Introduction
Flavonoids, which are polyphenolic compounds, have
been widely investigated for their antioxidant effects (1). Flavonoids have two classical
antioxidant structural components, including a B-ring catechol
group, which donates a hydrogen/electron to stabilize a radical
species, and a C2-C3 double bond conjugated with an oxo group at
C4, which binds transition metal ions, including iron and copper
(2,3). Luteolin (LUT), one of the most common
flavonoids found in plants in the form of glycosides, are
eventually metabolized by intestinal bacteria, cleaved and
glucuronated during uptake in the gut and metabolism in the
organism. As LUT and a number of its glycosides fulfill these two
structural requirements, it has been suggested that LUT possesses
antioxidant properties (4).
A number of signaling events are initiated and
driven by oxidative stress. Hydrogen peroxide
(H2O2) and other reactive oxygen species
(ROS) lead to oxidative stress, and are increased by several
stimulants, including ultraviolet (UV) irradiation (5–10),
which also decrease levels of anti-oxidant enzymes (11). These features exist in
chronologically aged human tissues, particularly skin. These two
factors increase ROS production, which leads to alterations in
genes, protein structure and function, finally leading the damage
of tissues, including the skin. During a process of energy
transfer, the superoxide anion, O2−, is produced from
endogenous UV-absorbing chromophores (12) into molecular oxygen. The superoxide
dismutase (SOD) catalyzes O2− to produce
H2O2 which can be converted to the reactive
hydroxyl radical, HO• (8). These
compounds are ROS, which can activate several downstream
proteins.
Human skin is usually exposed to several oxidants,
of which UV is the most common, causing ROS burst. Increasing
levels of harmful oxygen free radicals are implicated in the
pathogenesis of skin carcinoma, the mechanism of skin senescence
and other skin diseases (5). The
current focus on the bioactivity of the flavonoids is partly due to
the potential health benefits of the polyphenolic components
present within major dietary constituents. LUT, which is considered
to be one of the most important flavonoids, has been reported to
resist against several extraneous oxidants (2–4),
however, its antioxidant effects against UV remain to be fully
elucidated. The present study aimed to investigate the probability
of LUT scavenging the ROS induced by UVA irradiation in human skin
fibroblasts (HSFs), and to examine the potential mechanism to allow
improved skin protection.
Materials and methods
Cell culture
HSFs, isolated from the foreskins of children (age,
3–9) following circumcision surgery at the Guangzhou General
Hospital of Guangzhou Military Command (Guangzhou, China), were
routinely cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% newborn calf serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 4 mM glutamine, 100 U/ml
penicillin and 100 mg/ml streptomycin, following homogenization of
tissue. The cells were harvested with trypsin when they reached 80%
confluence and were seeded in a 6-well plate at a density of
1×105 cells/well at 37°C. Following incubation for 48 h,
the cultured medium was removed. The successfully cultured cells
were stored in a nitrogen canister. Cells between passages 4–10
were used in the subsequent experiments. The present study was
approved by the ethics committee of the Guangzhou General Hospital
of Guangzhou Military Command.
Reagents and antibodies
LUT, glutamine, penicillin, streptomycin, trypsin,
formaldehyde, glutaraldehyde, OsO4, uranyl acetate,
lead, SDS, doxorubicin (DOX), rhodamine123 [for the detection of
mitochondrial membrane potential (MMP)] and
dichlorodihydrofluorescein diacetate (DCFH-DA) were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
3-(4,5-dimethylthyl-thiazol-2-yl)-2,5-di-phenyltetrazolium bromide
(MTT) was purchased from Promega Corporation (Madison, WI, USA),
and DAPI and radioimmunoprecipitation lysis buffer were purchased
from Beyotime Institute of Biotechnology (Haimen, China). LUT and
DOX were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich). LUT
concentrations of 5, 20 and 40 µM were used in the
experiments. Unless otherwise specified in the figure legends,
values are expressed as concentrations in µM. Beclin 1 and
LC3 antibodies were obtained from Cell Signaling Technologies, Inc.
(Danvers, MA, USA). Primary antibodies against GAPDH, β-actin and
hypoxia-inducible factor (HIF)-1α, and the secondary
horseradish-peroxidase-labeled antibodies, were also purchased from
Beyotime Institute of Biotechnology.
Irradiation procedure
When the cells reached 80% confluency, they were
irradiated under a Solar UV Simulator (Oriel® Sol-UV-4;
Newport Corporation, Irvine, CA, USA). The radiation intensity was
measured using a UVX digital radiometer (Ultra-Violet Products,
Inc., Uplands, CA, USA) equipped with a UVX-310 sensor. The HSFs
were irradiated by 320-400 nm UVA in single or repetitive 7.2
J/cm2 low doses. The medium was removed and the cells
were washed twice with phosphate-buffered saline (PBS) prior to UV
irradiation. The cells were covered with a thin film of PBS during
UV exposure, and remained in culture in the maintenance medium
following irradiation for 10 min repeated 3 times. A control group
of cells were treated in a similar manner, however, these cells
were exposed to normal room lighting. All cells were incubated at
37°C and 5% CO2.
Cell viability assessment
To measure cell viability, the MTT method was used
(13). Prior to adding the MTT
working solution (5 mg/ml), the cells were seeded in 96-well plates
at a density of 5×105 cells per well overnight, and
treated with LUT and UVA, as indicated. Subsequently, the cells
were incubated in a CO2 incubator for 4 h. The medium
was then replaced with 150 µl DMSO (Sigma-Aldrich) to
completely dissolve the formazan crystals. The absorbance of each
well was then measured using a plate reader (iMark; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) at a test wavelength of 570
nm. Cell viability was calculated using the following equation:
Cell viability = absorbance of experiment samples / absorbance of
control) × 100%.
Apoptosis assay
Apoptosis was determined using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining
procedure. In brief, following treatment with UV irradiation and
incubation with LUT, the cells were collected and washed twice with
ice-cold PBS, followed by incubation with Annexin V-FITC and PI.
Fluorescence was measured using a BD FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) with an excitation wavelength
of 480 nm through a FL-1 filter (530 nm) and a FL-2 filter (585
nm).
Cellular ROS measurement
The dichloro-dihydro-fluorescein diacetate (DCFH-DA)
fluorescent dye (14) was used for
ROS analysis. In brief, following treatment with UVA with or
without LUT, the cells were collected and incubated with 10
µM DCFH-DA at 37°C for 30 min. Finally, the cells were
washed three times with PBS, and the fluorescence was measured
using a flow cytometer through an FL-1 filter with an excitation
wavelength of 480 nm.
Analysis of autophagy
Monodansylcadaverine (MDC) has been used as a tracer
for autophagic vacuoles previously (15). In the present study,
1×105/ml cells were seeded on coverslips overnight, and
then exposed to UVA with or without LUT, as described above, and
rinsed with PBS. The cells were then stained with 50 µM MDC
at 37°C for 1 h and examined using a flow cytometer.
Cell morphology assessment
Autophagic vascular organelles (AVOs) were examined
by staining the treated cells with MDC (Sigma-Aldrich) (15) for 30 min at 37°C. Following washing
of the cells with PBS, 4% formaldehyde was added to fix the cells
for 30 min. The cells were observed under a Nikon Intensilight
fluorescence microscope (Nikon Corporation, Tokyo, Japan) following
washing with PBS three times. To observe nuclear morphology, the
cells were incubated with DAPI for 10 min following fixation with
4% formaldehyde for 30 min and washing with PBS. The cells were
then observed under a fluorescence microscope following washing
with PBS three times.
For transmission electron microscopy, the cells were
fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4),
followed by 1% OsO4. Following dehydration, thin
sections (70 nm) were stained with uranyl acetate and lead for
observation under an electron microscope (JSM-6010LA; JEOL, Ltd.,
Tokyo, Japan) (16).
Western blot analysis
At a density of 1×107 cells/ml, the cells
were treated with either 10 µl 5% DMSO, LUT alone (0, 5, 20
and 40 µM), 10 ml DOX alone, or with a combination of LUT
and DOX at 37°C for 48 h, and were harvested at indicated time
points. Following a lysis procedure, the lysates were centrifuged
at 12,000 g for 15 min at 4°C. Bicinchoninic acid protein assay
reagent (Beyotime Institute of Biotechnology) was used to quantify
the protein concentrations of the supernatants. The protein (50 mg)
from each sample were separated by 30% SDS-PAGE and transferred to
a polyvinylidene fluoride membrane. The membrane was blocked with
5% non-fat milk and incubated with primary antibodies (dilution,
1:1,000) at room temperature for 2 h, then washed 3 times for 5 min
prior to incubation with secondary antibodies at room temperature
for 1 h. The bands were detected using a ChemiDoc Touch imaging
system (Bio-Rad Laboratories, Inc.).
Statistical analysis
The data are expressed as the mean ± standard
deviation and were analyzed using Student's t-test
(two-tailed) and SPSS 22.0 (IBM SPSS, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
LUT decreases UVA-induced cell death in
HSFs
Previous studies have indicated that LUT has
potential protective effects against exogenous oxidants in various
cell types, however, these protective effects have not been
investigated in HSFs exposed to UV irradiation (6,9,12).
To investigate the protective effects of LUT in human skin, the
present study first investigated the resistance of cells to cell
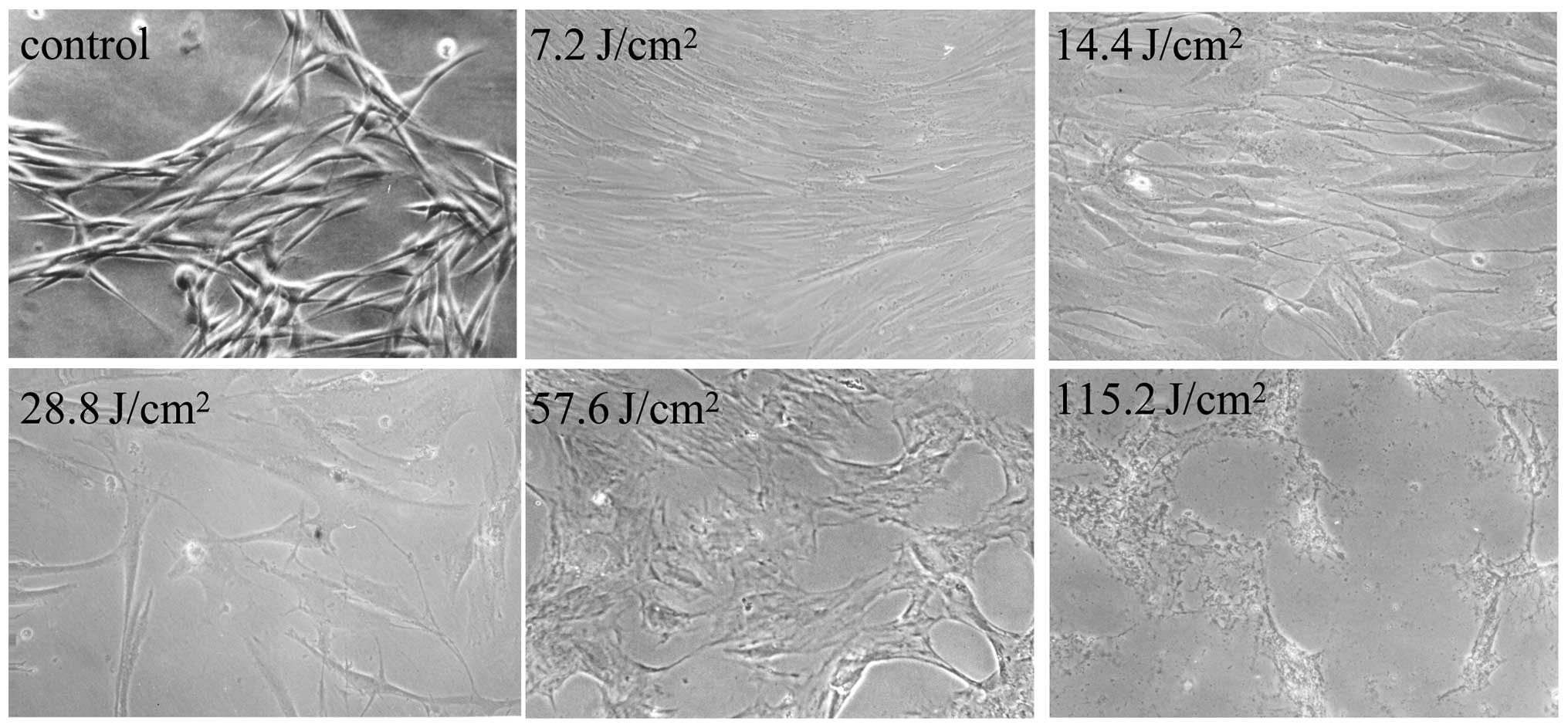
death induced by UVA irradiation (Fig.
1), following treatment of the cells with LUT at concentrations
ranging between 0 and 40 µM.
The results of the MTT analysis showed the toxic
effects of UVA towards the cells alone and following treatment with
LUT. As shown in Fig. 2A and B,
UVA irradiation induced HSF death in a dose-dependent manner. As
shown in Fig. 2C, the HSFs exposed
to UVA irradiation accompanied by incubation with LUT did not
exhibit significant cell death, compared with the HSFs treated with
UVA alone. These results indicated that LUT protected the HSFs from
UVA-induced death.
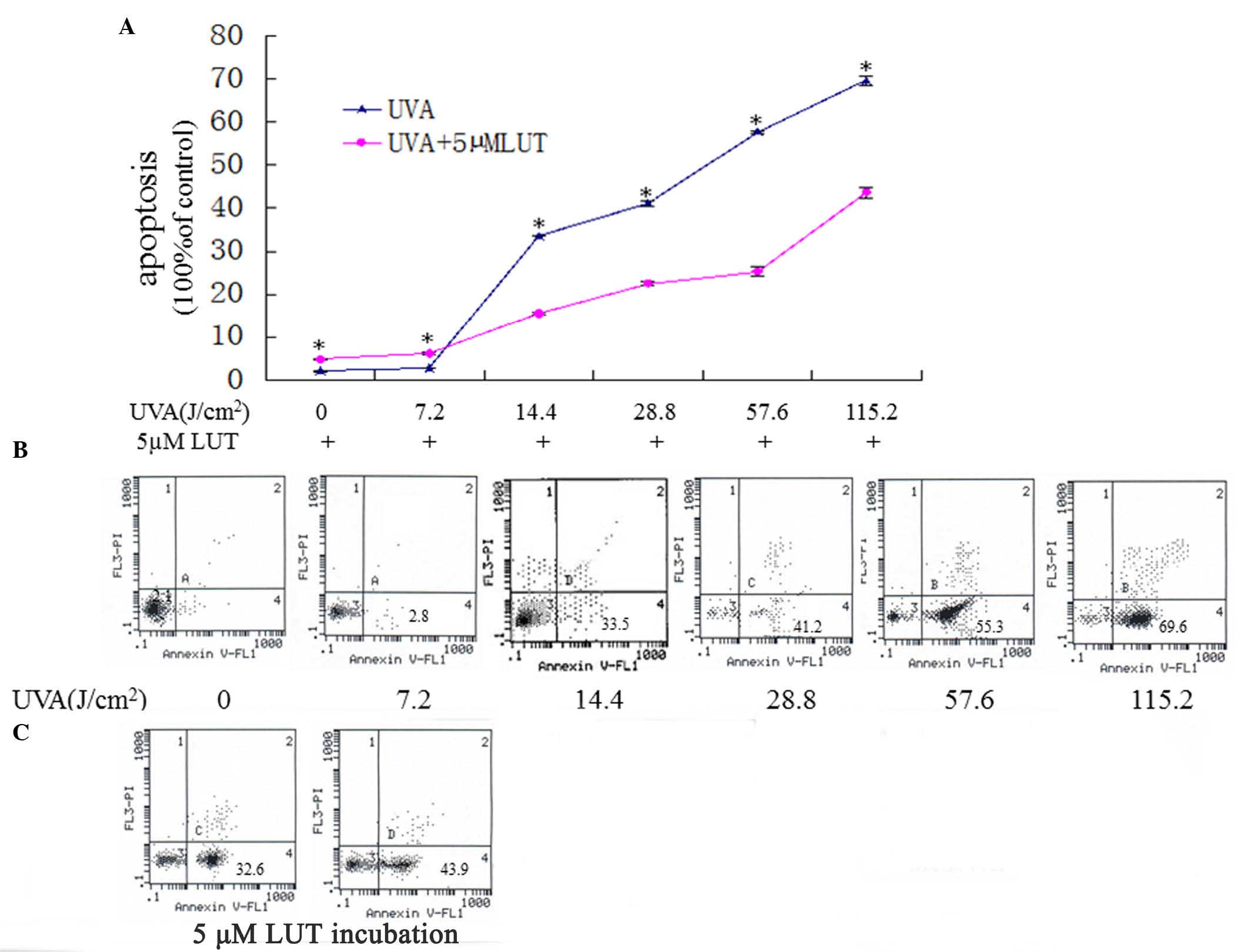 | Figure 2LUT decreases HSF apoptosis induced by
UVA. The HSFs were assigned into two groups, and were irradiated
with repetitive doses of UVA (0 J, 7.2, 14.4, 28.8, 57.6 and 115.2
J/cm2, respectively). One group of HSFs was irradiated
with UVA alone, the other group was irradiated with UVA and
incubated with 5 µM LUT. (A)
3-(4,5-dimethylthyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
analyses of the apoptosis in the two groups. Data are expressed as
the mean ± standard deviation (*P<0.05). (B and C)
Annexin V-FITC/PI staining for the detection of apoptosis. The
X-axis denotes Annexin V-FITC; the Y-axis denotes DNA content by
PI. The apoptosis of HSFs were elevated gradually with the
increasing doses of UVA while 5 µM LUT resisted HSFs
apoptosis induced by UVA. The experiment was repeated three times,
with representative results presented. HSFs, human skin
fibroblasts; PI, propidium iodide; FITC, fluorescein
isothiocyanate; UVA, ultraviolet A; LUT, luteolin. |
LUT induces resistance to UVA-induced
autophagy of HSFs
To observe the morphology of the HSFs following UVA
irradiation and LUT incubation, a light microscope (Fig. 1) and fluorescence microscope were
used to visualize the cells.
Autophagy, also termed non-apoptotic programmed cell
death (type II programmed cell death), involves a series of
biochemical steps, through which eukaryotic cell death is induced
through self degradation of their own cytoplasm and organelles
(17). To determine whether LUT
treatment decreases UVA-induced HSF autophagy, the cells were
observed under a fluorescence microscope to detect AVO formation
following staining with MDC. Punctuation of MDC-positive cells were
observed when treated with 5 µM LUT, compared with 40
µM LUT, as shown in Fig.
3A. As shown in Fig. 3B,
following treatment with the higher concentration of 40 µM
LUT, AVO formation decreased. The formation of AVOs decreased
sharply following 24 h of treatment with the combination of LUT. To
further demonstrate the induction of autophagy, electron microscopy
was performed, which is the Gold Standard method for confirmation
of autophagy. It was found that, in all treatments, incubation with
higher concentrations of LUT exhibited fewer AVOs (Fig. 3B). Almost no cell death was
observed in the untreated cells (Fig.
3C).
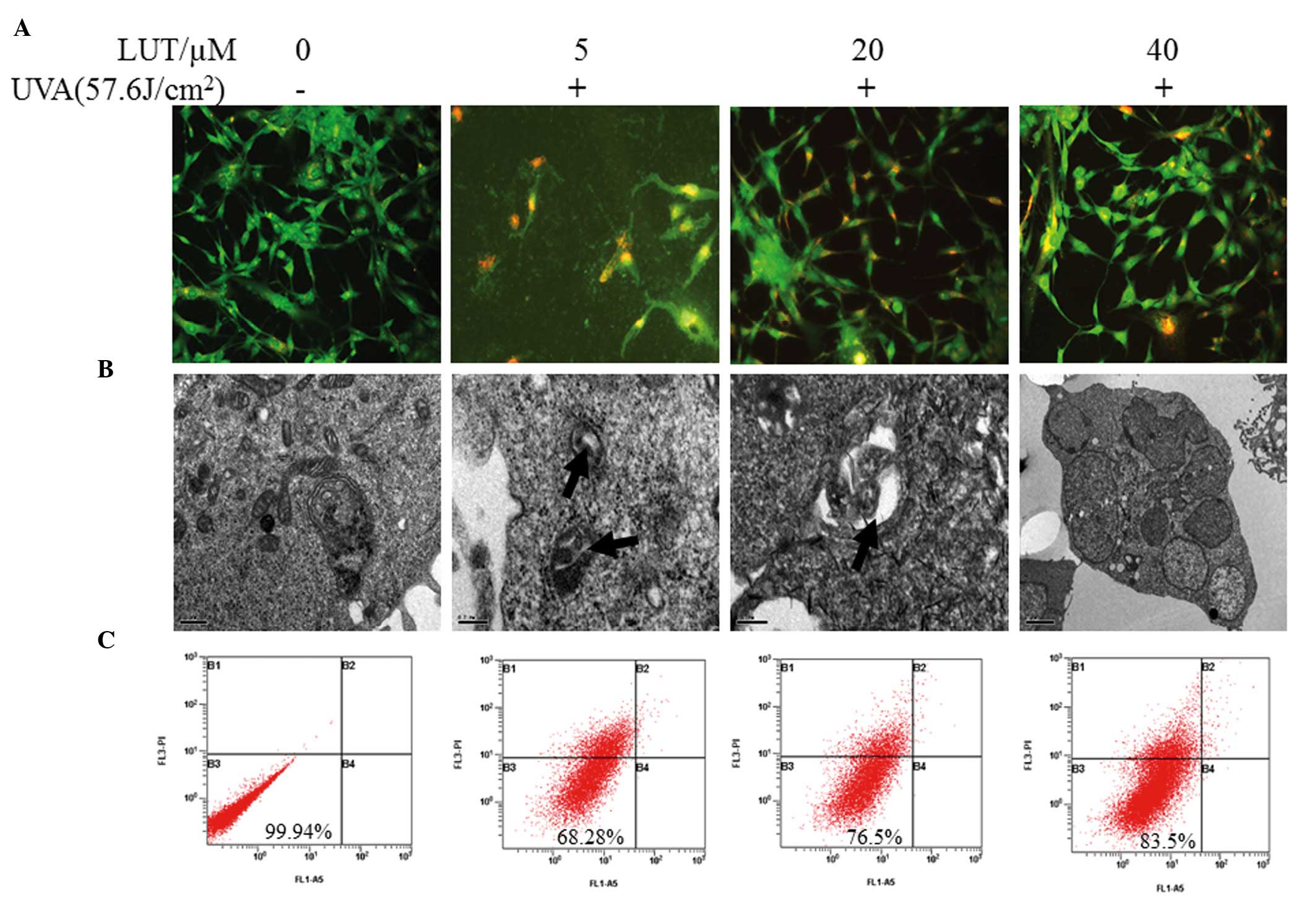 | Figure 3LUT decreases UVA-induced autophagy in
HSFs. Four groups of HSFs were incubated with 0, 5, 20 and 40
µM LUT, respectively. These groups of HSFs were irradiated
with 57.6 J/cm2 UVA. (A) Following treatment, the cells
were stained with MDC and observed using fluorescence microscopy to
detect the presence of MDC puncta. (B) Electron micrographs of HSFs
following treatment with LUT and irradiation with 57.6
J/cm2 UVA. Phosphate-buffered saline was used in
treatment as a bank control. Magnification, ×200. (C) Annexin
V-FITC and PI staining for apoptosis. The X-axis denotes Annexin
V-FITC; the Y-axis denotes DNA content by PI. The experiment was
repeated three times and the results are representative of the
three independent experiments. HSFs, human skin fibroblasts; PI,
propidium iodide; FITC, fluorescein isothiocyanate; UVA,
ultraviolet A; LUT, luteolin. |
LUT impairs the production of ROS induced
by UVA irradiation in HSFs
Cell redox status changes between the equilibration
of ROS and GSH. In addition, MMP disruption and cell apoptosis are
always associated with the generation of intracellular ROS and
depletion of GSH (18,19). Therefore, the present study
examined the levels of ROS in HSFs treated with UVA irradiation,
with and without incubation with LUT. The ROS levels were examined
by DCFH-DA. The rapid generation of ROS, which was between 1.89-
and 1.30-fold faster, compared with the control, was detected
following UVA treatment, as shown in Fig. 4. Incubation with LUT decreased
UVA-induced ROS production in the HSFs following treatment with 40
µM LUT, compared with the cells exposed to UVA alone. These
results indicated that LUT assisted in impairing the UVA-induced
increase in ROS levels in the HSFs.
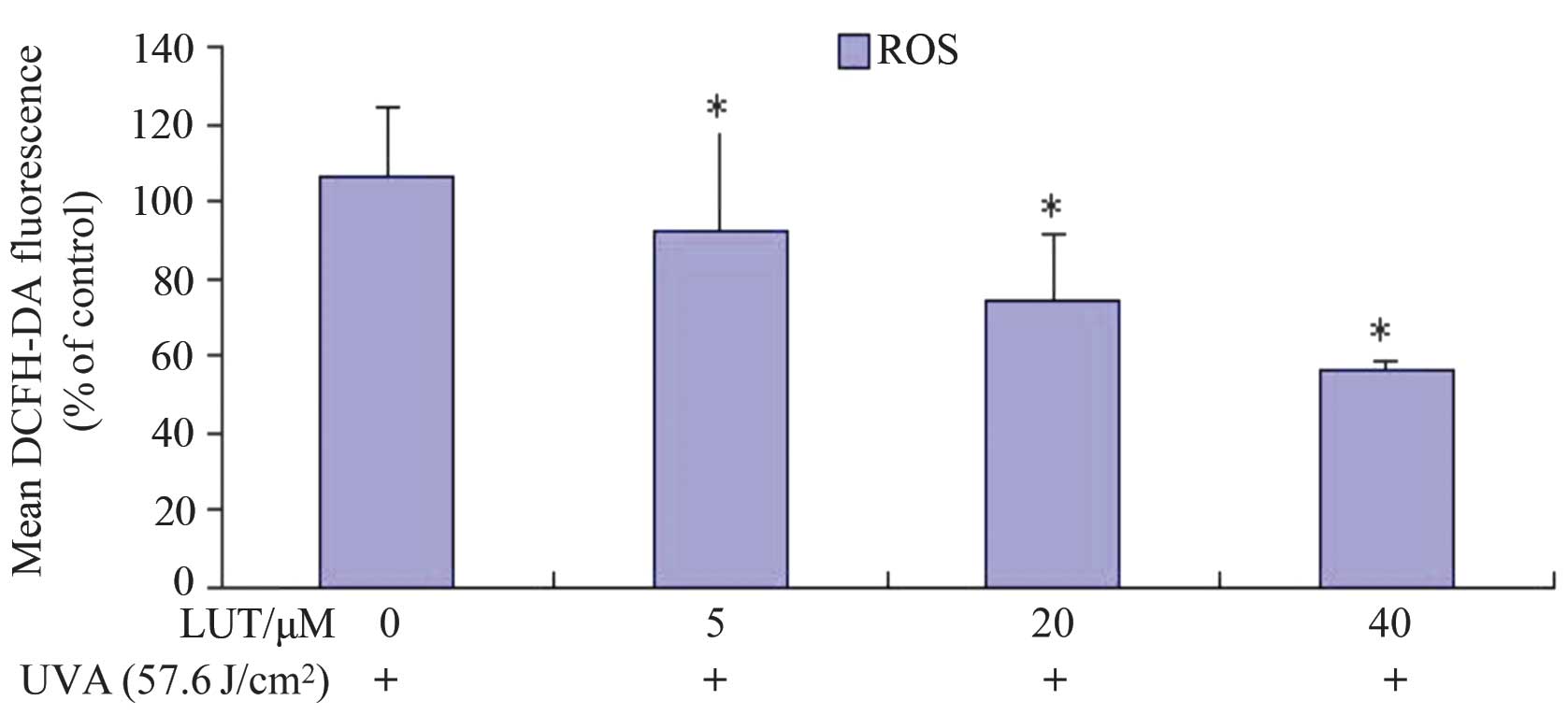 | Figure 4LUT decreases UVA-induced ROS in HSFs.
The HSFs were incubated with different doses of LUT (0, 5, 20 and
40 µM LUT, respectively) at a concentration of
1×106 cells/ml, and then exposed to 57.6
J/cm2 UVA. Following three washes in PBS, the cells were
incubated at 37°C for 30 min with 10 µM DCFH-DA in PBS. The
fluorescence was measured using flow cytometry (excitation
wavelengths, 488 nm; emission wavelengths, 525 nm). The analysis
was repeated three times, and the results are representative of the
three. Each bar represents the mean ± standard deviation of three
experiments (*P<0.05, vs. dimethyl sulfoxide
control). HSFs, human skin fibroblasts; UVA, ultraviolet A; LUT,
luteolin; ROS, reactive oxygen species; DCFH-DA,
dichlorodihydrofluorescein diacetate; PBS, phosphate-buffered
saline. |
Antioxidants protect HSFs from UVA
cytotoxicity
The present study used two ROS scavengers,
N-acetyl-cysteine (NAC), a well-known antioxidant and glutathione
(GSH) precursor, and catalase (CAT; an
H2O2-scavenging enzyme), to verify the
linkage between ROS generation and cell toxicity in UVA-induced HSF
cell death. Changes in MMP and cell toxicity were determined
following exposure of the HSFs to UVA irradiation and incubation
with either 1 mM NAC or 2,000 U/ml CAT for 30 min. As shown in
Fig. 5A, the loss of MMP was
inhibited by NAC and CAT, and the cells were protected from UVA
cytotoxicity. Hoechst staining and a trypan blue exclusion assay
indicated that NAC and CAT markedly reduced UVA-induced cell
apoptosis. These results suggested that cell apoptosis was
associated with ROS accumulation, followed by MMP disruption. As
shown in Fig. 5B, LUT had the same
effect as NAC and CAT, indicating that LUT exerted antioxidant
effects against UVA irradiation in the HSFs.
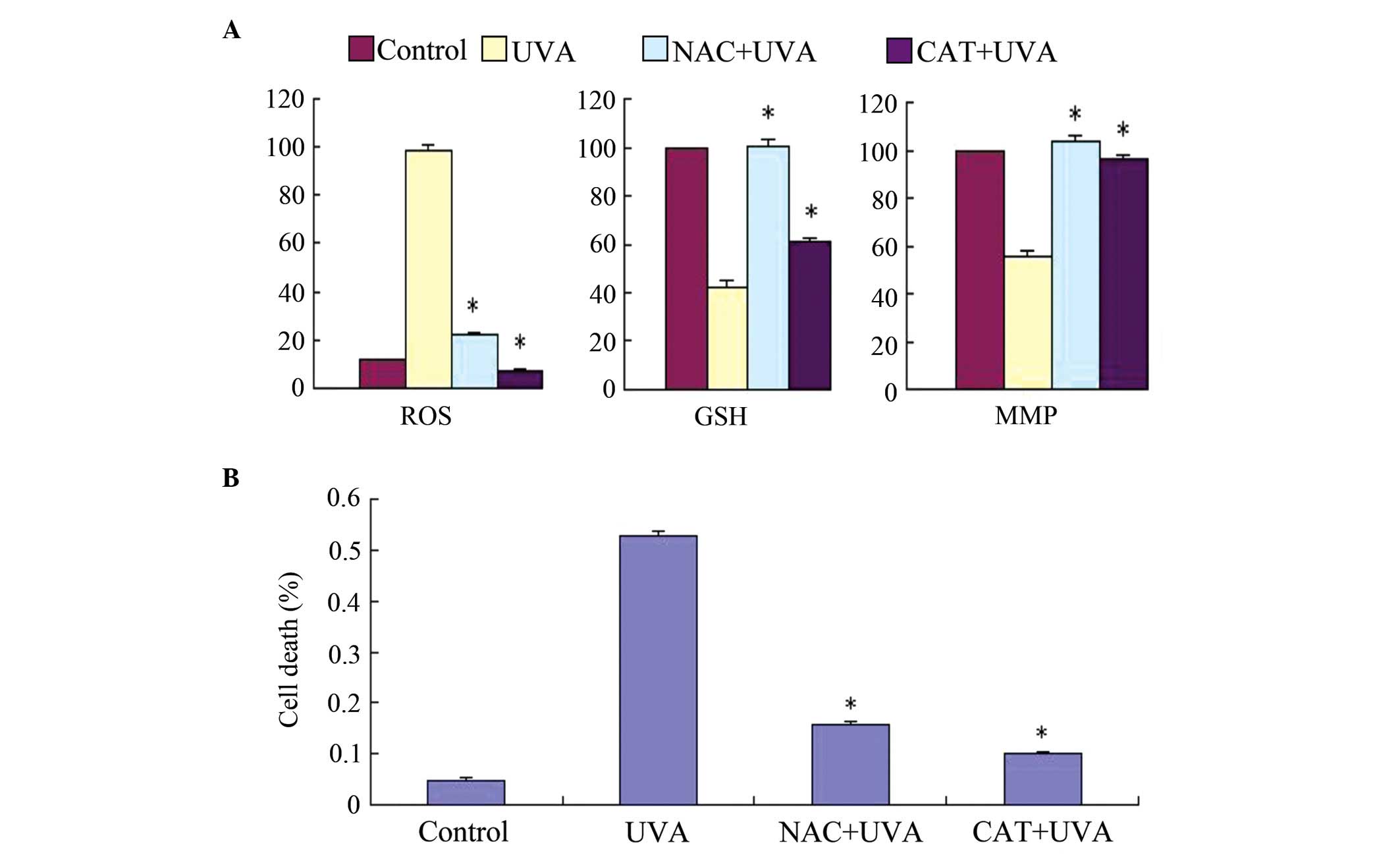 | Figure 5Effects of exogenous application of
NAC and CAT on UVA-induced ROS generation, GSH depletion, MMP
disruption and cell viability in HSFs. (A) Changes in ROS, GSH and
MMP were examined using flow cytometric analysis. (B) Cell death
rate was determined using a trypan blue exclusion assay. Control,
cells treated with solvent as control; UVA, cells treated with 57.6
J/cm2 UVA; CAT+UVA, cells treated with 57.6
J/cm2 UVA following incubation with 2,000 U/ml CAT for
30 min; NAC+UVA, cells treated with 57.6 J/cm2 UVA
following incubation with 1 mM NAC for 30 min. The data are
expressed as the mean ± standard deviation of three independent
experiments; *P<0.05, vs. cells treated with 57.6
J/cm2 UVA alone. HSFs, human skin fibroblasts; UVA,
ultraviolet A; LUT, luteolin; ROS, reactive oxygen species; NAC,
N-acetyl-cysteine; CAT, catalase; GSH, glutathione; MMP;
mitochondrial membrane potential. |
Expression of HIF-1α significantly
decreases following UVA irradiation in HSFs incubated with LUT
Several cellular activities, including glycolysis,
apoptosis, angiogenesis, metastasis and migration, are regulated by
HIF-1, which is particularly sensitive to oxygen levels in the cell
microenvironment (17,20–22).
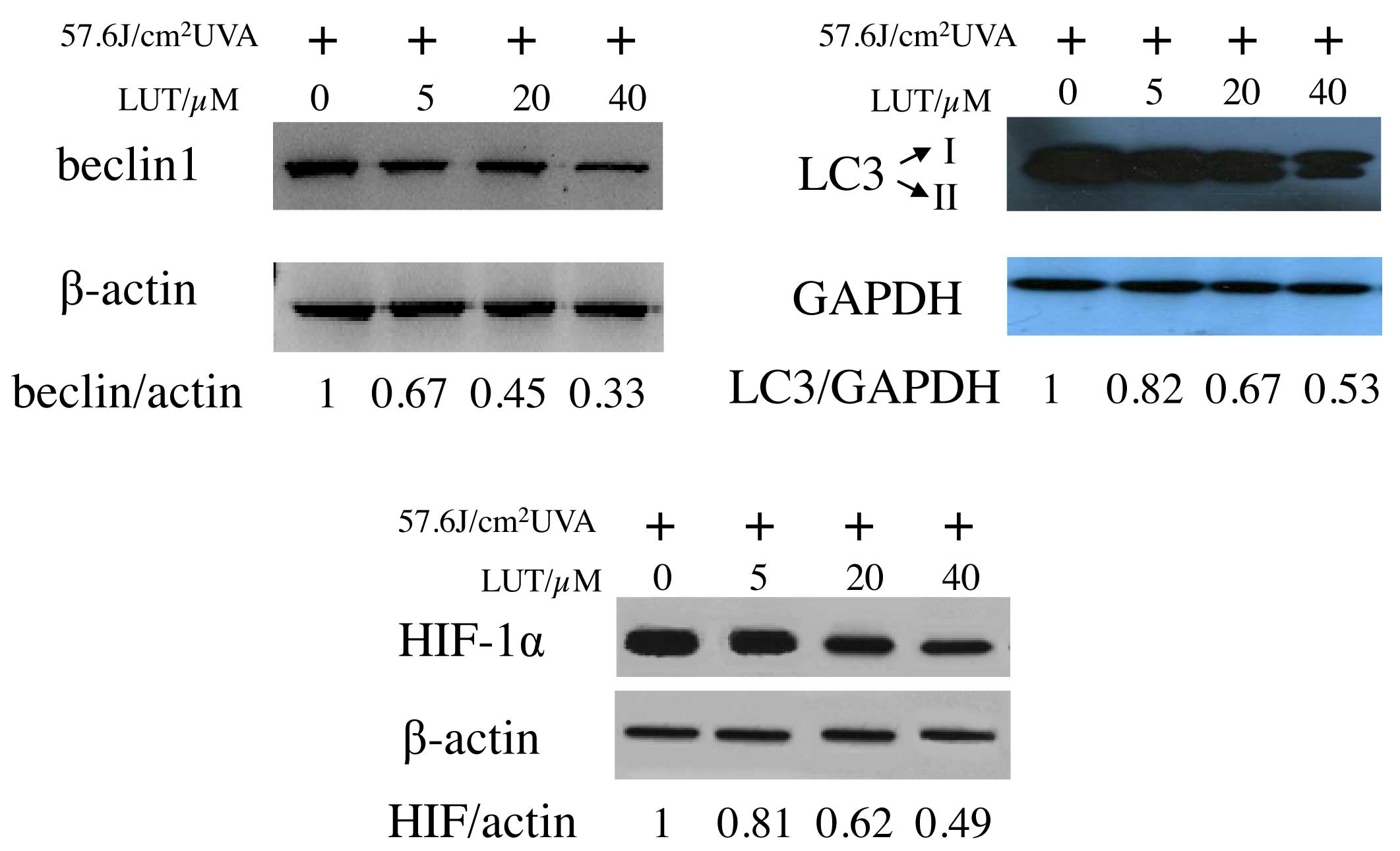
Beclin 1 induces autophagy and inhibits
tumorigenesis (22). To examine
the expression of beclin 1 in the LUT-treated HSFs following UVA
irradiation, western blot analysis was performed on the cell
lysates. As expected, decreased expression levels of beclin 1 were
detected following UVA exposure with LUT incubation (Fig. 6A), compared with the cells exposed
to UVA alone. The present study also analyzed LC3, an autophagic
marker, which is essential in the expansion of autophagosomes
(23,24). As shown in Fig. 6B, incubation with LUT in the
UVA-irradiated HSFs led to a decrease in the conversion of LC3 from
LC3-I, a soluble, cytoplasmic form, to LC3-II, a membrane-bound,
autophagosome-associated form. These results provided further
evidence for the increased reduction of autophagy by LUT treatment
in the UVA-irradiated HSFs.
Discussion
The antioxidant effect of LUT is due to several
mechanisms. Firstly, the specific structure of LUT enables it to
act as a ROS scavenger (23).
Secondly, ROS-generating oxidases, including xanthine oxidase
activity, can be inhibited by LUT through suppressing
O2•-formation (25). It
is already known that mitochondria are the primary site for ROS
generation, however, whether LUT affects ROS generation in this
manner in mammalian cells remains to be elucidated (26). Thirdly, intracellular antioxidant
enzymes, including superoxide dismutase, glutathione-S-transferase,
glutathione reductase and CAT may be protected or enhanced by LUT
(27–29). Finally, LUT impairs the oxidation
of several cellular components, possibly by inhibiting the activity
of the corresponding enzymes.
Previous studies have indicated that various cell
types show varying degrees of sensitivity to flavonoids (23–29).
Verschooten et al (30)
demonstrated that LUT decreases the damage induced by
UVB-irradiation in normal human keratinocytes, whereas no
photoprotective effects are observed in malignant
keratinocytes.
The present study found that LUT exerted a
protective effect on HSFs damaged by UVA. LUT was suggested to
scavenge UVA-induced ROS in the HSFs. Of note, the UVA-irradiated
HSFs endured substantial changes in autophagy when incubated with
LUT, and these changes were due to decreases in the levels of
ROS.
HIF-1α is an important transcriptional factor
induced by numerous oxidants. In the present study, HIF-1α was
down-regulated in the LUT-treated UVA-irradiated HSFs. It was
concluded that there are specific proteins downstream of HIF-1α,
which induced autophagy. This indicated the association of HIF-1α
with autophagy. Once the expression of HIF-1α decreased, autophagy
declines. LUT, acting as a potent ROS scavenger, markedly impaired
the production of HIF-1α induced by UVA. These results indicated
that LUT decreased the UVA-induced autophagy of the HSFs by
scavenging ROS.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 30972652), and the
National Natural Science Foundation of Guangdong Province (grant
no. 2013B021800053).
References
|
1
|
Seelinger G1, Merfort I, Wölfle U and
Schempp CM: Anti-carcinogenic effects of the flavonoid luteolin.
Molecules. 13:2628–2651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rice-Evans CA, Miller NJ and Paganga G:
Structure-antioxidant activity relationships of flavonoids and
phenolic acids. Free Radic Biol Med. 20:933–956. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mira L, Fernandez MT, Santos M, Rocha R,
Florêncio MH and Jennings KR: Interactions of flavonoids with iron
and copper ions: A mechanism for their antioxidant activity. Free
Radic Res. 36:1199–1208. 2002. View Article : Google Scholar
|
|
4
|
López-Lázaro M: Distribution and
biological activities of the flavonoid luteolin. Mini Rev Med Chem.
9:31–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masaki H, Atsumi T and Sakurai H:
Detection of hydrogen peroxide and hydroxyl radicals in murine skin
fibroblasts under UVB irradiation. Biochem Biophys Res Commun.
206:474–479. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jurkiewicz BA and Buettner GR: EPR
detection of free radicals in UV-irradiated skin: Mouse versus
human. Photochem Photobiol. 64:918–922. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barber LA, Spandau DF, Rathman SC, Murphy
RC, Johnson CA, Kelley SW, Hurwitz SA and Travers JB: Expression of
the platelet-activating receptor results in enhanced ultraviolet B
radiation-induced apoptosis in a human epidermal cell line. J Biol
Chem. 273:18891–18897. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brenneisen P, Wenk J, Klotz LO, Wlaschek
M, Briviba K, Krieg T, Sies H and Scharffetter-Kochanek K: Central
role of ferrous/ferric iron in the ultraviolet B
irradiation-mediated signaling pathway leading to increased
interstitial collagenase (matrix-degrading metalloproteinase
(MMP)-1) and stromelysin-1 (MMP-3) mRNA levels in cultured human
dermal fibroblasts. J Biol Chem. 273:5279–5287. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasui H and Sakurai H: Chemiluminescent
detection and imaging of reactive oxygen species in live mouse skin
exposed to UVA. Biochem Biophys Res Commun. 269:131–136. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang S, Chung JH, Lee JH, Fisher GJ, Wan
YS, Duell EA and Voorhees JJ: Topical N-acetyl cysteine and
genistein prevent ultraviolet-light-induced signaling that leads to
photoaging in human skin in vivo. J Invest Dermatol. 120:835–841.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamamoto Y: Role of active oxygen species
and antioxidants in photoaging. J Dermatol Sci. 27(Suppl 1): S1–S4.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanson KM and Simon JD: Epidermal
trans-urocanic acid and the UV-A-induced photoaging of the skin.
Proc Natl Acad Sci USA. 95:10576–10578. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Y, Wang CH and Gong XG:
Apoptosis-inducing effects of two anthraquinones from Hedyotis
diffusaWILLD. Biol Pharm Bull. 31:1075–1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Xu Z, Tan M, Su W and Gong X:
3-(4-(Benzo [d]thiazo l-2-yl)-1-phenyl-1H-pyrazol-3-yl) phenyl
acetate induced Hep G2 cell apoptosis through a ROS-mediated
pathway. Chem Biol Interact. 183:1832010. View Article : Google Scholar
|
|
15
|
Biederbick A, Kern HF and Elsässer HP:
Monodansylcadaverine (MDC) is a specific in vivo marker for
autophagic vacuoles. Eur J Cell Biol. 66:3–14. 1995.PubMed/NCBI
|
|
16
|
Gong K, Chen C, Zhan Y, Chen Y, Huang ZB
and Li WH: Autophagy-related gene7 (Atg7) and reactive oxygen
species/extracellular-signal-regulated kinase regulate
tetrandrine-induced autophagy in human hepatocellular carcinoma. J
Biol Chem. 287:35576–35888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maxwell PH, Dachs GU, Gleadle JM, Nicholls
LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW and Ratcliffe PJ:
Hypoxia-inducible factor-1 modulates gene expression in solid
tumors and influences both angiogenesis and tumor growth. Proc Natl
Acad Sci USA. 94:8104–8109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong Y, Liu X, Lee CP, Chua BH and Ho YS:
Attenuation of doxorubicin-induced contractile and mitochondrial
dysfunction in mouse heart by cellular glutathione peroxidase. Free
Radic Biol Med. 41:46–55. 2006. View Article : Google Scholar
|
|
19
|
Lopez E, Arce C, Oset-Gasque MJ, Cañadas S
and González MP: Cadmium induces reactive oxygen species generation
and lipid peroxidation in cortical neurons in culture. Free Radic
Biol Med. 40:940–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bardos JI and Ashcroft M: Negative and
positive regulation of HIF-1: A complex network. Biochim Biophys
Acta. 1755:107–120. 2005.PubMed/NCBI
|
|
21
|
Harris AL: Hypoxia-a key regulatory factor
in tumor growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Semenza GL: HIF-1, O2 and the
3PHDs: How animal cells signal hypoxia to the nucleus. Cell.
107:1–3. 2001. View Article : Google Scholar
|
|
23
|
Lien EJ, Ren S, Bui HH and Wang R:
Quantitative structure-activity relationship analysis of phenolic
antioxidants. Free Radic Biol Med. 26:285–294. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shimoi K, Masuda S, Furugori M, Esaki S
and Kinae N: Radioprotective effect of antioxidative flavonoids in
gamma-ray irradiated mice. Carcinogenesis. 15:2669–2672. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nagao A, Seki M and Kobayashi H:
Inhibition of xanthine oxidase by flavonoids. Biosci Biotechnol
Biochem. 63:1787–1790. 1999. View Article : Google Scholar
|
|
26
|
Sen N, Das BB, Ganguly A, Banerjee B, Sen
T and Majumder HK: Leishmania donovani: Intracellular ATP level
regulates apoptosis-like death in luteolin induced dyskinetoplastid
cells. Exp Parasitol. 114:204–214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leung HW, Kuo CL, Yang WH, Lin CH and Lee
HZ: Antioxidant enzymes activity involvement in luteolin-induced
human lung squamous carcinoma CH27 cell apoptosis. Eur J Pharmacol.
534:12–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manju V and Nalini N: Chemopreventive
potential of luteolin during colon carcinogenesis induced by 1,
2-dimethylhydrazine. Ital J Biochem. 54:268–275. 2005.
|
|
29
|
Harris GK, Qian Y, Leonard SS, Sbarra DC
and Shi X: Luteolin and chrysin differentially Inhibit
cyclooxygenase-2 expression and scavenge reactive oxygen species
but similarly inhibit prostaglandin-E2 formation in RAW 264.7
cells. J Nutr. 136:1517–1521. 2006.PubMed/NCBI
|
|
30
|
Verschooten L, Smaers K, Van Kelst S,
Proby C, Maes D, Declercq L, Agostinis P and Garmyn M: The
flavonoid luteolin increases the resistance of normal, but not
malignant keratinocytes, against UVB-induced apoptosis. J Invest
Dermatol. 130:2277–2285. 2010. View Article : Google Scholar : PubMed/NCBI
|