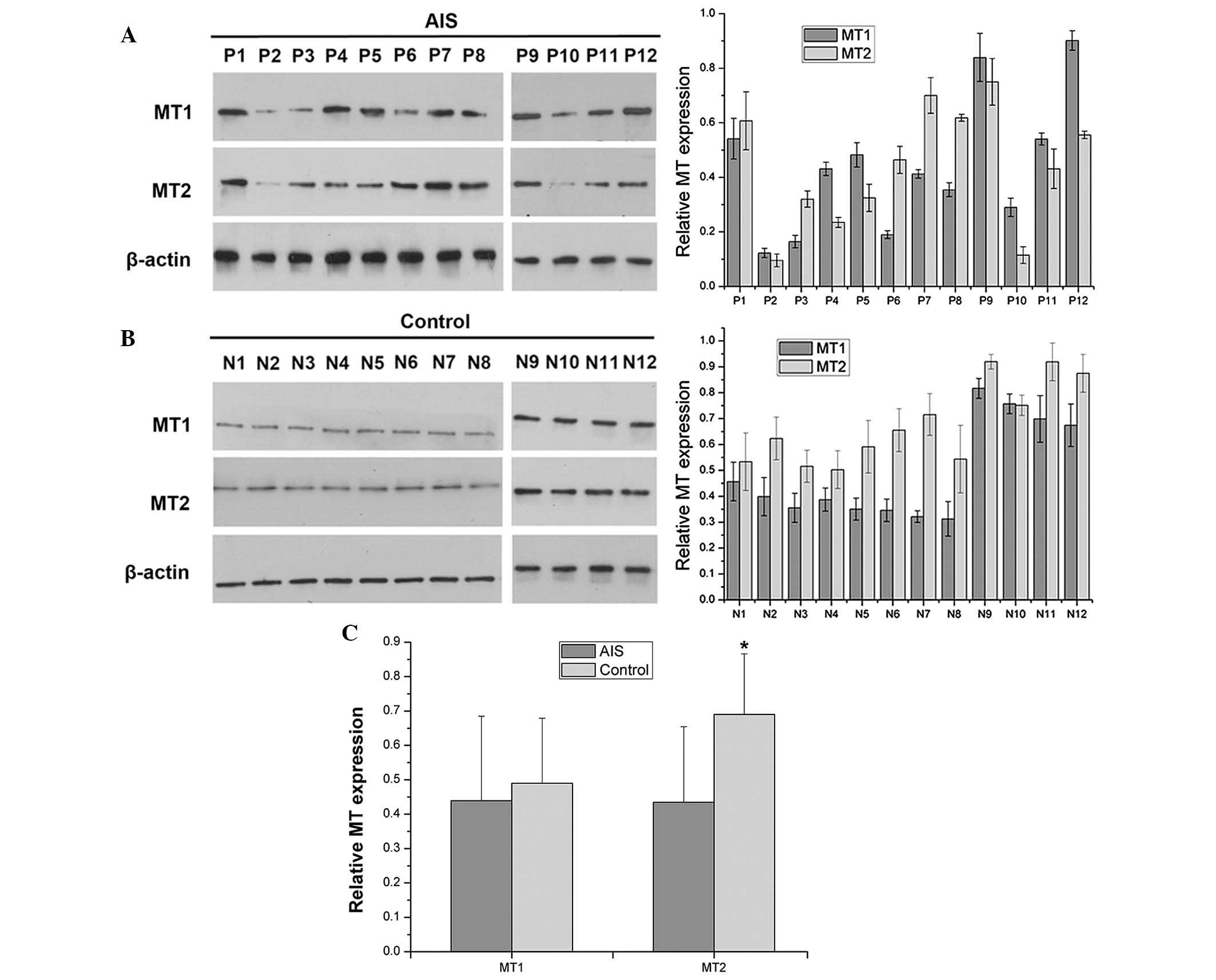 | Figure 1MTs expression in hMSCs. (A) hMSCs
isolated from 12 patients with AIS (P1-P12). Protein expression of
MT1 and MT2 was irregular in hMSCs from patients with AIS. hMSCs
from four subjects (P2, P3, P6, and P10) demonstrated low MT1
expression, and those from six subjects (P2-P5, P10, and P11)
exhibited low MT2 expression. Expression was extremely low in two
subjects (P2 and P10). (B) hMSCs isolated from 12 control subjects
(N1-N12). All hMSCs isolated from control subjects exhibited
uniform bands for MT1 and MT2. (C) Overall, MT2 demonstrated
reduced expression in the AIS group compared with control group.
n=12. *P<0.05 vs. control group. hMSCs, human
mesenchymal stem cells; AIS, adolescent idiopathic scoliosis; MT,
melatonin receptor. |
 | Table IIClinical data from patients with
AIS. |
Table II
Clinical data from patients with
AIS.
| Case | Diagnosis | Major curve
range | Sex | Age at surgery
(years) | Cobb angle (°) | Risser sign |
|---|
| P1 | AIS | T5-T11 | F | 14 | 57 | Grade III |
| P2 | AIS | T5-L1 | F | 14 | 51 | Grade II |
| P3 | AIS | T3-T11 | F | 15 | 66 | Grade III |
| P4 | AIS | T7-L3 | F | 19 | 68 | Grade V |
| P5 | AIS | T6-L2 | F | 14 | 75 | Grade II |
| P6 | AIS | T5-T11 | F | 15 | 72 | Grade IV |
| P7 | AIS | T10-L4 | F | 14 | 74 | Grade IV |
| P8 | AIS | T3-T10 | F | 16 | 70 | Grade IV |
| P9 | AIS | T7-L3 | M | 16 | 59 | Grade IV |
| P10 | AIS | T5-L2 | M | 16 | 55 | Grade IV |
| P11 | AIS | T4-T11 | M | 14 | 67 | Grade III |
| P12 | AIS | T6-L3 | M | 15 | 58 | Grade III |
 | Table IIIClinical data for control
subjects. |
Table III
Clinical data for control
subjects.
| Case | Diagnosis | Sex | Age at surgery
(years) |
|---|
| N1 | Fracture | F | 16 |
| N2 | Fracture | F | 17 |
| N3 | Musculoskeletal
tumor | F | 15 |
| N4 | Fracture | F | 18 |
| N5 | Fracture | F | 19 |
| N6 | Fracture | F | 24 |
| N7 | Fracture | F | 18 |
| N8 | Fracture | F | 15 |
| N9 | Fracture | M | 16 |
| N10 | Musculoskeletal
tumor | M | 17 |
| N11 | Fracture | M | 19 |
| N12 | Fracture | M | 19 |
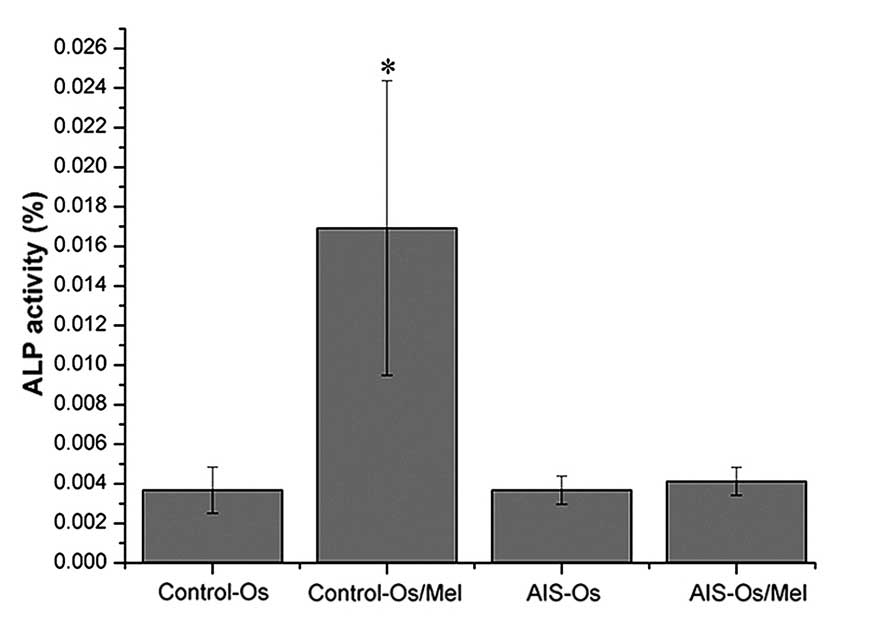
Lack of response of hMSCs to melatonin
treatment in osteo- genic differentiation in AIS patients
Following osteogenic differentiation for 12 days,
treatment with 50 nM melatonin significantly enhanced ALP activity
in normal control MSCs compared with untreated normal controls
(P=0.01685), indicating that these MSCs were sensitive to melatonin
during osteogenesis. By contrast, melatonin did not promote ALP
activity in the AIS groups (Fig.
2). Similarly, melatonin treatment enhanced the mRNA expression
levels of osteogenic marker genes, including ALP,
osteopontin, osteocalcin and RUNX2, in the normal control
group compared with the untreated normal control (P= 0.00472, P=
0.0177, P= 0.0285 and P=0.00726, respectively), however melatonin
exhibited no effect on the AIS groups (Fig. 3). Thus, MSCs from patients with AIS
appeared to be unresponsive to melatonin treatment during
osteogenesis.
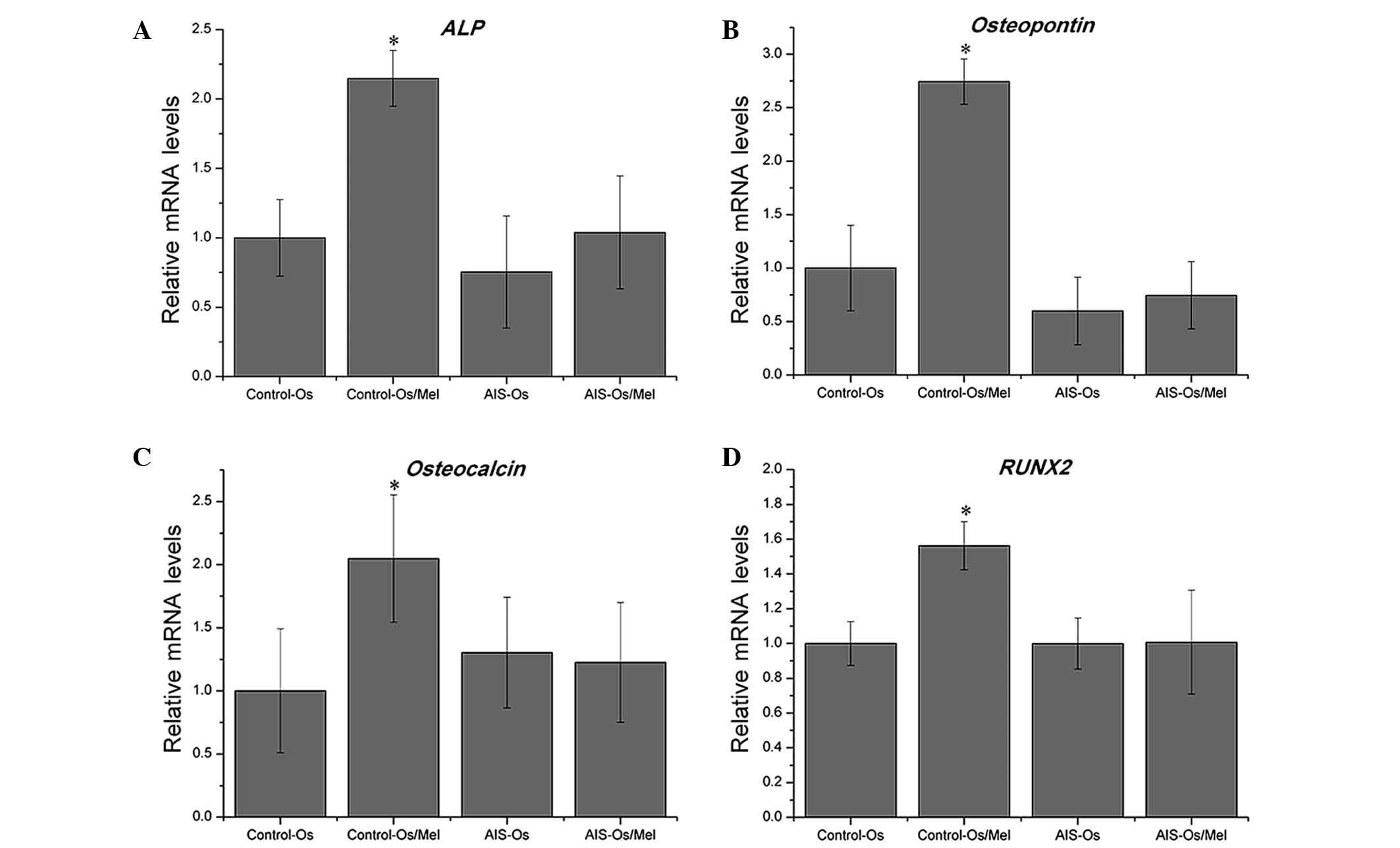 | Figure 3Effect of melatonin on osteogenic
differentiation and the expression of marker genes in human
mesenchymal stem cells. mRNA expression was measured at day 12, and
relative expression levels were calculated using the
2−ΔΔCt method. Melatonin treatment enhanced the
expression of osteogenic marker genes, including (A) ALP,
(B) osteopontin, (C) osteocalcin, and (D) RUNX2, in the
control groups, but not in the AIS groups. *P<0.05
vs. control-Os group. ALP, alkaline phosphatase; Os, osteogenesis;
AIS, adolescent idiopathic scoliosis; RUNX2, runt-related
transcription factor 2. |
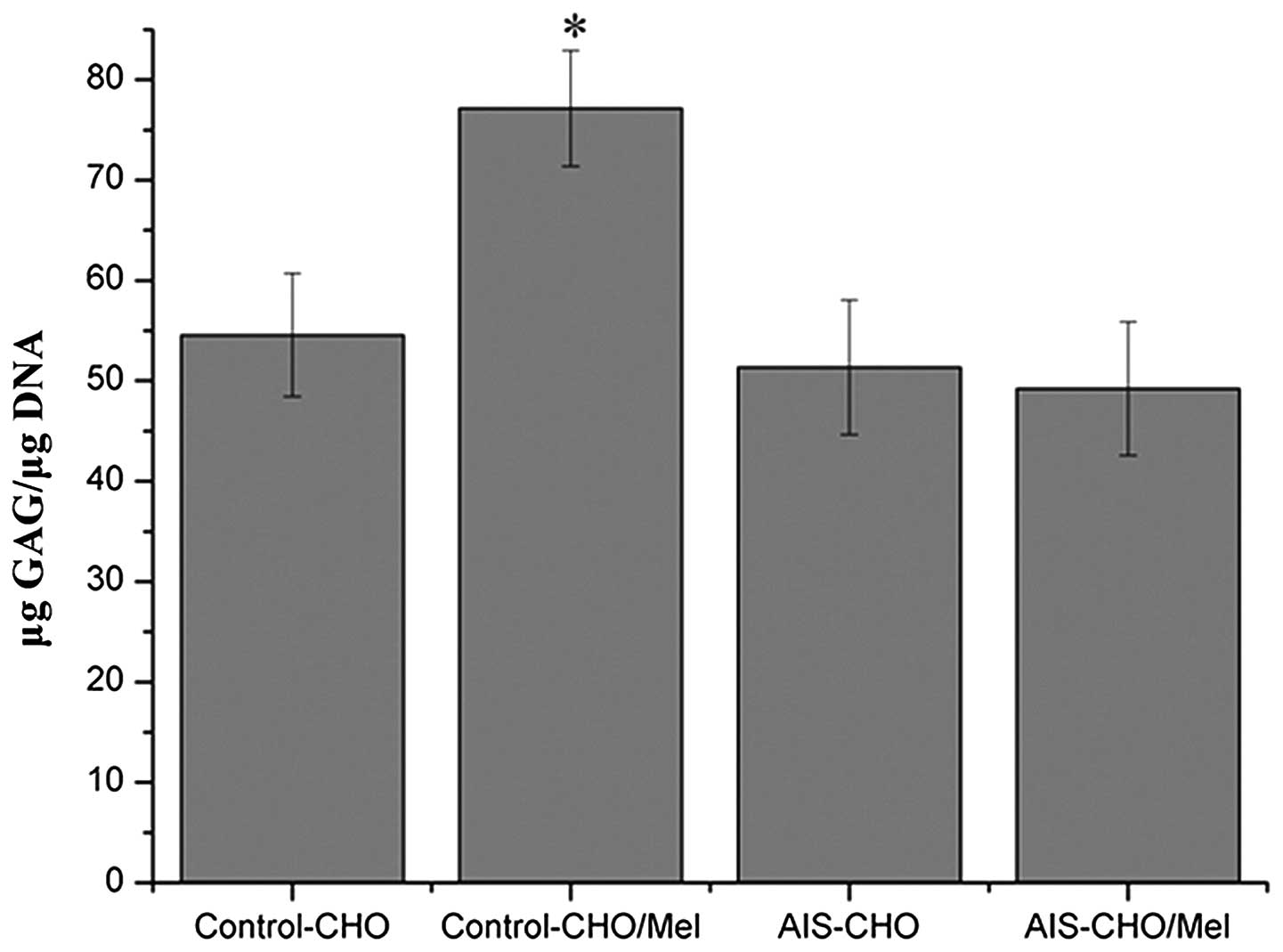
Lack of response of hMSCs to melatonin
treatment in chon-drogenic differentiation in AIS patients
During chondrogenic differentiation, melatonin
treatment increased GAG synthesis in normal control MSCs, which was
elevated significantly on day 21 compared with untreated normal
control cells (P=0.0318), however, melatonin exhibited no effect in
the AIS groups (Fig. 4).
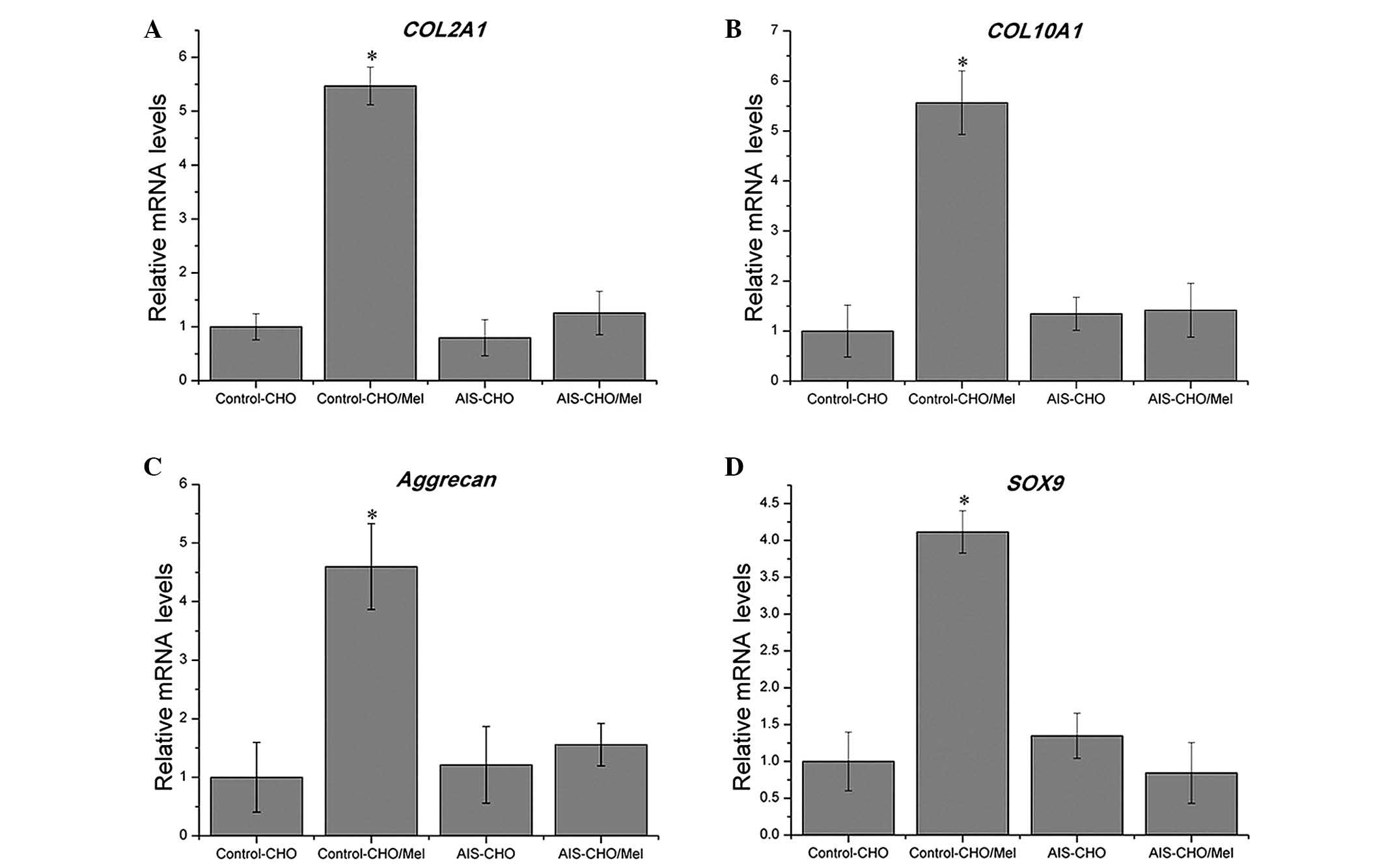
Consistent with the results of quantitative GAG analysis, during
chondrogenic differentiation, melatonin significantly upregulated
the mRNA expression levels of COL2A1, COL10A1,
aggrecan and SOX9 in the normal control group compared with
untreated cells (P=0.00415, P=0.01942, P=0.02773 and P=0.0069,
respectively), which are critical for chondrogenic differentiation,
however, no effect was observed in the AIS groups (Fig. 5). The SOX9 level was
marginally downregulated in the AIS-CHO/Mel group compared with the
AIS-CHO group, although this difference was not significant.
Discussion
This study investigated the effects of melatonin on
osteogenic and chondrogenic differentiation of hMSCs in patients
with AIS and control subjects. The lack of hMSC response to
melatonin during osteogenic and chondrogenic differentiation in AIS
samples may be attributed to alterations in the melatonin signaling
pathway.
MT1 and MT2, the two MT subtypes in mammals, are
members of the seven-transmembrane domain G protein-coupled
receptor family (24). Multiple
studies have demonstrated that an MT2 polymorphism is associated
with the occurrence of AIS and the severity of spinal curvature
(10,16,25,26).
Expression of MT2 mRNA is asymmetric in the bilateral paravertebral
muscles, with greater expression levels on the concave compared
with the convex side in patients with AIS (11). Wang et al (27) also demonstrated that mRNA
expression of MT2 was significantly reduced in growth plate
chondrocytes from patients with AIS compared with control subjects.
In the present study, MT2 expression was reduced in the AIS group
compared with control group. MT1 expression was lower in 4 subjects
with AIS and MT2 expression was lower in 6 subjects with AIS
(extremely low in 2 subjects). Subjects P2 and P10 demonstrated low
MT1 expression and extremely low MT2 expression, however, these
factors were not associated with AIS severity; these patients
demonstrated Cobb angles of 51° and 55°, respectively, which were
not the most severe in this sample population. However, in a
previous study MT2 expression was not detectable in the osteoblasts
of 4 out of 11 patients with AIS (15). Additionally, a previous study
demonstrated that longer arm span, as part of abnormal systemic
skeletal growth, was correlated with low MT2 expression in subjects
with AIS (28). Comprehensive
investigation of MT2, including RNA and protein expression, and
also the gene promoter polymorphism and signaling pathway analysis,
should be performed using hMSCs from subjects with AIS and controls
to further understand potential MT2 abnormalities.
Melatonin enhances ALP activity in the
differentiation processes of hMSCs cultured in osteogenic medium
via MT2 and the mitogen-activated protein kinase kinase
(MEK)/extracellular signal-regulated kinase 1/2 (ERK 1/2) signaling
cascade (29). Previously,
melatonin was demonstrated to stimulate proliferation and type I
collagen synthesis in human bone cells in vitro at maximal
stimulatory doses of 50–100 nM (30), and to promote osteoblast
differentiation and mineralization of matrix (31) through the bone morphogenetic
protein/ERK/Wnt signaling pathways (32), suggesting that this hormone is
involved in regulating bone growth. Furthermore, a previous study
reported that melatonin stimulated proliferation and ALP activity
during human osteoblastic differentiation in vitro, in a
dose-dependent manner at pharmacological concentrations (33). Melatonin suppresses the
proliferation and promotes the differentiation of rat dental
papilla cells, and enhances mineralized matrix formation (34). In the present study, melatonin
treatment led to increased expression of osteoblast differentiation
markers in control subjects, as demonstrated by ALP activity and
RT-qPCR assays. These results are in accordance with our previous
research (19,20). However, melatonin exhibited no
observable effect on hMSCs from subjects with AIS during osteogenic
differentiation. Man et al (15) also reported that melatonin was
unable to promote proliferation and differentiation of osteoblasts
in subjects with AIS, which might be associated with low bone
mineral density in these patients (35–38).
Proliferative and hypertrophic chondrocytes in the
anterior spinal columns of patients with AIS may affect spinal
curve development (39). Increased
numbers of these chondrocytes were observed in pinealectomized
(PNX) chickens, and rapid bone elongation was more pronounced in
chickens following PNX-induced osteoporosis, which may also
contribute to the development of scoliosis (40). The results of the present study
demonstrated that melatonin enhanced chondrogenic differentiation
of hMSCs in control subjects, in accordance with previous findings
(21), however, melatonin did not
affect chondrogenic differentiation in subjects with AIS. Abnormal
responses to melatonin during the proliferation and differentiation
of growth-plate chondrocytes in subjects with AIS have also been
reported previously (27).
Previous studies have suggested that AIS is
associated with the loss of coupling between endochondral and
membranous ossification during the developmental period (9,41–43).
Longitudinal growth, achieved mainly by endochondral ossification,
is more rapid than circumferential growth in vertebral bodies. By
contrast, circumferential growth in the vertebral bodies and
pedicles is slower in patients with AIS and achieved by membranous
ossification. Dissociation between longitudinal and circumferential
growth, resulting in overgrowth of the anterior column in the
scoliotic spine, may contribute to the development of AIS (9). The results of the present study
suggest that the melatonin signaling pathway is dysfunctional in
MSCs of patients with AIS, which may be important for abnormal
membranous and endochondral ossification during growth in these
subjects.
In the present study, a physiological concentration
of melatonin demonstrated functional abnormalities of the melatonin
signaling pathway in regulating hMSCs from patients with AIS, and
responses to pharmacological and high concentrations of melatonin
should be examined further. Furthermore, the mechanism underlying
the effects of MT2 on bone development in patients with AIS
requires further elucidation. IN the present study, there were
concerns regarding the in vitro culture of hMSCs from
primary cells and differentiation by osteogenesis or
chondrogenesis. Thus, future studies should explore differences in
treatment length and compare cellular responses to melatonin
following osteogenic and chondrogenic induction, and in osteoblasts
and chondrocytes from patients with AIS and controls.
To the best of our knowledge, the current report is
the first to describe the abnormal response of hMSCs to melatonin
during osteogenic and chondrogenic differentiation in patients with
AIS. These findings provide further evidence supporting the
presence of an abnormal systemic melatonin signaling pathway in
these patients, which may be associated with abnormal membranous
and endochondral skeletal growth. Notably, the present study
suggests a possible explanation for the modulating role of
melatonin via the MT2 receptor on abnormal osteogenic and
chondrogenic differentiation in patients with AIS, which may be a
mechanism underlying the etiopathogenesis of AIS.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81371908 and 81472039), the
Program for New Century Excellent Talents in University (grant no.
NCET-12-0564), and Fundamental Research Funds for the Central
Universities, Young Teachers Fund of Sun Yat-sen University (grant
no. 13YKPY23).
References
|
1
|
Hresko MT: Clinical practice. Idiopathic
scoliosis in adolescents. N Engl J Med. 368:834–841. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wise CA, Gao X, Shoemaker S, Gordon D and
Herring JA: Understanding genetic factors in idiopathic scoliosis,
a complex disease of childhood. Curr Genomics. 9:51–59. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Machida M: Cause of idiopathic scoliosis.
Spine (Phila Pa 1976). 24:2576–2583. 1999. View Article : Google Scholar
|
|
4
|
Cheung KM, Wang T, Qiu GX and Luk KD:
Recent advances in the aetiology of adolescent idiopathic
scoliosis. Int Orthop. 32:729–734. 2008. View Article : Google Scholar
|
|
5
|
Peng Y, Liang G, Pei Y, Ye W, Liang A and
Su P: Genomic polymorphisms of G-protein estrogen receptor 1 are
associated with severity of adolescent idiopathic scoliosis. Int
Orthop. 36:671–677. 2012. View Article : Google Scholar :
|
|
6
|
Kulis A, Goździalska A, Drag J, Jaśkiewicz
J, Knapik-Czajka M, Lipik E and Zarzycki D: Participation of sex
hormones in multifactorial pathogenesis of adolescent idiopathic
scoliosis. Int Orthop. 39:1227–1236. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chu WC, Lam WW, Chan YL, Ng BK, Lam TP,
Lee KM, Guo X and Cheng JC: Relative shortening and functional
tethering of spinal cord in adolescent idiopathic scoliosis? Study
with multiplanar reformat magnetic resonance imaging and
somatosensory evoked potential. Spine (Phila Pa 1976). 31:E19–E25.
2006. View Article : Google Scholar
|
|
8
|
Cheng JC, Hung VW, Lee WT, Yeung HY, Lam
TP, Ng BK, Guo X and Qin L: Persistent osteopenia in adolescent
idiopathic scoliosis-longitudinal monitoring of bone mineral
density until skeletal maturity. Stud Health Technol Inform.
123:47–51. 2006.
|
|
9
|
Guo X, Chau WW, Chan YL and Cheng JC:
Relative anterior spinal overgrowth in adolescent idiopathic
scoliosis. Results of disproportionate endochondral-membranous bone
growth. J Bone Joint Surg Br. 85:1026–1031. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu XS, Tang NL, Yeung HY, Lee KM, Hung
VW, Ng BK, Ma SL, Kwok RH, Qin L, Qiu Y and Cheng JC: Melatonin
receptor 1B (MTNR1B) gene polymorphism is associated with the
occurrence of adolescent idiopathic scoliosis. Spine (Phila Pa
1976). 32:1748–1753. 2007. View Article : Google Scholar
|
|
11
|
Qiu Y, Wu L, Wang B, Yu Y and Zhu Z:
Asymmetric expression of melatonin receptor mRNA in bilateral
paravertebral muscles in adolescent idiopathic scoliosis. Spine
(Phila Pa 1976). 32:667–672. 2007. View Article : Google Scholar
|
|
12
|
Lombardi G, Akoume MY, Colombini A, Moreau
A and Banfi G: Biochemistry of adolescent idiopathic scoliosis. Adv
Clin Chem. 54:165–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Azeddine B, Letellier K, Wang da S,
Moldovan F and Moreau A: Molecular determinants of melatonin
signaling dysfunction in adolescent idiopathic scoliosis. Clin
Orthop Relat Res. 462:45–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moreau A, Wang DS, Forget S, Azeddine B,
Angeloni D, Fraschini F, Labelle H, Poitras B, Rivard CH and
Grimard G: Melatonin signaling dysfunction in adolescent idiopathic
scoliosis. Spine (Phila Pa 1976). 29:1772–1781. 2004. View Article : Google Scholar
|
|
15
|
Man GC, Wang WW, Yeung BH, Lee SK, Ng BK,
Hung WY, Wong JH, Ng TB, Qiu Y and Cheng JC: Abnormal proliferation
and differentiation of osteoblasts from girls with adolescent
idiopathic scoliosis to melatonin. J Pineal Res. 49:69–77.
2010.PubMed/NCBI
|
|
16
|
Man GC, Wong JH, Wang WW, Sun GQ, Yeung
BH, Ng TB, Lee SK, Ng BK, Qiu Y and Cheng JC: Abnormal melatonin
receptor 1B expression in osteoblasts from girls with adolescent
idiopathic scoliosis. J Pineal Res. 50:395–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruder SP, Fink DJ and Caplan AI:
Mesenchymal stem cells in bone development, bone repair, and
skeletal regeneration therapy. J Cell Biochem. 56:283–294. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kelly DJ and Jacobs CR: The role of
mechanical signals in regulating chondrogenesis and osteogenesis of
mesenchymal stem cells. Birth Defects Res C Embryo Today. 90:75–85.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Su P, Xu C, Chen C, Liang A, Du
K, Peng Y and Huang D: Melatonin inhibits adipogenesis and enhances
osteogenesis of human mesenchymal stem cells by suppressing PPARγ
expression and enhancing Runx2 expression. J Pineal Res.
49:364–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Zhang J, Ling Y, Chen C, Liang A,
Peng Y, Chang H, Su P and Huang D: Sustained release of melatonin
from poly (lactic-co-glycolic acid) (PLGA) microspheres to induce
osteogenesis of human mesenchymal stem cells in vitro. J Pineal
Res. 54:24–32. 2013.
|
|
21
|
Gao W, Lin M, Liang A, Zhang L, Chen C,
Liang G, Xu C, Peng Y, Chen C, Huang D and Su P: Melatonin enhances
chondrogenic differentiation of human mesenchymal stem cells. J
Pineal Res. 56:62–70. 2014. View Article : Google Scholar
|
|
22
|
Zhang L, Su P, Xu C, Yang J, Yu W and
Huang D: Chondrogenic differentiation of human mesenchymal stem
cells: A comparison between micromass and pellet culture systems.
Biotechnol Lett. 32:1339–1346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
von Gall C, Stehle JH and Weaver DR:
Mammalian melatonin receptors: Molecular biology and signal
transduction. Cell Tissue Res. 309:151–162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu XS, Tang NL, Yeung HY, Cheng JC and
Qiu Y: Lack of association between the promoter polymorphism of the
MTNR1A gene and adolescent idiopathic scoliosis. Spine (Phila Pa
1976). 33:2204–2207. 2008. View Article : Google Scholar
|
|
26
|
Nelson LM, Ward K and Ogilvie JW: Genetic
variants in melatonin synthesis and signaling pathway are not
associated with adolescent idiopathic scoliosis. Spine (Phila Pa
1976). 36:37–40. 2011. View Article : Google Scholar
|
|
27
|
Wang WW, Man GC, Wong JH, Ng TB, Lee KM,
Ng BK, Yeung HY, Qiu Y and Cheng JC: Abnormal response of the
proliferation and differentiation of growth plate chondrocytes to
melatonin in adolescent idiopathic scoliosis. Int J Mol Sci.
15:17100–17114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yim AP, Yeung HY, Sun G, Lee KM, Ng TB,
Lam TP, Ng BK, Qiu Y, Moreau A and Cheng JC: Abnormal skeletal
growth in adolescent idiopathic scoliosis is associated with
abnormal quantitative expression of melatonin receptor, MT2. Int J
Mol Sci. 14:6345–6358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Radio NM, Doctor JS and Witt-Enderby PA:
Melatonin enhances alkaline phosphatase activity in differentiating
human adult mesenchymal stem cells grown in osteogenic medium via
MT2 melatonin receptors and the MEK/ERK (1/2) signaling cascade. J
Pineal Res. 40:332–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakade O, Koyama H, Ariji H, Yajima A and
Kaku T: Melatonin stimulates proliferation and type I collagen
synthesis in human bone cells in vitro. J Pineal Res. 27:106–110.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roth JA, Kim BG, Lin WL and Cho MI:
Melatonin promotes osteoblast differentiation and bone formation. J
Biol Chem. 274:22041–22047. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park KH, Kang JW, Lee EM, Kim JS, Rhee YH,
Kim M, Jeong SJ, Park YG and Kim SH: Melatonin promotes
osteoblastic differentiation through the BMP/ERK/Wnt signaling
pathways. J Pineal Res. 51:187–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Satomura K, Tobiume S, Tokuyama R,
Yamasaki Y, Kudoh K, Maeda E and Nagayama M: Melatonin at
pharmacological doses enhances human osteoblastic differentiation
in vitro and promotes mouse cortical bone formation in vivo. J
Pineal Res. 42:231–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu J, Zhou H, Fan W, Dong W, Fu S, He H
and Huang F: Melatonin influences proliferation and differentiation
of rat dental papilla cells in vitro and dentine formation in vivo
by altering mitochondrial activity. J Pineal Res. 54:170–178. 2013.
View Article : Google Scholar :
|
|
35
|
Burner WL III, Badger VM and Sherman FC:
Osteoporosis and acquired back deformities. J Pediatr Orthop.
2:383–385. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng JC and Guo X: Osteopenia in
adolescent idiopathic scoliosis. A primary problem or secondary to
the spinal deformity? Spine (Phila Pa 1976). 22:1716–1721. 1997.
View Article : Google Scholar
|
|
37
|
Cheng JC, Guo X and Sher AH: Persistent
osteopenia in adolescent idiopathic scoliosis. A longitudinal
follow up study. Spine (Phila Pa 1976). 24:1218–1222. 1999.
View Article : Google Scholar
|
|
38
|
Yu WS, Chan KY, Yu FW, Yeung HY, Ng BK,
Lee KM, Lam TP and Cheng JC: Abnormal bone quality versus low bone
mineral density in adolescent idiopathic scoliosis: A case-control
study with in vivo high-resolution peripheral quantitative computed
tomography. Spine J. 13:1493–1499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu F, Qiu Y, Yeung HY, Lee KM and Cheng
JC: Histomorphometric study of the spinal growth plates in
idiopathic scoliosis and congenital scoliosis. Pediatr Int.
48:591–598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aota Y, Terayama H, Saito T and Itoh M:
Pinealectomy in a broiler chicken model impairs endochondral
ossification and induces rapid cancellous bone loss. Spine J.
13:1607–1616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dickson RA, Lawton JO, Archer IA and Butt
WP: The pathogenesis of idiopathic scoliosis. Biplanar spinal
asymmetry. J Bone Joint Surg Br. 66:8–15. 1984.PubMed/NCBI
|
|
42
|
Parent S, Labelle H, Skalli W, Latimer B
and de Guise J: Morphometric analysis of anatomic scoliotic
specimens. Spine (Phila Pa 1976). 27:2305–2311. 2002. View Article : Google Scholar
|
|
43
|
Dayer R, Haumont T, Belaieff W and
Lascombes P: Idiopathic scoliosis: Etiological concepts and
hypotheses. J Child Orthop. 7:11–16. 2013. View Article : Google Scholar :
|