Introduction
Autistic spectrum disorder (ASD) is a highly
debilitating developmental neuropathology characterized by impaired
social interaction and communication, and is associated with
stereotypical and repetitive behaviors (1). An alteration in the genesis and
maturation of neural circuits appears to produce a failed
connection in several regions of the autistic brain (2). An imbalance between glutamate and
GABAergic systems has been suggested and a strong bias to the
excitatory neurotransmission may be characteristic of an ASD brain
(3). Although ASD etiology remains
elusive, it is clearly multifactorial. Genetic studies demonstrate
a high degree of heterogeneity and as many as 100 genes or genomic
imbalances have been implicated in this neuropathology (4). Between genetic factors, certain
monogenetic types of autism are associated with mutations in genes
for structural synaptic proteins; predominantly the postsynaptic
cell adhesion proteins, including neuroligin 3 and 4, and their
presynaptic partner neurexin 1, and the gene for postsynaptic
density scaffolding protein SHANK3 (5–8).
Genes for functional synaptic proteins are also mutated in ASD,
including the postsynaptic ubiquitin UBE3A ligase in Angelman's
patients (9,10) or calcium dependent activator
protein (CAPS2/CADPS2), which is involved in neurotrophin secretion
(11). Additional evidence from
previous genetic disorders not included in ASD, but presenting
autistic signs, involves a particular signaling pathway regulating
local protein synthesis in the dendritic compartment (12,13).
Tuberous sclerosis complex (TSC), a genetic type of epilepsy with
autistic signs, presents with dominant mutations in TSC1 or TSC2
genes that produce over-activation of the mammalian target of
rapamycin kinase (mTOR) (14). By
contrast, fragile X-mental retardation is a genetic type of mental
disability with autistic signs, where the FMRP gene is
transcriptionally repressed, causing exaggerated protein synthesis
dependent-long term depression (15,16).
More recent evidence directly links autistic mutations with
synaptic translation. Phosphatase and tensin homolog (PTEN) is a
tumor suppressor gene that regulates TSC1/2, and individuals with
germline mutations in PTEN exhibit macrocephaly and autistic
behavior (17–19). In other autistic cases, an
activating mutation in the promotor region of the eukaryotic
initiation factor 4E (EIF4E) gene, the rate-limiting component of
eukaryotic translation, has been described (20). EIF4E is the final effector in a
signaling pathway mediated by TSC/mTOR and is regulated, between
others, by PTEN and FMRP that permits the generation of new
proteins in a precise spatio-temporal manner for neural plasticity
occurring during development and maturation of brain circuits.
Therefore, alteration of dendritic protein synthesis appears to be
a common defect in genetic autism (21). Glutamatergic neurotransmission is
an important regulator of this pathway. In particular, glutamate
metabotropic receptor type 5 (mGluR5), a member of the class I
family, has a probed role in dendritic translation. For example, in
fragile X-mental retardation, FMRP absence produces an excessive
dendritic protein synthesis following the activation of mGluR5,
resulting in synaptic pruning defects (22). However, a large number of autistic
cases appear to have an environmental origin and embryonic
exposition to the antiepileptic sodium valproate (VPA) in rats is a
well-validated experimental model of autism (23). In this model, an altered
development of neural circuits has been established. In sensorial
and prefrontal cortical areas, a hyper-connectivity,
hyper-reactivity and hyper-plasticity has been reported (24-26).
Notably, a mis-connection implicating over-connectivity in local
sort distance circuits and under-connection in the long distance
circuits, is now recognized as a key characteristic in the autistic
brain (2). However, the molecular
mediators that cause this mis-connected brain in
environmentally-induced autism are poorly investigated, even though
increases in the NMDA receptor and CamKII levels have been reported
in the rodent VPA model of autism (27). In addition, a role for mGluR5 has
been suggested in mediating the repetitive and anxious-like
behaviors in the VPA model of autism (28); however, the role of other members
of class I metabotropic glutamate receptor as mGluR1 is poorly
investigated. In the context of a supposed alteration in synaptic
protein synthesis in autism, the present study investigated if
upstream regulators of this process, including mGluRs, are located
at the point of convergence between genetic and non-genetic autism.
The aim of the present study was to analyze the immunoreactivity
for mGluR1a and mGluR5 in the hippocampus of rats treated with VPA
in the prenatal period.
Materials and methods
Animals
A total of 24 pregnant Sprague-Dawley rats (Faculty
of Chemical and Pharmaceutical Sciences, Universidad de Chile,
Santiago, Chile) were housed in 25×45×15 cm cages under standard
conditions (21°C and 12 h light-dark cycle) with free access to
food and water. To generate the animal model of autism, pregnant
female rats received a single intraperitoneal injection of 450
mg/kg VPA (Sigma-Aldrich, St. Louis, MO, USA) on embryonic day 12.5
and the control group received a saline solution injection.
Offspring were weaned at postnatal day 21 (P21) when males and
females were separated and housed in groups of 4–5 littermates.
Only male rats were used in behavioral and immunohistochemical
studies. Efforts were made to minimize both the number of animals
used and their suffering. All procedures were approved by the local
Ethics Committee of the Science and Technology National Commission
(CONICYT) in compliance with the National Institutes of Health
Guide for Care and Use of Laboratory Animals (8th
edition).
Behavioral tests
All test were performed between 9:00 and 15:00, and
where recorded by a Logiteck camera located 1.5 m from the test
surface. For behavioral studies, sequential tests were performed
considering increased anxiogenic behavior with a 48 h inter-test
period. A limit of three tests was conducted on each animal.
Open-field was always the first test and Y-maze or plus-maze was
the last. To reduce suffering, Y-maze and plus-maze were never
performed by the same animal. Between tests the apparatus was
cleaned with 5% ethanol and every test was performed with 60 db of
white noise. All videos where analyzed using ANY-maze software
(Stoelting, Wood Dale, IL, USA) by an operator in a blinded manner,
with the exception of the Y-Maze, which was analyzed manually in a
blinded manner.
Open field
Locomotor and exploratory activity was evaluated in
a 60×60 cm open field arena. The animal was placed in the center of
the illuminated arena and was allowed 5 min to explore the arena.
Total distance, mean speed, and time and frequency of grooming
behavior were then determined.
Elevated plus maze
The elevated plus maze protocol was performed with
P30 rats, as described previously (29). The arms of the apparatus measured
40×10 cm and the enclosed arms where limited by walls of 30 cm
high. The open arms have a 5 mm elevation in the edges to enhance
the exploratory activity (30).
The arms were elevated 50 cm from the floor and a camera was
recording from the upside. To start the test, the animal was placed
in the center of the apparatus facing to an open arm (31) and exploration was recorded during 5
min.
Three chamber social test
A three-chamber social test was performed in an
apparatus consisting of three 30×22 cm chambers of transparent
acrylic adjoining, each separated by 10×10 cm doors, as described
previously by Moy et al (32). The animal was allowed to explore
the central camera for 5 min and then the doors conducting to the
lateral chambers were opened. One of the lateral chambers houses an
animal of the same litter (familiar congener) and the other chamber
was void. The social behavior of the animal was recorded for 10
min.
Y-Maze
The spatial memory-Y-Maze was performed, as
described by Conrad et al (33), and consisted in three 46×13×32 cm
arms maze disposed at angles of 120 degrees between them. The test
consisted of two stages. In the first test, one animal was allowed
to explore only two arms of the maze for 15 min and was
subsequently returned to its home cage. The second stage was
performed 4 h later, but this time the animal is allowed to explore
the three arms during a 5 min duration. The initial arm was
alternated between animals to avoid preference for a direction
(34).
Immunohistochemistry
For immunohistochemical analysis, males were
euthanized using isoflurane (Baxter, Shanghai, China) and
sacrificed by decapitation on P30. Whole brain tissue blocks were
dissected briefly following decapitation and fixed overnight in 4%
paraformaldehyde in phosphate buffered saline (PBS; pH 7.4).
Following this, the tissue sections were cryoprotected in 30%
sacarose. For free-floating immunostaining, 40 µm coronal sections
were obtained in a cryostat Microm HM525 (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The tissue sections were treated for 45
min with 0.3% H2O2 at room temperature to
block endogenous peroxidase activity and were subsequently blocked
for 1 h at room temperature in blocking buffer (3% bovine serum
albumin with 0.4% Triton X-100 in PBS). The tissue sections were
incubated in the following primary antibodies overnight at room
temperature: Rabbit anti-mGluR5 (1:100) and rabbit anti-mGluR1a
(1:100), both from Thermo Fisher Scientific, Inc. The sections were
subsequently rinsed and incubated for 2 h at room temperature with
Biotin-SP-Conjugated anti-rabbit immunoglobulin G (H+L) (1:1,000;
Jackson ImmunoResearch Labs, West Grove, PA, USA), followed by a 1
h incubation at room temperature with avidin/biotin horseradish
peroxidase complex using a Vectastain ABC kit (Vector Laboratories,
Burlingame, CA, USA). Finally, immunoreactivity was detected using
ImmPACT DAB peroxidase substrate (Vector Laboratories).
Image analysis
Digital microphotographs were captured using a Carl
Zeiss Axiolab E microscope with a digital camera (Cannon EOS Rebel
T3) and immunoreactivity quantification was performed using the
public software ImageJ (NIH, Bethesda, MD, USA). In order to
evaluate possible localization difference in the immunoreactivity
between control and VPA groups, the present study quantified the
optical density of the different hippocampal layers and corrected
by the background in a no staining zone in the same slice. In
addition, data from the experimental group were standardized for
the control value in the same zone in order to minimize technical
artefacts in immunostaining procedures performed on different
days.
Statistical analysis
All data obtained from the behavioral test were
analyzed by one-way analysis of variance, followed by Bonferroni
post-hoc test. The statistical analysis of densitometric
immunoreactivity was performed using the non-parameric Mann-Whitney
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Behavioral studies
Social behavior
The three chamber social test is a valid behavioral
probe to analyze probable alterations in social approach or
avoidance behavior (32). The
present study evaluated the time spent in each one of the tree
chambers, starting with the experimental animal in the central
chamber and facing a familiar congener on one of the lateral
chambers, while the other lateral chamber was empty. The expected
normal behavior is a preference for spending the time in the
occupied chamber even with the center and non-occupied lateral
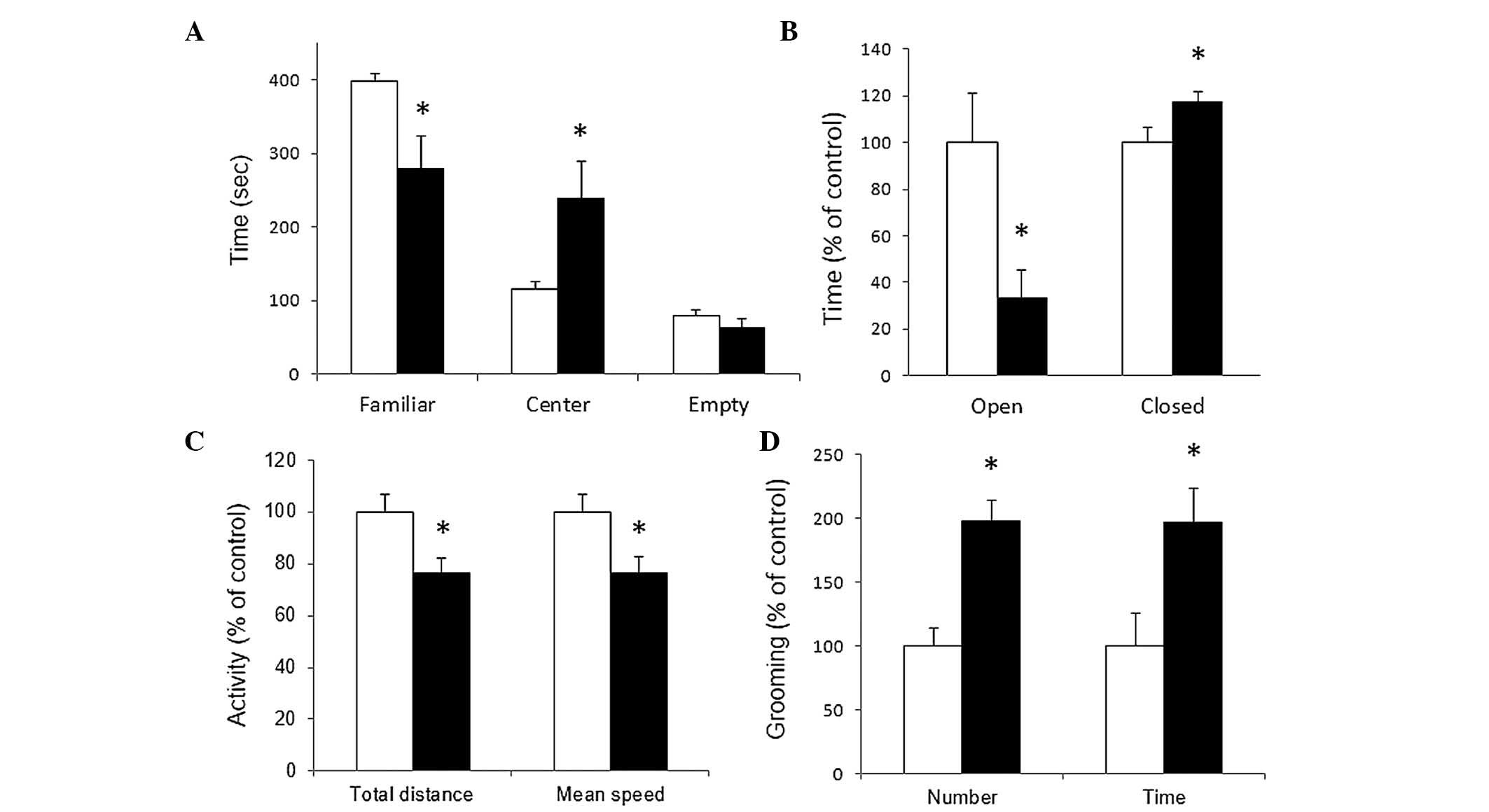
chamber. As shown in Fig. 1A,
control rats exhibited a preference for the occupied chamber
(398.1±10.9 sec in familiar zone vs. 116.6±9.1 sec in center;
P≤0.001). For the VPA-treated group, this preference is lost
demonstrating no statistically significant difference between the
time spent in occupied lateral chamber compared with the center or
the empty zone (279.9±43.6 sec in occupied zone vs. 239.1±49.4 sec
in center). This result demonstrated social deficit in the VPA
group, a major characteristic of autism.
Anxiety
Anxious behavior was evaluated using the elevated
plus maze, where experimental animals can freely explore two
protected and two unprotected elevated arms disposed
perpendicularly from each other. The present study quantified
accumulated time spent in open or closed arms by VPA-treated rats
and have expressed the data as a percentage of the time spent for
the control group in the respective arms. As shown in Fig. 1B, VPA rats spent less time, in a
statistically significant manner, in the exposed open arms and more
time in the protected closed arms compared with that of the control
group (100±21.4% in control vs. 33.5±11.7% in VPA for open arms,
P≤0.05; 100±6.2% in control vs. 117.5±4.3% in VPA for closed arms,
P≤0.05). This result demonstrated anxious behavior in VPA-treated
animals, also an important characteristic of autism.
Locomotor activity
General locomotor activity representing exploratory
conduct was evaluated by quantifying distance and speed in an open
field arena. As shown in Fig. 1C,
a statistically significant reduction of ~20% was observed in both
the total distance and the mean speed produced by VPA prenatal
treatment (100±7% in control vs. 76.4±5.9% in VPA for total
distance, P≤0.05; 100±7.1% in control vs. 76.5±6% in VPA for mean
speed, P≤0.05).
Repetitive behavior
Other major characteristic of autism are repetitive
and stereotypical behavior. In the same session of open field
arena, the present study quantified the frequency of grooming
events and accumulated time spent in that stereotypical conduct. As
shown in Fig. 1D, VPA prenatal
treatment increased by ~100% the occurrence and the time spent in
grooming conduct during the open field session (100±14.2% in
control vs. 197.8±16.7% in VPA for grooming frequency, P≤0.001;
100±25.6% in control vs. 196±27.3% in VPA for time spent, P≤0.05).
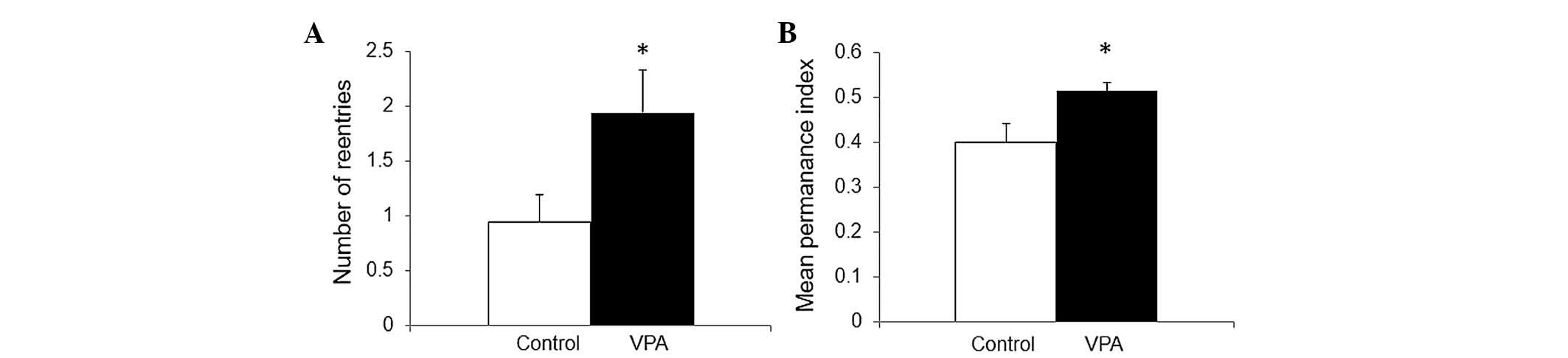
In addition, another way to demonstrate the repetitive conduct
reported in the VPA model, was the number of reentries in the same
arm during the Y-maze test, a test designed to evaluate spatial
memory (35). As shown in Fig. 2A, VPA prenatal treatment doubled
the number of reentries during the second phase in a two-trial
Y-maze (0.94±0.26 in control vs. 1.94±0.4 in VPA, P≤0.05), in
accord with data previously reported (36).
Spatial memory
Spatial memory was evaluated with a two trial Y-maze
test. In the first trial, animals have access to only two arms in a
three arms maze. At 4 h later, in the second trial, the animals
have access to the three arms and the normal expected conduct is a
preference for the novel arm. Memory was evaluated by the mean time
permanence index, expressing the proportion of the average time
spent in the novel arm in relation to the average time spent in the
novel plus the known arm. As shown in Fig. 2B, a moderate increase of spatial
memory was produced by VPA prenatal treatment (0.4±0.04 in control
vs. 0.5±0.02 in VPA, P≤0.05).
Immunohistochemical study
mGluR5 expression
Immunohistochemical analysis for mGluR5 in
hippocampal formation showed intense labeling in all dendritic
fields, while cellular layers displayed less staining in control
and VPA-treated rats (Fig. 3A and
B). Semi-quantitative analysis of mGluR5 immunoreactivity
labeling intensity demonstrated no effect of treatment in any
subfields and layers of the hippocampus analyzed (Fig. 3C).
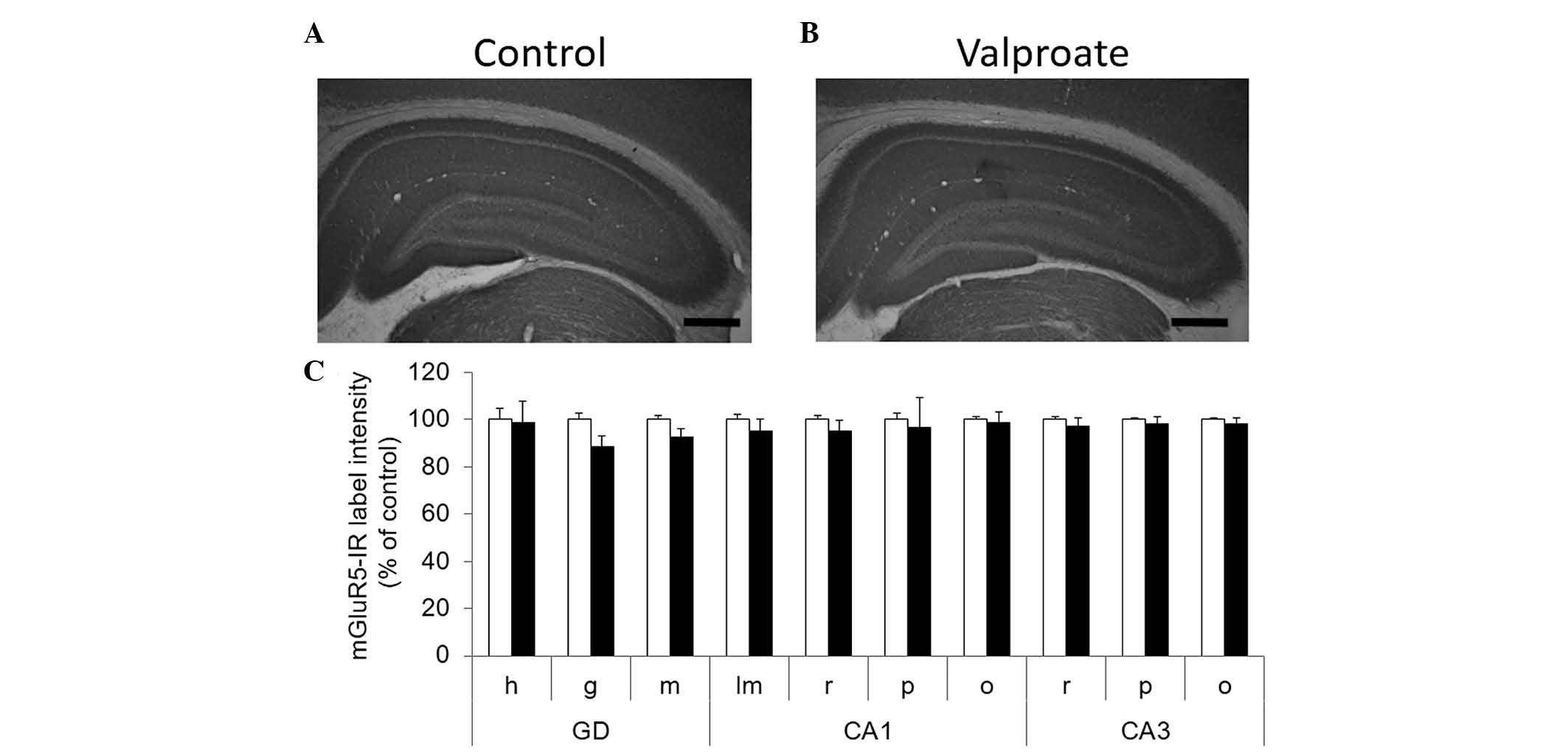 | Figure 3No effect was observed in hippocampal
mGluR5 immunoreactivity following prenatal valproate treatment.
Digital microphotographs showing immunohistochemical staining for
mGluR5 in dorsal hippocampal formation from postnatal day 30 (A)
control and (B) valproate treated animals (scale bar, 500 µm). (C)
The label intensity of digital microphotographs was analyzed in the
different layers of hippocampal subfields and were expressed as a
percentage of the control. The data are presented as the mean ±
standard error of the mean (n=6). The control is represented by
white bars and the valproate treated animals are represented by
black bars. The data were analyzed using Mann-Withney test. h,
hilus; g, granular; m, molecular; lm, lacunosum moleculare; r,
radiatum; p, pyramidal; o, oriens; a, alveus; GD, dentate gyrus;
CA, Ammon's horn. |
mGluR1a expression
Immunoreactive staining for mGluR1a exhibited a
distinctive pattern in the hippocampal subfields, staining a number
of cellular processes in all dendritic stratus of the hippocampus
(hilus of dentate gyrus, stratum radiatum and stratum oriens of CA1
and CA3). A less intense staining was evident in the cellular
layers (stratum pyramidale and stratum granulosum) (Fig. 4). The most intense label intensity
was found in the stratum alveus of CA1 and in its limits with
stratum oriens, as shown in Fig.
4A. In this layer, somatic and dendritic elements were stained,
which most probably corresponded to the alveus interneurons and
their profuse dendritic network, as previously reported (37,38).
The second most intense staining was found in the hilus of dentate
gyrus, immediately adjacent to granular cell layer (Fig. 4C). VPA-treated rats exhibited
clearly more intense staining in these two later regions, CA1
alveus and hilus (Fig. 4A and B).
Semi-quantitative analyses revealed a statistically significant
increase of mGluR1a immunoreactivity labeling intensity in CA1
stratum alveus (100±6.8 5% in control vs. 130.9±7.5% in VPA,
P≤0.05) and hilus of dentate gyrus (100±6.3% in control vs.
131.6±8.8% in VPA, P≤0.05). These results indicate that treatment
with VPA increased mGluR1a immunoreactivity in selected areas of
the hippocampus without altering mGluR5.
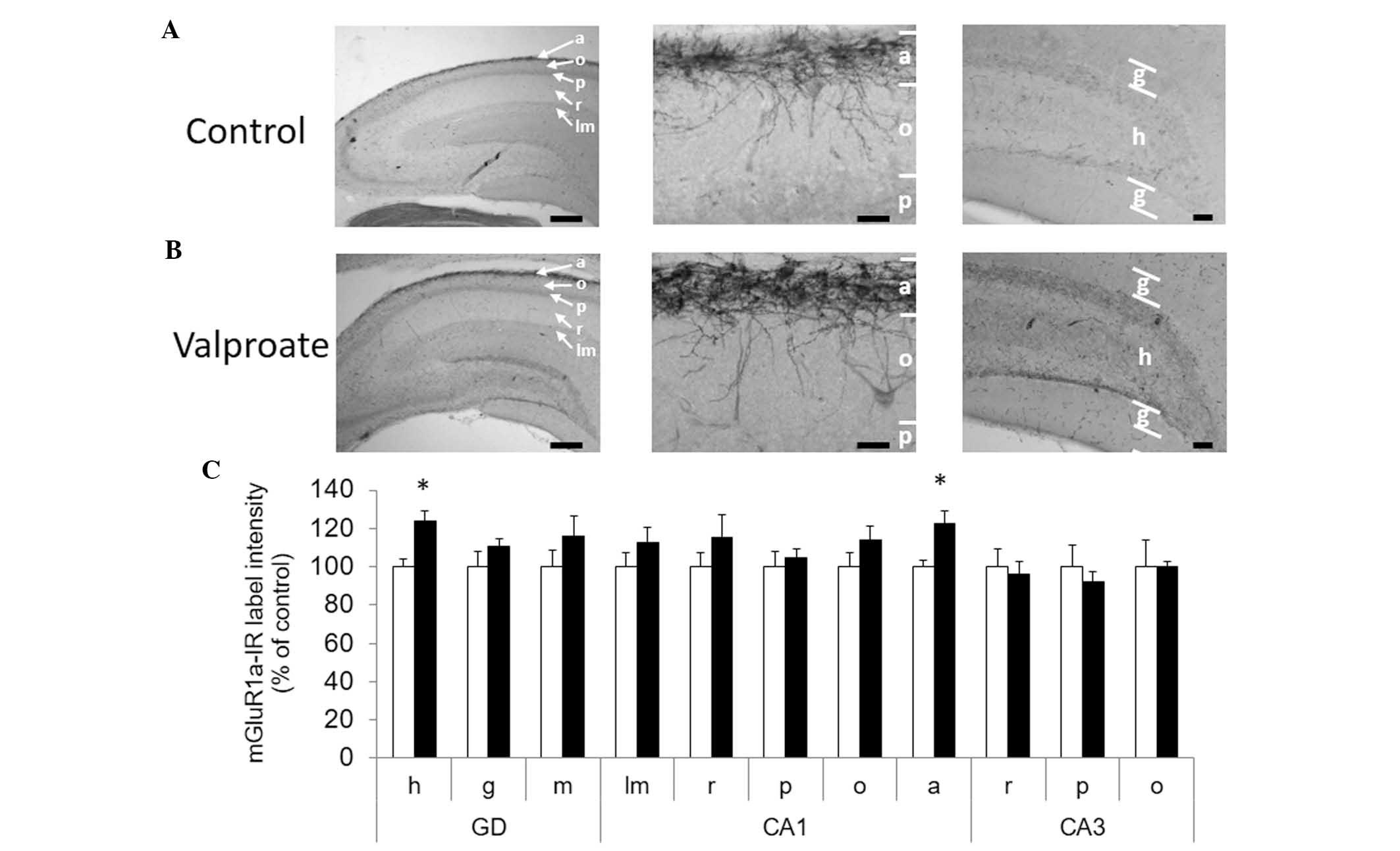 | Figure 4Increase of mGluR1a-immunoreactivity
in gyrus dentate hilus and CA1 alveus subfields of hippocampus
induced by prenatal valproate treatment. Digital microphotographs
showing immunohistochemical staining for mGluR1a in dorsal
hippocampal formation from postnatal day 30 (A) control and (B)
valproate treated animals (scale bar, 500 µm). Detail of CA1
oriens/alveus limit from control and valproate treated animals
(left panels). Detail of dentate gyrus from control and VPA-treated
animals (middle panels). The right panels are the left panel images
at a higher magnification (scale bar, 50 µm). (C) Label intensity
of digital microphotographs was analyzed in the different layers of
hippocampal subfields and expressed as a percentage of the control.
The data are presented as the mean ± standard error of the mean
(*P<0.05). The control is represented by white bars
and the valproate treated animals are represented by black bars.
The data were analyzed using the Mann-Withney test. h, hilus; g,
granular; m, molecular; lm, lacunosum moleculare; r, radiatum; p,
pyramidal; o, oriens; a, alveus; GD, dentate gyrus; CA, Ammon's
horn. |
Discussion
Animal models of autism are characterized by social
deficit, anxiety, and repetitive and stereotypical behaviors
(39). In the present study,
treatment of the rat with a unique dose of 450 mg/kg VPA on
gestational day 12.5 produced the expected triad of signs. At P30,
VPA-exposed rats exhibited social deficit in the three chamber
social test, an anxious profile in the elevated plus maze and
repetitive behavior, quantified as self-grooming conducts or
re-entries during the Y-maze performance. Additionally, all
VPA-treated rats exhibited a crooked tail phenotype from the
earliest postnatal stages of development, a mild teratogenic
effect, usually observed in the VPA model of autism (40). All characteristics in the
experimental group confirmed this as an autistic-like model of
study. The immunoreactivity of mGluR5 and mGluR1a in the
hippocampal region of P30 male control and VPA-treated rats were
assessed. The most important finding reported in the present study
was the increased mGluR1a immunoreactivity in the stratum alveus of
CA1 and in the hilus of dentate gyrus subfields of hippocampus
produced by prenatal treatment with VPA, without changes in mGluR5
immunostaining.
In the open field arena, a hypokinetic condition
that is consistent with a reduction in exploratory activity was
observed, as has been previously reported by others in VPA-treated
rats using the same test (41).
This was also demonstrated previously with a motor alteration, and
a balance and coordination deficit was reported in the rotarod and
vertical pole test (42). Motor
alteration reinforces the idea that biological mechanisms
underlying autism affect synaptic development in diverse brain
circuits. Based on genetic evidence, synaptic protein synthesis
regulated by the mTOR pathway appears to be a point of convergence
between several types of autism and affecting different brain
regions, where an apparent increase in mTOR signaling may be the
common pathophysiological marker. In effect, in the TSC+/−rodent, a
hyperactive mTOR causes altered autophagy that most likely mediates
a synaptic pruning deficit, parallel to these observed in an
autistic child (43). However, a
recent report demonstrated decreased mTOR signaling in the temporal
cortex of prenatally treated VPA rats and in human postmortem
fusiform gyrus of idiopathic autism (44). These authors suggest that a
bidirectional alteration in the mTOR signaling pathway can be
prejudicial to synaptic development and not only its exacerbation.
Another interpretation is that in VPA-induced autism exists with an
increased synaptic protein synthesis independent of mTOR, and that
decreased mTOR signaling may be a compensatory change to excessive
activation of a different pathway. Thus, it is fundamental in the
VPA model to evaluate other possible regulators of synaptic protein
synthesis.
Upstream regulators of synaptic protein synthesis
initiated by the mTOR pathway or in parallel to it are metabotropic
glutamate receptors. Their relevance to autistic behavior has been
clearly highlighted in fragile X-mental disability, a neurological
disorder with autistic signs (45). In the mouse model for fragile
X-syndrome, a hypersensitivity to mGluR5 stimulation and increased
Erk activation, causing increased synaptic protein synthesis in the
hippocampus, was demonstrated (46). In agreement with that previous
study, the mGluR5 receptor antagonist,
2-methyl-6-phenylethyl-pyrididine, reduces increased self-grooming
and marble burying, however, no anxiety in VPA-treated mice
(28). However, in that previous
report, anxiety was evaluated by open field test and no social
interaction was assessed. Nonetheless, the role of the other member
of Class I mGluR receptor, mGluR1, has rarely been studied in
environment-induced autism. In this context, it is important that
mGluR5 and mGlu1 receptors have no redundant roles in the
hippocampus as they differentially regulate CA1 pyramidal cell
function (47).
The present study have analyzed mGluR5 and mGluR1a
immunoreactivity in the hippocampal region of P30 male rats treated
with VPA on embryonic day 12.5. The most important finding was the
increased mGluR1a immunoreactivity in the stratum alveus, including
the limit oriens/alveus, of the CA1 field of the hippocampus and in
the hilus of dentate gyrus produced by prenatal treatment with VPA.
These class I mGluR immunoreactive cells in CA1 oriens/alveus
certainly correspond to inhibitory GABAergic interneurons (38). The hippocampus has a high diversity
of inhibitory interneurons, which form complex feedback and
feedforward circuits. These are fundamental for hippocampal
functions and, at least four different types of interneurons have
been described in the oriens/alveus region, according to their
electrical responses to class I mGluRs agonist, trans-(1S,
3R)-1-aminocyclopentane-1, 3-dicarboxylic acid (ACPD) (48). Oriens/alveus interneurons type I
express mGluR1, mGluR5 and somatostatin, while type II express
mGluR1 and calbindin, and type III express only mGluR5. Type IV
also express class I mGluRs, but they do not respond to ACPD
(48). Judging by its profuse
mGluR1a-immunoreactive dendritic arborization, the cells with
increased labeling in the CA1 oriens/alveus of VPA-treated animals
may correspond to type I or II. Notably, some of the oriens/alveus
interneurons I, II and IV project to the CA1 stratum lacunosum
moleculare and are termed O-LM interneurons (38,49).
In functional terms, O-LM interneurons type I and type II have an
interesting function, collaborating with the septal area (50) in the hippocampal theta rhythmic
oscillations, which are involved in several neurobiological
processes, including locomotion, defense, affect, learning and
memory (51). In the context of
autistic signs, the function for O-LM cells in the exclusion of
aversive stimuli during hippocampal contextual representation in
the fear-learning paradigm is interesting (52). The increased mGluR1a
immunoreactivity in the alveus/oriens region can represents
mGluR1a-expressing O-LM interneurons innervating the last portion
of pyramidal dendrites laying in the lacunosum moleculare region.
Therefore, in this way, increased mGluR1a-mediated activity of O-LM
interneurons can produce altered inhibition, most likely increased,
in the distal portion of apical dendritic tree of CA1 pyramidal
neurons. Those distal ramifications, also termed tufts, lie in the
stratum lacunosum moleculare and modulates proximal synapsis of the
same neurons lying in stratum radiatum (53). Following this line of reasoning,
and knowing that the stratum radiatum and lacunosum moleculare
represents distinct CA1 input pathways, it was expected that an
altered activity of O-LM interneurons can exert long-lasting
heterosynaptic effects altering the interplay between two
functionally and spatially distinct pathways, affecting finally the
network properties. This alteration can affect the role of O-LM
interneurons in theta oscillations affecting the functions in which
CA1 O-LM interneurons-mediated feedback circuits are involved.
Therefore, that simple change in the mGluR1a immunoreactivity may
have important consequences for the hippocampal integration and
diverse neurobiological functions. In this context, the role of
O-LM interneurons and hippocampal theta oscillations in defense,
affect and, in particular, contextual fear learning deserves
special attention. The suggested function of O-LM interneurons in
the exclusion of aversive stimuli of hippocampal dependent fear
learning (52) and the probed role
of mGluR1 in long term plasticity of the same cells (37) suggests that the increases in
mGluR1a immunoreactivity reported in the present study may be
relevant to explain certain specific signs of autism.
Increased immunoreactivity for mGluR1a in the hilus
may corresponds to hilar interneurons and it may have important
consequences for hippocampal functioning. Granular cells of the
dentate gyrus receive the major hippocampal inputs coming from the
entorhinal cortex and subsequently, mossy fibers from granular
cells innervate proximal dendrites of pyramidal neurons in CA3. In
addition, mossy fibers also innervate profusely interneurons in the
hilus of dentate gyrus and in the CA3. Therefore, granular layer
activation by entorhinal inputs produces a rather general reduction
of CA3 pyramidal cell excitability, a phenomena that likely aids to
filter memory information (54).
The majority of hilar GABAergic neurons are mGluR1a- and substance
P-immunoreactive, and small type terminals of mossy fibers
innervate almost all of these (55). In addition, a part of hilar mGluR1
immunoreactive inter-neurons are somatostatin-positive and form a
feedback loop from the hilar region to the outer molecular layer of
dentate gyrus (55,56). Thus, increased mGluR1a
immunoreactivity in the hilus can affect forward and feedback
inhibitory loops, modulating the hippocampal information
processing.
By contrast, increased mGluR1a immunoreactivity may
be a result of increased transcription or altered mRNA processing,
translation or stability. In base to these results, the present
study failed to determine the origin of increased mGluR1a
immunoreactivity, but suggested an over-functioning of that
specific type of class I mGluR, in line with the previous evidence
for the involvement of this family of receptors in autism. Analysis
of mRNA is required and it is interesting to determinate mGluR1a
and mGluR1b levels since an interesting cooperative function
between 1a and 1b isoforms has been previously demonstrated
(57). In addition, the present
study cannot discard the role of mGluR5 in the VPA model of autism
and the possible cooperative signaling between homodimers of
metabotropic glutamate receptors 1 and 5 is highly interesting
(58).
In conclusion, the results of the present study
suggested that prenatal treatment with VPA produces mGluR1a
over-functioning in hippocampal interneurons of juvenile rats. This
likely alters distinct regulatory loops affecting hippocampal
processing that may be associated with autistic signs. The
functional involvement of mGluR1a in autism models require further
research as a possible novel pharmacological target in autism.
Acknowledgments
The present study was supported by the National
Found for Scientific and Technological Research (nos. 1110855 and
1120528).
References
|
1
|
Frith U and Happé F: Autism spectrum
disorder. Curr Biol. 15:R786–R790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wass S: Distortions and disconnections:
Disrupted brain connectivity in autism. Brain Cogn. 75:18–28. 2011.
View Article : Google Scholar
|
|
3
|
Blatt GJ: The neuropathology of autism.
Scientifica (Cairo). 2012:7036752012.
|
|
4
|
Betancur C: Etiological heterogeneity in
autism spectrum disorders: More than 100 genetic and genomic
disorders and still counting. Brain Res. 1380:42–77. 2011.
View Article : Google Scholar
|
|
5
|
Jamain S, Quach H, Betancur C, Råstam M,
Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M,
Gillberg C, et al: Mutations of the X-linked genes encoding
neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet.
34:27–29. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moessner R, Marshall CR, Sutcliffe JS,
Skaug J, Pinto D, Vincent J, Zwaigenbaum L, Fernandez B, Roberts W,
Szatmari P and Scherer SW: Contribution of SHANK3 mutations to
autism spectrum disorder. Am J Hum Genet. 81:1289–1297. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Durand CM, Betancur C, Boeckers TM,
Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg
IC, Anckarsäter H, et al: Mutations in the gene encoding the
synaptic scaffolding protein SHANK3 are associated with autism
spectrum disorders. Nat Genet. 39:25–27. 2007. View Article : Google Scholar :
|
|
8
|
Hung AY, Futai K, Sala C, Valtschanoff JG,
Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, et al:
Smaller dendritic spines, weaker synaptic transmission, but
enhanced spatial learning in mice lacking Shank1. J Neurosci.
28:1697–1708. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dindot SV, Antalffy BA, Bhattacharjee MB
and Beaud: The Angelman syndrome ubiquitin ligase localizes to the
synapse and nucleus and maternal deficiency results in abnormal
dendritic spine morphology. Hum Mol Genet. 17:111–118. 2008.
View Article : Google Scholar
|
|
10
|
Kishino T, Lalande M and Wagstaff J:
UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 15:70–73.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sadakata T, Washida M, Iwayama Y, Shoji S,
Sato Y, Ohkura T, Katoh-Semba R, Nakajima M, Sekine Y, Tanaka M, et
al: Autistic-like phenotypes in Cadps2-knockout mice and aberrant
CADPS2 splicing in autistic patients. J Clin Invest. 117:931–943.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costa-Mattioli M, Sossin WS, Klann E and
Sonenberg N: Translational control of long-lasting synaptic
plasticity and memory. Neuron. 61:10–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoeffer CA and Klann E: mTOR signaling: At
the crossroads of plasticity, memory and disease. Trends Neurosci.
33:67–75. 2010. View Article : Google Scholar
|
|
14
|
de Vries PJ and Howe CJ: The tuberous
sclerosis complex proteins-a GRIPP on cognition and
neurodevelopment. Trends Mol Med. 13:319–326. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feng Y, Zhang F, Lokey LK, Chastain JL,
Lakkis L, Eberhart D and Warren ST: Translational suppression by
trinucleotide repeat expansion at FMR1. Science. 268:731–734. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koekkoek SK, Yamaguchi K, Milojkovic BA,
Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F,
Bakker CE, et al: Deletion of FMR1 in Purkinje cells enhances
parallel fiber LTD, enlarges spines, and attenuates cerebellar
eyelid conditioning in Fragile X syndrome. Neuron. 47:339–352.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Butler MG, Dasouki MJ, Zhou XP,
Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton
R, Pilarski R and Eng C: Subset of individuals with autism spectrum
disorders and extreme macrocephaly associated with germline PTEN
tumour suppressor gene mutations. J Med Genet. 42:318–332. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goffin A, Hoefsloot LH, Bosgoed E, Swillen
A and Fryns JP: PTEN mutation in a family with Cowden syndrome and
autism. Am J Med Genet. 105:521–524. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zori RT, Marsh DJ, Graham GE, Marliss EB
and Eng C: Germline PTEN mutation in a family with Cowden syndrome
and Bannayan-Riley-Ruvalcaba syndrome. Am J Med Genet. 80:399–402.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neves-Pereira M, Müller B, Massie D,
Williams JH, O'Brien PC, Hughes A, Shen SB, Clair DS and
Miedzybrodzka Z: Deregulation of EIF4E: A novel mechanism for
autism. J Med Genet. 46:759–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kelleher RJ IIIrd and Bear MF: The
autistic neuron: Troubled translation? Cell. 135:401–406. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kao DI, Aldridge GM and Greenough WT:
Altered mRNA transport, docking and protein translation in neurons
lacking fragile X mental retardation protein. Proc Natl Acad Sci
USA. 107:15601–15606. 2010. View Article : Google Scholar
|
|
23
|
Markram K, Rinaldi T, La Mendola D, Sandi
C and Markram H: Abnormal fear conditioning and amygdala processing
in an animal model of autism. Neuropsychopharmacology. 33:901–912.
2008. View Article : Google Scholar
|
|
24
|
Silva GT, Le Bé JV, Riachi I, Rinaldi T,
Markram K and Markram H: Enhanced long-term microcircuit plasticity
in the valproic acid animal model of autism. Front Synaptic
Neurosci. 1:12009.PubMed/NCBI
|
|
25
|
Rinaldi T, Perrodin C and Markram H:
Hyper-connectivity and hyper-plasticity in the medial prefrontal
cortex in the valproic acid animal model of autism. Front Neural
Circuits. 2:42008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rinaldi T, Silberberg G and Markram H:
Hyperconnectivity of local neocortical microcircuitry induced by
prenatal exposure to valproic acid. Cereb Cortex. 18:763–770. 2008.
View Article : Google Scholar
|
|
27
|
Rinaldi T, Kulangara K, Antoniello K and
Markram H: Elevated NMDA receptor levels and enhanced postsynaptic
long-term potentiation induced by prenatal exposure to valproic
acid. Proc Natl Acad Sci USA. 104:13501–13506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mehta MV, Gandal MJ and Siegel SJ:
mGluR5-antagonist mediated reversal of elevated stereotyped,
repetitive behaviors in the VPA model of autism. PLoS One.
6:e260772011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
WalfA AA and Frye CA: The use of the
elevated plus maze as an assay of anxiety-related behavior in
rodents. Nat Protoc. 2:322–328. 2007. View Article : Google Scholar
|
|
30
|
Fernandes C and File SE: The influence of
open arm ledges and maze experience in the elevated plus-maze.
Pharmacol Biochem Behav. 54:31–40. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pellow S, Chopin P, File SE and Briley M:
Validation of open:Closed arm entries in an elevated plus-maze as a
measure of anxiety in the rat. J Neurosci Methods. 14:149–167.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moy SS, Nadler JJ, Perez A, Barbaro RP,
Johns JM, Magnuson TR, Piven J and Crawley JN: Sociability and
preference for social novelty in five inbred strains: An approach
to assess autistic-like behavior in mice. Genes Brain Behav.
3:287–302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Conrad CD, Grote KA, Hobbs RJ and
Ferayorni A: Sex differences in spatial and non-spatial Y-maze
performance after chronic stress. Neurobiol Learn Mem. 79:32–40.
2003. View Article : Google Scholar
|
|
34
|
Conrad CD, Galea LA, Kuroda Y and McEwen
BS: Chronic stress impairs rat spatial memory on the Y maze, and
this effect is blocked by tianeptine pretreatment. Behav Neurosci.
110:1321–1334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dellu F, Mayo W, Cherkaoui J, Le Moal M
and Simon H: Two-trial memory task with automated recording: Study
in young and aged rats. Brain Res. 588:132–139. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Edalatmanesh MA, Nikfarjam H, Vafaee F and
Moghadas M: Increased hippocampal cell density and enhanced spatial
memory in the valproic acid rat model of autism. Brain Res.
1526:15–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lapointe V, Morin F, Ratté S, Croce A,
Conquet F and Lacaille JC: Synapse-specific mGluR1-dependent
long-term potentiation in interneurones regulates mouse hippocampal
inhibition. J Physiol. 15:125–135. 2004. View Article : Google Scholar
|
|
38
|
Shigemoto R, Kinoshita A, Wada E, Nomura
S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S and
Mizuno N: Differential presynaptic localization of metabotropic
glutamate receptor subtypes in the rat hippocampus. J Neurosci.
17:7503–7522. 1997.PubMed/NCBI
|
|
39
|
Patterson PH: Modeling autistic features
in animals. Pediatr Res. 69:34R–40R. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim KC, Kim P, Go HS, Choi CS, Yang SI,
Cheong JH, Shin CY and Ko KH: The critical period of valproate
exposure to induce autistic symptoms in Sprague-Dawley rats.
Toxicol Lett. 201:137–142. 2010. View Article : Google Scholar
|
|
41
|
Schneider T and Przewłocki R: Behavioral
alterations in rats prenatally exposed to valproic acid: Animal
model of autism. Neuropsychopharmacology. 30:80–89. 2005.
View Article : Google Scholar
|
|
42
|
Kim JE, Shin MS, Seo TB, Ji ES, Baek SS,
Lee SJ, Park JK and Kim CJ: Treadmill exercise ameliorates motor
disturbance through inhibition of apoptosis in the cerebellum of
valproic acid-induced autistic rat pups. Mol Med Rep. 8:327–334.
2013.PubMed/NCBI
|
|
43
|
Tang G, Gudsnuk K, Kuo SH, Cotrina ML,
Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto
A, et al: Loss of mTOR-dependent macroautophagy causes
autistic-like synaptic pruning deficits. Neuron. 83:1131–1143.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nicolini C, Ahn Y, Michalski B, Rho JM and
Fahnestock M: Decreased mTOR signaling pathway in human idiopathic
autism and in rats exposed to valproic acid. Acta Neuropathol
Commun. 3:32015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bear MF, Huber KM and Warren ST: The mGluR
theory of fragile X mental retardation. Trends Neurosci.
27:370–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Osterweil EK, Krueger DD, Reinhold K and
Bear MF: Hypersensitivity to mGluR5 and ERK1/2 leads to excessive
protein synthesis in the hippocampus of a mouse model of fragile X
syndrome. J Neurosci. 30:15616–15627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mannaioni G, Marino MJ, Valenti O,
Traynelis SF and Conn PJ: Metabotropic glutamate receptors 1 and 5
differentially regulate CA1 pyramidal cell function. J Neurosci.
21:5925–5934. 2001.PubMed/NCBI
|
|
48
|
van Hooft JA, Giuffrida R, Blatow M and
Monyer H: Differential expression of group I metabotropic glutamate
receptors in functionally distinct hippocampal interneurons. J
Neurosci. 20:3544–3551. 2000.PubMed/NCBI
|
|
49
|
Kullmann DM: Interneuron networks in the
hippocampus. Curr Opin Neurobiol. 21:709–716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Stewart M and Fox SE: Do septal neurons
pace the hippocampal theta rhythm? Trends Neurosci. 13:163–168.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chee SS, Menard JL and Dringenberg HC: The
lateral septum as a regulator of hippocampal theta oscillations and
defensive behavior in rats. J Neurophysiol. 113:1831–1841. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lovett-Barron M, Kaifosh P, Kheirbek MA,
Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV
and Losonczy A: Dendritic inhibition in the hippocampus supports
fear learning. Science. 343:857–863. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Han E and Heinemann S: Distal dendritic
inputs control neuronal activity by heterosynaptic potentiation of
proximal inputs. J Neurosci. 33:1314–1325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Scharfman HE and Myers CE: Hilar mossy
cells of the dentate gyrus: A historical perspective. Front Neural
Circuits. 6:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Acsády L, Kamondi A, Sík A, Freund T and
Buzsáki G: GABAergic cells are the major postsynaptic targets of
mossy fibers in the rat hippocampus. J Neurosci. 18:3386–3403.
1998.PubMed/NCBI
|
|
56
|
Baude A, Nusser Z, Roberts JD, Mulvihill
E, McIlhinney RA and Somogyi P: The metabotropic glutamate receptor
(mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal
subpopulations as detected by immunogold reaction. Neuron.
11:771–787. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Techlovská S, Chambers JN, Dvořáková M,
Petralia RS, Wang YX, Hájková A, Nová A, Franková D, Prezeau L and
Blahos J: Metabotropic glutamate receptor 1 splice variants mGluR1a
and mGluR1b combine in mGluR1a/b dimers in vivo. Neuropharmacology.
86:329–336. 2014. View Article : Google Scholar :
|
|
58
|
Sevastyanova TN and Kammermeier PJ:
Cooperative signaling between homodimers of metabotropic glutamate
receptors 1 and 5. Mol Pharmacol. 86:492–504. 2014. View Article : Google Scholar : PubMed/NCBI
|