Introduction
The distal-less homeobox (Dlx) gene family contains
six members (Dlx1-6), which are organized into three convergent
pairs (Dlx1/2, Dlx3/4 and Dlx5/6), located on chromosome 2 in mice,
and exhibit a nesting expression pattern in the first and second
branchial arch region (1,2). Dlx1 and 2 and are expressed in the
epithelium and the cranial neural crest cells (CNCCs) derived from
mesenchyme in the maxillary and mandibular processes (2,3).
Previous studies based on knock-out mutant mice revealed that a
null mutant of Dlx2 may lead to malformation in craniofacial
tissues, including maxilla, nasal bone, auditory ossicle and
maxillary teeth. The 'Dlx family' regionalize the jaw primordium
with Dlx1/2 regulating upper jaw development and Dlx5/6 regulating
the development of the lower jaw (2,4–8). A
previous study also determined that an overexpression mutant of
Dlx2 leads to sorting and aggregation of CNCCs (9). Additionally, it has been demonstrated
that Dlx2 overexpression may result in ectopic skeletal and
cartilaginous elements, in the maxillary and mandibular region
in ovo (10). Our previous
study also revealed that overexpressing Dlx2 in neural crest cells
(NCCs) in mice, resulted in midfacial clefts, nasal and
premaxillary hypoplasia and spinal deformities (11).
The mandibular condyle in mammals is a primary site
of mandible growth and a major element of the temporal-mandibular
joint (TMJ) (12,13). The mandibular condylar cartilage is
ontogenetically characterized as a secondary cartilage, and is
important for the development and function of TMJ (14). Although numerous factors are
involved in the development of the mandibular condyle and certain
genes have been previously demonstrated to have interactions with
the Dlx gene family (14,15), to the best of our knowledge, the
exact molecular mechanisms regulating the emergence and cellular
organization of the condylar cartilage and the degradation of
condylar cartilage remain to be fully elucidated.
Regarding the effects of overexpression of Dlx2 in
the craniofacial region, previous studies in virus-infected ovo or
transgenic mice have determined that the overexpression of Dlx2
primarily affects the development of tissues from the maxillary
arch (10,11). The comprehensive effects of
overexpression of Dlx2 on mammalian mandibular condyle remains to
be fully elucidated. The aim of the present study was to assess the
effects of overexpression of Dlx2 on postnatal condyle
development.
Materials and methods
Mouse strains
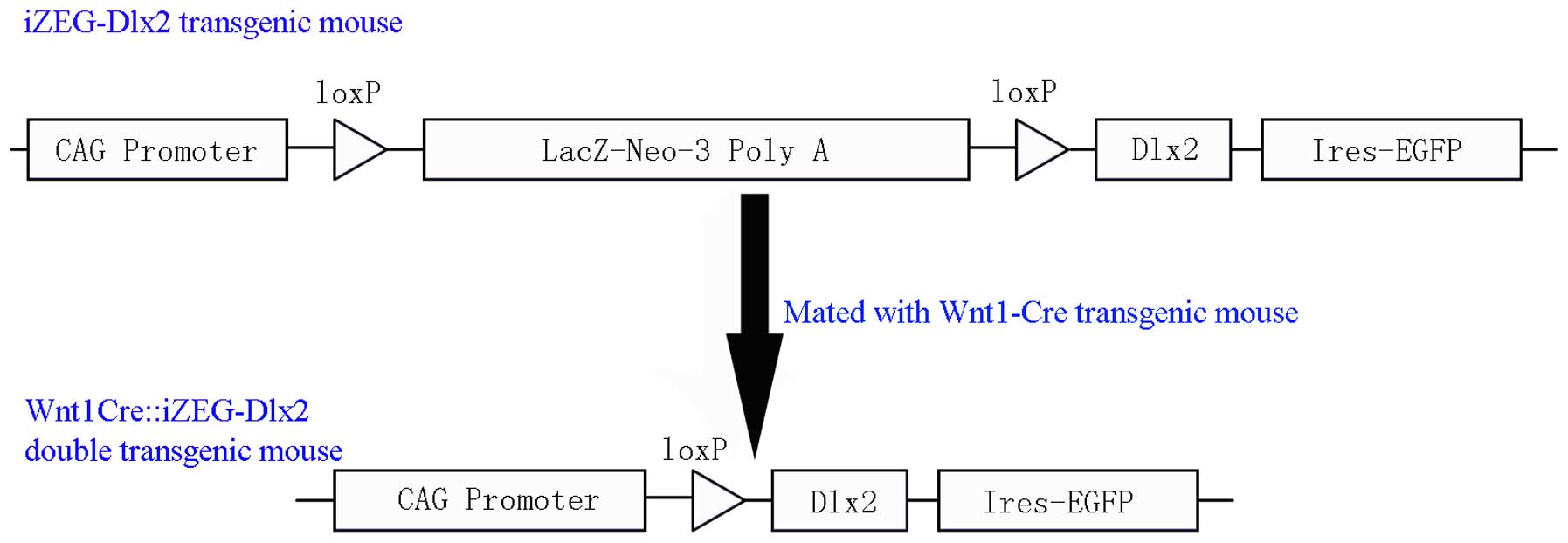
Dlx2 C57BL/6J genetic background conditional
overexpression transgenic mice (iZEG-Dlx2) were bred in Department
of Oral and Cranio-Maxillofacial Surgery, Shanghai Ninth People's
Hospital (Shanghai, China), and Wnt1-Cre transgenic mice were
obtained from the Jackson Laboratory (Bar Harbor, Maine, USA).
iZEG-Dlx2 transgenic mice were further bred with Wnt1-Cre
transgenic mice to obtain the mice, which specifically overexpress
Dlx2 in NCC derived tissues (Wnt1Cre::iZEG-Dlx2), as previously
described (Fig. 1) (11). All animal experimental procedures
were performed in compliance with the guidelines of the National
Institute of Health in the United States and Institutional Animal
Care and Use Committees of the Shanghai Ninth People's Hospital,
Shanghai Jiao Tong University School of Medicine, and were reviewed
and approved by the Ethical Committees of the Shanghai Ninth
People's Hospital, Shanghai Jiao Tong University School of
Medicine, Shanghai, China (approval no, 2013-7).
Mandible preparations
The mice (age, 90 days; mean weight, 25.2 g) were
housed in a pathogen-free condition with a 14/10-h light/dark
cycle, in conditions of 18–23°C and 40–60% humidity. Mice were
euthanized by carbon dioxide. All mice were used for analysis
regardless of gender. The mandibles of eight P90 Wnt1Cre::iZEG-Dlx2
and control mice were separated from the surrounding tissue, and
were transferred to 95% ethanol for 48 h. The tissues were
subsequently stained with Alizarin red and Alcian blue, as
previously described (11).
Subsequently, the stained/unstained condyle was transferred to a
mixed solution of glycerol and water (50:50) for image capturing
under an integrated microscope.
Histological analysis
The condyles of P90 Wnt1Cre::iZEG-Dlx2 and control
mice were dissected, followed by fixation in 4% paraformaldehyde
for 24 h at room temperature and demineralization in 0.5 M EDTA for
14–20 days also at room temperature. The tissues were embedded in
paraffin with a section at thickness of 5 µm, then stained
with hematoxylin and eosin (H&E) or Alizarin red/Alcian blue,
as previously described (11),
followed by mounting with resinous mounting medium for image
capturing on an integrated microscope.
Micro-computed tomography (CT) scans
The skulls/mandibles of the P90 control and
Wnt1Cre::iZEG-Dlx2 mice were fixed with 4% paraformaldehyde at room
temperature for 24 h. Next, the tissues were scanned using an
eXplore Locus MicroCT scanner (GE Healthcare Life Sciences,
Milwaukee, WI, USA). The slice thickness used for micro CT scans
was 10 µm. Reconstruction of 3D skulls and bone mineral
density (BMD) calculations were performed using GE MicroView
software version 2.2 (GE Healthcare Life Sciences).
Immunohistochemistry
The condyles of the P90 control and
Wnt1Cre::iZEG-Dlx2 mice were demineralized and embedded in
paraffin, and sectioned at a thickness of 5 µm. The
immunohistochemistry process was performed as previously described
(11,16). Briefly, antigen retrieval for
slides was performed with a bone antigen restoration liquid kit
(Sunteam Biotech Co., Ltd., China) and subsequently, the samples
were blocked for 60 min with 3% bovine serum albumin in
phosphate-buffered saline (PBS) containing 0.2% Triton-X-100.
Subsequently, the sections were incubated with anti-Dlx2 (1:100;
Abcam, Cambridge, UK; cat. no. ab18188), anti-osteocalcin (OCN;
1:200; Abcam; cat. no. ab93876) or anti-msh homeobox 2 (Msx2;
1:100; Abcam; cat. no. ab69058) overnight at 4°C. Following
incubation, donkey anti-rabbit secondary antibodies (AlexaFluor
488-conjugated; Thermo Fisher Scientific, Inc.; cat. no. A21206)
were diluted in PBS (1:300) and incubated for 60 min at room
temperature. Finally, the slides were mounted with Vectashield
mounting medium containing 4′,6-diamidino-2-phenylindole
(Invitrogen; Thermo Fisher Scientific, Inc.) was used to mount the
cover-slips. Images were capture under a fluorescence
microscope.
Statistical analysis
All data were expressed as the mean ± standard
deviation. The difference between experimental groups was assessed
by independent Student's t-test using SPSS software version 18.0
(IBM SPSS, Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Establishment of Wnt1Cre::iZEG-Dlx2 mouse
strain
Dlx2 (C57BL/6J genetic background) conditional
overexpression transgenic mice (iZEG-Dlx2) were bred in our
laboratory. The iZEG-Dlx2 transgenic mice were mated with Wnt1-Cre
transgenic mice to obtain mice, which specifically overexpress Dlx2
in NCC-derived tissues (Wnt1Cre::iZEG-Dlx2). This was performed as
previously described in detail (Fig.
1) (11).
Morphology of condyles of P90
Wnt1Cre::iZEG-Dlx2 mice
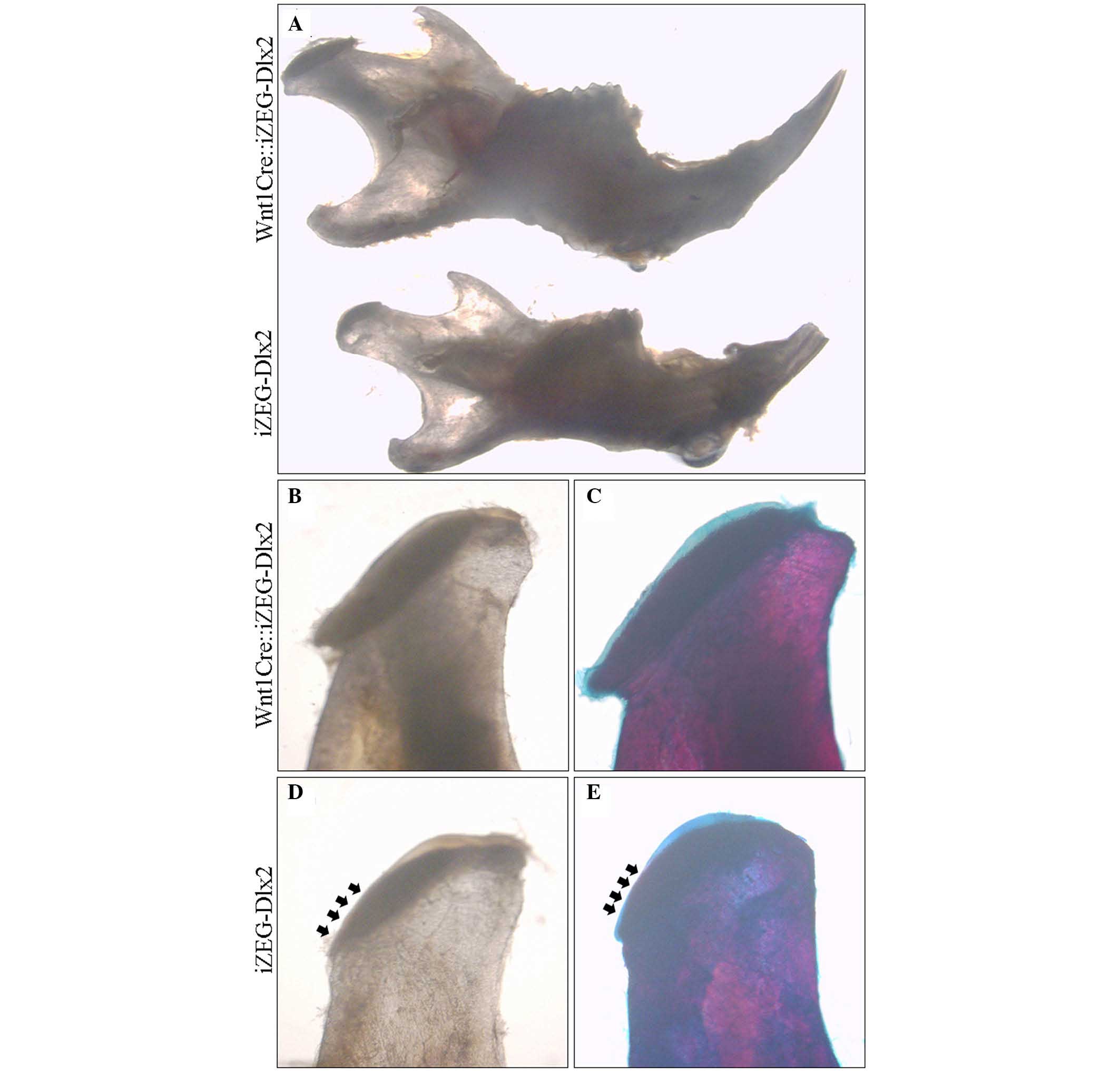
Observation under an integrated microscope
demonstrated that P90 Wnt1Cre::iZEG-Dlx2 mice exhibited smaller
mandible and condyle, and the condylar cartilage cap was malformed
and thinner compared with the control group (Fig. 2). At P90, the reconstructed 3D
images from micro-CT highlighted a cross-bite malocclusion, and
bone defect in the mandible of Wnt1Cre::iZEG-Dlx2 mice (Fig. 3A and B). The 3D images and sagittal
sections also showed osteoporosis in the condylar bone when
compared with the control group (Fig.
3C and D). BMD analysis based on micro-CT examination suggested
a significant decrease in BMD in the condylar bone in P90
Wnt1Cre::iZEG-Dlx2 mice compared with control mice (P<0.01;
Fig. 3E). This demonstrated that
osteoporosis occurred in the condyle, and implied that
overexpression of Dlx2 may lead to condyle degradation or abnormal
development.
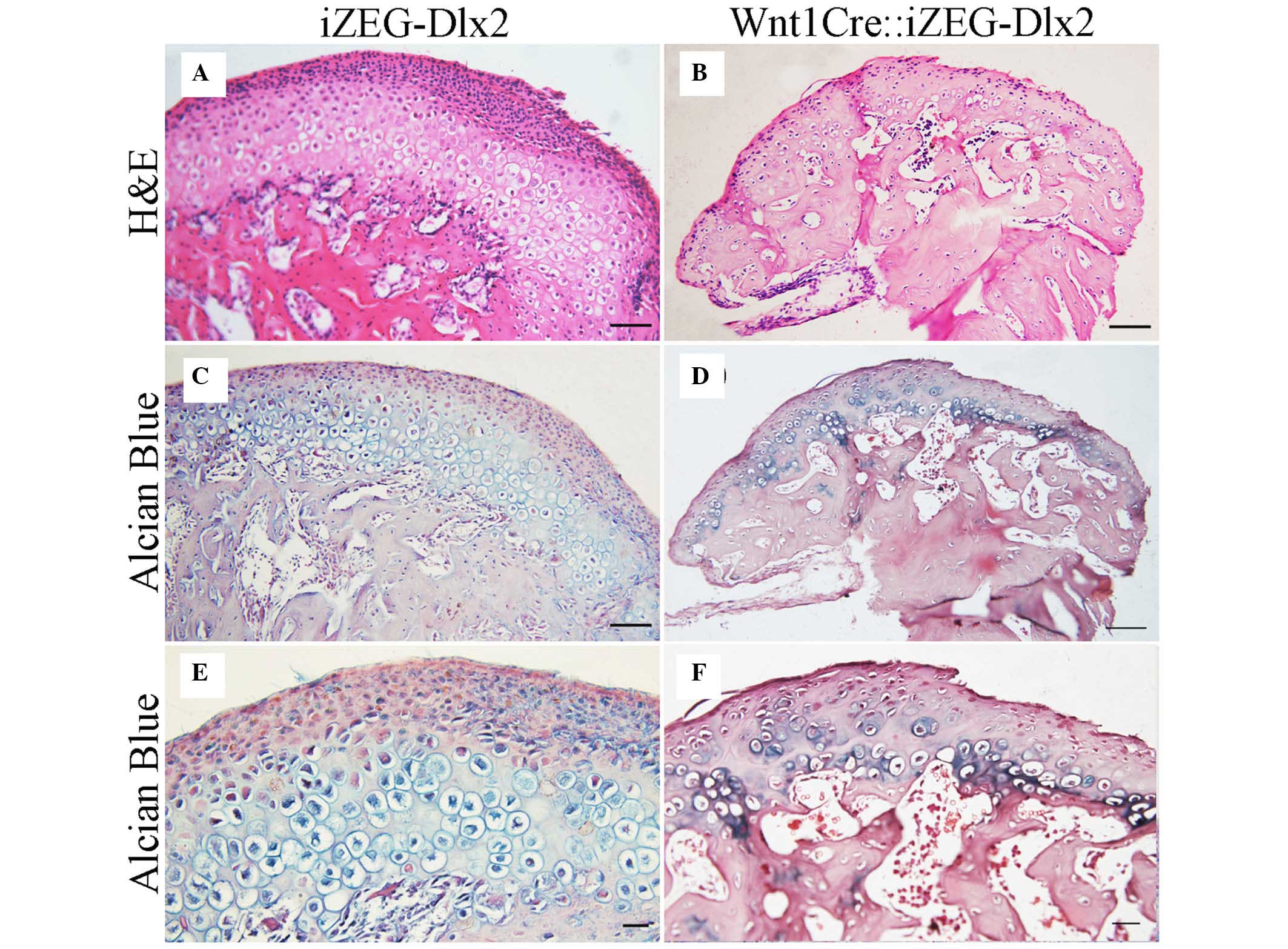
Histological appearance of condyle of P90
Wnt1Cre::iZEG-Dlx2 mice
The condyle of P90 Wnt1Cre::iZEG-Dlx2 mice was
analyzed in detail. H&E and Alizarin red/Alcian blue staining
of coronal sections indicated that the Wnt1Cre::iZEG-Dlx2 mice
exhibited an irregular condylar cartilage structure and a uneven
condylar cartilage surface. Condylar cartilage were disorganized
and narrower, irregular in size and lacked a regular arrangement of
four layers, including resting layer, proliferative layer,
hypertrophic layer and mineralized zone. Additionally, less
hypertrophic chondrocytes were observed in the condylar cartilage
in P90 Wnt1Cre::iZEG-Dlx2 mice (Fig.
4). Bone loss was also evident in the condylar bone region when
compared with the control group (Fig.
4). The phenotype demonstrated was observed in six condyle from
three mice. Although the three mice exhibited similar phenotype,
the severity was not consistent in each mouse; therefore, the
expression pattern of Dlx2 may not be the same across the different
mice.
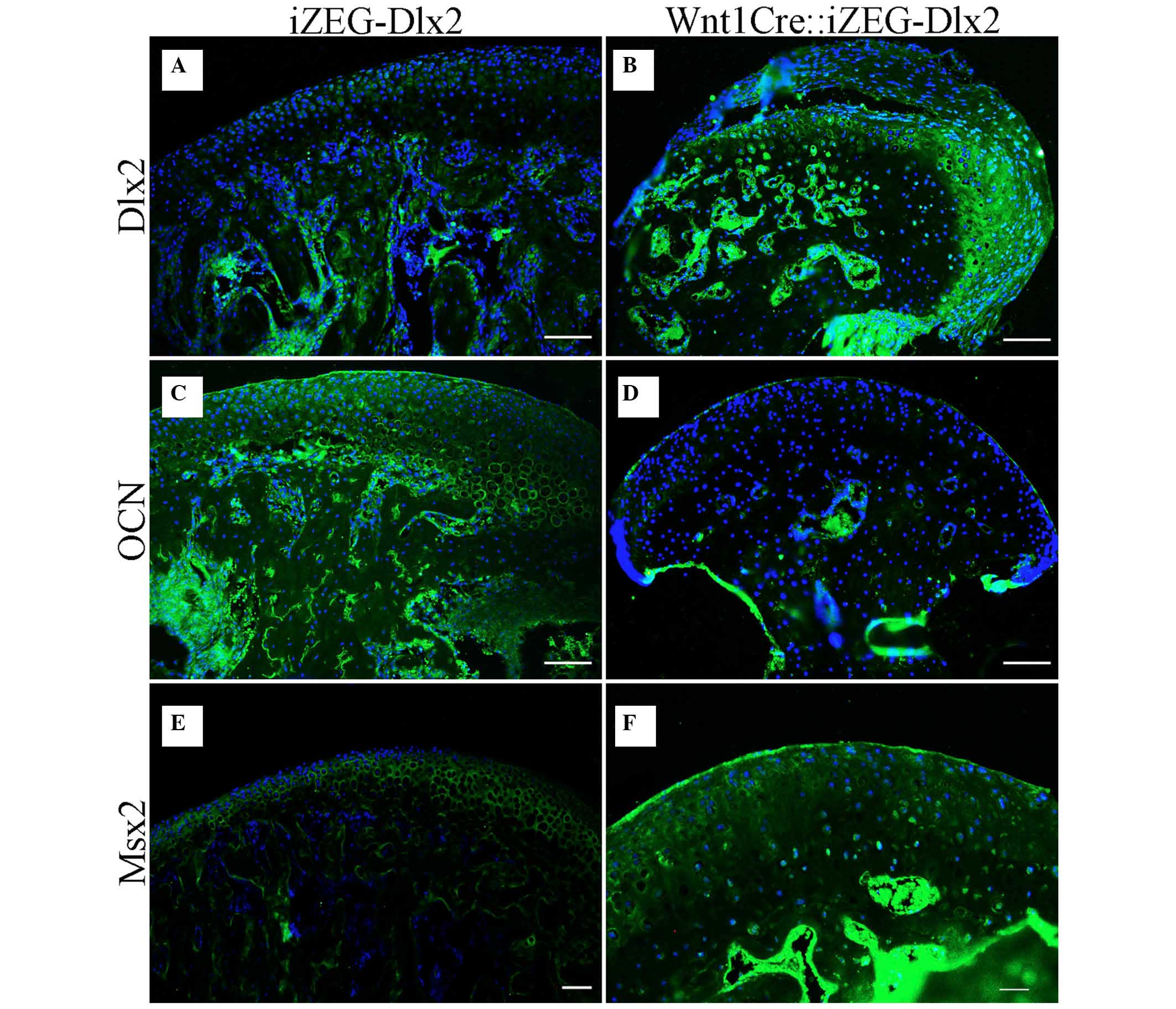
Expression of Dlx2, OCN and Msx2 in the
condylar region in P90 Wnt1Cre::iZEG-Dlx2 mice
Immunofluorescence staining also indicated that Dlx2
expression levels were higher in the condylar region in P90
Wnt1Cre::iZEG-Dlx2 mice (Fig. 5A and
B). The expression levels of OCN, a marker of osteogenesis,
were downregulated in the condylar cartilage and bone region in P90
Wnt1Cre::iZEG-Dlx2 mice (Fig. 5C and
D). Therefore, it is possible that osteogenesis has been
impaired in the condylar region. The expression of Msx2, important
for craniofacial bone development, which had been identified to
interact with Dlx2 in previous studies (17–20),
was upregulated in the condylar region, particularly in the resting
layer and mineralized zone (Fig. 5E
and F). Dlx2 may interact with Msx2 in the process of condyle
development or degradation.
Discussion
Previous studies have determined that inactivation
of Dlx1 and Dlx2 results in defects in the upper jaw, whereas
inactivation of Dlx5 and Dlx6 leads to homeotic transformation of
the lower jaw into an upper jaw (2). Therefore, the Dlx family may
regionalize the jaw primordium, and Dlx1/2 regulate upper jaw
development, whereas Dlx5/6 control the lower jaw (6–8). The
present study was the first, to the best of our knowledge, to
describe the effect of overexpression of Dlx2 on postnatal condyle
degradation, and revealed that the Dlx2 overexpression in NCCs in
mice may lead to postnatal condyle malformation and osteoporosis.
The present study revealed that Dlx2 had effect on the lower jaw.
It is possible that the Dlx1/2 vs. Dlx5/6 proteins exert unique
roles in specifying maxilla and mandible (qualitative hypothesis),
whereas higher levels of total Dlx protein in mandible can disrupt
the development of condyle (quantitative hypothesis) (6). Previous studies also revealed that
Dlx6 activity in lower jaw development is partially shared by
Dlx1/2. Additionally, Dlx1/2 function is partially redundant with
Dlx5/6 in regulating mandibular gene expression, which may further
support the findings of the present study (6). The differences in expression level,
pattern or timing in the transgenic mice may also contribute to
this phenotype.
In condyles overexpressing Dlx2, an irregular
histological structure was observed; the resting layer was thinner
and more likely to undergo premature maturation and hypertrophy. As
a consequence, condylar morphology and growth are limited, possibly
due to reduced chondroprogenitor cell proliferation and impaired
osteogenesis. Lower expression levels of OCN in condyle of the mice
model overexpressing Dlx2 confirm this hypothesis, which is similar
to Ihh and Cilia mutant mice (17,18).
Our previous study also determined that overexpression of Dlx2
disturbs the nasal bone/cartilage maxilla development, and leads to
irregular histological structure of the spinal cartilage (11). In addition, previous studies have
demonstrated that Dlx2 overexpression may result in ectopic
skeletal and cartilaginous elements in the maxillary and mandibular
region in ovo (10). Taken
together, it may be suggested that Dlx2 is important for the
development of bone and cartilage.
The present study also indicated an upregulation of
Msx2 in the condylar region of Wnt1Cre::iZEG-Dlx2 mice, which was
similar to our previous findings in the craniofacial region
(11). Previous studies revealed
that Msx2 is a downstream gene of Dlx2, and heterodimerization of
Msx2 with Dlx2 results in functional antagonism. In addition, Msx2
is important for craniofacial development, and overexpression of
Msx2 impedes osteoblast differentiation and triggers multiple
craniofacial defects (19–21). Therefore, overexpressed Dlx2 may
interact with Msx2 to regulate the condyle degradation.
Osteoarthritis (OA), typically characterized as
progressive cartilage degradation and subchondral bone changes, is
a severe pathological change in the condyle of patients with severe
temporomandibular disorder (TMD) (22,23).
Previous studies have indicated that low BMD and increased bone
turnover in the early stages of OA in the knee joint, and the
inhibition of bone resorption for treating OA, which implied that
abnormal subchondral bone remodeling is crucial for the development
of OA (23,24). In the present study, the Dlx2
overexpressed postnatal condyle resulted in degraded cartilage and
subchondral bone osteoporosis, which was similar to a previous
study that reported OA transgenic mice overexpressed transforming
growth factor-β in the subchondral bone (24). Therefore, the present findings
implied that overexpression of Dlx2 may contribute to TMJ OA, and
present a possible TMJ OA model for future studies.
In conclusion, Dlx2 overexpression in NCCs may lead
to postnatal condyle malformation, subchondral bone degradation and
irregular histological structure of condylar cartilage.
Additionally, Dlx2 overexpression affected postnatal mandible
development and may be used in future TMJ OA model animal studies.
The exact molecular mechanisms underlying the effect observed in
the present study must be investigated in the future.
Acknowledgments
The present study was supported by the National
Nature Science Foundation of China (nos. 81300842 and 81271122),
Emerging Cutting-Edge Technology Joint Research Project of Shanghai
Municipal Hospital (no. SHDC12013103) and the Program for
Innovation Research Team of Shanghai Municipal Education
Commission.
References
|
1
|
Panganiban G and Rubenstein JL:
Developmental functions of the Distal-less/Dlx homeobox genes.
Development. 129:4371–4386. 2002.PubMed/NCBI
|
|
2
|
Depew MJ, Simpson CA, Morasso M and
Rubenstein JL: Reassessing the Dlx code: The genetic regulation of
branchial arch skeletal pattern and development. J Anat.
207:501–561. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thomas BL, Liu JK, Rubenstein JL and
Sharpe PT: Independent regulation of Dlx2 expression in the
epithelium and mesenchyme of the first branchial arch. Development.
127:217–224. 2000.
|
|
4
|
Qiu M, Bulfone A, Martinez S, Meneses JJ,
Shimamura K, Pedersen RA and Rubenstein JL: Null mutation of Dlx-2
results in abnormal morphogenesis of proximal first and second
branchial arch derivatives and abnormal differentiation in the
forebrain. Genes Dev. 9:2523–2538. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiu M, Bulfone A, Ghattas I, Meneses JJ,
Christensen L, Sharpe PT, Presley R, Pedersen RA and Rubenstein JL:
Role of the Dlx homeobox genes in proximodistal patterning of the
branchial arches: Mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter
morphogenesis of proximal skeletal and soft tissue structures
derived from the first and second arches. Dev Biol. 185:165–184.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeong J, Li X, McEvilly RJ, Rosenfeld MG,
Lufkin T and Rubenstein JL: Dlx genes pattern mammalian jaw
primordium by regulating both lower jaw-specific and upper
jaw-specific genetic programs. Development. 135:2905–2916. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beverdam A, Merlo GR, Paleari L, Mantero
S, Genova F, Barbieri O, Janvier P and Levi G: Jaw transformation
with gain of symmetry after Dlx5/Dlx6 inactivation: Mirror of the
past? Genesis. 34:221–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Depew MJ, Lufkin T and Rubenstein JL:
Specification of jaw subdivisions by Dlx genes. Science.
298:381–385. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McKeown SJ, Newgreen DF and Farlie PG:
Dlx2 over-expression regulates cell adhesion and mesenchymal
condensation in ecto-mesenchyme. Dev Biol. 281:22–37. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gordon CT, Brinas IM, Rodda FA, Bendall AJ
and Farlie PG: Role of Dlx genes in craniofacial morphogenesis:
Dlx2 influences skeletal patterning by inducing ectomesenchymal
aggregation in ovo. Evol Dev. 12:459–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dai J, Kuang Y, Fang B, Gong H, Lu S, Mou
Z, Sun H, Dong Y, Lu J, Zhang W, et al: The effect of
overexpression of Dlx2 on the migration, proliferation and
osteogenic differentiation of cranial neural crest stem cells.
Biomaterials. 34:1898–1910. 2013. View Article : Google Scholar
|
|
12
|
Shibukawa Y, Young B, Wu C, Yamada S, Long
F, Pacifici M and Koyama E: Temporomandibular joint formation and
condyle growth require Indian hedgehog signaling. Dev Dyn.
236:426–434. 2007. View Article : Google Scholar
|
|
13
|
Michikami I, Fukushi T, Honma S, Yoshioka
S, Itoh S, Muragaki Y, Kurisu K, Ooshima T, Wakisaka S and Abe M:
Trps1 is necessary for normal temporomandibular joint development.
Cell Tissue Res. 348:131–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oka K, Oka S, Sasaki T, Ito Y, Bringas P
Jr, Nonaka K and Chai Y: The role of TGF-beta signaling in
regulating chondrogenesis and osteogenesis during mandibular
development. Dev Biol. 303:391–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Owtad P, Park JH, Shen G, Potres Z and
Darendeliler MA: The biology of TMJ growth modification: A review.
J Dent Res. 92:315–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai J, Wang J, Lu J, Zou D, Sun H, Dong Y,
Yu H, Zhang L, Yang T, Zhang X, et al: The effect of co-culturing
costal chondrocytes and dental pulp stem cells combined with
exogenous FGF9 protein on chondrogenesis and ossification in
engineered cartilage. Biomaterials. 33:7699–7711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kinumatsu T, Shibukawa Y, Yasuda T,
Nagayama M, Yamada S, Serra R, Pacifici M and Koyama E: TMJ
development and growth require primary cilia function. J Dent Res.
90:988–994. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ochiai T, Shibukawa Y, Nagayama M, Mundy
C, Yasuda T, Okabe T, Shimono K, Kanyama M, Hasegawa H, Maeda Y, et
al: Indian hedgehog roles in post-natal TMJ development and
organization. J Dent Res. 89:349–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alappat S, Zhang ZY and Chen YP: Msx
homeobox gene family and craniofacial development. Cell Res.
13:429–442. 2003. View Article : Google Scholar
|
|
20
|
Diamond E, Amen M, Hu Q, Espinoza HM and
Amendt BA: Functional interactions between Dlx2 and lymphoid
enhancer factor regulate Msx2. Nucleic Acids Res. 34:5951–5965.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Iwata J, Hacia JG, Suzuki A, Sanchez-Lara
PA, Urata M and Chai Y: Modulation of noncanonical TGF-β signaling
prevents cleft palate in Tgfbr2 mutant mice. J Clin Invest.
122:873–885. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang XD, Kou XX, He DQ, Zeng MM, Meng Z,
Bi RY, Liu Y, Zhang JN, Gan YH and Zhou YH: Progression of
cartilage degradation, bone resorption and pain in rat
temporomandibular joint osteoarthritis induced by injection of
iodoacetate. PLoS One. 7:e450362012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Embree M, Ono M, Kilts T, Walker D,
Langguth J, Mao J, Bi Y, Barth JL and Young M: Role of subchondral
bone during early-stage experimental TMJ osteoarthritis. J Dent
Res. 90:1331–1338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiao K, Zhang M, Niu L, Yu S, Zhen G, Xian
L, Yu B, Yang K, Liu P, Cao X and Wang M: Overexpressed TGF-β in
subchondral bone leads to mandibular condyle degradation. J Dent
Res. 93:140–147. 2014. View Article : Google Scholar
|