Introduction
Alzheimer's disease (AD) is the most common
age-associated disorder, accounting for ~60–80% of all cases of
dementia (1). Previous studies
have shown that atrophy of the hippocampus and amygdala may occur
in AD, even at the preclinical stages (2–4). AD
is typically characterized by a progressive loss of memory,
impairment of higher cognitive functions and major degeneration in
the brain cortex. This degeneration includes the production and
deposition of β-amyloid (Aβ) peptide, intracellular neurofibrillary
tangles (5,6) and extensive neuronal cell death
(7) in specific cortical and
subcortical zones. AD shares a number of common pathological
features with other neurodegenerative diseases, including activated
apoptotic biochemical cascades, up-regulated oxidative stress
levels, abnormal protein processing, and so forth (8). Age-associated oxidative insults have
been associated with neurodegenerative diseases, including AD and
Parkinson's disease (9,10). Aβ peptide fragments are capable of
inducing neuronal cell death directly or indirectly (11–13).
In addition, transgenic mice with mutant amyloid precursor protein
are considered a valuable animal model to test preventative and
therapeutic interventions for AD due to the occurrence of
biochemical, behavioral and histopathological changes that are
similar to those observed in patients with AD (14). Although current therapeutic
candidates for the treatment of AD that are similar to
cholinesterase inhibitors such as meserine (15) and memantine (16) may modestly improve memory and
cognitive function in this transgenic mouse model, these drugs do
not show disease-modifying effects in patients. To date, the
available therapies for AD only serve the purpose of ameliorating
disease symptoms, and there are no effective therapeutic approaches
that address the underlying pathological processes of AD (17).
Neuregulin-1 (Nrg1), a protein encoded by the
NRG1 gene, has been identified as an active epithelial
growth factor (EGF) family member (18). At least 31 isoforms and six types
of Nrg1, including Nrg1α and Nrg1β, types I to VI, have been
identified, due to alternative splicing (19). These types, or isoforms, perform a
broad spectrum of functions. Nrg1 has been implicated in glioma
malignancy (20), gastrointestinal
systems (21) and prolactin
secretion (22–24). Specific direct binding of Nrg1 to
ErbB receptors, including ErbB3 and ErbB4 (25,26),
activates a diverse set of biological processes, including
myelination, neurite outgrowth, cell proliferation, differentiation
and protection against apoptosis (27,28).
Nrg1, as well as its receptor ErbB tyrosine kinase, is expressed in
the developing nervous system and the adult brain, where they exert
a key role in regulating the development and regeneration of the
nervous system (29–31). Nrg1 has been reported to prevent
brain injury following stroke (32), and to exert a protective role for
dopaminergic neurons in a mouse model of Parkinson's disease
(33). A burgeoning body of
evidence suggests that Nrg1 is associated with traumatic brain
injury (34) and AD (35).
Given the alteration of Nrg1 signaling in patients
with AD and the protective role of Nrg1 in the lesioned nervous
system (29,30), it was hypothesized that Nrg1 may
exert a preventive role in the maintenance of cell
survival-associated signaling under the pathological conditions
present in AD. In the present study, it has been shown that the
levels of Nrg1 are altered in response to hydrogen peroxide
(H2O2)- or Aβ1–42-induced
oxidative stress and neuronal damage in an attempt to protect the
cortical neurons from abnormal changes in cell signaling. Notably,
exogenous Nrg1 was revealed to have a pivotal role in preventing
neurons from oxidative damage and in triggering changes in
Nrg1-ErbB signaling in response to the harmful situation. These
results demonstrated that Nrg1 signaling is perturbed under the
pathological conditions of AD, and this alteration may be partially
reversed by the exogenous application of Nrg1. Taken together,
these data indicate a neuroprotective role of Nrg1 against
pathological damage during the development of AD.
Materials and methods
Tissue microarray
Human brain tissue microarray containing
4-μm-thick cortical tissues was purchased from Shaanxi
Chaoying Biotechnology Co., Ltd. (BN 126; Xi'an, Shanxi, China). In
addition, human brain frontal lobe sections from a normal
individual (cat. no. ab4304; Abcam, Cambridge, MA, USA) and from a
patient with AD (cat. no. ab4582; Abcam) at a thickness of 5
μm were used.
Animals
Female and male C57BL/6 mice (n=20; age, 3 months)
were purchased from Guangdong Medical Laboratory Animal Center
(Foshan, Guangdong, China) and maintained in the animal center of
Shantou University Medical College (SUMC). All the animals were
housed in the SUMC animal center at 25°C in a reversed 12/12 h
dark-light cycle, and food and water were provided ad
libitum. All experiments conducted on animals were reviewed and
approved by the Animal Ethics Committee of SUMC and the authorities
of the Guangdong Province. All efforts were made to minimize the
suffering of animals and to reduce the number of animals used in
these experiments.
Preparation of recombinant Nrg1β (rNrg1β)
and oligomeric Aβ1–42
The Escherichia coli-derived rNrg1β (CYT-407;
ProSpec-Tany TechnoGene Ltd., Ness Ziona, Israel) was dissolved in
phosphate-buffered saline (PBS, pH 7.4) and used for in
vitro cell culture experiments.
Synthetic Aβ1–42 powder (cat.
no.1932-2-15; ChinaPeptides Co., Ltd., Shanghai, China) was
dissolved in 0.1% dimethyl sulfoxide and diluted 1:100 in
Dulbecco's modified Eagle's medium (DMEM)/F-12 culture medium
(HyClone™; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
diluted solution was subsequently incubated at 4°C for 24 h prior
to centrifugation at 14,000 g for 10 min. The supernatant was used
as oligomeric Aβ1–42 for in vitro cell culture
experiments.
Primary culture of the mouse cortical
neurons
Mouse frontal cortical tissues were obtained from
postnatal C57BL/6 mice on day 0 (P0) and crudely homogenized by
chopping following the removal of the vessels and meninges. The
tissues were kept on ice in DMEM/F-12 culture medium (HyClone™;
Thermo Fisher Scientific, Inc.) without serum, and subsequently
digested with 0.125% trypsin (Solarbio Biotech Corp., Beijing,
China) at 37°C in a humidified 5% CO2 atmosphere for 30
min. The finely separated cortical neurons were seeded in a volume
of 200 μl at a density of 2×105 cells per well in
48-well cell culture plates pre-coated with 100 μg/ml
poly-D-lysine (C0312; Beyotime Institute of Biotechnology,
Shanghai, China). Cells were cultured in DMEM/F-12 culture medium
supplemented with 10% fetal bovine serum (FBS; Sijiqing Biotech
Corp., Hangzhou, China) and 1% penicillin/streptomycin (Solarbio
Biotech Corp.) for 6 h to enable cell adhesion to the plates. The
medium was subsequently aspirated and replaced with Neurobasal-A
(cat. no. 21103-049; Gibco; Thermo Fisher Scientific, Inc.) culture
medium supplemented with 2% B-27 (cat. no. 17504-001, Gibco, Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin.
To investigate changes in Nrg1 signaling in
vitro at the protein level, the primary cortical neurons were
treated using two different paradigms: i) The cortical neurons were
maintained at 37°C in a humidified 5% CO2 atmosphere for
24 h, and subsequently the culture medium was replaced with
Neurobasal-A medium containing H2O2 at
various concentrations: 0, 1, 2.5, 5, 10 and 20 μM for 24 h;
ii) the cells were cultured for 6 h, 1, 3, 6 and 10 days. Cells
cultured for 6 h were used as a control (0 days). At the indicated
time points, whole-cell lysates were collected.
To study the neuroprotective role of rNrg1β in
regulating Nrg1 signaling at the protein level, cortical neurons
were utilized with two different cell models: i) Cortical neurons
were treated with 0, 5 or 10 nM rNrg1β for 2 h, followed by an
exposure to 2.5 μM H2O2 for 24 h; ii)
after a 24-h incubation period, cells were treated with 0, 5 or 10
nM rNrg1β for 2 h prior to incubation with a sublethal dose of 10
μM oligomeric Aβ1–42 for 24 h. Finally,
whole-cell lysates were collected for western blotting.
Immunofluorescence staining
Paraffin-embedded human brain cortical tissue
microarray and human frontal cortical sections from a normal
individual and a patient with AD were deparaffinized, rehydrated
via a graded array of ethanol to PBS, and antigen retrieval was
then performed using 10 mM citrate buffer (pH 6.0) for 40 min.
Non-specific protein binding sites were blocked by incubation with
10% normal donkey serum diluted in PBS at room temperature (RT,
25°C) for 1 h. The sections were incubated at 4°C overnight with
mouse monoclonal anti-Nrg1 antibody (1:200, cat. no. MS-272-P1,
Thermo Fisher Scientific, Inc.) without or with either rabbit
polyclonal anti-phosphorylated (p)ErbB4 antibody (1:200, cat. no.
sc-33040, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or
rabbit polyclonal anti-pNeu antibody (1:200, cat. no. sc-12352-R,
Santa Cruz Biotechnology, Inc.). Mouse monoclonal anti-β-III
tubulin (1:1,000, cat. no. sc-80016, Santa Cruz Biotechnology,
Inc.) was applied to indicate the mouse cortical neurons. Following
washing with PBS three times (5 min each wash), the samples were
incubated with a donkey anti-mouse secondary antibody conjugated to
Dylight™ 488 (1:1,000) and a donkey anti-rabbit secondary antibody
conjugated to Dylight™ 594 (1:1,000) at RT in the dark for 90 min.
The samples were finally mounted using ProLong® Gold
Antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI; P36935,
Gibco; Thermo Fisher Scientific, Inc.). Double-immunofluorescence
images were acquired using an Olympus laser confocal system
(FV-1000; Olympus, Tokyo, Japan). DAPI was excited at 405 nm.
Dylight™ 488 and Dylight™ 594 were excited at 488 and 594 nm,
respectively, in a multi-track configuration.
The protein levels of Nrg1, pNeu and pErbB4 in the
human brain cortical tissue microarray were evaluated using
integrated fluorescence intensity (IFI). The IFI at each tissue
point was obtained using the MultiImage Light Cabinet CY3 filter
for Nrg1, and the CY5 filter of the FluorChem HD2 gel-imaging
system for pErbB4 or pNeu (Alpha Innotech, San Leandro, CA, USA).
The IFI was analyzed using Image Tool II software 3.0 (University
of Texas Health Science Center, San Antonio, TX, USA).
Immunohistochemical staining
Deparaffinized human frontal cortical sections from
a normal individual and a patient with AD were rehydrated via a
graded array of ethanol to PBS. Subsequently, hit-induced antigen
retrieval was performed in citrate buffer (10 mM, pH 6.0) at 95°C
for 40 min, followed by cooling down to RT for at least 60 min.
Sections were then incubated in a 3% H2O2
solution for endogenous peroxidase clearance at RT for 10 min.
Sections were subsequently washed in PBS for 5 min three times.
Following blocking in 10% PBS-buffered normal goat serum for 30
min, sections were incubated with primary antibodies, including
mouse monoclonal anti-Nrg1 antibody (1:200, cat. no. MS-272-P1,
Thermo Fisher Scientific, Inc.), rabbit polyclonal anti-pErbB4
antibody (1:200, cat. no. sc-33040, Santa Cruz Biotechnology,
Inc.), rabbit polyclonal anti-pNeu antibody (1:200, cat. no.
sc-12352-R, Santa Cruz Biotechnology, Inc.) and rabbit polyclonal
anti-Aβ1–42 antibody (1:1,000, cat. no. ab39377, Abcam)
overnight at 4°C, followed by incubation with an Enhanced Polymer
DAB Detection kit (cat. no. PV-900; ZSGB-Bio, Beijing, China) and
an AEC kit (cat. no. ZLI-9036; ZSGB-Bio). Stained sections were
mounted on slides, dehydrated and sealed with coverslips using a
commercial water-soluble mounting kit (cat. no. AR1018; Boster
Biological Technology, Wuhan, China). Counterstaining was performed
with Mayer's hematoxylin in certain of the tissue sections.
Congo red reagent (cat. no. DG0025, Beijing Leagene
Biotech. Co., Ltd., Beijing, China) was used to confirm the
formation of amyloid plaques in frontal lobe sections from a
patient with AD, according to the manufacturer's protocol.
Western blotting analysis
Tissue lysates of the frontal lobe from either
normal adults (cat. no. ab29969, Abcam) or the patient with AD
(cat. no. ab29971, Abcam) and the whole-cell lysates from cultured
mouse cortical neurons were mixed with 20% sample loading buffer
[0.125 M Tris/HCl (pH 6.8), 20% glycerol, 10% sodium
dodecylsulfate, 0.1% bromophenol blue and 5% β-mercaptoethanol].
All samples were heated for 15 min at 95°C. Protein samples were
resolved using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and subsequently electroblotted onto polyvinylidene
difluoride membranes (Millipore Corp., Billerica, USA). Incubation
in 5% non-fat milk or bovine serum albumin diluted in Tris/HCl
saline buffer supplemented with 0.1% Tween-20 (TBST, pH 7.4) for 1
h was used to block non-specific protein-binding sites. Membranes
were incubated with antibodies specific for mouse monoclonal
anti-Nrg1 antibody (1:1,000, cat. no. MS-272-P1, Thermo Fisher
Scientific, Inc.), rabbit polyclonal anti-pErbB4 antibody (1:500,
cat. no. sc-33040, Santa Cruz Biotechnology, Inc.), rabbit
polyclonal anti-ErbB4 antibody (1:1,000, cat. no. sc-283, Santa
Cruz Biotechnology, Inc.), rabbit polyclonal anti-pNeu antibody
(1:500, cat. no. sc-12352-R, Santa Cruz Biotechnology, Inc.), mouse
monoclonal anti-Neu antibody (1:1,000, cat. no. sc-33684, Santa
Cruz Biotechnology, Inc.), mouse monoclonal anti-phosphorylated
extracellular signal-regulated kinase 1/2 (anti-pErk1/2) antibody
(1:1,000, cat. no. sc-7383, Santa Cruz Biotechnology, Inc.), mouse
monoclonal anti-Erk1/2 antibody (1:1,000, cat. no. sc-135900, Santa
Cruz Biotechnology, Inc.), mouse monoclonal anti-pAkt1 antibody
(1:1,000, cat. no. sc-81433, Santa Cruz Biotechnology, Inc.), mouse
monoclonal anti-Akt1 antibody (1:1,000, cat. no. sc-55523, Santa
Cruz Biotechnology, Inc.) and mouse monoclonal
anti-glyceraldehyde-3-phosphate dehydrogenase (GADPH) antibody
(1:1,000, cat. no. sc-365062, Santa Cruz Biotechnology, Inc.)
overnight at 4°C. After washing the membrane with TBST three times
at RT (5 min each wash), membranes were further incubated with
horseradish peroxidase-conjugated goat anti-mouse secondary
antibody (1:1,000, cat. no. BA1051, Boster Biological Technology,
Wuhan, China) or anti-rabbit secondary antibody (1:1,000, cat. no.
BA1055, Boster Biological Technology) for 1 h. Subsequently,
membranes were washed in TBST three times (5 min each wash) at RT.
The immunoreactive bands were visualized using an enhanced
chemiluminescence kit (Bio-Rad Laboratories, Richmond, CA, USA) and
an imaging system (Alpha Innotech, San Leandro, CA, USA). The
signal intensity was quantified using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analyses were performed with SPSS 17.0
software (Chicago, IL, USA). Data were expressed as the mean ±
standard error of the mean and analyzed with one-way analysis of
variance (ANOVA) with Tukey's post-hoc test for independent
samples. P<0.05 was considered to indicate a statistically
significant difference.
Results
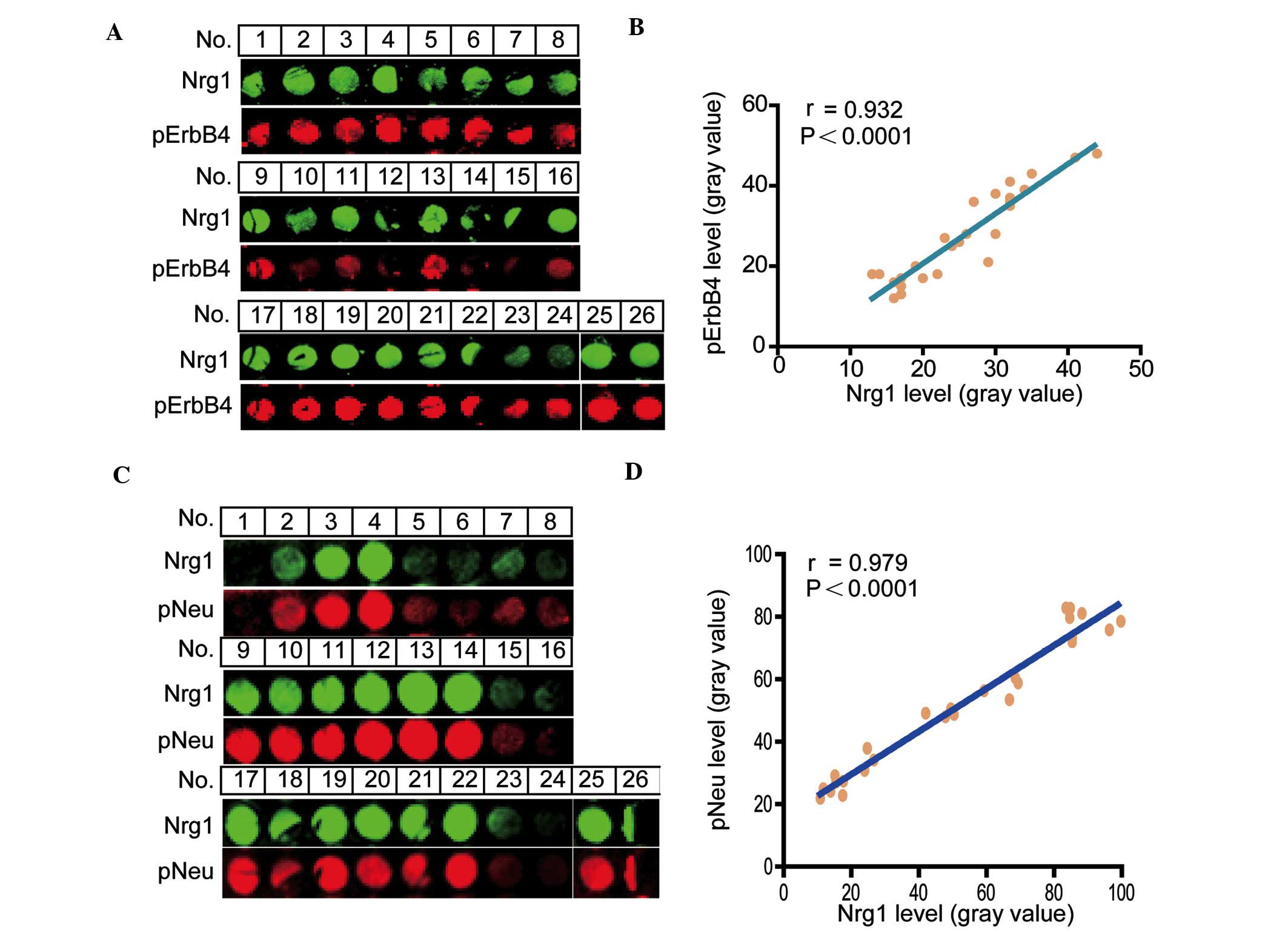
Co-immunostaining correlation analysis of
Nrg1 with the phosphorylation levels of ErbB4 and Neu receptors in
a human cortical tissue microarray
To determine a functional correlative relationship
between the level of Nrg1 and the phosphorylation levels of either
the ErbB4 or the Neu receptors, the co-localization of Nrg1 with
either pErbB4 or pNeu receptors was examined. The signal intensity
for Nrg1 with pErbB4 receptors was revealed by double
immunofluorescence (Fig. 1A), and
a positive correlation between Nrg1 and pErbB4 (r=0.932,
P<0.0001) (Fig. 1B) was
identified. Similarly, the signal intensity for Nrg1 with Neu
receptors was also revealed by double immunofluorescence (Fig. 1C) and an apparent positive
correlation between Nrg1 and pNeu (r=0.979, P<0.0001) (Fig. 1D) was revealed in the human
cortical region.
Detection of Nrg1 signaling in the
frontal lobe of a human AD brain
Congo red staining was used to confirm the formation
of the amyloid plaques in the frontal lobe of the brain of a human
patient with AD. The results demonstrated that there were numerous
amyloid plaques distributed in the frontal lobe of an AD brain,
whereas no amyloid plaque was detected in the normal control
(Fig. 2A). In addition,
immunohistochemical staining also revealed the formation of
Aβ1–42 positive plaques in the frontal cortical zone,
whereas few Aβ1–42 positive plaques were found in the
normal individual (Fig. 2A).
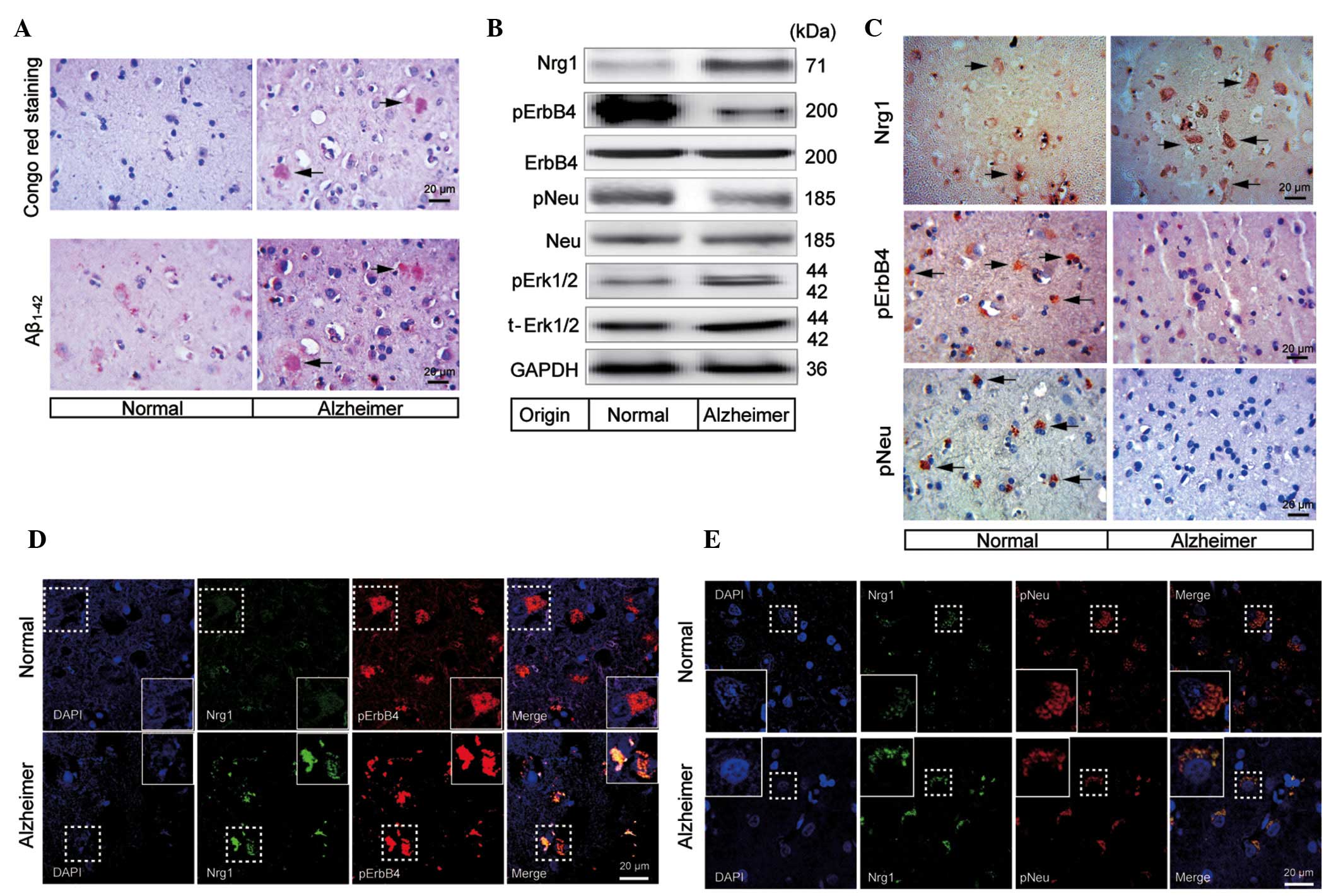 | Figure 2Changes in the Nrg1 signaling pathway
molecules in the frontal lobe of a human AD brain. (A) Congo red
staining and abnormal aggregation of Aβ1–42, indicating
the formation of the amyloid plaques (indicated by the arrowheads)
in the frontal cortical gray matter of a human AD brain. The scale
bar represents 20 μm. (B) Western blotting analysis of Nrg1,
phosphorylation levels of ErbB4, Neu and Erk1/2 in the frontal lobe
of a human AD patient brain. (C) Immunohistochemical detection of
Nrg1, pErbB4 and pNeu (indicated by the arrowheads) in the frontal
cortical gray matter from either the normal indivdual or the human
AD patient. Double immunofluorescence staining images are shown for
co-localization of Nrg1 with either (D) pErbB4 or (E) pNeu in the
frontal cortical gray matter from either the normal individual or
the human AD patient. The scale bar represents 20 μm. AD,
Alzheimer's disease; Aβ, β-amyloid; Nrg1, neuregulin 1; pErbB4,
phosphorylated ErbB4; pNeu, phosphorylated Neu; Erk,
extracellular-signal regulated kinase; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. |
To study the changes in Nrg1 signaling in the
frontal cortex of an AD brain, western blotting was used to
determine the protein levels of Nrg1 and the phosphorylation levels
of ErbB4 and Neu. The protein level of Nrg1 was increased in the
frontal cortical gray matter from an AD brain (Fig. 2B). In contrast, the levels of
pErbB4 and pNeu showed a tendency towards a decrease when compared
with a normal control. Notably, the phosphorylation level of
Erk1/2, which is involved in downstream Nrg1-ErbB signaling, was
increased when compared with that in the normal control (Fig. 2B).
To further investigate changes in Nrg1 signaling
under the conditions of AD, Nrg1, pErbB4 and pNeu in the frontal
cortical gray matter from a patient with AD and a normal control
were immunohistochemically stained. A tendency for there to be an
increased level of Nrg1 was observed in the gray matter of an AD
brain compared with a normal control (Fig. 2C). By contrast, the staining
intensities for pErbB4 and pNeu were clearly reduced (Fig. 2C).
To evaluate the expression and potential
co-localization of Nrg1 with pErbB4 or pNeu, co-immunostaining of
Nrg1 with these receptors was performed using the frontal cortical
tissue from an AD brain. Co-localization of Nrg1 (green) with
pErbB4 (red) or with pNeu (red) was observed. In the human AD
brain, a tendency towards an up-regulation of the levels of Nrg1
was observed, whereas the levels of pErbB4 and pNeu were not
up-regulated compared with those of normal brain tissue (Fig. 2D and E). In addition, pErbB4 and
pNeu were detected in a smaller number of the neuronal cells in the
AD-affected cerebral cortex, which may explain, in part, the
reduced levels of the two molecules observed in the western
blots.
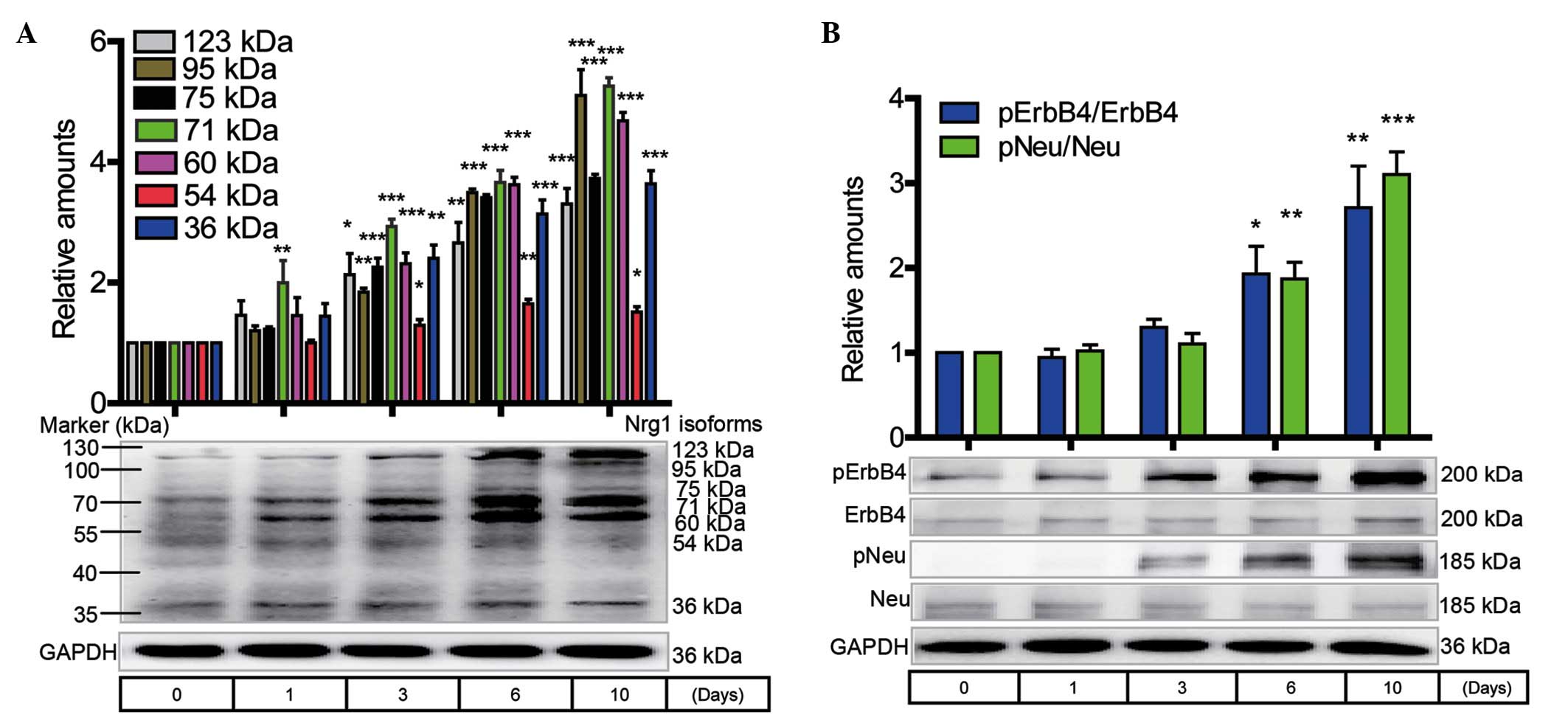
Western blotting analysis of Nrg1 isoform
expression and the ErbB receptor phosphorylation level in primary
cortical neurons during the progression of cell senescence
The primary cortical neurons were routinely cultured
for 1, 3, 6 and 10 days without any treatment. The expression of
Nrg1 isoforms, including the 123-, 95-, 75-, 71-, 60-, 54- and
36-kDa variants, showed a time-dependent increase that reached
statistical significance at 3, 6 and 10 days when compared with the
0-day control (Fig. 3A). A marked
increase in the protein levels of the pNeu and pErbB4 receptors was
observed at 1 to 10 days when compared with the 0-day control
(Fig. 3B).
Investigation of the protective role of
Nrg1β in primary cortical neuronal cultures oxidatively stressed by
H2O2
To determine the role of Nrg1 in preventing
oxidative insults at the protein level, western blotting was
employed to analyze the protein level of Nrg1 and the
phosphorylation levels of Neu and ErbB4 receptors in primary
cortical neurons in response to a 24 h treatment with
H2O2 at concentrations ranging from 0 to 20
μM. Multiple isoforms of Nrg1, including the 123-, 71-, 60-,
54- and 36-kDa variants, were expressed in the primary cortical
neurons. Compared with the vehicle control, all Nrg1 isoforms
showed a dose-dependent decrease in response to the gradually
increased concentrations of H2O2 (Fig. 4A). In comparison with the vehicle
control, the expression of Nrg1 was down-regulated, accompanied by
a marked reduction in the receptor levels of pNeu and pErbB4
(Fig. 4B).
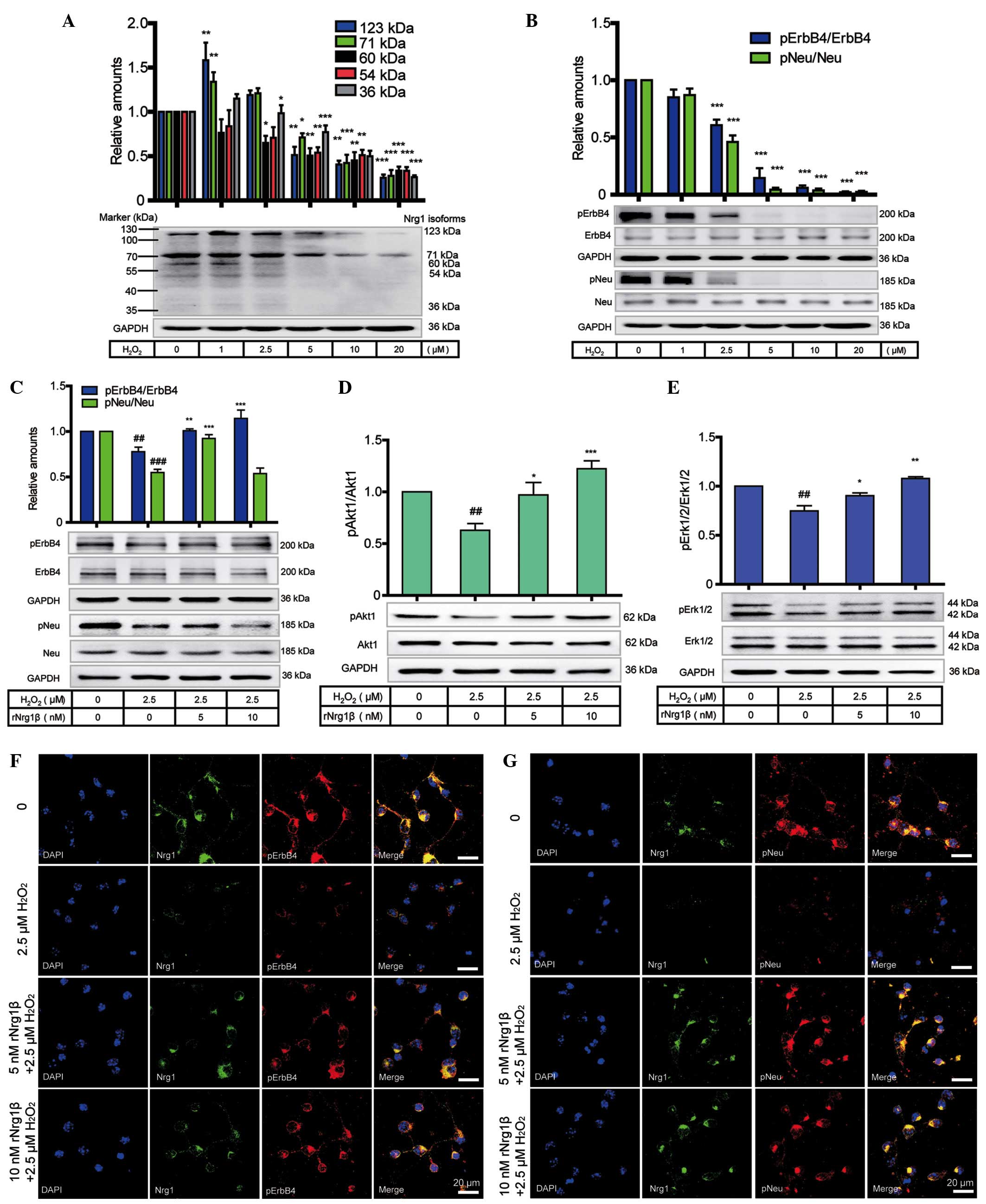 | Figure 4Protective role of rNrg1β in primary
mouse cortical neurons in response to oxidative stress and neuraxon
damage. (A) Protein levels of Nrg1 isoforms and (B) phosphorylation
levels of Neu and ErbB4 receptors in primary cortical neurons after
a 24 h treatment with 0-20 μM H2O2
(n=6, one-way ANOVA with Tukey's post-hoc test; data are expressed
as the mean ± SEM). *P<0.05, **P<0.01
and ***P<0.001 compared with the control group. (C)
The phosphorylation levels of Neu and ErbB4, and (D) the pAkt1
levels and (E) the pErk1/2 levels in primary cortical neurons
pretreated with rNrg1β at a concentration of 5 or 10 nM for 2 h
prior to a 24-h treatment with 2.5 μM oligomeric
H2O2 (n=5, one-way ANOVA with Tukey's
post-hoc test; data are expressed as the mean ± SEM).
##P<0.01 and ###P<0.001 vs. the vehicle
control and *P<0.05, **P<0.01 and
***P<0.001 vs. the H2O2-treated
group. Double immunofluorescence staining of Nrg1 with either (F)
pErbB4 or (G) pNeu in the cortical neurons pretreated with rNrg1β
at a concentration of 5 or 10 nM for 2 h prior to a 24 h treatment
with 2.5 μM oligomeric H2O2. Scale
bars=20 μm. ANOVA, analysis of variance; SEM, standard error
of the mean; H2O2, hydrogen peroxide; rNRG1β,
recombinant neuregulin 1β, pErbB4, phosphorylated ErbB4; pNeu,
phosphorylated Neu; pErk1/2, phosphorylated extracellular-regulated
signal kinase 1/2; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
Furthermore, in order to explore the role of rNrg1β
in alleviating oxidative stress and axonal damage, western blotting
was conducted to evaluate whether the Nrg1-ErbB signaling pathway
was involved in the preventive mechanism on exposure to
H2O2. Based on the data from a previous study
(Chen et al, unpublished) a concentration of 2.5 μM
was adopted as the optimal concentration of
H2O2 for treatment of the cortical neurons
following pretreatment with 0, 5 or 10 nM rNrg1β for 2 h. It was
revealed that the levels of pErbB4 and pNeu were markedly decreased
following treatment with H2O2, and this
effect was reversed on addition of rNrg1β, to a maximal extent at
10 nM for pErbB4 and at 5 nM for pNeu (Fig. 4C). The levels of Akt1 and Erk1/2
activation exhibited a similar trend to that of pErbB4 (Fig. 4D and E).
Double immunofluorescence staining was subsequently
used to further confirm these observations. It was revealed that,
compared with non-stressed neurons,
H2O2-treated neurons exhibited diminished
levels of neurite outgrowth, with the detection of reduced levels
of Nrg1 and of pNeu or pErbB4. In contrast, pretreatment with 5 and
10 nM rNrg1β prior to H2O2 exposure was able
to partially reverse these effects by increasing the levels of both
pNeu and pErbB4, with more clearly recognizable effects observed at
a concentration of 10 nM (Fig. 4F and
G).
The preventive role of rNrg1β in
counteracting the effects of Aβ1–42 on mouse cortical
neurons
The protective role of rNrg1β in mouse cortical
neurons treated with Aβ1–42 was subsequently
investigated. Western blotting was utilized to evaluate the
influence of rNrg1β pretreatment on the phosphorylation levels of
Neu and ErbB4 and on the downstream signaling pathways in primary
cortical neurons following a 24 h incubation with 10 μM
oligomeric Aβ1–42. Administration of 10 μM
oligomeric Aβ1–42 significantly downregulated the protein levels of
several Nrg1 isoforms, including the 60 kDa and 36 kDa Nrg1,
whereas pretreatment with 5 nM rNrg1β counteracted the effects of
Aβ1–42 by increasing the Nrg1 isoforms. By contrast,
pretreatment with 10 nM rNrg1β showed no apparent effects on the
function of Aβ1–42 (Fig.
5A). It was also demonstrated that the relative levels of
pErbB4 and pNeu were markedly decreased following treatment with
Aβ1–42, and this effect on pErbB4 was compensated for by
pretreatment with 10 nM rNrg1β and on pNeu by pretreatment with 5
nM rNrg1β (Fig. 5B). In addition,
pAkt1 levels were increased, and pErk levels were decreased when
treated with Aβ1–42 alone (Fig. 5C and D). However, rNrg1β
pretreatment upregulated the levels of pAkt1 and pErk in
Aβ1–42-challenged cortical neurons (Fig. 5C and D).
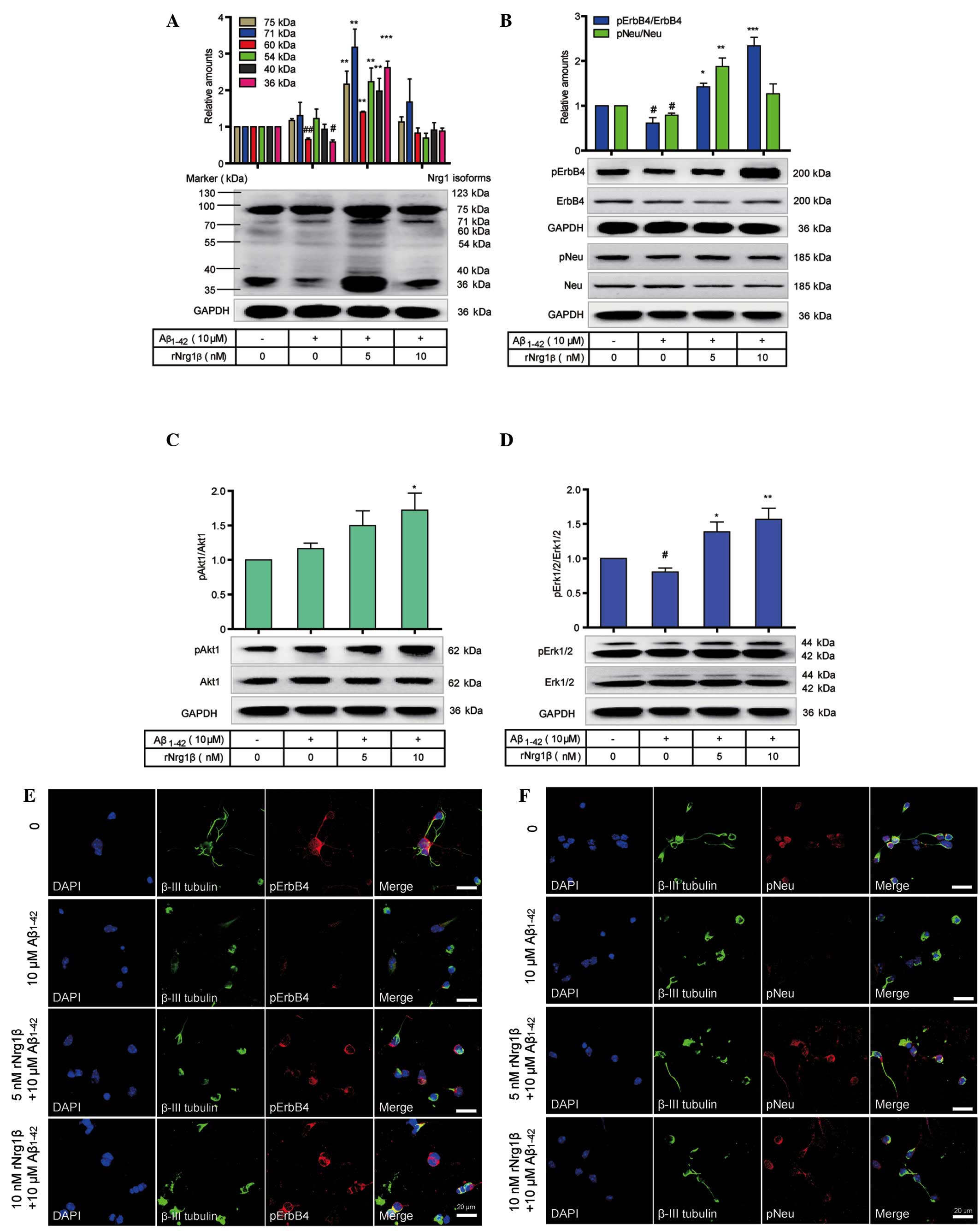 | Figure 5Effects of rNrg1β pretreatment on the
Nrg1 signaling in primary mouse cortical neurons exposed to
Aβ1–42. The (A) relative levels of the Nrg1 isoforms,
(B) phosphorylation levels of Neu and ErbB4, (C) pAkt1 levels and
(D) pErk1/2 levels in primary cortical neurons pretreated with
rNrg1β at a concentration of 5 or 10 nM for 2 h prior to a 24-h
treatment with 10 μM oligomeric Aβ1–42 are shown
(n=5, one-way analysis of variance with Tukey's post-hoc test; data
are expressed as the mean ± standard error of the mean). Double
immunofluorescence staining of β-III tubulin with either (E) pErbB4
or (F) pNeu in the cortical neurons pretreated with rNrg1β at a
concentration of 5 or 10 nM for 2 h prior to a 24 h treatment with
10 μM oligomeric Aβ1–42. Scale bars=20 μm.
rNRG1β, recombinant neuregulin 1β, pErbB4, phosphorylated ErbB4;
pNeu, phosphorylated Neu; pErk1/2, phosphorylated
extracellular-regulated signal kinase 1/2; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; Aβ, amyloid-beta; DAPI,
4′,6-diamidino-2-phenylindole. #P<0.05 and
##P<0.01 vs. vehicle control and
*P<0.05, **P<0.01 and
***P<0.001 compared with the control group. |
We subsequently applied double immunofluorescence
staining to further confirm these observations. It was observed
that, compared with non-stressed neurons, Aβ1–42-treated
neurons demonstrated a diminished neurite outgrowth, with reduced
levels of pNeu or pErbB4 detected. In contrast, pretreatment with 5
or 10 nM rNrg1β prior to Aβ1–42 exposure was able to
partially reverse these effects by increasing the levels of both
pNeu and pErbB4, with the most marked effects being observed with
10 nM rNrg1β for pErbB4 and 5 nM rNrg1β for pNeu (Fig. 5E and F).
Discussion
It is widely acknowledged that the Nrg1-ErbB
signaling pathway exerts a crucial role in multiple biological
processes, including cell differentiation, organ development and
tumorigenesis. Receptors for Nrg1 signaling undergo phosphorylation
of their cytoplasmic tyrosine residues, which elicits downstream
effects and biological responses (36). The binding of the ligand results in
the dimerization and activation of ErbB receptors. Phosphorylation
of the intracellular domains creates docking sites for adaptor
proteins, including growth factor receptor-bound protein 2 (Grb2)
and Shc for the activation of the Erk pathway, and p85 for the
activation of the phosphoinositide 3-kinase pathway (37). A previous study revealed an
association between Nrg1 and ErbB4 immunoreactivity and the
formation of neuritic plaques in patients with AD in a transgenic
animal model of AD (35). In the
present study, a linear correlation was observed between Nrg1 and
the phosphorylation of Neu and ErbB4 receptors in a normal human
cortical tissue microarray. To elucidate the exact mechanism by
which Nrg1 contributes to AD development, two cell models were
applied. Based on our results using cortical neurons under the
pathological conditions of AD, multiple isoforms of Nrg1 were
altered, including the 123-, 95-, 75-, 71-, 60-, 54-, 40- and
36-kDa proteins. These bands represent alternatively spliced
products of the NRG1 gene, post-translationally modified
forms of the proteins, and/or a shedding of the ectodomains from
the initial precursors. All isoforms of Nrg1 contain an
epidermal growth factor (EGF)-like signaling domain that is
required for activation of the receptors (38). In addition, the interaction of Nrg1
with its receptors was shown to be associated with the activation
of intracellular signaling pathways that are associated with the
development and regeneration of the nervous system (29,30).
In the present study, it has been demonstrated that the changes in
Nrg1 isoform expression and receptor phosphorylation are highly
influenced by the pathological conditions observed in AD. Thus,
expression changes in Nrg1 isoforms appear to be associated with
the pathological development of AD, suggesting that Nrg1 may be a
critical molecule in the development of AD.
Oxidative stress plays an essential role in the
onset and development of AD (39,40),
and cellular oxidative stress levels are increased in vulnerable
regions of the AD brain (41,42).
The brain is particularly sensitive to oxidative stress due to
special cellular features, including a large dependence on
oxidative phosphorylation for energy production, low antioxidant
concentrations, low levels of membrane lipids and high levels of
iron, which are associated with free radical injury (43–45).
Previous studies reported that Nrg1 was up-regulated following
nerve injury, and it served as an essential agent to protect the
neurons from ischemic damage (34,46,47).
In addition, Nrg proteins attenuated the release of free radicals
and protected neuronal cells from
H2O2-induced apoptosis (48,49).
In the present study, the protein levels of multiple Nrg1 isoforms
and the phosphorylation of their receptors were observed to
increase in a time-dependent manner. Changes in Nrg1 signaling in
cortical neurons exposed to oxidative stress were further
investigated. It was observed that protein levels of Nrg1, and the
phosphorylation of its receptors, were down-regulated in response
to high concentrations of H2O2. Although the
Nrg1 protein level was up-regulated at low concentrations of
H2O2 compared with the control, no
up-regulation of receptor phosphorylation was identified. This
suggested that the interactions between Nrg1 and the ErbB receptors
were perturbed under conditions of oxidative stress. By contrast,
exogenous rNrg1β was able to protect the cortical neurons from
oxidative stress and neuraxonal damage via the up-regulation of the
Nrg1-ErbB cell signaling pathway. Cui et al (50) proposed that endogenous Nrg1 was
increased in response to the production of Aβ to protect the
neurons against damage. However, the injured neurons were not
capable of expressing sufficient Nrg1β1 to adapt to prolonged
damage, which ultimately led to apoptosis. Increased oxidative
stress occurs in response to increased Aβ levels (51); therefore, in the present study, it
was originally hypothesized that the up-regulation of endogenous
Nrg1 in primary cortical neurons exposed to
H2O2 may be an initial, local protective
response against abnormal cell signaling. However, the cortical
neurons were unable to express sufficient Nrg1 over time,
eventually resulting in the dysfunction of Nrg1 signaling. These
results suggest the existence of an intrinsic self-protective
mechanism in which injured cortical neurons may adapt to, and
automatically counteract, neuronal injury.
Aβ is associated with the generation of reactive
oxygen species, which cause cell damage, apoptosis, mitochondrial
dysfunction and the peroxidation of membrane lipids (52,53).
In addition, the accumulation of Aβ peptides has been identified as
a key step in the multiple pathogenic changes associated with
neurodegeneration and dementia (54,55).
Previous studies demonstrated that the neurotoxicity induced by
Aβ1–42 may lead to apoptotic cell death (56), and that Aβ is able to disrupt
signaling pathways, including those involving Erk1/2 and Akt in the
primary rat cortical neurons (57,58).
In the present study, it was observed that exposure of primary
cortical neurons to Aβ1–42 caused an up-regulation in
the level of Nrg1 protein and in Akt1 phosphorylation, and a
down-regulation of Neu/ErbB4 phosphorylation and pErk1/2 levels.
In vitro studies have demonstrated that Nrg1β treatment may
protect neuronal cells (59–62).
In the present study, Aβ1–42 treatment increased the
levels of Nrg1 and Akt1 phosphorylation, and decreased the
phosphorylated levels of ErbB4, Neu and Erk1/2. Moreover, the
Aβ1–42-induced reduction in the levels of pErbB4, pNeu,
pAkt1 and pErk1/2 was antagonized by rNrg1β pretreatment.
Recombinant human Nrg1 contains an EGF-like domain that is
essential for the phosphorylation-dependent activation of Neu/ErbB4
receptors (63). Nrg1 is able to
signal to target cells via interactions with transmembrane tyrosine
kinase receptors of the ErbB family. The interaction of Nrg1 with
ErbB receptors may result in the dimerization of receptors,
tyrosine phosphorylation, and activation of intracellular signaling
pathways (59,64). Activation of ErbB4 by Nrg1 may
induce a marked increase in ErbB4 phosphorylation (65) and lead to a sustained activation of
Akt and Erk (66). In addition,
the Akt and Erk1/2 signaling cascades have an essential role in
regulating gene expression and in preventing apoptosis (67). A wide spectrum of in vivo
and in vitro studies have demonstrated that phosphorylation
of Erk facilitates cell survival (68), and that the dephosphorylation of
Akt is involved in the development of AD (58,69,70).
These results indicated that Nrg1 signaling in mouse cortical
neurons is altered in response to the accumulation of Aβ,
suggesting that Nrg1 may function as a crucial candidate for the
prevention and treatment of AD.
In view of these observations, it was our hypothesis
that, although Nrg1 is up-regulated in cortical neurons during the
early stages of AD to protect against abnormal changes in cell
signaling, the phosphorylation levels of its receptors are
relatively less responsive due to some unknown interrupting
factors. As a consequence, Nrg1 signaling is not able to function
properly when ErbB4 is not adequately activated. In addition,
sufficient levels of Nrg1 are not expressed when the damage is
prolonged, thus failing to prevent the development and progression
of AD. Notably, the present study revealed that pretreatment of
neural cells with rNrg1β partially reversed the neurotoxicity of
Aβ1–42. These findings have provided a foundational
basis for Nrg1 signaling as a potential therapeutic target for the
prevention, and possibly the treatment, of AD.
Acknowledgments
We would like to thank the National Natural Science
Foundation of China (grant nos. 81171138 and 81471279 to W-J. Z.)
for support. This work was also supported by a Talent Support Grant
from Shantou University Medical College (grant no. 250122 0118 to
W-J. Z.).
References
|
1
|
Alzheimer's Association: 2013 Alzheimer's
disease facts and figures. Alzheimers Dement. 9:208–245. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Golebiowski M, Barcikowska M and Pfeffer
A: Magnetic resonance imaging-based hippocampal volumetry in
patients with dementia of the Alzheimer type. Dement Geriatr Cogn
Disord. 10:284–288. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heun R, Mazanek M, Atzor KR, Tintera J,
Gawehn J, Burkart M, Gänsicke M, Falkai P and Stoeter P:
Amygdala-hippocampal atrophy and memory performance in dementia of
Alzheimer type. Dement Geriatr Cogn Disord. 8:329–336. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fox NC, Warrington EK, Freeborough PA,
Hartikainen P, Kennedy AM, Stevens JM and Rossor MN: Presymptomatic
hippocampal atrophy in Alzheimer's disease. A longitudinal MRI
study. Brain. 119:2001–2007. 1996. View Article : Google Scholar
|
|
5
|
Querfurth HW and LaFerla FM: Alzheimer's
disease. N Engl J Med. 362:329–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
D'Aniello A, Fisher G, Migliaccio N,
Cammisa G, D'Aniello E and Spinelli P: Amino acids and
transaminases activity in ventricular CSF and in brain of normal
and Alzheimer patients. Neurosci Lett. 388:49–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Selkoe DJ: Alzheimer's disease results
from the cerebral accumulation and cytotoxicity of amyloid
beta-protein. J Alzheimers Dis. 3:75–80. 2001.
|
|
8
|
Culmsee C and Landshamer S: Molecular
insights into mechanisms of the cell death program: Role in the
progression of neurodegenerative disorders. Curr Alzheimer Res.
3:269–283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Honda K, Casadesus G, Petersen RB, Perry G
and Smith MA: Oxidative stress and redox-active iron in Alzheimer's
disease. Ann N Y Acad Sci. 1012:179–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jenner P: Oxidative stress in Parkinson's
disease. Ann Neurol. 53(Suppl 3): S26–S36; discussion S36–S38.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su JH, Anderson AJ, Cummings BJ and Cotman
CW: Immunohistochemical evidence for apoptosis in Alzheimer's
disease. Neuroreport. 5:2529–2533. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yagami T, Ueda K, Asakura K, Sakaeda T,
Nakazato H, Kuroda T, Hata S, Sakaguchi G, Itoh N, Nakano T, et al:
Gas6 rescues cortical neurons from amyloid beta protein-induced
apoptosis. Neuropharmacology. 43:1289–1296. 2002. View Article : Google Scholar
|
|
13
|
Wang DM, Li SQ, Zhu XY, Wang Y, Wu WL and
Zhang XJ: Protective effects of hesperidin against amyloid-β (Aβ)
induced neurotoxicity through the voltage dependent anion channel 1
(VDAC1)-mediated mitochondrial apoptotic pathway in PC12 cells.
Neurochem Res. 38:1034–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schenk D, Barbour R, Dunn W, Gordon G,
Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al:
Immunization with amyloid-beta attenuates Alzheimer-disease-like
pathology in the PDAPP mouse. Nature. 400:173–177. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shao BY, Xia Z, Xie Q, Ge XX, Zhang WW,
Sun J, Jiang P, Wang H, Le WD, Qiu ZB, et al: Meserine, a novel
carbamate AChE inhibitor, ameliorates scopolamine-induced dementia
and alleviates amyloidogenesis of APP/PS1 transgenic mice. CNS
Neurosci Ther. 20:165–171. 2014. View Article : Google Scholar
|
|
16
|
Liu MY, Wang S, Yao WF, Zhang ZJ, Zhong X,
Sha L, He M, Zheng ZH and Wei MJ: Memantine improves spatial
learning and memory impairments by regulating NGF signaling in
APP/PS1 transgenic mice. Neuroscience. 273:141–151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sadowski M and Wisniewski T: Disease
modifying approaches for Alzheimer's pathology. Curr Pharm Des.
13:1943–1954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ritch PS, Carroll SL and Sontheimer H:
Neuregulin-1 enhances survival of human astrocytic glioma cells.
Glia. 51:217–228. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mei L and Xiong WC: Neuregulin 1 in neural
development, synaptic plasticity and schizophrenia. Nat Rev
Neurosci. 9:437–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao WJ and Schachner M: Neuregulin 1
enhances cell adhesion molecule l1 expression in human glioma cells
and promotes their migration as a function of malignancy. J
Neuropathol Exp Neurol. 72:244–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao WJ: The expression and localization
of neuregulin-1 (Nrg1) in the gastrointestinal system of the rhesus
monkey. Folia Histochem Cytobiol. 51:38–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao W and Ren SG: Neuregulin-1 (Nrg1) is
mainly expressed in rat pituitary gonadotroph cells and possibly
regulates prolactin (PRL) secretion in a juxtacrine manner. J
Neuroendocrinol. 23:1252–1262. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao W, Shen Y and Ren S: Endogenous
expression of Neuregulin-1 (Nrg1) as a potential modulator of
prolactin (PRL) secretion in GH3 cells. Cell Tissue Res.
344:313–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao WJ, Jiang Q and Mei JP:
Neurohypophyseal Neuregulin 1 is derived from the hypothalamus as a
potential prolactin modulator. Neuroendocrinology. 102:288–299.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson TR, Lee DY, Berry L, Shames DS and
Settleman J: Neuregulin-1-mediated autocrine signaling underlies
sensitivity to HER2 kinase inhibitors in a subset of human cancers.
Cancer Cell. 20:158–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao WJ and Ren SG: Endogenous
neuregulin-1 expression in the anterior pituitary of female
Wistar-Furth rats during the estrous cycle. Nan Fang Yi Ke Da Xue
Xue Bao. 31:921–927. 2011.In Chinese. PubMed/NCBI
|
|
27
|
Liu J and Kern JA: Neuregulin-1 activates
the JAK-STAT pathway and regulates lung epithelial cell
proliferation. Am J Respir Cell Mol Biol. 27:306–313. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Puricelli L, Proietti CJ, Labriola L,
Salatino M, Balañá ME, Aguirre Ghiso J, Lupu R, Pignataro OP,
Charreau EH, Bal de Kier Joffé E and Elizalde PV: Heregulin
inhibits proliferation via ERKs and phosphatidyl-inositol 3-kinase
activation but regulates urokinase plasminogen activator
independently of these pathways in metastatic mammary tumor cells.
Int J Cancer. 100:642–653. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Falls DL: Neuregulins: Functions, forms,
and signaling strategies. Exp Cell Res. 284:14–30. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stefansson H, Sigurdsson E,
Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S,
Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al:
Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet.
71:877–892. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Britsch S: The neuregulin-I/ErbB signaling
system in development and disease. Adv Anat Embryol Cell Biol.
190:1–65. 2007.PubMed/NCBI
|
|
32
|
Xu Z, Croslan DR, Harris AE, Ford GD and
Ford BD: Extended therapeutic window and functional recovery after
intraarterial administration of neuregulin-1 after focal ischemic
stroke. J Cereb Blood Flow Metab. 26:527–535. 2006. View Article : Google Scholar
|
|
33
|
Carlsson T, Schindler FR, Höllerhage M,
Depboylu C, Arias-Carrión O, Schnurrbusch S, Rösler TW, Wozny W,
Schwall GP, Groebe K, et al: Systemic administration of
neuregulin-1β1 protects dopaminergic neurons in a mouse model of
Parkinson's disease. J Neurochem. 117:1066–1074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tokita Y, Keino H, Matsui F, Aono S,
Ishiguro H, Higashiyama S and Oohira A: Regulation of neuregulin
expression in the injured rat brain and cultured astrocytes. J
Neurosci. 21:1257–1264. 2001.PubMed/NCBI
|
|
35
|
Chaudhury AR, Gerecke KM, Wyss JM, Morgan
DG, Gordon MN and Carroll SL: Neuregulin-1 and erbB4
immunoreactivity is associated with neuritic plaques in Alzheimer
disease brain and in a transgenic model of Alzheimer disease. J
Neuropathol Exp Neurol. 62:42–54. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yarden Y and Sliwkowski MX: Untangling the
ErbB signalling network. Nat Rev Mol Cell Biol. 2:127–137. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mei L and Nave KA: Neuregulin-ERBB
signaling in the nervous system and neuropsychiatric diseases.
Neuron. 83:27–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kanzaki H, Mizobuchi S, Obata N, Itano Y,
Kaku R, Tomotsuka N, Nakajima H, Ouchida M, Nakatsuka H, Maeshima K
and Morita K: Expression changes of the neuregulin 1 isoforms in
neuropathic pain model rats. Neurosci Lett. 508:78–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maiese K, Li F and Chong ZZ:
Erythropoietin in the brain: Can the promise to protect be
fulfilled? Trends Pharmacol Sci. 25:577–583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mattson MP: Pathways towards and away from
Alzheimer's disease. Nature. 430:631–639. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chong ZZ, Li F and Maiese K: Oxidative
stress in the brain: Novel cellular targets that govern survival
during neurodegenerative disease. Prog Neurobiol. 75:207–246. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mattson MP: Oxidative stress, perturbed
calcium homeostasis, and immune dysfunction in Alzheimer's disease.
J Neurovirol. 8:539–550. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Facecchia K, Fochesato LA, Ray SD, Stohs
SJ and Pandey S: Oxidative toxicity in neurodegenerative diseases:
Role of mitochondrial dysfunction and therapeutic strategies. J
Toxicol. 2011:6837282011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Viegas CM, Tonin AM, Zanatta A, Seminotti
B, Busanello EN, Fernandes CG, Moura AP, Leipnitz G and Wajner M:
Impairment of brain redox homeostasis caused by the major
metabolites accumulating in
hyperornithinemia-hyperammonemia-homocitrullinuria syndrome in
vivo. Metab Brain Dis. 27:521–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Herbert V, Shaw S, Jayatilleke E and
Stopler-Kasdan T: Most free-radical injury is iron-related: It is
promoted by iron, hemin, holoferritin and vitamin C, and inhibited
by desferoxamine and apoferritin. Stem Cells. 12:289–303. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Guo WP, Fu XG, Jiang SM and Wu JZ:
Neuregulin-1 regulates the expression of Akt, Bcl-2, and Bad
signaling after focal cerebral ischemia in rats. Biochem Cell Biol.
88:649–654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist
J, Loeb JA and Bennett DL: Neuregulin-ErbB signaling promotes
microglial proliferation and chemotaxis contributing to
microgliosis and pain after peripheral nerve injury. J Neurosci.
30:5437–5450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Erlich S, Goldshmit Y, Lupowitz Z and
Pinkas-Kramarski R: ErbB-4 activation inhibits apoptosis in PC12
cells. Neuroscience. 107:353–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dimayuga FO, Ding Q, Keller JN, Marchionni
MA, Seroogy KB and Bruce-Keller AJ: The neuregulin GGF2 attenuates
free radical release from activated microglial cells. J
Neuroimmunol. 136:67–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cui W, Tao J, Wang Z, Ren M, Zhang Y, Sun
Y, Peng Y and Li R: Neuregulin1beta1 antagonizes apoptosis via
ErbB4-dependent activation of PI3-kinase/Akt in APP/PS1 transgenic
mice. Neurochem Res. 38:2237–2246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu W, Bonnet M, Farso M, Ma K, Chabot JG,
Martin E, Torriglia A, Guan Z, McLaurin J, Quirion R and Krantic S:
The expression of apoptosis inducing factor (AIF) is associated
with aging-related cell death in the cortex but not in the
hippocampus in the TgCRND8 mouse model of Alzheimer's disease. BMC
Neurosci. 15:732014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cecchi C, Fiorillo C, Baglioni S,
Pensalfini A, Bagnoli S, Nacmias B, Sorbi S, Nosi D, Relini A and
Liguri G: Increased susceptibility to amyloid toxicity in familial
Alzheimer's fibroblasts. Neurobiol Aging. 28:863–876. 2007.
View Article : Google Scholar
|
|
53
|
Miranda S, Opazo C, Larrondo LF, Muñoz FJ,
Ruiz F, Leighton F and Inestrosa NC: The role of oxidative stress
in the toxicity induced by amyloid beta-peptide in Alzheimer's
disease. Prog Neurobiol. 62:633–648. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hardy JA and Higgins GA: Alzheimer's
disease: The amyloid cascade hypothesis. Science. 256:184–185.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Karran E, Mercken M and De Strooper B: The
amyloid cascade hypothesis for Alzheimer's disease: An appraisal
for the development of therapeutics. Nat Rev Drug Discov.
10:698–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Thangnipon W, Puangmalai N,
Chinchalongporn V, Jantrachotechatchawan C, Kitiyanant N,
Soi-Ampornkul R, Tuchinda P and Nobsathian S: N-benzylcinnamide
protects rat cultured cortical neurons from β-amyloid
peptide-induced neurotoxicity. Neurosci Lett. 556:20–25. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen TJ, Wang DC and Chen SS: Amyloid-beta
interrupts the PI3K-Akt-mTOR signaling pathway that could be
involved in brain-derived neurotrophic factor-induced Arc
expression in rat cortical neurons. J Neurosci Res. 87:2297–2307.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tong L, Balazs R, Thornton PL and Cotman
CW: Beta-amyloid peptide at sublethal concentrations downregulates
brain-derived neurotrophic factor functions in cultured cortical
neurons. J Neurosci. 24:6799–6809. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Parkinson DB, Langner K, Namini SS, Jessen
KR and Mirsky R: beta-Neuregulin and autocrine mediated survival of
Schwann cells requires activity of Ets family transcription
factors. Mol Cell Neurosci. 20:154–167. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu Z, Jiang J, Ford G and Ford BD:
Neuregulin-1 is neuroprotective and attenuates inflammatory
responses induced by ischemic stroke. Biochem Biophys Res Commun.
322:440–446. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xu Z, Ford GD, Croslan DR, Jiang J, Gates
A, Allen R and Ford BD: Neuroprotection by neuregulin-1 following
focal stroke is associated with the attenuation of ischemia-induced
pro-inflammatory and stress gene expression. Neurobiol Dis.
19:461–470. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu Y, Yu Y, Schachner M and Zhao W:
Neuregulin 1-β regulates cell adhesion molecule L1 expression in
the cortex and hippocampus of mice. Biochem Biophys Res Commun.
441:7–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
An T, Zhang Y, Huang Y, Zhang R, Yin S,
Guo X, Wang Y, Zou C, Wei B, Lv R, et al: Neuregulin-1 protects
against doxorubicin-induced apoptosis in cardiomyocytes through an
Akt-dependent pathway. Physiol Res. 62:379–385. 2013.PubMed/NCBI
|
|
64
|
Burden S and Yarden Y: Neuregulins and
their receptors: A versatile signaling module in organogenesis and
oncogenesis. Neuron. 18:847–855. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Vaskovsky A, Lupowitz Z, Erlich S and
Pinkas-Kramarski R: ErbB-4 activation promotes neurite outgrowth in
PC12 cells. J Neurochem. 74:979–987. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Di Segni A, Farin K and Pinkas-Kramarski
R: ErbB4 activation inhibits MPP+-induced cell death in
PC12-ErbB4 cells: Involvement of PI3K and Erk signaling. J Mol
Neurosci. 29:257–267. 2006. View Article : Google Scholar
|
|
67
|
McCubrey JA, Steelman LS, Abrams SL, Lee
JT, Chang F, Bertrand FE, Navolanic PM, Terrian DM, Franklin RA,
D'Assoro AB, et al: Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT
pathways in malignant transformation and drug resistance. Adv
Enzyme Regul. 46:249–279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Junttila MR, Li SP and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar
|
|
69
|
Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL,
Lo SC, Bru-Mercier G, Dickinson B, Scullion S, Sheng M,
Collingridge G and Cho K: Aβ(1-42) inhibition of LTP is mediated by
a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat
Neurosci. 14:545–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ma T, Du X, Pick JE, Sui G, Brownlee M and
Klann E: Glucagon-like peptide-1 cleavage product GLP-1(9-36) amide
rescues synaptic plasticity and memory deficits in Alzheimer's
disease model mice. J Neurosci. 32:13701–13708. 2012. View Article : Google Scholar : PubMed/NCBI
|