Introduction
Glioma is most commonly observed in adults, and has
high severity and fatality rates. Due to the heterogeneity and
abnormality of the tumor cells, glioma is rarely curable using
current therapeutic methods, which include surgery and
chemoradiotherapy (1). During the
past decade, the median lifetime for patients with glioma is ≤12
months. The majority of previous studies on glioma were conducted
from the perspectives of histopathology and molecular biology
(2). Recently, large amounts of
evidence has demonstrated that in addition to glioma tumor cells
that exhibit strong multiplication capacity and invasiveness, a set
of cells, termed glioma stem cells, are also present in glioma
tissue and exhibit similar characteristics to neural stem cells,
such as infinite proliferation, self-renewing capacity and
multi-lineage differentiation (3).
Studies on microRNAs (miRNAs) began early in 1993.
The first miRNA was identified in Caenorhabditis elegans
(4). miRNAs are a type of
non-coding single stranded RNA with length of 19–25 nucleotides
(5). With the progressing of
molecular biology, studies demonstrated that miRNAs are involved in
the occurrence and development of tumors (6). miRNAs may function as tumor
suppressor genes to lower the activities of oncogenes, and also may
oncogenes that lower the activities of tumor suppressors (6). miRNAs can regulate the relative
expressions gene associated with tumor development (7). Mutations, deficiency, translocation
and abnormality of mutual regulation of miRNAs may cause abnormal
expression of miRNA target genes. miRNA-16 (miR-16) is located in
chromosome 13ql4, which has been demonstrated to be associated with
human tumors in a previous study (8). As a tumor suppressor gene, miR-16
expression is downregulated by regulating BMI1 proto-oncogene in
mantle cell lymphoma side population cells, reducing tumor volume
(9). miR-16 reduces the degree of
malignancy of glioma by downregulating nuclear factor-κB (NF-κB)
and matrix metalloproteinase (MMP-9), inhibiting invasiveness of
glioma cell lines. Upregulation of miR-16 inhibits the expression
of B-cell lymphoma 2 (BCL2), to promote tumor cell apoptosis
(10).
Apigenin is a plant flavonoid compound present in
numerous fruits, vegetables, beans and tea leaves (11). Previous studies demonstrated that
it exerts various biological activities and pharmacological
effects, including anticancer, anti-inflammation, antioxidant,
anti-viral and immunoregulatory effects (12,13).
Previous studies of apigenin reported that it exerts cytotoxic
effects on various cancer cells, including breast, lung, liver and
prostate cancer (11,14–16).
In the present study, the in vitro effects of apigenin on
glioma were investigated and the results indicate that apigenin may
provide a novel therapeutic approach for the treatment of
glioma.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) was
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Fetal bovine serum (FBS) was purchased from Gibco (Thermo Fisher
Scientific, Inc.). Cell Counting Kit-8 (CCK-8) solution was
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China). Annexin V-fluorescein isothiocyanate (FITC)/propidium
iodide Apoptosis Detection kit was purchased from BestBio
Biotechnology Co., Ltd. (Shanghai, China). Protein lysis buffer and
Bradford protein assay were purchased from Zhongshan Jinqiao
Biotechnology Co., Ltd., (Beijing, China).
Cell culture
Human U87 glioma cells were obtained from the Cell
Bank Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China), and were cultured in DMEM supplemented with 10%
FBS and 100 U penicillin/ml, 100 mg streptomycin/ml at 37°C in a
humidified atmosphere of 5% CO2.
Cell viability
The effect of apigenin on U87 cell viability was
determined using the CCK-8 kit. U87 cells were seeded onto 96-well
plates (1×103 cells/well) and then incubated for 24 h
following treatment with different concentrations (0, 1, 5, 10, 20,
30 and 40 μg/ml) of apigenin (Nanjing Pu Yi Biological
Technology, Co., Ltd., Nanjing, China). Following treatment with
apigenin, 10 μl thawed CCK-8 solution was added to each well
and incubated for 4 h at 37°C. Subsequently, cell viability of was
measured using the Varioskan Flash Multimode reader (Thermo Fisher
Scientific, Inc.) at 450 nm with a reference wavelength of 600
nm.
Cell apoptosis
The effect of apigenin (0, 10, 20 and 30
μg/ml) on U87 apoptotic cell death was determined using the
Annexin V-FITC/propidium iodide Apoptosis Detection kit according
to the manufacturer's instructions and were analyzed by flow
cytometry (Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of miR-16 and MMP-9
expression
Total RNA was isolated from U87 cells treated with
apigenin using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and cDNA was generated from 2 μg RNA using
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.) at 42°C for 1 h and 72°C for 5 min. miR-16 and
MMP-9 relative expression levels were examined using SYBR Green
RT-qPCR (LightCycler 480 Roche, Switzerland). The primers used are
presented in Table I. The
thermocycling conditions were 30 cycles of denaturation at 94°C for
15 sec, annealing at 57°C for 15 sec and extension at 72°C for 30
sec. The expression of miR-16 was quantified using the
2−ΔΔCq method (17).
 | Table IOligonucleotide of primers of target
genes. |
Table I
Oligonucleotide of primers of target
genes.
| Gene | Primer (5′-3′) |
|---|
| miR-16 | |
| Sense | GCGGCA
ACCCGTAGATCCGAA |
| Antisense |
GTGCAGGGTCCGAGGT |
| U6 RNA | |
| Sense |
CTCGCTTCGGCAGCACA |
| Antisense | AACGCT
TCACGAATTTGCGT |
| MMP-9 | |
| Sense |
CCCTGCGTATTTCCATTCAT |
| Antisense |
ACCCCACTTCTTGTCAGCGTC |
| β-actin | |
| Sense |
AAGCCTAAGGCCAACCGTGAAAAG |
| Antisense |
TCAATGAGGTAGTCTGTCAGGT |
Western blot analysis of NF-κB and BCL-2
expression
U87 cells treated with apigenin were washed with
ice-cold phosphate-buffered saline (PBS) and lysed in ice-cold
lysis buffer (Zhongshan Jinqiao Biotechnology, Ltd.) for 30 min.
The samples were centrifuged at 23,000 × g at 4°C for 10 min. The
supernatant was collected and the protein concentration was
determined using the Bradford protein assay. Total protein (50
μg) was separated with 12% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was blocked with 5% non-fat milk then incubated
with anti-NF-κB (cat. no. sc-109) and BCL-2 (cat. no. sc-783)
primary antibodies at a dilution of 1:1,000 (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight.
Subsequently, the membrane was washed with 0.1% Tween 20 in PBS and
incubated with horseradish peroxidase-conjugated secondary antibody
(1:5,000; Beyotime Institute of Biotechnology, Haimen, China; cat.
no. A0208) for 2 h at room temperature. The protein bands were
developed using horseradish peroxidase (Beyotime Institute of
Biotechnology) and expression was determined using densitometry
with Fujifilm Multi Gauge software, version 3.0 (Fujifilm, Tokyo,
Japan).
Anti-miR-16 plasmid transfection
Anti-miR-16 plasmid (5′-CGCCAAUAUUUACGUGCUGCUA-3′)
and negative control plasmid (5′-CAGUACUUUUGUGUAGUACAA-3′). were
synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) and
transfected into U87 cells using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions.
Statistical analysis
Data are presented as the mean ± standard error and
statistical analysis was performed where appropriate with the
two-tailed Student's t-test using SPSS software (version 17.0;
SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
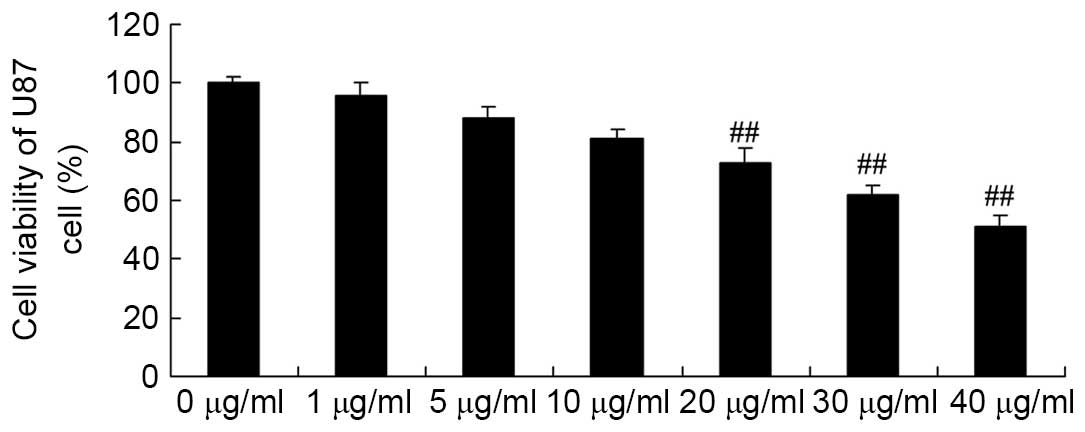
Apigenin inhibits U87 cell viability
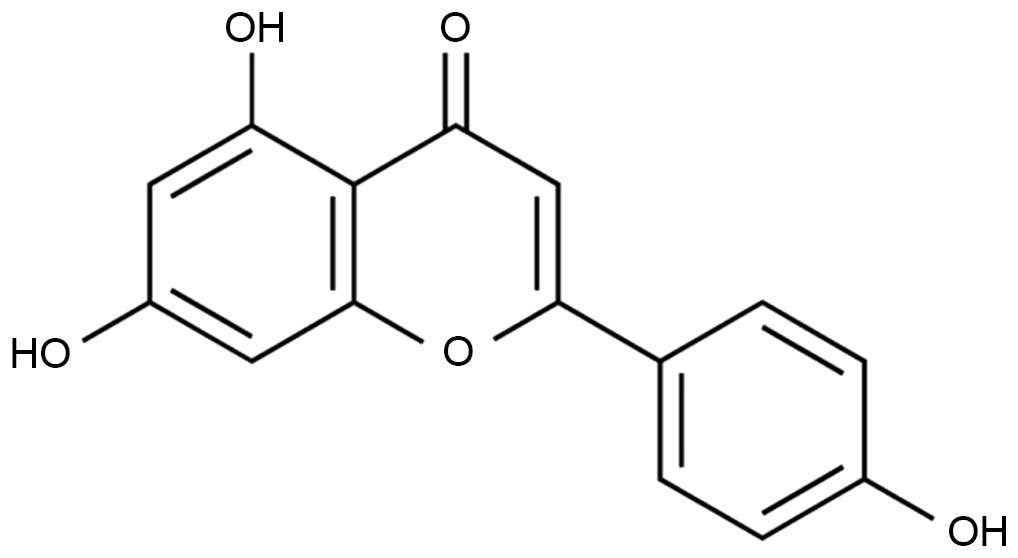
The chemical structure of apigenin is presented in
Fig. 1. The effects of apigenin on
U87 cell viability were determined. As demonstrated in Fig. 2, apigenin (20, 30, and 40
μg/ml) significantly decreased cell viability in a
dose-dependent manner compared with the control group (P=0.0081,
P=0.005 and P=0.0004, respectively).
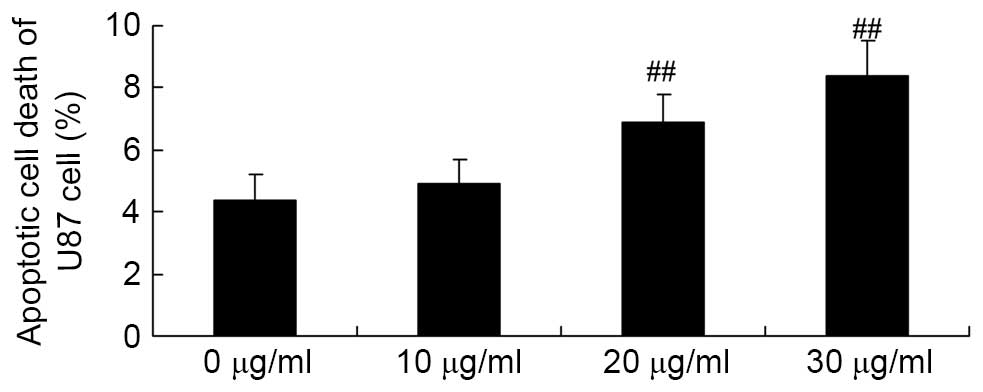
Apigenin induces apoptotic cell death of
U87 cell
Flow cytometry analysis was used to investigate the
effect of apigenin on apoptotic cell death of U87 cells. The
results demonstrated that 30 and 40 μg/ml apigenin
significantly induced apoptotic cell death of U87 cells in a
dose-dependent manner compared with the 0 μg/ml apigenin
group (P=0.0032 and P=0.0007, respectively; Fig. 3).
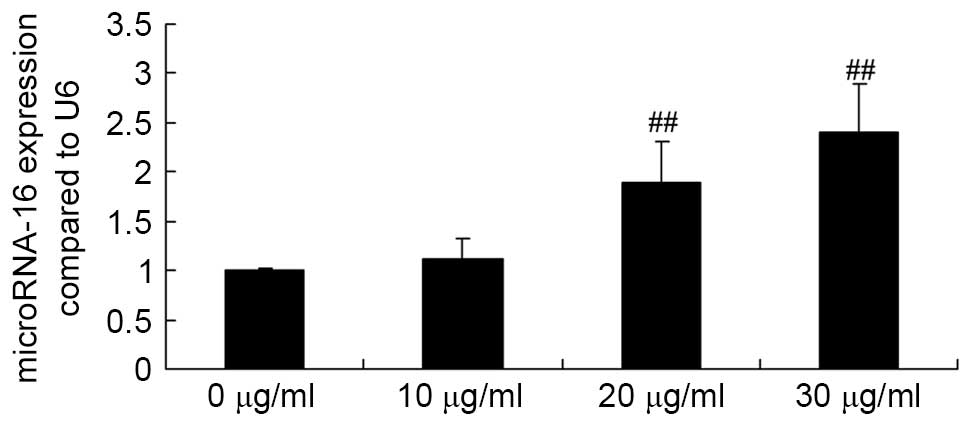
Apigenin promotes miR-16 expression in
U87 cells
To determine the underlying mechanism that mediates
the effect of apigenin on U87 cells, the effect of apigenin on
miR-16 expression in U87 cells was examined. Briefly, U87 cells
were treated with 0, 10, 20 and 30 μg/ml apigenin for 24 h,
then subjected to RT-qPCR analysis. When U87 cells were treated
with 20 or 30 μg/ml of apigenin, miR-16 expression was
significantly increased compared with the control group (P=0.0005
and P<0.0001, respectively; Fig.
4).
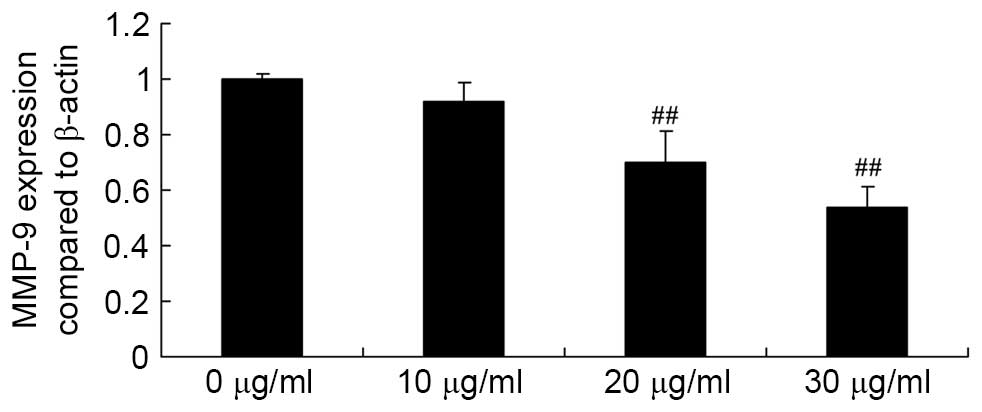
Apigenin inhibits MMP-9 expression in U87
cells
Subsequently, the effect of apigenin on MMP-9 gene
expression in U87 cells was determined by performing RT-qPCR
analysis. The assay results demonstrated that exposure of U87 cells
to 20 and 30 μg/ml apigenin for 24 h resulted in an
significant decrease in MMP-9 gene expression levels compared with
untreated control cells (P=0.0057 and P=0.0022, respectively;
Fig. 5).
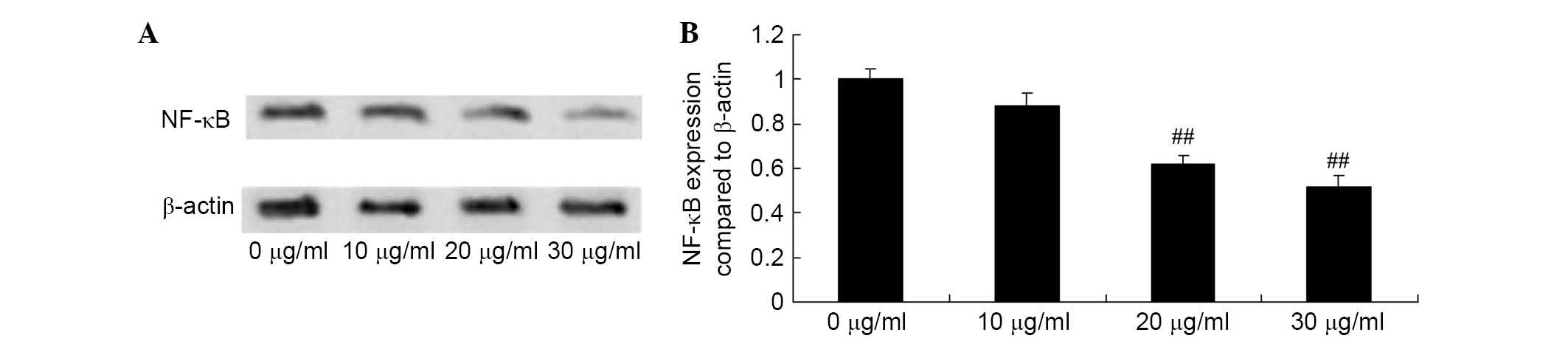
Apigenin inhibits NF-κB expression in U87
cells
To determine the effect of apigenin on NF-κB levels,
western blotting was performed to detect NF-κB protein expression
in U87 cells. The results of western blot analysis demonstrated
that NF-κB protein expression in U87 cells was significantly
reduced by treatment with 20 and 30 μg/ml of apigenin for 24
h compared with the control group (P=0.0042 and P=0.0013,
respectively; Fig. 6).
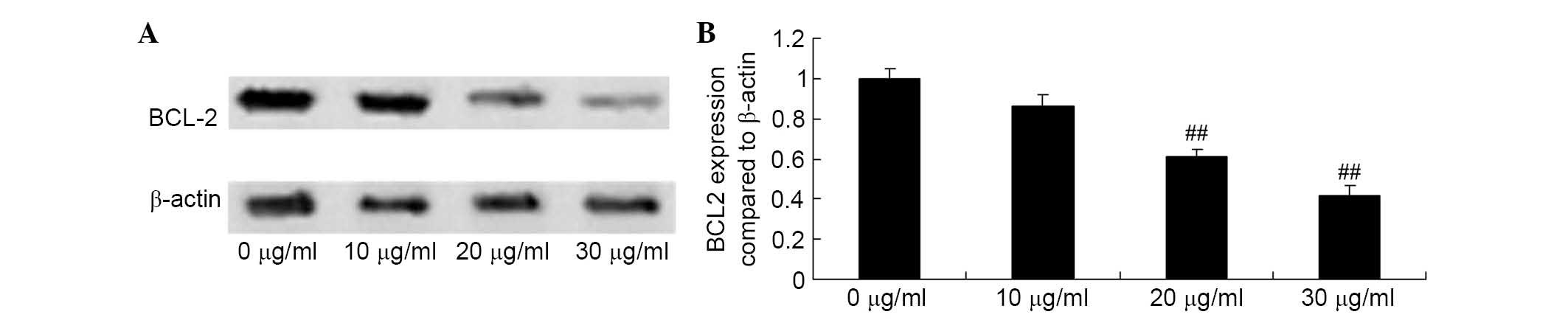
Apigenin inhibits BCL2 expression of U87
cell
The loss of BCL2 protein expression is an early
event in apoptosis. Thus, the present study assessed the effect of
apigenin on BCL2 protein expression using western blot analysis. As
demonstrated in Fig. 7, when U87
cells were incubated with 20 and 30 μg/ml apigenin for 24 h,
BCL2 protein expression was significantly suppressed compared with
the control group (P=0.0031 and P=0.0009, respectively).
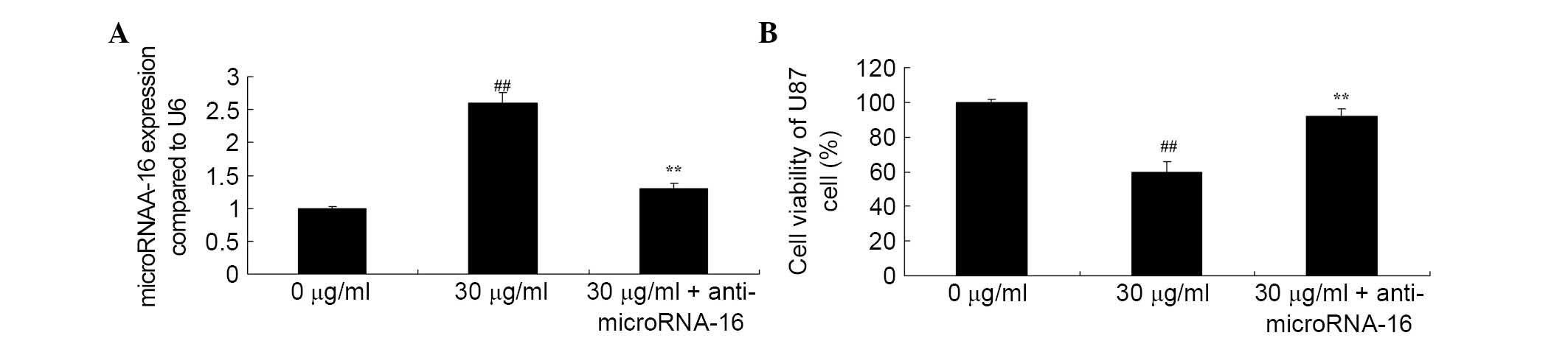
Anti-miR-16 reverses the effect of
apigenin on U87 cell viability
Whether anti-miR-16 reverses the effect of apigenin
on U87 cell viability was investigated. The results demonstrated
that anti-miR-16 inhibited apigenin-induced miR-16 gene expression
(P=0.0027; Fig. 8A) and increased
apigenin-suppressed viability of U87 cells compared with 30
μg/ml apigenin treatment (P=0.0047; Fig. 8B).
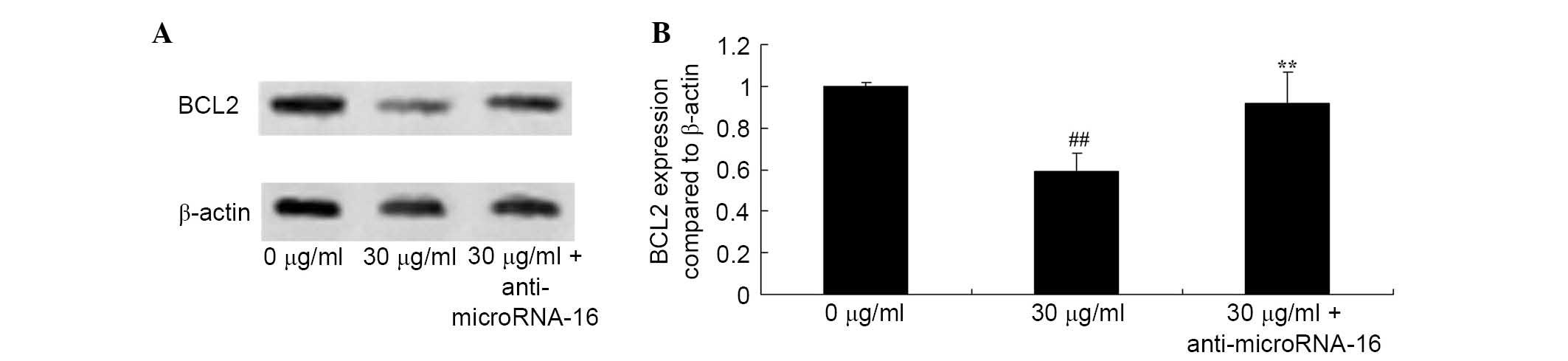
Anti-miR-16 reverses the effect of
apigenin on BCL2 expression in U87 cells
To determine the molecular events involved in BCL2
expression in apigenin-induced apoptosis of U87 cells, the effect
of anti-miR-16 on the level of BCL2 protein expression in U87 cells
was analyzed. The results demonstrated that anti-miR-16
significantly increased BCL2 protein expression in U87 cell
compared with 30 μg/ml apigenin treatment (P=0.0022;
Fig. 9).
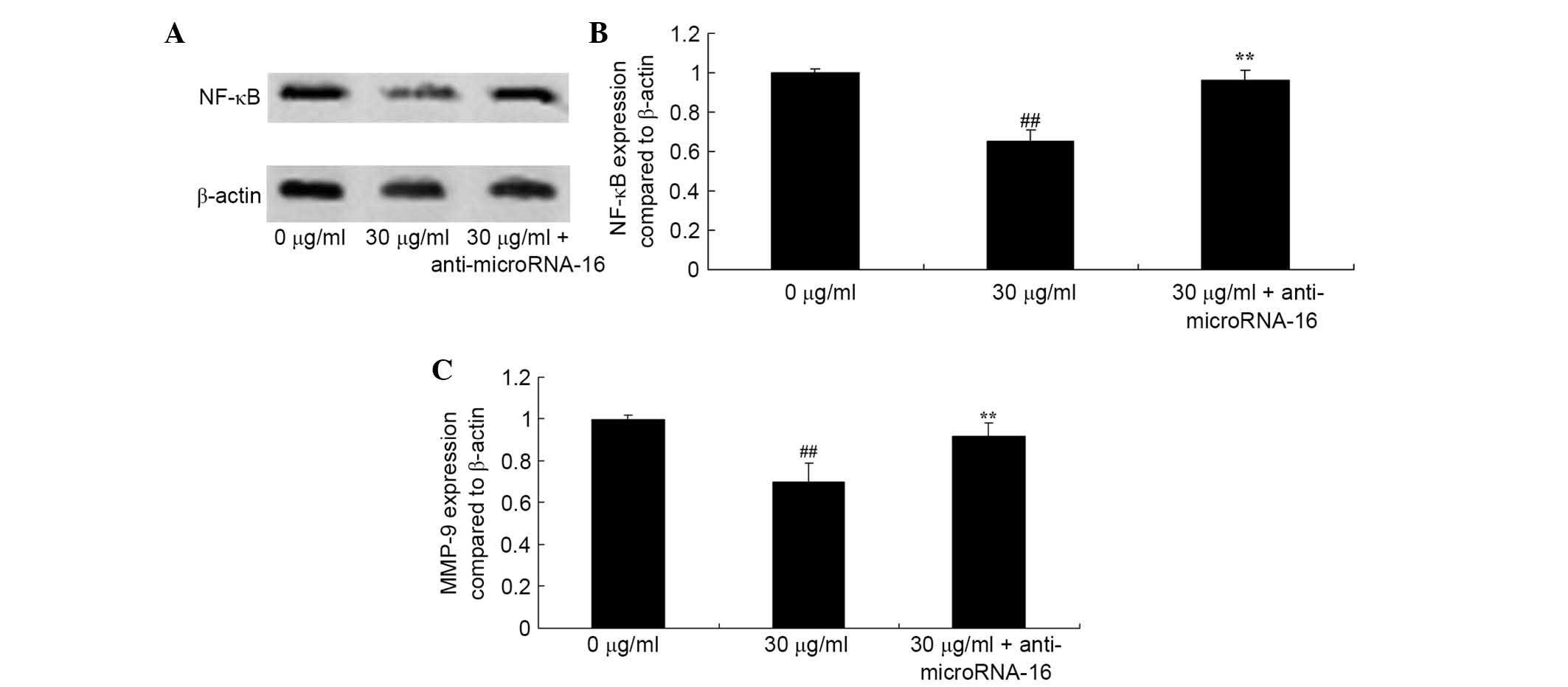
Anti-miR-16 reverses the effect of
apigenin on NF-κB/MMP-9 expression in U87 cells
To identify whether miR-16 is involved in the effect
of apigenin on NF-κB/MMP-9 expression in U87 cells, the effect of
abti-miR-16 on the protein expression levels of NF-κB and MMP-9
were determined. As demonstrated in Fig. 10, apigenin-inhibited NF-κB/MMP-9
levels were significantly increased by anti-miR-16 compared with 30
μg/ml apigenin (P=0.0072 and P=0.0041; Fig. 10).
Discussion
Glioma is a common tumor with high severity
(18). Compared with malignant
tumors in other locations, glioma is rarely transferred to sites
out of the central nervous system (1). Additionally, glioma exhibits strong
invasion capacity, and can infiltrate from primary lesions to other
cerebral tissue or metastasize to distant sites far from the
primary lesions (19). As a
result, the edges of the primary tumor are unclear. Combined with
its strong invasive capacities, mortality rates of patients with
glioma are high (20).
Consequently, even following treatment, including surgery and
chemoradiotherapy, survival periods of patients with glioma ≤15
months (21). It is particularly
important to identify novel molecules that participate in the
metastasis and invasion of glioma. The present study demonstrated
that apigenin significantly decreased cell viability and induced
apoptotic cell death of U87 cells in a dose-dependent manner.
Previous studies demonstrated that apigenin inhibits the
proliferation of human bladder cancer (11), ovarian cancer cells (14) and lung cancer (15).
Additionally, multiple studies have demonstrated
that miRNAs are important in tumor physiological and pathological
processes, including growth, proliferation, apoptosis, lipid
metabolism, hormone secretion and tumorigenesis (4). miRNAs can be regarded as oncogenes or
tumor suppressor genes (7).
Particularly, studies have reported that miRNAs are involved in
cell differentiation, proliferation and invasion (5). A previous study reported that miR-16
reduced the metastasis and invasion of glioma cells by inhibiting
MMP-9 (22). Additionally, another
previous report demonstrated that increased miR-16 inhibited the
proliferation of glioma and accelerated its apoptosis (23). To the best of our knowledge, the
current study demonstrated for the first time that apigenin
significantly increased miR-16 levels in U87 cells. Yang et
al (22) previously reported
that miR-16 reduced glioma cell growth via suppression of BCL2 and
the NF-κB/MMP9 signaling pathway.
The BCL2 genes are localized at chromosome 18q21 and
the encoded protein products can reverse the apoptosis-promoting
effects of c-myc proteins, stabilize mitochondrial function and
structures, and disrupt the activity of caspase enzymes via
antagonism of p53 proteins (24).
Thus, BCL2 inhibits cell apoptosis induced by various factors and
participates in the dynamic equilibrium that regulates
proliferation and apoptosis (25).
Abnormal increases in BCL2 expression causes cells with abnormal
changes to avoid apoptosis (25).
Increased accumulation of genetic abnormal events is a prerequisite
of cell transformation and tumorigenesis (26). Infiltrative growth is a distinctive
feature of glioma and the invasive capability is proportional to
its severity. Studies using glioma cell lines in vitro
demonstrated that BCL2 promoted the synthesis, secretion and
activity of MMPs to increase the infiltrative capacities of glioma
cells (27,28). The present study demonstrated that
treatment with apigenin significantly suppressed BCL2 protein
expression in U87 cells. Additionally, Shukla and Gupta (16) concluded that apigenin induced
apoptosis of DU145 human prostate carcinoma cells by altering the
BCL2 associated X protein/BCL2 ratio (16).
Previous findings have demonstrated that NF-κB is a
important factor during inflammation (29). As one of most important
transcription factors discovered in recent years, NF-κB was named
as it sequence-specifically binds with enhancer κB of κ light chain
of B cell immunoglobulins (30).
NF-κB is involved in vascularization and tumor spread through
regulation of vascular endothelial growth factor and interleukin-8;
it promotes the transcription of MMPs to degrade extracellular
matrix (31). Thus, NF-κB may
promote tumor infiltration to surrounding tissues and the
metastasis of breast cancer cells (31). The current study identified that
apigenin significantly reduced NF-κB protein expression in U87
cells. Chang et al (32)
demonstrated that apigenin protects against adjuvant-induced
arthritis via inhibiting the purinergic receptor P2X 7/NF-κB
pathway.
A study investigating chronic myeloid leukemia by Li
et al (23) demonstrated
that miR-15a and miR-16-1 expression was increased and BCL2 was
decreased in glioma cells. miR-15a and miR-16-1 negatively regulate
BCL2 expression at the post-transcriptional level. BCL2 was
demonstrated to be a target gene of miR-16, which significantly
inhibited the 3′UTR region of BCL2 transcripts (22). A previous in vivo experiment
demonstrated that miR-16 directly inhibited protein expressions of
BCL2 to induce early apoptosis of glioma cells in human brain
(22). The altered activity of
NF-κB/MMP-9 signaling pathways in brain glioma promoted invasion
(33). In vitro experiments
demonstrated that, as a cancer suppressor gene, miR-16 reduced the
proliferation and invasion of brain glioma via inhibiting the gene
expressions NF-κB and MMP-9 (22).
In the present study, apigenin suppressed the NF-κB/MMP9 signaling
pathway in U87 cells. Palmieri et al (12) reported that apigenin inhibits the
TNFα-induced expression of MMP-9. Additionally, the current study
demonstrated that anti-miR-16 reversed the effect of apigenin on
cell viability, BCL2 protein expression and the NF-κB/MMP-9 pathway
in U87 cells. Yang et al (22) reported that miR-16 reduced the
proliferation of glioma cells via suppression of BCL2 expression
and NF-κB/MMP9 signaling.
In conclusion, the present study demonstrated that
apigenin reduced glioma viability through increased expression of
miR-16, and suppression of BCL-2 and NF-κB/MMP-9. These results
suggested that apigenin may represent a valuable cancer therapeutic
option for the treatment of glioma.
References
|
1
|
Okada H, Kalinski P, Ueda R, Hoji A,
Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK,
et al: Induction of CD8+ T-cell responses against novel
glioma-associated antigen peptides and clinical activity by
vaccinations with (alpha)-type 1 polarized dendritic cells and
polyinosinic-polycytidylic acid stabilized by lysine and
carboxymethylcellulose in patients with recurrent malignant glioma.
J Clin Oncol. 29:330–336. 2011. View Article : Google Scholar
|
|
2
|
Diez Valle R, Slof J, Galvan J, Arza C,
Romariz C and Vidal C; VISIONA study researchers: Observational,
retrospective study of the effectiveness of 5-aminolevulinic acid
in malignant glioma surgery in Spain (The VISIONA study).
Neurologia. 29:131–138. 2014.
|
|
3
|
Morrison LC, McClelland R, Aiken C,
Bridges M, Liang L, Wang X, Di Curzio D, Del Bigio MR, Taylor MD
and Werbowetski-Ogilvie TE: Deconstruction of medulloblastoma
cellular heterogeneity reveals differences between the most highly
invasive and self-renewing phenotypes. Neoplasia. 15:384–398. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ouyang Q, Xu L, Cui H, Xu M and Yi L:
MicroRNAs and cell cycle of malignant glioma. Int J Neurosci.
126:1–9. 2016. View Article : Google Scholar
|
|
5
|
Wang BC and Ma J: Role of MicroRNAs in
Malignant Glioma. Chin Med J (Engl). 128:1238–1244. 2015.
View Article : Google Scholar
|
|
6
|
Hu E, Wang D, Zhang X, Li J, Hu Y, Gong H
and Liu E: Four common polymorphisms in microRNAs and the risk of
adult glioma in a chinese case-control study. J Mol Neurosci.
51:933–940. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feng SY, Dong CG, Wu WK, Wang XJ, Qiao J
and Shao JF: Lentiviral expression of anti-microRNAs targeting
miR-27a inhibits proliferation and invasiveness of U87 glioma
cells. Mol Med Rep. 6:275–281. 2012.PubMed/NCBI
|
|
8
|
Venturutti L, Cordo Russo RI, Rivas MA,
Mercogliano MF, Izzo F, Oakley RH, Pereyra MG, De Martino M,
Proietti CJ, Yankilevich P, et al: MiR-16 mediates trastuzumab and
lapatinib response in ErbB-2-positive breast and gastric cancer via
its novel targets CCNJ and FUBP1. Oncogene. May 9–2016.Epub ahead
of print. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen RW, Bemis LT, Amato CM, Myint H, Tran
H, Birks DK, Eckhardt SG and Robinson WA: Truncation in CCND1 mRNA
alters miR-16-1 regulation in mantle cell lymphoma. Blood.
112:822–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han J and Chen Q: MiR-16 modulates
temozolomide resistance by regulating BCL-2 in human glioma cells.
Int J Clin Exp Pathol. 8:12698–12707. 2015.
|
|
11
|
Shi MD, Shiao CK, Lee YC and Shih YW:
Apigenin, a dietary flavonoid, inhibits proliferation of human
bladder cancer T-24 cells via blocking cell cycle progression and
inducing apoptosis. Cancer Cell Int. 15:332015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Palmieri D, Perego P and Palombo D:
Apigenin inhibits the TNFalpha-induced expression of eNOS and MMP-9
via modulating Akt signalling through oestrogen receptor
engagement. Mol Cell Biochem. 371:129–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Liu X, Sun C, Yang J, Wang L,
Gong L and Jing Y: Apigenin attenuates experimental autoimmune
myocarditis by modulating Th1/Th2 cytokine balance in mice.
Inflammation. 39:678–686. 2016. View Article : Google Scholar
|
|
14
|
Suh YA, Jo SY, Lee HY and Lee C:
Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor
tyrosine kinases by apigenin circumvent taxol resistance in ovarian
cancer cells. Int J Oncol. 46:1405–1411. 2015.
|
|
15
|
Bruno A, Siena L, Gerbino S, Ferraro M,
Chanez P, Giammanco M, Gjomarkaj M and Pace E: Apigenin affects
leptin/leptin receptor pathway and induces cell apoptosis in lung
adenocarcinoma cell line. Eur J Cancer. 47:2042–2051. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shukla S and Gupta S: Molecular mechanisms
for apigenin-induced cell-cycle arrest and apoptosis of hormone
refractory human prostate carcinoma DU145 cells. Mol Carcinog.
39:114–126. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leylabadlo HE, Yekani M and Ghotaslou R:
Helicobacter pylori hopQ alleles (type I and II) in gastric cancer.
Biomed Rep. 4:601–604. 2016.PubMed/NCBI
|
|
18
|
Solomon MT, Selva JC, Figueredo J, Vaquer
J, Toledo C, Quintanal N, Salva S, Domíngez R, Alert J, Marinello
JJ, et al: Radiotherapy plus nimotuzumab or placebo in the
treatment of high grade glioma patients: Results from a randomized,
double blind trial. BMC Cancer. 13:2992013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morokoff A, Ng W, Gogos A and Kaye A:
Molecular subtypes, stem cells and heterogeneity: Implications for
personalised therapy in glioma. J Clin Neurosci. 22:1219–1226.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sizoo EM, Pasman HR, Dirven L, Marosi C,
Grisold W, Stockhammer G, Egeter J, Grant R, Chang S, Heimans JJ,
et al: The end-of-life phase of high-grade glioma patients: A
systematic review. Support Care Cancer. 22:847–857. 2014.
View Article : Google Scholar
|
|
21
|
Siu A, Wind JJ, Iorgulescu JB, Chan TA,
Yamada Y and Sherman JH: Radiation necrosis following treatment of
high grade glioma-a review of the literature and current
understanding. Acta Neurochir (Wien). 154:191–201. 2012. View Article : Google Scholar
|
|
22
|
Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al: MicroRNA-16 inhibits
glioma cell growth and invasion through suppression of BCL2 and the
nuclear factor-kappaB1/MMP9 signaling pathway. Cancer Sci.
105:265–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Ling N, Bai Y, Dong W, Hui GZ, Liu
D, Zhao J and Hu J: MiR-16-1 plays a role in reducing migration and
invasion of glioma cells. Anat Rec (Hoboken). 296:427–432. 2013.
View Article : Google Scholar
|
|
24
|
Wang Q, Li A, Wang H and Wang J: Knockdown
of apoptosis repressor with caspase recruitment domain (ARC)
increases the sensitivity of human glioma cell line U251MG to
VM-26. Int J Clin Exp Pathol. 5:555–561. 2012.PubMed/NCBI
|
|
25
|
Jia G, Wang Q, Wang R, Deng D, Xue L, Shao
N, Zhang Y, Xia X, Zhi F and Yang Y: Tubeimoside-1 induces glioma
apoptosis through regulation of Bax/Bcl-2 and the ROS/Cytochrome
C/Caspase-3 pathway. Onco Targets Ther. 8:303–311. 2015.PubMed/NCBI
|
|
26
|
Li CL, Chang L, Guo L, Zhao D, Liu HB,
Wang QS, Zhang P, Du WZ, Liu X, Zhang HT, et al: beta-elemene
induces caspase-dependent apoptosis in human glioma cells in vitro
through the upregulation of Bax and Fas/FasL and downregulation of
Bcl-2. Asian Pac J Cancer Prev. 15:10407–10412. 2014. View Article : Google Scholar
|
|
27
|
Wick W, Wild-Bode C, Frank B and Weller M:
BCL-2-induced glioma cell invasiveness depends on furin-like
proteases. J Neurochem. 91:1275–1283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al: MicroRNA-16 inhibits
glioma cell growth and invasion through suppression of BCL2 and the
nuclear factor-kappaB1/MMP9 signaling pathway. Cancer Sci.
105:265–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu LB, Jiang J, Zhu XP, Wang TF, Chen XY,
Luo QF, Shu Y, Liu ZL and Huang SH: Knockdown of Aurora-B inhibits
osteosarcoma cell invasion and migration via modulating
PI3K/Akt/NF-kappaB signaling pathway. Int J Clin Exp Pathol.
7:3984–3991. 2014.
|
|
30
|
Du Z, Whitt MA, Baumann J, Garner JM,
Morton CL, Davidoff AM and Pfeffer LM: Inhibition of type I
interferon-mediated antiviral action in human glioma cells by the
IKK inhibitors BMS-345541 and TPCA-1. J Interferon Cytokine Res.
32:368–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kesanakurti D, Chetty C, Bhoopathi P,
Lakka SS, Gorantla B, Tsung AJ and Rao JS: Suppression of MMP-2
attenuates TNF-alpha induced NF-kappaB activation and leads to JNK
mediated cell death in glioma. PLoS One. 6:e193412011. View Article : Google Scholar
|
|
32
|
Chang X, He H, Zhu L, Gao J, Wei T, Ma Z
and Yan T: Protective effect of apigenin on Freund's complete
adjuvant-induced arthritis in rats via inhibiting P2X7/NF-κB
pathway. Chem Biol Interact. 236:41–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang L, Wu J, Yang Y, Liu L, Song L, Li J
and Li M: Bmi-1 promotes the aggressiveness of glioma via
activating the NF-kappaB/MMP-9 signaling pathway. BMC Cancer.
12:4062012. View Article : Google Scholar : PubMed/NCBI
|