Introduction
Bacillus Calmette-Guérin (BCG), a vaccine
incorporated into routine childhood vaccination schedules globally,
is administered to neonates and children worldwide (1). In addition to the prevention of
tuberculosis, BCG has been used as an immune modulator and even as
immunotherapy in certain non-infectious diseases (2–4).
Furthermore, recent clinical and experimental studies have revealed
that BCG has a neuroprotective role in several central nervous
system (CNS) pathological conditions, including clinically isolated
syndrome, multiple sclerosis, Parkinson's disease and experimental
autoimmune encephalomyelitis (5–7).
Early-life immune activation has been
well-established to regulate the programming of brain development
and influence behavior in later life, predominantly using three
animal models of early-life immune activation: Polycytidylic acid,
Escherichia coli and lipopolysaccharide (LPS) (8,9).
However, whether neonatal BCG vaccination as immune
pre-conditioning has consequences in the CNS, and its function in
adulthood, remains to be elucidated.
There are two patterns through which early-life
immune activation may affect behavior in later life (10). In one pattern, early-life immune
activation is able to directly modulate or disrupt development
(11,12). In the other pattern, early-life
immune activation can influence the immune system and alter the
neuroimmune response to a subsequent immune challenge in adulthood
(13,14). Therefore, direct and indirect
later-life effects on the brain and behavior were assessed in the
present study.
Adult LPS administration has been demonstrated to
induce behavior impairments and neuroinflammation in rodents
(15,16), and LPS has also been used for adult
immune challenge in early-life-infected rodents (17,18).
Therefore, LPS injection was used as an adult immune challenge in
the present study to investigate the potential indirect effects of
neonatal BCG vaccination.
Sickness, depression and anxiety-like behaviors were
observed within 24 h following LPS injection. Additionally, certain
molecules associated with behavior and immune activation were
examined, including cytokines, neurotrophins, 5-hydroxytryptamine
(5-HT) and corticosterone, in the brain and/or serum.
Materials and methods
Animals and study design
The present study was approved by the Sun Yat-Sen
University (SYSU) Institute Research Ethics Committee (Guangzhou,
China) and was strictly performed according to the UK Animals
(Scientific Procedures) Act, 1986 (19). Newborn litters of C57BL/6 mice were
obtained from the SYSU Laboratory Animal Center (Guangzhou, China)
and were reared under specific pathogen-free conditions. The mice
were housed at a temperature of 25°C in 60% relative humidity on a
12-h light/dark cycle (lights on between 6:00 AM and 6:00 PM), and
allowed free access to food and water. A total of four groups were
used in the study: BCG/LPS, BCG alone, LPS alone and control (CON)
groups. The mice were administered with BCG (BCG/LPS and BCG
groups) or PBS (LPS and CON groups) at birth. At 12 weeks old, the
mice were administered LPS (BCG/LPS and LPS groups) or PBS (BCG and
CON groups).
For each of the four behavioral tests, social
exploratory behavior test (SEB), open field test (OFT), forced
swimming test (FST) and tail suspension test (TST), a total of 16
newborn litters of C57BL/6 mice were used and 64 male pups were
selected (4 pups/litter). The pups from each litter were
distributed randomly into the 4 groups (1 pup/litter/group; total
16 pups/group). For bodyweight and food intake measurements, a
total of 10 newborn litters were used and 40 male pups were
selected (4 pups/litter). The pups from each litter were
distributed randomly into the 4 groups (1 pup/litter/group; total
10 pups/group). For the remaining experiments, a total of 6 newborn
litters were used and 24 male pups were selected (4 pups/litter).
The pups from each litter were distributed randomly into the 4
groups (1 pup/litter/group; total 6 pups/group). Pups were weaned
at 3 weeks old. In the SEB paradigm, 4-week-old male juvenile
conspecifics were used.
Neonatal immunization and adult immune
challenge
Freeze-dried living BCG (D2-BP302 strain; Biological
Institute of Shanghai, Shanghai, China) was dissolved in PBS. BCG
was administered to mice at birth, imitating the age at which
vaccination is performed in human infants. Each newborn mouse in
the BCG/LPS and BCG groups was injected subcutaneously in the back
with 25 µl/mouse of BCG suspension containing 105
colony forming units (CFU), as previously described (20); mice in the LPS and CON groups were
injected with PBS in an identical manner. At 12 weeks old, each
mouse in the BCG/LPS and LPS groups was injected with 0.33 mg/kg
LPS (Sigma-Aldrich, St. Louis, MO, USA) intraperitoneally, and mice
in the BCG and CON groups were injected with PBS in an identical
manner. This dosage of LPS induces a proinflammatory cytokine
response in the peripheral nervous system and brain, resulting in
mild transient sickness behavior in adult mice (15).
SEB test
The mice were subjected to the SEB test 30 min prior
to, and 4, 8 and 24 h following, LPS injection. The test was
performed as described previously (15). To assess the motivation to perform
SEB, a novel juvenile conspecific was placed into the home cage of
the test subject for 10 min. Behavior was videotaped and the total
time spent by the test subject in social investigation (including
anogenital sniffing and trailing) was calculated from the video
records by a trained observer in a blinded manner. SEB was
represented as the quantity of time spent by the test subject
investigating the juvenile.
Body weight and food intake
measurement
Body weight alteration and food intake of mice
within the 24 h following LPS injection were calculated from body
weight and food weight measured immediately prior to and 24 h
following the LPS injection. Food intake was estimated according to
a previously described method (21).
OFT
The OFT tests were performed 2.5 h following the LPS
injection. This time interval was selected based on a previous
study by Wang et al (22).
The animals were individually placed in a plexiglass cubicle
(40×40×38 cm). The spontaneous locomotor activity for each animal
was recorded for 30 min by the Flex-Field activity system (San
Diego Instruments, San Diego, CA, USA). The number of beam breaks
by each mouse were counted automatically by the system. The
apparatus was thoroughly cleaned with 70% ethanol following each
trial.
FST
The mice were subjected to the FST task 30 min prior
to, and 4, 8 and 24 h following, LPS injection. The animals were
individually forced to swim for 6 min in an open cylindrical
container (diameter, 10 cm; height, 30 cm), containing 20 cm of
water (depth) at 22±1°C. The total duration of immobility was
recorded during the final 5-min period and was analyzed by a video
tracking system EthoVision (Noldus Information Technology B.V.,
Wageningen, Netherlands).
TST
The mice were subjected to the TST task 30 min prior
to, and 4, 8, and 24 h following, LPS injection. In a soundproof
room, each mouse was suspended upside down by their tails for 6
min. The total duration of immobility was measured during the final
5 min period.
5-Bromo-2-deoxyuridine (BrdU) labeling
and tissue preparation
Together with LPS (in BCG/LPS and LPS mice) or PBS
(in BCG and CON mice) injection, one dose of BrdU (50 mg/kg;
Sigma-Aldrich, St. Louis, MO, USA) was injected into the mice. A
repeat dose of BrdU was administered 12 h later. The mice were
anesthetized with 10% chloral hydrate (0.3 ml per mouse, i.p.;
Melone Pharmaceutical Co., Ltd., Dalian, China) and perfused
transcardially with 4% paraformaldehyde a total of 24 h following
the initial BrdU injection. The brains were excised and
subsequently fixed overnight in 4% paraformaldehyde at 4°C, and
dehydrated with 30% sucrose at 4°C for 72 h. Then, following
freezing at -20°C, the brains were sliced into serial coronal
sections (40 µm) on a freezing microtome (Leica SM2000 R;
Leica Microsystems GmBH, Wetzlar, Germany). Serial coronal sections
(40 µm) were collected and were stained for BrdU+
cells.
Immunofluorescence and cell
quantification
Specimens were incubated in 2-N HCl for 30 min at
37°C and were subsequently blocked with PBS containing 1% bovine
serum albumin and 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO,
USA) for 1 h at 37°C. Sections were subsequently incubated with a
primary monoclonal rat anti-BrdU antibody (1:500; catalog no., OBT
0030; AbD Serotec, Raleigh, NC, USA) overnight at 4°C, followed by
incubation with the secondary antibody (Alexa Fluor 594-conjugated
polyclonal donkey anti-rat; 1:400; Invitrogen; Thermo Fisher
Scientific, Waltham, MA, USA; catalog no., A-21209).
BrdU+ cells in the unilateral dentate gyrus (DG) of each
animal were counted using a Stereo Investigator stereology system
(MBF Bioscience, Williston, VT, USA). The actual section thickness
was measured, and the top and bottom guard zones were defined to
avoid oversampling. Measurements were finished in an equidistant
series of six coronal sections spanning the DG in its rostrocaudal
extension. Representative confocal micrographs were obtained with a
Zeiss LSM 710 confocal laser-scanning microscope (Carl Zeiss AG,
Oberkochen, Germany).
ELISA
Serum was separated from trunk blood 24 h following
the LPS injection by centrifugation at 4,000 × g for 5 min and was
subsequently stored at −20°C until use. Serum corticosterone levels
were measured using ELISA kits (Corticosterone ELISA kit; EIAab
Science Co, Ltd., Wuhan, China), according to the manufacturer's
instructions. The concentrations of interleukin (IL)-1β, interferon
(IFN)-γ, tumor necrosis factor (TNF)-α, IL-6, IL-4, IL-10,
brain-derived neurotrophic factor (BDNF) and insulin-like growth
factor (IGF)-1 in several brain zones were determined using
commercially available ELISA assays, according to the instructions
supplied by the manufacturer (Mouse IL-1β ELISA set, Mouse IFN-γ
(AN-18) ELISA set, Mouse IL-4 ELISA set, Mouse IL-10 ELISA set; all
purchased from BD Pharmingen™; BD Biosciences, Franklin Lakes, NJ,
USA Mouse TNF-α ELISA kit, Mouse IL-6 ELISA kit, Mouse BDNF ELISA
kit, Mouse IGF-1 ELISA kit; all purchased from EIAab Science Co,
Ltd.).
High-performance liquid chromatography
analyses of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA)
Brain samples were weighed and subsequently
homogenized in 0.5 ml ice-cold solution of 0.1 M perchloric acid,
containing 0.1% cysteine, and were centrifuged at 20,817 × g for 20
min at 4°C. The standard solution or sample was then injected onto
the column (5 µm; 4.6×150 mm2). The separation
was performed on a reversed-phase Hypersil BDS-C18 column (Elite
Analytical Instruments Co., Ltd., Dalian, China) in an isocratic
elution mode using a mobile phase consisting of 85 mM citric acid
and 100 mM sodium acetate buffer (pH 4.0), containing 8% methanol,
3 mM sodium heptane-1-sulphonate and 0.2 mM EDTA, at a flow rate of
0.8 ml/min. The levels of 5-HT and 5-HIAA were expressed in ng/mg
tissue weight (wet).
Statistical analyses
All the data were processed using SPSS version 17.0
for Windows (SPSS, Inc., Chicago, IL, USA). The data are expressed
as mean ± standard error. Data from the SEB, FST and TST were
analyzed using three-way (BCGxLPSxtime) repeated measures analysis
of variance (ANOVA) followed by Bonferroni post-hoc test. Data from
the remaining tests were analyzed using two-way (BCGxLPS) ANOVA
followed by Bonferroni post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Neonatal BCG vaccination alleviates
LPS-induced sickness behavior in adulthood
SEB was measured 30 min prior to, and 4, 8 and 24 h
following, LPS injection. A three-way ANOVA revealed the
significant effects of BCG (F1,60=12.10; P<0.001),
LPS (F1,60=141.58; P<0.001) and time
(F3,180=52.87; P<0.001), and the significant
interactions of BCGxLPS (F1,60=15.13; P<0.001),
BCGxtime (F3,180=2.74; P=0.0448), LPSxtime
(F3,180=49.12; P<0.001) and BCGxLPSxtime
(F3,180= 4.26; P=0.0062). Subsequent analyses revealed
that adult LPS treatment decreased the social behavior of mice (LPS
vs. CON group, P<0.001) and that neonatal BCG vaccination
significantly attenuated LPS-induced sickness behavior (BCG/LPS vs.
LPS group, P<0.001; Fig.
1A).
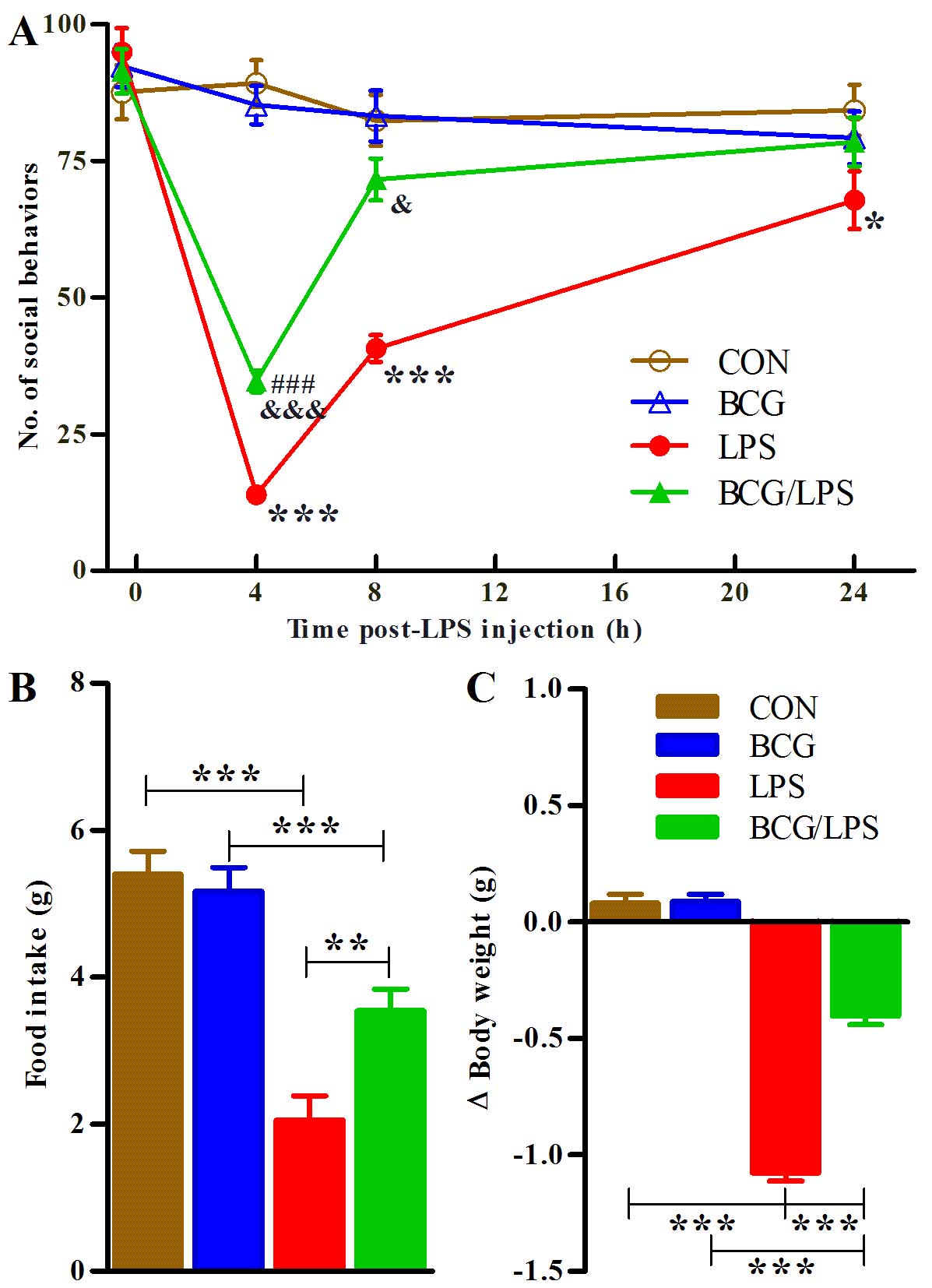 | Figure 1Neonatal BCG vaccination alleviates
LPS-induced sickness behavior in adulthood. (A) Social exploratory
behavior was measured 30 min prior to the LPS injection and 4, 8,
and 24 h later. The data were analyzed using three-way
(BCGxLPSxtime) repeated measures ANOVA followed by Bonferroni
post-hoc test. The data represent the mean ± SE (n=16). In a
different experiment, (B) food intake and (C) body weight changes
were measured within 24 h of the LPS injection. The symbol 'Δ' in
'Δ Body weight' means alteration in body weight. The data were
analyzed using two-way (BCGxLPS) ANOVA followed by Bonferroni
post-hoc test. The bars represent the mean ± SE (n=10). The
symbols '*', '#' and '&' indicate significant
differences compared with the CON mice, BCG group and LPS group,
respectively. The single, double and triple symbols indicate
P<0.05, P<0.01 and P<0.001, respectively (post-hoc
differences). The experiment was repeated twice with similar
results. BCG, Bacillus Calmette-Guérin; LPS, lipopolysaccharide;
ANOVA, analysis of variance; SE, standard error; CON, control. |
Food intake within the 24 h subsequent to LPS
injection was analyzed using two-way (BCGxLPS) ANOVA. There were
significant effects of LPS (F1,36=62.35; P<0.001) and
interaction of BCGxLPS (F1,36=7.47; P=0.0097).
Subsequent analyses revealed that adult LPS treatment decreased the
food intake (LPS vs. CON group, P<0.001) and that neonatal BCG
vaccination significantly attenuated the LPS-induced decrease
(BCG/LPS vs. LPS group, P=0.0019; Fig.
1B).
A two-way ANOVA of body weight change within the
initial 24 h following LPS injection revealed a significant effect
of BCG (F1,36=561.57; P<0.001), LPS
(F1,36=95.25; P<0.001) and interaction of BCGxLPS
(F1,36=91.38; P<0.001). Subsequent analyses revealed
that adult LPS treatment decreased the body weight of the mice (LPS
vs. CON group, P<0.001) and that neonatal BCG vaccination
significantly attenuated the LPS-induced decrease (BCG/LPS vs. LPS
group, P<0.001; Fig. 1C).
Notably, neonatal BCG vaccination alone had a
non-significant impact on social behavior, food intake and body
weight in adulthood compared with the CON group (P-values for
comparisons between the BCG and CON groups for social behavior:
P=0.3212; food intake: P=0.6041; and body weight: P=0.8881;
Fig. 1).
Neonatal BCG vaccination weakens
LPS-induced depression and anxiety-like behaviors in adulthood
The mice were subjected to the FST and TST tasks 30
min prior to, and 4, 8 and 24 h following, LPS injection. A
three-way (BCGxLPSxtime) ANOVA of the FST data revealed significant
effects of BCG (F1,60=31.42; P<0.001), LPS
(F1,60=125.44; P<0.001) and time
(F3,180=36.13; P<0.001), and significant interactions
of BCGxLPS (F1,60=28.26; P<0.001), BCGxtime
(F3,180=3.01; P=0.0316), LPSxtime
(F3,180=36.26; P<0.001) and BCGxLPSxtime
(F3,180=4.86; P=0.0028). Subsequent analyses revealed
that adult LPS treatment increased the immobility time of mice (LPS
vs. CON group, P<0.001) and that neonatal BCG vaccination
significantly attenuated the LPS-induced increase (BCG/LPS vs. LPS
group, P<0.001; Fig. 2A).
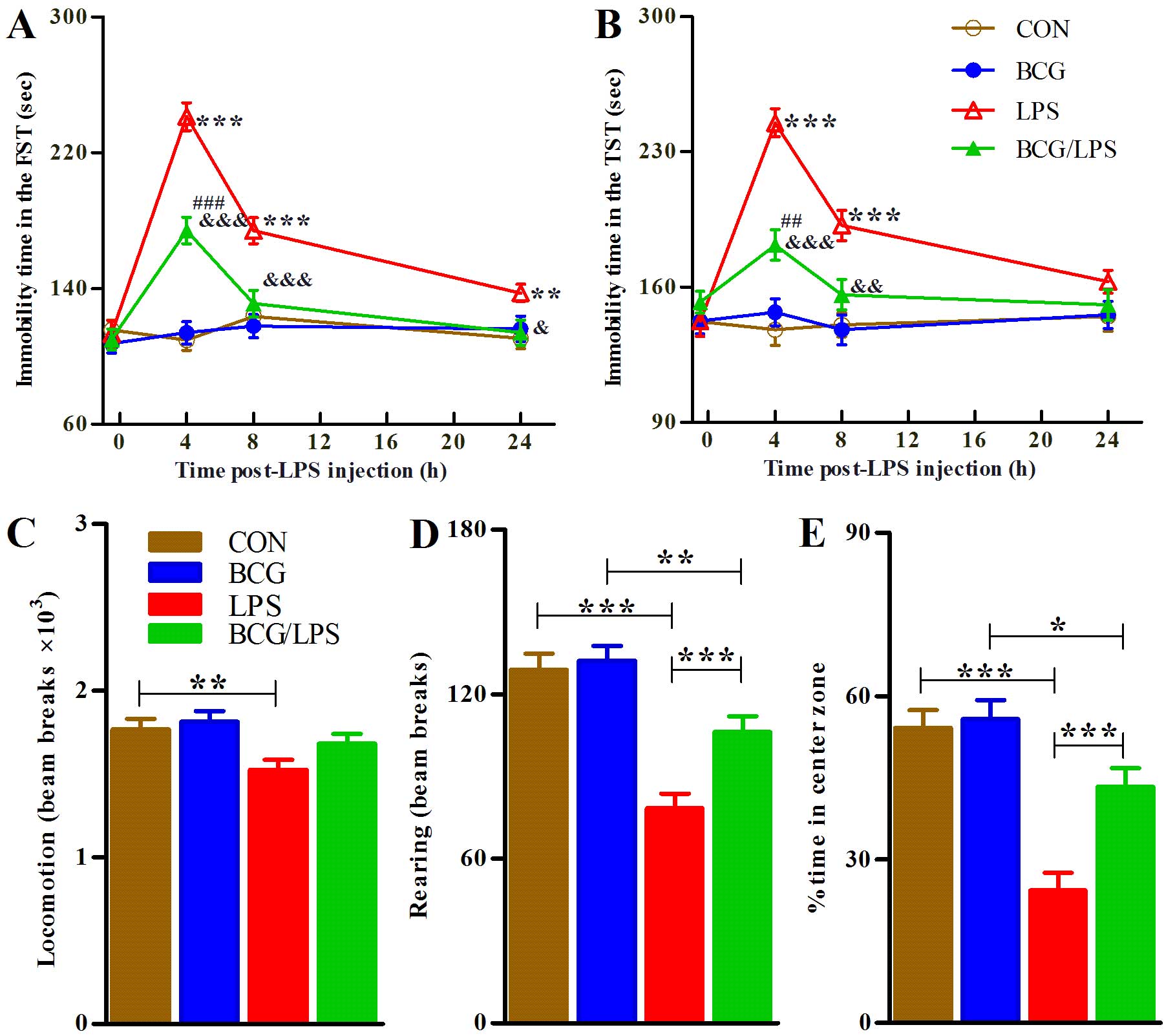 | Figure 2Neonatal BCG vaccination alleviates
LPS-induced depression and anxiety-like behavior in adulthood. The
mice were subjected to the FST and TST tasks 30 min prior to LPS
injection and 4, 8, and 24 h later. The bars represent the mean
immobility time spent in the (A) FST and (B) TST tasks. The data
were analyzed using three-way (BCGxLPSxtime) repeated measures
ANOVA followed by Bonferroni post-hoc test. The mice were
subjected to the OFT task 2.5 h subsequent to LPS injection. The
bars represent the mean (C) locomotor activities, (D) rearing
activities and the (E) proportion of center zone activities by mice
during the 30-min test. For each of the three behavior tests, a new
set of animals were used to avoid possible disturbances. The data
represent the mean ± SE (n=16) and were analyzed using two-way
ANOVA followed by Bonferroni post-hoc test. The symbols
'*', '#' and '&' indicate significant differences
compared with the CON mice, BCG group and LPS group, respectively.
The single, double and triple symbols indicate P<0.05, P<0.01
and P<0.001, respectively (post-hoc differences). The
experiment was repeated twice with similar results. BCG, Bacillus
Calmette-Guérin; LPS, lipopolysaccharide; FST, forced swimming
test; TST, tail suspension test; ANOVA, analysis of variance; OFT,
open field test; SE, standard error; CON, control. |
Analyses of the TST data revealed significant
effects of BCG (F1,60=11.83, P=0.0011), LPS
(F1,60=81.77, P<0.001) and time
(F3,180=15.07, P<0.001), and significant interactions
of BCGxLPS (F1,60=16.17, P<0.001), BCGxtime
(F3,180=3.77, P=0.0117), LPSxtime
(F3,180=16.32, P<0.001) and BCGxLPSxtime
(F3,180=5.56, P=0.0011). Subsequent analyses revealed
that adult LPS treatment increased the immobility time of mice (LPS
vs. CON group, P<0.001) and that neonatal BCG vaccination
attenuated the LPS-induced increase (BCG/LPS vs. LPS group,
P<0.001; Fig. 2B).
The mice were subjected to the OFT task 4 h
subsequent to the LPS injection, and locomotor activities, rearing
activities and the proportion of center area activities in total
were recorded for 30 min. A two-way ANOVA revealed significant
effects of BCG (rearing activities, F1,60=7.01;
P=0.0103; center proportion, F1,60=4.01; P=0.0497) and
LPS (locomotor activities, F1,60=7.86; P=0.0067; rearing
activities, F1,60=31.58; P<0.001; center proportion,
F1,60=30.61; P<0.001) and interaction of BCGxLPS
(center proportion, F1,60=4.25; P= 0.0435). Subsequent
analyses revealed that LPS injection alone induced a marginal, but
significant, decrease in locomotor activities (LPS vs. CON group,
P<0.01) and that neonatal BCG vaccination did not significantly
affect the locomotor activities, regardless of adult LPS treatment
(BCG vs. CON group, P=0.601; BCG/LPS vs. LPS group, P=0.092).
Subsequent analyses for the other indices of OFT revealed that
adult LPS treatment induced decreases in locomotion, rearing
activities (LPS vs. CON group, P<0.001) and center proportion
activities (LPS vs. CON group, P<0.001). Neonatal BCG
vaccination attenuated the LPS-induced decreases in rearing
activities (BCG/LPS vs. LPS group, P<0.001) and center
proportion (BCG/LPS vs. LPS group, P<0.001; Fig. 2C–E).
Notably, neonatal BCG vaccination alone
insignificantly impacted these indices in the FST and TST tasks in
adulthood compared with the CON group (Fig. 2).
Neonatal BCG vaccination alleviates the
LPS-induced impairment in hippocampal cell proliferation
There were significant effects of BCG
(F1,20= 6.761; P= 0.0171) and, LPS
(F1,20=70.485; P<0.001) and interaction of BCGxLPS
(F1,20=6.645; P=0.0180). Subsequent analyses revealed
that adult LPS treatment decreased the number of BrdU+
cells (LPS vs. CON group, P<0.001) and that neonatal BCG
vaccination attenuated LPS-induced impairment (BCG/LPS vs. LPS
group, P=0.0016), although neonatal BCG vaccination alone caused no
significant effect (BCG vs. CON group, P=0.9875; Fig. 3).
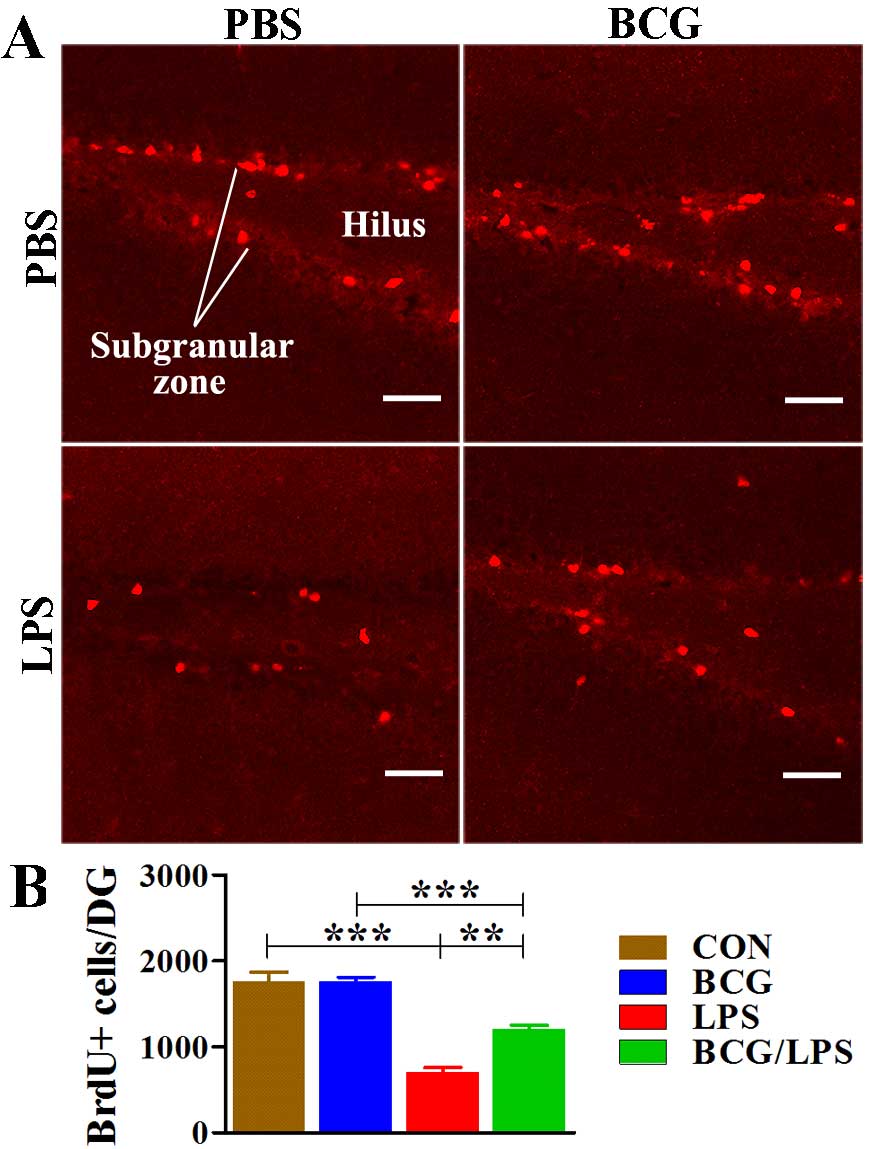 | Figure 3Neonatal BCG vaccination alleviates
LPS-induced impairment in hippocampal cell proliferation. (A)
Representative confocal micrographs of DG in the CON (upper left
panel), BCG (upper right panel), LPS (lower left panel) and BCG/LPS
groups (lower right panel). (B) The bars represent the mean ± SE of
the cell number in each group. The data represent the mean ± SE
(n=6) and were analyzed using two-way analysis of variance followed
by Bonferroni post-hoc test. ** and
*** indicate P<0.01 and P<0.001, respectively
(post-hoc differences). Scale bar=50 µm. The
experiment was repeated twice with similar results. BCG, Bacillus
Calmette-Guérin; LPS, lipopolysaccharide; DG, dentate gyrus; CON,
control; SE, standard error; PBS, phosphate-buffered saline; BrdU,
5-bromo-2-deoxyuridine. |
Neonatal BCG vaccination reduces the
LPS-induced proinflammatory cytokine response in serum and the
brain
LPS treatment induced proinflammatory cytokine
expression in serum and the brain. Neonatal BCG vaccination reduced
the LPS-induced proinflammatory responses in the serum and brain,
although neonatal BCG vaccination alone resulted in no significant
alteration in cytokine expression in serum or the brain (Fig. 4). The statistical analyses of
two-way ANOVA for cytokines are presented in Table I.
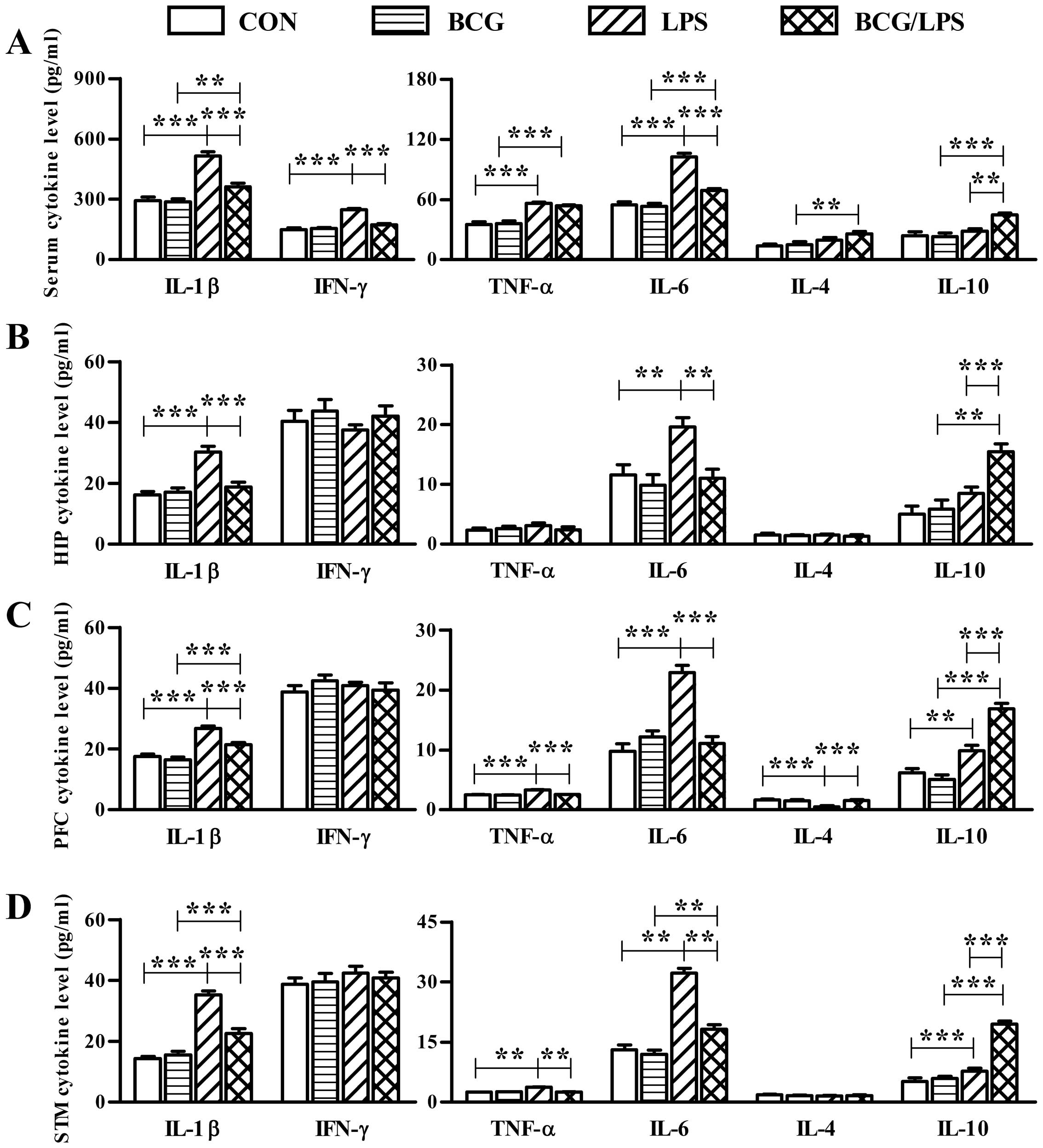 | Figure 4Neonatal BCG vaccination alleviates
the LPS-induced proinflammatory cytokine response in the serum and
brain. Cytokine levels (in the serum or brain tissue supernatent)
were assessed 4 h subsequent to LPS injection. The bars represent
the mean cytokine levels in (A) serum, (B) hippocampus, (C)
prefrontal cortex and (D) striatum. The data represent the mean ±
SE (n=6) and were analyzed using two-way analysis of variance
followed by Bonferroni post-hoc test. ** and
*** indicate P<0.01 and P<0.001, respectively
(post-hoc differences). The experiment was repeated three
times with similar results. BCG, Bacillus Calmette-Guérin; LPS,
lipopolysaccharide; CON, control; SE, standard error; HIP,
hippocampus; PFC, prefrontal cortex; STM, striatum; IL,
interleukin; IFN-γ, interferon-γ; TNF-α, tumor necrosis
factor-α. |
 | Table IStatistics of two-way ANOVA for
cytokine levels. |
Table I
Statistics of two-way ANOVA for
cytokine levels.
| A, Serum |
|---|
|
|---|
| Effect | IL-1
| IFN-γ
| TNF-α
| IL-6
| IL-4
| IL-10
|
|---|
| F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value |
|---|
| LPS | 71.211 | <0.001 | 77.466 | <0.001 | 87.367 | <0.001 | 131.466 | <0.001 | 12.026 | 0.002 | <0.001 | 0.480 |
| BCG | 20.089 | <0.001 | 26.583 | <0.001 | 0.061 | 0.807 | 40.382 | <0.001 | 2.267 | 0.148 | 0.022 | 0.235 |
| LPSx BCG | 17.506 | <0.001 | 37.445 | <0.001 | 0.771 | 0.390 | 34.140 | <0.001 | 0.873 | 0.361 | 0.011 | 0.280 |
| B, Hippocampus |
|---|
|
|---|
| Effect | IL-1
| IFN-γ
| TNF-α
| IL-6
| IL-4
| IL-10
|
|---|
| F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value |
|---|
| LPS | 28.702 | <0.001 | 0.527 | 0.476 | 0.490 | 0.492 | 7.613 | 0.012 | 0.086 | 0.772 | 6.071 | 0.023 |
| BCG | 12.465 | 0.002 | 1.598 | 0.221 | 0.266 | 0.612 | 9.555 | 0.006 | 0.280 | 0.602 | 16.989 | 0.001 |
| LPSx BCG | 17.200 | <0.001 | 0.032 | 0.860 | 0.997 | 0.330 | 4.237 | 0.053 | 0.023 | 0.882 | 3.701 | 0.069 |
| C, Prefrontal
cortex |
|---|
|
|---|
| Effect | IL-1
| IFN-γ
| TNF-α
| IL-6
| IL-4
| IL-10
|
|---|
| F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value |
|---|
| LPS | 83.658 | <0.001 | 0.055 | 0.817 | 64.270 | <0.001 | 26.645 | <0.001 | 10.327 | 0.004 | 91.744 | <0.001 |
| BCG | 17.569 | <0.001 | 0.382 | 0.543 | 52.466 | <0.001 | 16.361 | 0.001 | 7.210 | 0.014 | 13.041 | 0.002 |
| LPSx BCG | 7.815 | 0.011 | 1.734 | 0.203 | 45.444 | <0.001 | 37.311 | <0.001 | 12.725 | 0.002 | 24.538 | <0.001 |
| D, Striatum |
|---|
|
|---|
| Effect | IL-1
| IFN-γ
| TNF-α
| IL-6
| IL-4
| IL-10
|
|---|
| F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value |
|---|
| LPS | 121.607 | <0.001 | 1.125 | 0.301 | 170.844 | <0.001 | 124.154 | <0.001 | 0.947 | 0.342 | 114.203 | <0.001 |
| BCG | 21.254 | <0.001 | 0.030 | 0.864 | 158.212 | <0.001 | 44.275 | <0.001 | 0.140 | 0.712 | 67.975 | <0.001 |
| LPSx BCG | 29.638 | <0.001 | 0.296 | 0.592 | 190.450 | <0.001 | 31.515 | <0.001 | 1.004 | 0.328 | 52.345 | <0.001 |
In the serum, the LPS group exhibited significantly
increased levels of IL-1β, IFN-γ, TNF-α and IL-6 compared with the
CON group; the BCG/LPS group exhibited significantly reduced levels
of IL-1β, IFN-γ and IL-6 and increased levels of IL-10 compared
with the LPS group (Fig. 4A). In
the hippocampus, the LPS group exhibited significantly increased
levels of IL-1β and IL-6 compared with the CON group; the BCG/LPS
group exhibited significantly reduced levels of IL-1β and IL-6 and
increased levels of IL-10 compared with the LPS group (Fig. 4B). In the prefrontal cortex, the
LPS group exhibited significantly increased levels of IL-1β, TNF-α,
IL-6 and IL-10 and reduced levels of IL-4 compared with the CON
group; the BCG/LPS group exhibited significantly reduced levels of
IL-1β, TNF-α and IL-6 and increased levels of IL-4 and IL-10
compared with the LPS group (Fig.
4C). In the striatum, the LPS group exhibited significantly
increased levels of IL-1β, TNF-α, IL-6 and IL-10 compared with the
CON group; the BCG/LPS group exhibited significantly reduced levels
of IL-1β, TNF-α, and IL-6 and increased levels of IL-10 compared
with the LPS group (Fig. 4D).
In addition, the levels of IFN-γ in serum, the
levels of IL-1β and IL-6 in the hippocampus, the levels of TNF-α,
IL-6 in the prefrontal cortex and the levels of TNF-α in the
striatum of the BCG/LPS group were as low as their levels in the
BCG group (Fig. 4). Therefore,
neonatal BCG vaccination completely prevented LPS-induced increased
expression of certain proinflammatory cytokines in the serum or
specific zones of the brain. In addition, compared with the BCG
group, the levels of the anti-inflammatory cytokines, IL-4 and
IL-10, in serum and the brain were elevated in the BCG/LPS group,
although adult LPS treatment alone had a marginal and inconsistent
influence on the levels of IL-4 and IL-10 (Fig. 4).
Neonatal BCG vaccination weakens the
LPS-induced decrease in BDNF and IGF-1 levels in the brain
Adult LPS treatment reduced levels of the
neurotrophins BDNF and IGF-1 in some or all of the three brain
zones investigated. Neonatal BCG vaccination weakened these
LPS-induced decreases, although neonatal BCG vaccination alone did
not significantly alter the levels of these neurotrophins (Fig. 5). The statistical analysis of
two-way ANOVA are presented in Table
II.
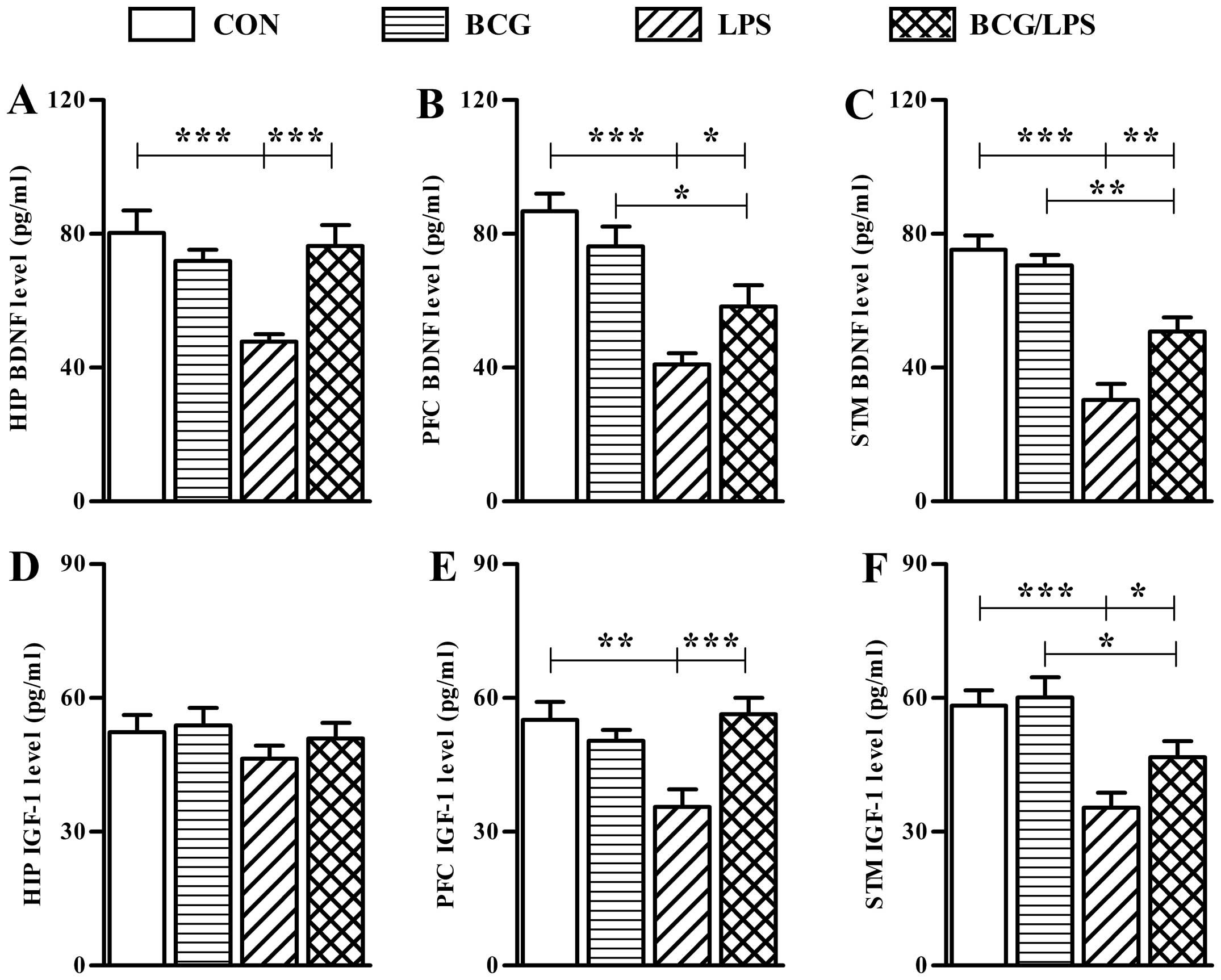 | Figure 5Neonatal BCG vaccination alleviates
LPS-induced decreases in BDNF and IGF-1 levels in the brain. These
molecues were examined 4 h subsequent to LPS injection. The bars
represent the mean levels of BDNF in the (A) hippocampus, (B)
prefrontal cortex and (C) striatum. The bars represent the mean
levels of IGF-1 in the (D) hippocampus, (E) prefrontal cortex and
(F) striatum. The data are expressed as the mean ± SE (n=6) and
were analyzed using two-way analysis of variance followed by
Bonferroni post-hoc test. *, ** and
*** indicate P<0.05, P<0.01 and P<0.001,
respectively (post-hoc differences). The experiment was
repeated three times with similar results. BCG, Bacillus
Calmette-Guérin; LPS, lipopolysaccharide; CON, control; BDNF,
brain-derived neurotrophic factor; IGF-1, insulin-like growth
factor-1; SE, standard error; HIP, hippocampus; PFC, prefrontal
cortex; STM, striatum. |
 | Table IIStatistics of two-way ANOVA for
neurotrophins, 5-HT turnover and 5-HT levels. |
Table II
Statistics of two-way ANOVA for
neurotrophins, 5-HT turnover and 5-HT levels.
| A, Hippocampus |
|---|
|
|---|
| Effect | BDNF
| IGF-1
| 5-HIAA/5-HT
| 5-HT
|
|---|
| F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value |
|---|
| LPS | 7.751 | 0.011 | 1.545 | 0.228 | 65.638 | 0.000 | 39.781 | 0.000 |
| BCG | 4.082 | 0.057 | 0.714 | 0.408 | 6.445 | 0.020 | 8.429 | 0.009 |
| LPSx BCG | 13.601 | 0.001 | 0.175 | 0.680 | 3.460 | 0.078 | 6.003 | 0.024 |
| B, Prefrontal
cortex |
|---|
|
|---|
| Effect | BDNF
| IGF-1
| 5-HIAA/5-HT
| 5-HT
|
|---|
| F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value |
|---|
| LPS | 35.191 | 0.000 | 3.601 | 0.072 | 57.328 | 0.000 | 51.183 | 0.000 |
| BCG | 0.426 | 0.522 | 5.057 | 0.036 | 5.315 | 0.032 | 11.952 | 0.002 |
| LPSx BCG | 6.630 | 0.018 | 12.430 | 0.002 | 7.378 | 0.013 | 9.378 | 0.006 |
| C, Striatum |
|---|
|
|---|
| Effect | BDNF
| IGF-1
| 5-HIAA/5-HT
| 5-HT
|
|---|
| F-value | P-value | F-value | P-value | F-value | P-value | F-value | P-value |
|---|
| LPS | 59.356 | 0.000 | 23.287 | 0.000 | 41.972 | 0.000 | 35.541 | 0.000 |
| BCG | 3.497 | 0.076 | 3.055 | 0.096 | 4.963 | 0.038 | 2.025 | 0.170 |
| LPSx BCG | 9.024 | 0.007 | 1.595 | 0.221 | 7.906 | 0.011 | 5.044 | 0.036 |
The LPS group exhibited significantly reduced BDNF
levels in the hippocampus, prefrontal cortex and striatum compared
with the CON group; the BCG/LPS group exhibited significantly
increased BDNF levels in all three zones compared with the LPS
group (Fig. 5). The LPS group
exhibited significantly reduced IGF-1 levels in prefrontal cortex
and striatum compared with the CON group; the BCG/LPS group
exhibited significantly increased IGF-1 levels in the prefrontal
cortex and striatum compared with the LPS group (Fig. 5). In addition, the levels of BDNF
in the hippocampus and IGF-1 in the striatum of the BCG/LPS group
were as high as their levels in the BCG group (Fig. 5).
Neonatal BCG vaccination reduces the
LPS-induced increased 5-HT turnover and decreased 5-HT levels in
the brain
Adult LPS treatment increased 5-HT turnover and
decreased 5-HT levels in the brain. Neonatal BCG vaccination
lessened these LPS-induced alterations, although neonatal BCG
vaccination alone did not result in any significant alteration in
these indices (Fig. 6A–F). The
statistical analysis of two-way ANOVA are presented in Table II.
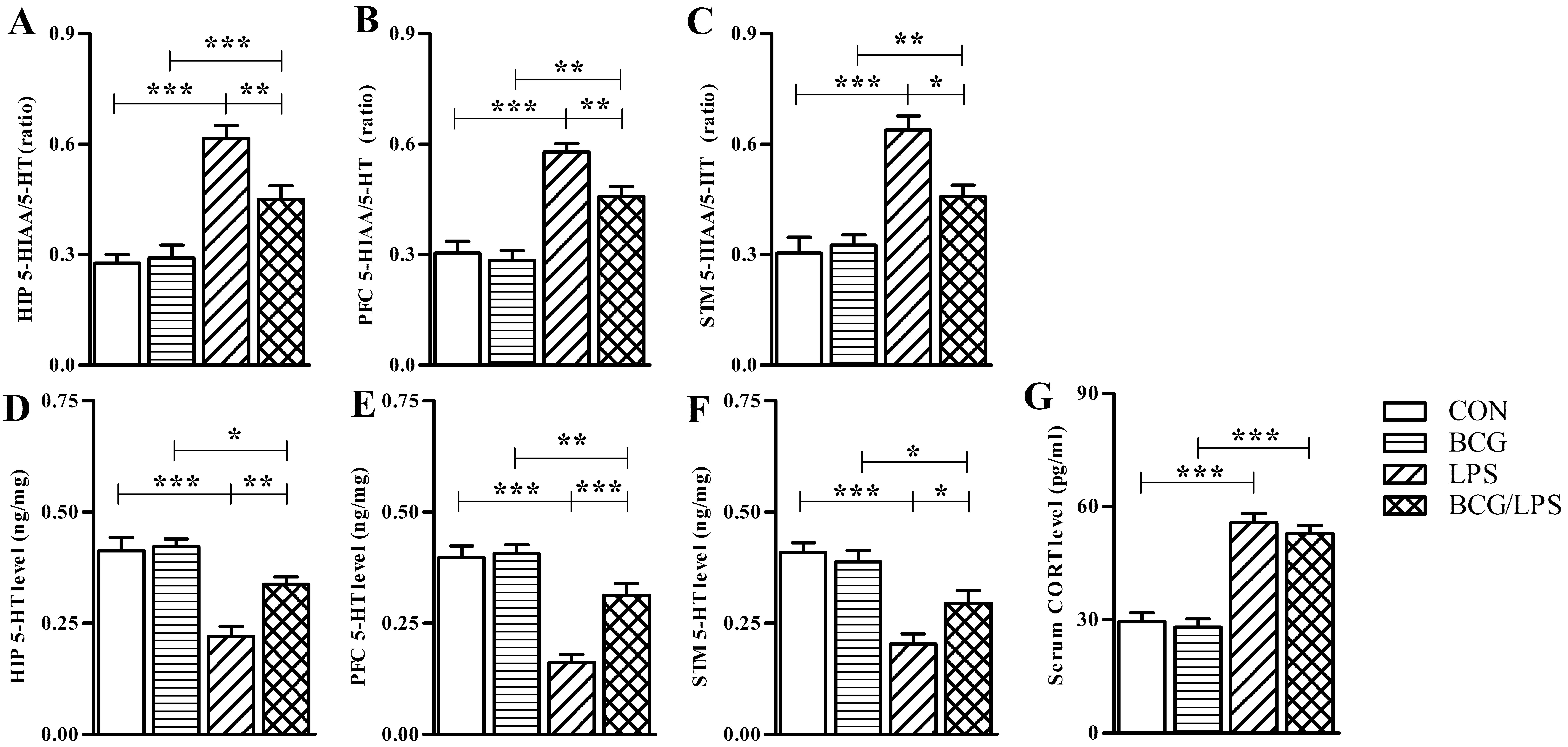 | Figure 6Neonatal BCG vaccination alleviates
LPS-induced 5-HT metabolism disorder in the brain without altering
the peripheral corticostrone levels. These molecues were examined 4
h subsequent to LPS injection. The bars represent the mean ratio of
5-HIAA to 5-HT in the (A) hippocampus, (B) prefrontal cortex and
(C) striatum. The bars represent the mean levels of 5-HT in the (D)
hippocampus, (E) prefrontal cortex and (F) striatum. (G) The bars
represent the mean levels of corticosterone in the serum. The data
are expressed as the mean ± SE (n=6) and were analyzed using
two-way analysis of variance followed by Bonferroni post-hoc
test. *, ** and *** indicate
P<0.05, P<0.01 and P<0.001, respectively (post-hoc
differences). The experiment was repeated three times with similar
results. BCG, Bacillus Calmette-Guérin; LPS, lipopolysaccharide;
CON, control; 5-HT, 5-hydroxytryptamine; 5-HIAA,
5-hydroxyindoleacetic acid; CORT, corticosterone; SE, standard
error; HIP, hippocampus; PFC, prefrontal cortex; STM, striatum. |
The LPS group exhibited a significantly increased
ratio of 5-HIAA to 5-HT (5-HIAA/5-HT) and decreased 5-HT levels in
the brain compared with the CON group. The BCG/LPS group had
significantly decreased 5-HIAA/5-HT and increased 5-HT levels in
the brain compared with the LPS group (Fig. 6A–F).
Neonatal BCG vaccination did not affect
the LPS-induced increase in corticosterone levels in serum or the
brain
A two-way ANOVA for corticosterone levels revealed a
significant effect of LPS (F1,20=120.942; P<0.001),
non-significant effect of BCG (F1,20=0.842; P=0.3697)
and non-significant interaction of BCGxLPS (F1,20=0.092;
P=0.7650). Subsequent analyses revealed that the BCG group
exhibited no significant alteration in the corticosterone levels in
the serum compared with the CON group (Fig. 6G). The LPS group had significantly
increased corticosterone levels in the serum compared with the CON
group, and no significant differences were observed in the
corticosterone levels in the serum between the BCG/LPS and LPS
groups (Fig. 6G).
Discussion
The results of the present study reveal that
neonatal BCG vaccination alleviates LPS-induced neurobehavioral
impairments and neuroinflammation in adult mice. Neonatal BCG
vaccination alleviated LPS-induced sickness, depression and
anxiety-like behaviors, as well as hippocampal proliferation
impairment. Furthermore, LPS-induced decreases in neurotrophins and
5-HT levels in brain were also alleviated by neonatal BCG
vaccination. In addition, neonatal BCG vaccination reduced the
pro-inflammatory responses induced by adult LPS challenge in the
periphery and brain.
Intraperitoneal LPS treatment has been confirmed to
induce a series of acute physiopathological and psychological
disorders in rodents. Previous studies have demonstrated decreased
social exploratory behavior and food intake in rodents
intraperitoneally administered with LPS (15,23),
while others have reported LPS-induced depression and anxiety-like
behaviors (24,25). Furthermore, LPS may acutely inhibit
the proliferation of stem cells in the DG in adult rodents
(26,27). The results from the LPS group in
the present study confirmed these widely reported neurobehavioral
impairments.
Immune activation by LPS may induce a large release
of cytokines in the periphery and brain, particularly
proinflammatory cytokines, including IL-1β, IL-6, TNF-α and IFN-γ
(15). These proinflammatory
cytokines may affect the functioning of the brain (28) and mediate sickness behavior
syndrome (29). For example, Wang
et al (22) reported that
an intraperitoneal injection of LPS in mice resulted in clear
impairments in performance of the OFT task, associated with
increased expression of IL-1β, IL-6 and TNF-α in the brain. IL-10
and IL-4 are considered anti-inflammatory cytokines and have
neuroprotective effects (30,31).
In the present study, LPS treatment induced proinflammatory
responses in the serum and brain. The increases in proinflammatory
cytokine levels by adult LPS treatment were reduced significantly
by neonatal BCG vaccination, and in certain cases cytokine levels
in the serum or specific brain zones were completely prevented from
increasing. These findings reveal that neonatal priming of the
immune system by BCG results in a reduced proinflammatory response
to a subsequent LPS challenge in adulthood, possibly explaining why
neonatal BCG vaccination alleviates LPS-induced behavior
impairments.
Notably, in the present study the levels of IL-10 in
the LPS group demonstrated a tendency to increase in serum and all
three brain zones compared with the CON group. IL-10 is one of the
regulatory T cell (Tregs) associated cytokines that may be released
by various immune cells when inflammation occurs (32). Stumhofer et al (33) reported that IL-6 induced signal
transducers and activators of transcription 3-mediated T cell
production of IL-10. McGeachy et al (34) reported that IL-6 drives the
production of IL-10 by T cells and prevents the T helper 17
cell-mediated pathology. The increased IL-10 levels may inhibit
inflammatory pathologies and avoid autoimmune impairments (35).
Notably, in the present study the BCG/LPS group
exhibited increased IL-10 levels in the periphery and brain
compared with the BCG and LPS groups. This observation aids
explanation of the reduced levels of proinflammatory cytokines in
the BCG/LPS group. How neonatal BCG vaccination increases IL-10
production in response to adult LPS treatment remains to be
elucidated. However, studies concerning the non-specific effects of
BCG on the immune system support the anti-inflammatory role of BCG
(36–38). Epidemiological studies have
indicated that BCG vaccination exerted a positive non-specific
effect on overall childhood mortality, which cannot be attributed
to the prevention of tuberculosis fatalities (39,40).
Additionally, the capacity of BCG to induce Tregs in vivo
has been widely reported (36). In
a study by Madura Larsen et al (37), dendritic cells (DCs) were generated
from peripheral blood mononuclear cells and cultured with LPS or
LPS/BCG in vitro. The study reported that BCG-exposed DCs
were able to induce IL-10-producing T cells. In a separate study,
neonatal BCG vaccination was observed to ameliorate
allergen-induced local inflammation and increase the number of
cluster of differentiation 4 (CD4)+CD25+ Treg
cells and IL-10 expression (38).
These findings suggest that BCG may interact with DCs directly to
result in an accumulation of IL-10-producing T cells.
DCs derived from BCG-infected mononuclear cells
produce IL-4, which may be associated with the failure of the BCG
vaccination against tuberculosis (41). This suggests that IL-4-producing
DCs may have an anti-inflammatory role. In the present study,
increased expression of IL-4 was observed in serum and the
prefrontal cortex of the BCG/LPS compared with the LPS mice,
suggesting that increased IL-4 may contribute to the inhibition of
LPS-induced inflammation.
BDNF, IGF-1 and 5-HT in the brain were identified as
the most important molecules for maintaining health-mood status,
and decreases in their levels, as well as an increase in 5-HT
turnover, are associated with depression (42,43).
In the present study, behavior impairments and alterations in
neurotrophins and 5-HT levels in LPS mice were consistent with
these reports. Furthermore, neonatal BCG vaccination improved the
LPS-induced neurochemical disorders. This alleviation, together
with the changes in proinflammatory cytokine levels, may assist
with explaining the effects of BCG on behavior and proliferation
observed in the present study.
Activity of the hypothalamic-pituitary-adrenal (HPA)
axis is another important aspect of the complicated pathophysiology
of sickness-like behavior and depression. HPA axis hyperactivity
may be one of the mechanisms underlying depression development
(44). Serum levels of
corticosterone are one marker of the HPA axis activation in rodents
(45). As reported in our previous
study, LPS treatment increased serum corticosterone levels in mice
(46). However, in the present
study neonatal BCG vaccination did not influence the LPS-induced
elevation of serum corticosterone levels, suggesting that neonatal
BCG vaccination lessened the LPS-induced neurobehavioral
impairments through its priming effects on inflammatory responses
in the periphery and brain, independent of the HPA axis.
How peripheral cytokines exert their influence on
the brain and its function remains to be fully elucidated.
Periphery-derived cytokines may permeate across the blood-brain
barrier and directly affect the neuronal activities (47). Additionally, crosstalk may occur
between cytokines in the brain and resident immune cells (such as
microglia), causing the latter to change their phenotype by, for
example, altering their secretion of local neuromodulative
molecules, including cytokines and neurotrophins (48,49).
These neuromodulative molecules and local cells combine to regulate
the neuroimmune niche and therefore affect brain functions
(43,50).
There are numerous studies reporting the influence
of previous exposure to a specific antigen on subsequent immune
responses to other antigen(s), including i) the effects of neonatal
exposure to immunogen and subsequent autism/schizophrenia-like
behavior; ii) the target disease-specific effects of a previous
vaccination on the subsequent immune responses to unrelated
antigens; and iii) the phenomenon of 'original antigenic sin'.
Previous studies have demonstrated that early life immune
activation by Escherichia coli led to CNS alterations at
behavioral, cellular and molecular levels following adult LPS
challenge (10,14,17).
It has additionally been observed that these later life
consequences were mediated primarily by CNS resident immune cells,
including microglia and astrocytes that were primed and thus
equipped with altered abilities when responding to subsequent
immune stimuli (10,14,17).
A previous study revealed that BCG vaccination enhanced the
immunogenicity of subsequent influenza vaccination in healthy
volunteers through enhanced proinflammatory leukocyte responses
(51). Original antigenic sin
describes the failure to mount effective antibody responses to
virus variants in a previously virus-infected host (52). The similarity of the prior and
subsequent antigens and the hyperresponsiveness of memory immune
cells are thought to be the underlying reasons for this phenomenon
(52). Similar mechanisms to those
identified by previous studies may apply in the present study, as
BCG-priming altered the expression of cytokines and neurotrophins
in the brain, which are produced and/or regulated by microglia and
astrocytes (10,14,17).
The mechanism concerning mainly peripheral cytokine responses may
also underlie the findings of the present study, as indicated by
the altered serum cytokines levels (51). However, the present study observed
abrogation, rather than enhancement, of immunogenicity. This is
understandable as BCG induces a mild, physiological immune
activation (unlike early life pathology) and LPS exposure resulted
in marked neuroinflammation (unlike later life vaccination)
(10,14,17,51).
Original antigenic sin may not apply in the present study as it
occurs during antibody responses to viral antigens (52).
The present study revealed a neuroprotective role
for neonatal BCG vaccination in the presence of later-life
neuroinflammation regardless of the lack of direct neurobehavioral
effects in adulthood. Notably, there are certain previous studies
reporting that rodents receiving BCG in adulthood develop a
depression-like phenotype (53–55).
In addition, previous studies reporting BCG depression-like effects
identified that BCG activated indoleamine 2,3-dioxygenase, which is
a tryptophan-catabolizing enzyme, and decreased brain 5-HT levels
(54–56). In the present study, BCG/LPS mice
exhibited a lower 5-HT turnover and higher levels of 5-HT in the
brain compared with the LPS group, suggesting that neonatal BCG
vaccination may assist in maintaining normal 5-HT metabolism in
adulthood. This plausible inconsistency may result from numerous
factors, particularly the dosage and age for vaccination, and the
time interval between BCG vaccination and behavioral tests. In the
previous studies, doses of ≥107 CFU were used, as these
higher doses were able to elicit clear sickness responses (53,55).
By contrast, a lower dose (105 CFU) was selected in the
present study to avoid inducing health impairment. BCG-induced
depressive-like behavior and evident bodyweight loss have been
verified as dose-dependent, and are induced only by ≥107
CFU (57). Additionally, sickness
and depression-like behaviors were observed in the mice within 1
month of adult BCG vaccination in the previous studies (53–55).
In the present study, newborn mice were vaccinated and their
behaviors were tested at 12 weeks old. During longer time intervals
across the significant postnatal development span, the organism may
experience complex and sufficient self-adjustment to reverse the
neurobehavioral effects of the neonatal vaccination, and if there
are any, they are not detectable. Furthermore, other factors,
including vaccination routes (intraperitoneally in previous studies
and subcutaneously in the present study) may also contribute.
Therefore, no contradiction exists between the current data and
previous studies concerning adult models of depression by BCG.
Sirén et al (58) injected adult male Sprague-Dawley
rats with BCG through the tail vein. LPS was then injected into the
lateral cerebral ventricle 2 weeks later. This previous study
reported that the incidence of paralysis and fatality in response
to LPS was increased in BCG-primed rats. There are various
methodological differences in the study by Sirén et al
compared with the present study: The species (rats vs. mice in the
present study), the age of vaccination (adult vs. neonates), the
time interval between BCG vaccination and LPS injection (2 vs. 12
weeks), the route of injection of BCG (through tail vein vs.
subcutaneous) and LPS (into the lateral cerebral ventricle vs.
intraperitoneally). Among the differences, the injection route and
time interval may be the most responsible for the neurobehavioral
effects of BCG/LPS administration in animals. As Sirén et al
(58), described, 13% of rats were
paralyzed or succumbed following injection of 300 µg LPS
into the lateral cerebral ventricle. However, a similar (~1.2
mg/kg) or lower (330 µg/kg, as used in the present study)
dosage of LPS injected intraperitoneally does not result in
paralysis or fatality (15,59).
In addition, Sirén et al (58) stated that no paralysis or fatality
resulted from BCG/LPS treatment when the time interval between BCG
vaccination and LPS injection was <1 or >4 weeks. All these
findings suggest that different dosages and protocols of BCG/LPS
treatment may lead to varying effects on neurobehavior.
BCG is administered worldwide to human infants who
may then suffer more or less during their later life from bacterial
infection, including with LPS-producing bacteria. Therefore, there
are numerous individuals who receive neonatal BCG immunization and
suffer LPS exposure in adulthood. Mice in the present study also
experienced neonatal BCG immunization and LPS-challenge in
adulthood, meaning the model used in the present study may be
comparable to humans. Furthermore, the BCG used in the present
study is identical to the vaccine administered to humans and the
age, dosage and route of vaccination in the present study are also
similar to those for humans. Therefore, it is worth investigating
if neonatal BCG vaccination exerts similar effects on brain
development and behavior in humans. These results may not be
limited to infection with LPS/LPS-producing bacteria. The present
study provides a basis for further examination and provides a
useful animal model for investigating the neurobehavioral effects
of physiological neonatal immune activation.
In conclusion, the neonatal BCG vaccination
alleviated the neurobehavioral impairments and neuroinflammation
induced by exposure of adult mice to LPS. The results of the
present study reveal the protective effect of BCG on the CNS
following exposure to LPS, and encourage further study to
investigate the use of immunoregulatory therapy for the treatment
of neuropsychiatric disorders.
Abbreviations:
|
5-HT
|
5-hydroxytryptamines
|
|
5-HIAA
|
5-hydroxyindoleacetic acid
|
|
BCG
|
Bacillus Calmette-Guérin
|
|
BDNF
|
brain-derived neurotrophic factor
|
|
BrdU
|
5-bromo-2-deoxyuridine
|
|
CFU
|
colony forming units
|
|
CNS
|
central nervous system
|
|
CON
|
control
|
|
DCs
|
dendritic cells
|
|
DG
|
dentate gyrus
|
|
FST
|
forced swimming test
|
|
HPA
|
hypothalamic-pituitary-adrenal
|
|
IFN-γ
|
interferon-γ
|
|
IGF-1
|
insulin-like growth factor-1
|
|
IL-1β
|
interleukin-1β
|
|
IL-4
|
interleukin-4
|
|
IL-6
|
interleukin-6
|
|
LPS
|
lipopolysaccharide
|
|
OFT
|
open field test
|
|
PBS
|
phosphate-buffered saline
|
|
ANOVA
|
analysis of variance
|
|
SEB
|
social exploratory behavior
|
|
SYSU
|
Sun Yat-Sen University
|
|
TNF-α
|
tumor necrosis factor-α
|
|
Tregs
|
regulatory T cells
|
|
TST
|
tail suspension test
|
Acknowledgments
The authors would like to thank Dr. Zejie Zuo, Dr.
Yingying Wu and Ms. Yunlong Xu (SYSU) for their invaluable
comments. The authors would also like to thank Technician Qunfang
Yuan (SYSU) for her technical instruction. The present study was
supported by the National Natural Science Foundation of China
(grant no. 31371130), the Special Foundation of Education
Department of Guangdong Province (grant no. 2010-036) and the
Medical Scientific Research Foundation of Guangdong Province, China
(grant no. 2013-159).
References
|
1
|
Centers for Disease Control and Prevention
(CDC): Global routine vaccination coverage-2012. MMWR Morb Mortal
Wkly Rep. 62:858–861. 2013.
|
|
2
|
Gandhi NM, Morales A and Lamm DL: Bacillus
Calmette-Guérin immunotherapy for genitourinary cancer. BJU Int.
112:288–297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Petrovsky N: Immunomodulation with
microbial vaccines to prevent type 1 diabetes mellitus. Nat Rev
Endocrinol. 6:131–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stewart JH IV and Levine EA: Role of
bacillus Calmette-Guérin in the treatment of advanced melanoma.
Expert Rev Anticancer Ther. 11:1671–1676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ristori G, Romano S, Cannoni S, Visconti
A, Tinelli E, Mendozzi L, Cecconi P, Lanzillo R, Quarantelli M,
Buttinelli C, et al: Effects of Bacille Calmette-Guerin after the
first demyelinating event in the CNS. Neurology. 82:41–48. 2014.
View Article : Google Scholar :
|
|
6
|
Laćan G, Dang H, Middleton B, Horwitz MA,
Tian J, Melega WP and Kaufman DL: Bacillus Calmette-Guerin
vaccine-mediated neuroprotection is associated with regulatory
T-cell induction in the
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of
Parkinson's disease. J Neurosci Res. 91:1292–1302. 2013. View Article : Google Scholar
|
|
7
|
Lee J, Reinke EK, Zozulya AL, Sandor M and
Fabry Z: Mycobacterium bovis bacille Calmette-Guérin infection in
the CNS suppresses experimental autoimmune encephalomyelitis and
Th17 responses in an IFN-gamma-independent manner. J Immunol.
181:6201–6212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bilbo SD and Schwarz JM: Early-life
programming of later-life brain and behavior: A critical role for
the immune system. Front Behav Neurosci. 3:142009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spencer SJ, Heida JG and Pittman QJ: Early
life immune challenge-effects on behavioural indices of adult rat
fear and anxiety. Behav Brain Res. 164:231–238. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bilbo SD and Schwarz JM: The immune system
and developmental programming of brain and behavior. Front
Neuroendocrinol. 33:267–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bitanihirwe BK, Peleg-Raibstein D, Mouttet
F, Feldon J and Meyer U: Late prenatal immune activation in mice
leads to behavioral and neurochemical abnormalities relevant to the
negative symptoms of schizophrenia. Neuropsychopharmacology.
35:2462–2478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Winter C, Djodari-Irani A, Sohr R,
Morgenstern R, Feldon J, Juckel G and Meyer U: Prenatal immune
activation leads to multiple changes in basal neurotransmitter
levels in the adult brain: Implications for brain disorders of
neurodevelopmental origin such as schizophrenia. Int J
Neuropsychopharmacol. 12:513–524. 2009. View Article : Google Scholar
|
|
13
|
Bland ST, Beckley JT, Young S, Tsang V,
Watkins LR, Maier SF and Bilbo SD: Enduring consequences of
early-life infection on glial and neural cell genesis within
cognitive regions of the brain. Brain Behav Immun. 24:329–338.
2010. View Article : Google Scholar :
|
|
14
|
Bilbo SD, Barrientos RM, Eads AS,
Northcutt A, Watkins LR, Rudy JW and Maier SF: Early-life infection
leads to altered BDNF and IL-1beta mRNA expression in rat
hippocampus following learning in adulthood. Brain Behav Immun.
22:451–455. 2008. View Article : Google Scholar
|
|
15
|
Henry CJ, Huang Y, Wynne A, Hanke M,
Himler J, Bailey MT, Sheridan JF and Godbout JP: Minocycline
attenuates lipopolysaccharide (LPS)-induced neuroinflammation,
sickness behavior, and anhedonia. J Neuroinflammation. 5:152008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rostami F, Oryan S, Ahmadiani A and
Dargahi L: Morphine preconditioning protects against LPS-induced
neuroinflam-mation and memory deficit. J Mol Neurosci. 48:22–34.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bilbo SD, Levkoff LH, Mahoney JH, Watkins
LR, Rudy JW and Maier SF: Neonatal infection induces memory
impairments following an immune challenge in adulthood. Behav
Neurosci. 119:293–301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bilbo SD, Biedenkapp JC, Der-Avakian A,
Watkins LR, Rudy JW and Maier SF: Neonatal infection-induced memory
impairment after lipopolysaccharide in adulthood is prevented via
caspase-1 inhibition. J Neurosci. 25:8000–8009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hollands C: The animals (scientific
procedures) act 1986. Lancet. 2:32–33. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen H, Huang H, Wang J, Ye S, Li W, Wang
K, Zhang G and Wang P: Neonatal vaccination with Bacillus
Calmette-Guérin elicits long-term protection in mouse-allergic
responses. Allergy. 63:555–563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin S, Thomas TC, Storlien LH and Huang
XF: Development of high fat diet-induced obesity and leptin
resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord.
24:639–646. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YJ, Zheng YL, Lu J, Chen GQ, Wang XH,
Feng J, Ruan J, Sun X, Li CX and Sun QJ: Purple sweet potato color
suppresses lipopolysaccharide-induced acute inflammatory response
in mouse brain. Neurochem Int. 56:424–430. 2010. View Article : Google Scholar
|
|
23
|
Yirmiya R: Endotoxin produces a
depressive-like episode in rats. Brain Res. 711:163–174. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frenois F, Moreau M, O'Connor J, Lawson M,
Micon C, Lestage J, Kelley KW, Dantzer R and Castanon N:
Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining
within the mouse extended amygdala, hippocampus and hypothalamus,
that parallel the expression of depressive-like behavior.
Psychoneuroendocrinology. 32:516–531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sulakhiya K, Kumar P, Gurjar SS, Barua CC
and Hazarika NK: Beneficial effect of honokiol on
lipopolysaccharide induced anxiety-like behavior and liver damage
in mice. Pharmacol Biochem Behav. 132:79–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujioka H and Akema T: Lipopolysaccharide
acutely inhibits proliferation of neural precursor cells in the
dentate gyrus in adult rats. Brain Res. 1352:35–42. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bachstetter AD, Jernberg J, Schlunk A,
Vila JL, Hudson C, Cole MJ, Shytle RD, Tan J, Sanberg PR, Sanberg
CD, et al: Spirulina promotes stem cell genesis and protects
against LPS induced declines in neural stem cell proliferation.
PloS One. 5:e104962010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pollmächer T, Haack M, Schuld A,
Reichenberg A and Yirmiya R: Low levels of circulating inflammatory
cytokines-do they affect human brain functions? Brain Behav Immun.
16:525–532. 2002. View Article : Google Scholar
|
|
29
|
Konsman JP, Parnet P and Dantzer R:
Cytokine-induced sickness behaviour: Mechanisms and implications.
Trends Neurosci. 25:154–159. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao W, Xie W, Xiao Q, Beers DR and Appel
SH: Protective effects of an anti-inflammatory cytokine,
interleukin-4, on motoneuron toxicity induced by activated
microglia. J Neurochem. 99:1176–1187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spera PA, Ellison JA, Feuerstein GZ and
Barone FC: IL-10 reduces rat brain injury following focal stroke.
Neurosci Lett. 251:189–192. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saraiva M and O'Garra A: The regulation of
IL-10 production by immune cells. Nat Rev Immunol. 10:170–181.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stumhofer JS, Silver JS, Laurence A,
Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O'Shea JJ and
Hunter CA: Interleukins 27 and 6 induce STAT3-mediated T cell
production of interleukin 10. Nat Immunol. 8:1363–1371. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McGeachy MJ, Bak-Jensen KS, Chen Y, Tato
CM, Blumenschein W, McClanahan T and Cua DJ: TGF-beta and IL-6
drive the production of IL-17 and IL-10 by T cells and restrain T
(H)-17 cell-mediated pathology. Nat Immunol. 8:1390–1397. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O'Garra A, Barrat FJ, Castro AG, Vicari A
and Hawrylowicz C: Strategies for use of IL-10 or its antagonists
in human disease. Immunol Rev. 223:114–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lagranderie M and Guyonvarc'h PM: The
interplay between bacillus Calmette-Guérin and Treg cells and its
role to prevent or cure inflammatory diseases. Expert Rev Clin
Immunol. 10:741–745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Madura Larsen J, Benn CS, Fillie Y, van
der Kleij D, Aaby P and Yazdanbakhsh M: BCG stimulated dendritic
cells induce an interleukin-10 producing T-cell population with no
T helper 1 or T helper 2 bias in vitro. Immunology. 121:276–282.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li Q and Shen HH: Neonatal bacillus
Calmette-Guérin vaccination inhibits de novo allergic inflammatory
response in mice via alteration of CD4+CD25+ T-regulatory cells.
Acta Pharmacol Sin. 30:125–133. 2009. View Article : Google Scholar
|
|
39
|
Kristensen I, Aaby P and Jensen H: Routine
vaccinations and child survival: Follow up study in Guinea-Bissau,
West Africa. BMJ. 321:1435–1438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roth A, Gustafson P, Nhaga A, Djana Q,
Poulsen A, Garly ML, Jensen H, Sodemann M, Rodriques A and Aaby P:
BCG vaccination scar associated with better childhood survival in
Guinea-Bissau. Int J Epidemiol. 34:540–547. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Martino A, Sacchi A, Sanarico N, Spadaro
F, Ramoni C, Ciaramella A, Pucillo LP, Colizzi V and Vendetti S:
Dendritic cells derived from BCG-infected precursors induce
Th2-like immune response. J Leukoc Biol. 76:827–834. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stepanichev M, Dygalo NN, Grigoryan G,
Shishkina GT and Gulyaeva N: Rodent models of depression:
Neurotrophic and neuro-inflammatory biomarkers. Biomed Res Int.
2014:9327572014. View Article : Google Scholar
|
|
43
|
Godbout JP, Moreau M, Lestage J, Chen J,
Sparkman NL, O'Connor J, Castanon N, Kelley KW, Dantzer R and
Johnson RW: Aging exacerbates depressive-like behavior in mice in
response to activation of the peripheral innate immune system.
Neuropsychopharmacology. 33:2341–2351. 2008. View Article : Google Scholar
|
|
44
|
Pariante CM and Lightman SL: The HPA axis
in major depression: Classical theories and new developments.
Trends Neurosci. 31:464–468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu Q, Yi LT, Pan Y, Wang X, Li YC, Li JM,
Wang CP and Kong LD: Antidepressant-like effects of the mixture of
honokiol and magnolol from the barks of Magnolia officinalis in
stressed rodents. Prog Neuropsychopharmacol Biol Psychiatry.
32:715–725. 2008. View Article : Google Scholar
|
|
46
|
Munhoz CD, Sorrells SF, Caso JR, Scavone C
and Sapolsky RM: Glucocorticoids exacerbate
lipopolysaccharide-induced signaling in the frontal cortex and
hippocampus in a dose-dependent manner. J Neurosci. 30:13690–13698.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Banks WA: Blood-brain barrier transport of
cytokines: A mechanism for neuropathology. Curr Pharm Des.
11:973–984. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Schwartz M and Shechter R: Systemic
inflammatory cells fight off neurodegenerative disease. Nat Rev
Neurol. 6:405–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yirmiya R and Goshen I: Immune modulation
of learning, memory, neural plasticity and neurogenesis. Brain
Behav Immun. 25:181–213. 2011. View Article : Google Scholar
|
|
50
|
Combrinck MI, Perry VH and Cunningham C:
Peripheral infection evokes exaggerated sickness behaviour in
pre-clinical murine prion disease. Neuroscience. 112:7–11. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Leentjens J, Kox M, Stokman R, Gerretsen
J, Diavatopoulos DA, van Crevel R, Rimmelzwaan GF, Pickkers P and
Netea MG: BCG vaccination enhances the immunogenicity of subsequent
influenza vaccination in healthy volunteers: A randomized,
placebo-controlled pilot study. J Infect Dis. 212:1930–1938. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Midgley CM, Bajwa-Joseph M, Vasanawathana
S, Limpitikul W, Wills B, Flanagan A, Waiyaiya E, Tran HB, Cowper
AE, Chotiyarnwong P, et al: An in-depth analysis of original
antigenic sin in dengue virus infection. J Virol. 85:410–421. 2011.
View Article : Google Scholar :
|
|
53
|
Kelley KW, O'Connor JC, Lawson MA, Dantzer
R, Rodriguez-Zas SL and McCusker RH: Aging leads to prolonged
duration of inflammation-induced depression-like behavior caused by
Bacillus Calmette-Guérin. Brain Behav Immun. 32:63–69. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Moreau M, André C, O'Connor JC, Dumich SA,
Woods JA, Kelley KW, Dantzer R, Lestage J and Castanon N:
Inoculation of Bacillus Calmette-Guerin to mice induces an acute
episode of sickness behavior followed by chronic depressive-like
behavior. Brain Behav Immun. 22:1087–1095. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Vijaya Kumar K, Rudra A, Sreedhara MV,
Siva Subramani T, Prasad DS, Das ML, Murugesan S, Yadav R, Trivedi
RK, Louis JV, et al: Bacillus Calmette-Guérin vaccine induces a
selective serotonin reuptake inhibitor (SSRI)-resistant depression
like phenotype in mice. Brain Behav Immun. 42:204–211. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Dantzer R, O'Connor JC, Lawson MA and
Kelley KW: Inflammation-associated depression: From serotonin to
kynurenine. Psychoneuroendocrinology. 36:426–436. 2011. View Article : Google Scholar :
|
|
57
|
O'Connor JC, Andrú C, Wang Y, Lawson MA,
Szegedi SS, Lestage J, Castanon N, Kelley KW and Dantzer R:
Interferon-gamma and tumor necrosis factor-alpha mediate the
upregulation of indoleamine 2,3-dioxygenase and the induction of
depressive-like behavior in mice in response to bacillus
Calmette-Guerin. J Neurosci. 29:4200–4209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Siren AL, McCarron R, Wang L, Garcia-Pinto
P, Ruetzler C, Martin D and Hallenbeck JM: Proinflammatory cytokine
expression contributes to brain injury provoked by chronic monocyte
activation. Mol Med. 7:219–229. 2001.PubMed/NCBI
|
|
59
|
Sekio M and Seki K:
Lipopolysaccharide-induced depressive-like behavior is associated
with α1-adrenoceptor dependent downregulation of the
membrane GluR1 subunit in the mouse medial prefrontal cortex and
ventral tegmental area. Int J Neuropsychopharmacol. 18:182014.
|




















