Introduction
A graying society is an indispensable and
significant link during humthean indispensable (1). A graying society is a population
structure in which the aging population has reached or exceeded a
certain proportion (1). According
to the traditional standard of the United Nations, a region where
the population of individuals aged >60 years old is 10% of the
total population can be regarded as entering into a graying society
(2). However, in the current
standard, it refers to a population of individuals, in which those
aged >65 years old represent 7% of the total population
(3). In 2002, China became a
graying society. As age increases, the elderly are more susceptible
to various types of diseases due to their unique physiological
characteristics, which introduce severe challenges for medical
treatment and the social insurance industry (4). It is important to seriously consider
these problems, and there is interest in postoperative cognitive
dysfunction (POCD), specifically in gerontal patients (5).
Isoflurane has lasting and severe effects on
cognitive function. In particular, it can affect learning and
memory function in sea horses (4,6).
Isoflurane is a novel inhalation anesthetic, which is frequently
used clinically (7). It can
inhibit cholinergic neurons from transmitting signals and induces
anesthetic effects via the N-methyl-D aspartic acid receptor, which
affect learning and memory function (7,8).
Ginseng is a type of traditional Chinese medicine,
which is widely used for the promotion of health and treatment of
diseases (9). Ginseng, marten and
cartialgenous are also collectively known as ̔the three treasures̓
in northeast China. There are several ginsenosides, including Ro,
Ra, Ral-2, Rbl-3, Rc, Rd, Re, Rf and Rg1 (10). Of the ginseng glycosides,
ginsenoside Rgl has anti-oxidative and anti-apoptotic functions and
other neuroprotective effects (10). Previous experiments have indicated
that ginsenoside Rg1 can improve, consolidate and recover memory in
cognitive impairment caused by quinolinic acid, Pb, dexamethasone,
anisodine, ethylene and ethyl alcohol (11,12).
In addition, it can improve impairment of learning and memory
function caused by scopolamine and morphine (13). The aims of the present study were
to detect whether the neuroprotective effect of ginsenoside Rg1
prevents cognitive impairment induced by isoflurane anesthesia via
antioxidant, anti-inflammatory and anti-apoptotic effects, and
whether these are mediated by the phosphoinositide 3-kinase
(PI3K)/AKT/glycogen synthase kinase-3β (GSK-3β) pathway in aged
rats.
Materials and methods
Reagents
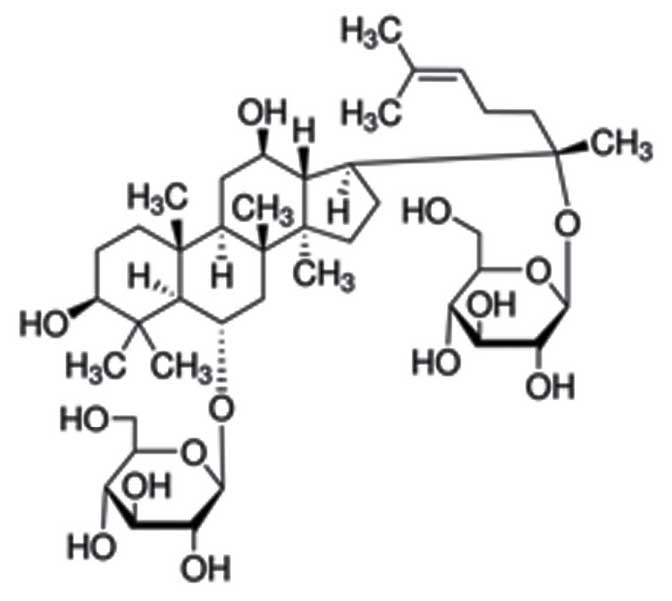
Ginsenoside Rg1 (≥90% in high performance liquid
chromatography) was purchased from Sigma-Aldrich (St. Louis, MO,
USA; its chemical structure is shown in Fig. 1. Isoflurane, the interleukin
(IL)-1β kit (cat. no. H002), IL-6 kit (cat. no. H007),
malondialdehyde (MDA) kit (cat. no. A003-1), caspase-3 kit (cat.
no. H076) and glutathione (GSH) kit (cat. no. A006-2) were
purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China). The bicinchoninic acid assay (BCA) assay was purchased from
Wuhan Boster Bioengineering Co., Ltd. (Wuhan, China).
Animals and ethical statement
Male, 3-month-old Sprague-Dawley (SD) rats were
purchased from the Medical and Laboratory Animal Center of Beijing
Jishuitan Hospital (Beijing, China) and housed at a temperature
(23±1°C) in a light-controlled room (12-h light/dark cycle), with a
relative humidity of 50–70% and free access to water and food. All
SD rats were treated, according to the guidelines of the Guide for
the Care and Use of Laboratory Animals of Beijing Jishuitan
Hospital and the study was approved by the ethics committee of
Beijing Jishuitan Hospital.
Exposure to anesthesia and grouping
The rats were randomly divided into three groups:
Control, isoflurane and ginsenoside Rg1 (n=10 in each group). The
SD rats in the ginsenoside Rg1 group were treated with 20 mg/kg
ginsenoside Rg1 for 7 days (14)
by intraperitoneal injection. After 12 h, the rats in the
isoflurane and ginsenoside Rg1 groups were exposed to 1.3%
isoflurane (Nanjing Jiancheng Bioengineering Institute) in a
humidified 30% oxygen carrier gas for 4 h. The SD rats in the
control group were exposed to humidified 30% oxygen without
isoflurane for 4 h.
Morris water maze performance
A round pool, with a diameter of 150 cm and depth of
50 cm, was filled with 24°C opaque water to the top of a movable,
clear 15-cm-diameter platform (1.5 cm above water). Motion
detection software (Actimetrics software, Evanston, IL, USA;
version ACT-556) was used to record the swimming motions and
analyze the results. Each rat was wiped dry and kept warm, and
returned to their cage with free access to food. Following
isoflurane exposure, trials were performed for 4 days, and the
spatial information of every rat was analyzed. The pool had a dark
black surround, which was used to prevent confounding visual cues.
All rats were placed in a fixed position in the swimming pool,
facing the wall, on the 4 days. The rats were allowed to find the
platform (in the third quadrant) for 20 sec, prior to being removed
from the pool. The swim speed and the time of escape latency were
recorded. Less time taken for a rat to reach the platform indicated
a higher learning ability.
Detection of levels of MDA, GSH, IL-1β,
IL-6 and caspase-3 using ELISA
Following isoflurane exposure, the rats in each
group were sacrificed and the hippocampi were dissected for
measurement of the protein levels of IL-1β, IL-6, MDA, GSH and
caspase-3 using ELISA kits (Nanjing Jiancheng Bioengineering
Institute).
Western blot analysis of the protein
expression of AKT, GSK-3β, p21WAF1/CIP1 and p53
Hippocampal tissues were collected and were
homogenized with phenylmethanesulfonyl fluoride containing
radioimmunoprecipitation assay buffer and protease inhibitor
cocktail (EDTA-free) on ice. The supernatant were collected and
centrifuged at 13,000 × g at 4°C for 30 min, following which the
BCA method (Wuhan Boster Bioengineering, Wuhan, China) was used to
analyze protein concentrations. The protein (50 mg) was separated
by SDS-PAGE and transferred onto polyvinylidene fluoride membranes
(Wuhan Boster Biological Technology, Wuhan, China). The membranes
were blocked using 5% skim milk powder and incubated overnight at
4°C with anti-AKT (1:500; cat. no. sc-5298; Santa Cruz
Biotechnology, Inc., Dallax, TX, USA), anti-phosphorylated (p)-AKT
(1:500; cat. no. sc-293125; Santa Cruz Biotechnology, Inc.),
anti-GSK-3β (1:2,000; cat. no. 9337; Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-p21WAF1/CIP1 (1:2,000; cat. no. 2947;
Cell Signaling Technology, Inc.), anti-p53 (1:2,000; cat. no. 2524;
Cell Signaling Technology, Inc.) and anti-β-actin antibodies
(1:500; cat. no. BM0626; Wuhan Boster Biological Technology). The
membranes were then incubated with anti-mouse secondary antibody
(1:5,000; cat. no. BM2002; Wuhan Boster Biological Technology) at
37°C for 1 h and visualized using an enhanced chemiluminescence
detection system (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and expression was quantified using a gel inage analysis system
(Thermo Fisher Scientific, Inc.).
Statistical analysis
All data are presented as the mean ± standard error
of the mean, and analysis was performed using SPSS 17.0 (SPSS,
Inc., Chicago, IL, USA). One-way analysis of variance was used for
comparison of mean values across the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Neuroprotective effects of ginsenoside
Rg1 on cognitive function in isoflurane anesthesia-exposed aged
rats
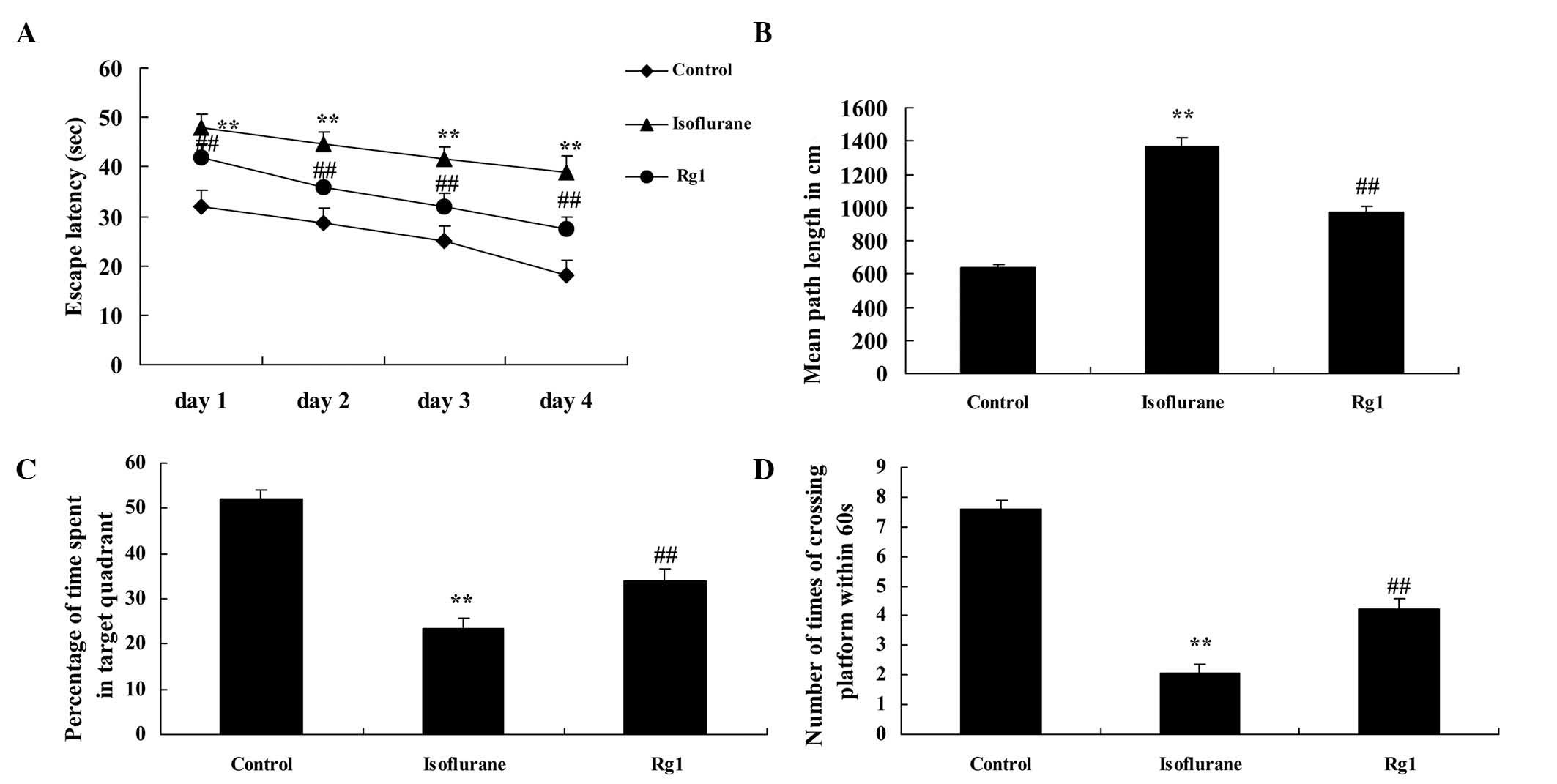
As shown in Fig. 2A and
B, the escape latency and path length of the control group were
lower, compared with those of the isoflurane anesthesia group
following the Morris water sessions. Ginsenoside Rg1 effectively
inhibited the increased escape latency and path length in the aged
rats exposed to isoflurane anesthesia (Fig. 2A and B). The mean swim speed and
swimming durations were significantly inhibited in the aged rats
exposed to isoflurane anesthesia, compared with the control group
(Fig. 2C and D). However,
ginsenoside Rg1 effectively elevated the suppression of mean swim
speed and extended the duration of swimming in the rats exposed to
isoflurane anesthesia (Fig. 2C and
D).
Antioxidant effects of ginsenoside Rg1 in
aging rats exposed to isoflurane anesthesia
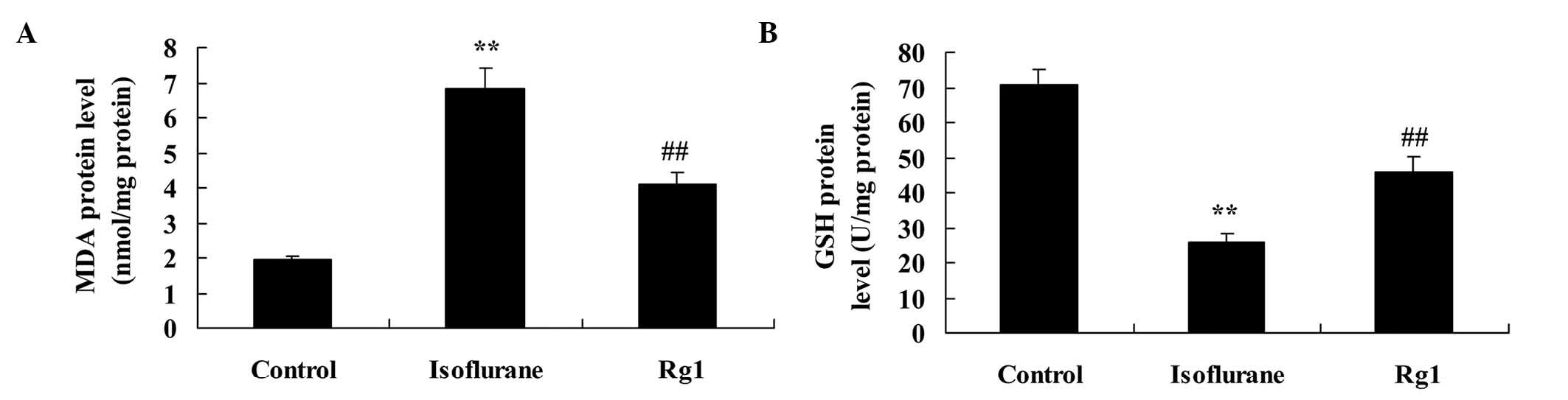
As shown in Fig. 3A and
B, the rats in the isoflurane groups had significantly
increased protein levels of MDA and decreased protein levels of
GSH, compared with the control group. Treatment with ginsenoside
Rg1 markedly reduced the elevated MDA protein level and inhibited
GSH protein level in the rats exposed to isoflurane anesthesia
(Fig. 3A and B).
Anti-inflammatory effect of ginsenoside
Rg1 in aging rats exposed to isoflurane anesthesia
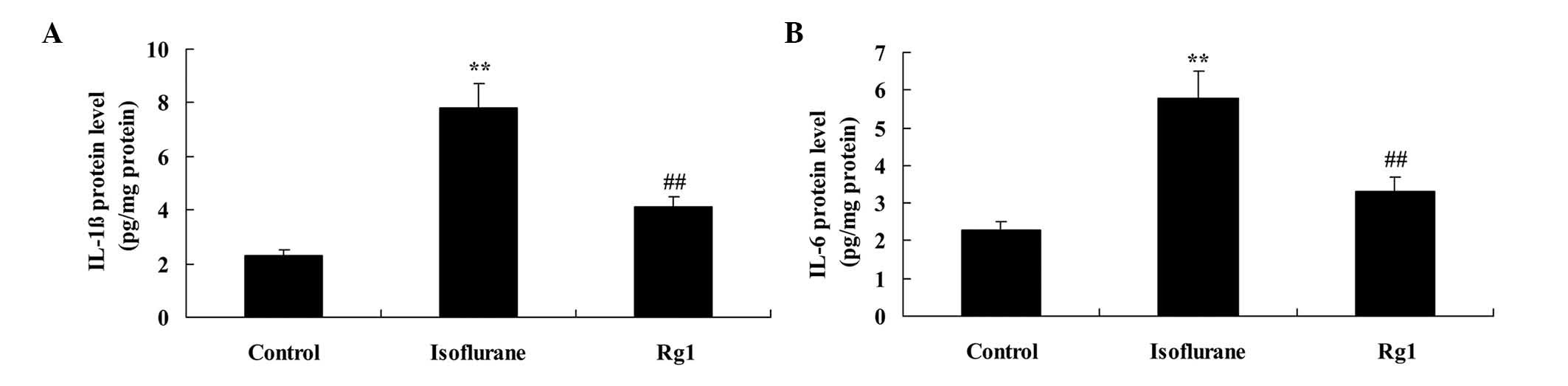
The results of the present study revealed increases
in the protein levels of IL-1β and IL-6 in the isoflurane
anesthesia group, compared with the control group (Fig. 4A and B). However, the
isoflurane-induced protein levels of IL-1β and IL-6 were
significantly weakened by pretreatment with ginsenoside Rg1
(Fig. 4A and B).
Anti-apoptotic effect of ginsenoside Rg1
in aging rats exposed to isoflurane anesthesia
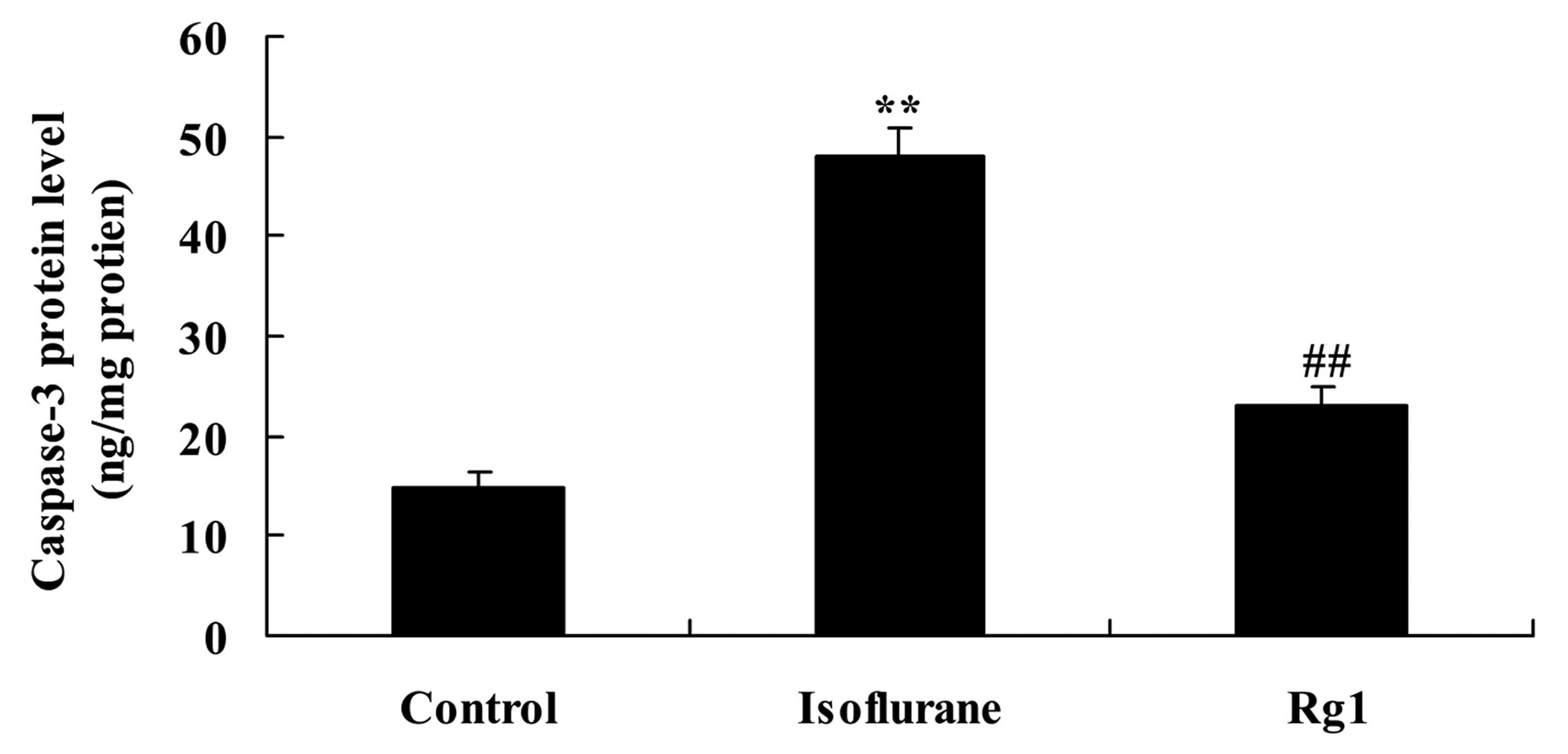
Isoflurane exposure significantly increased the
protein level of caspase-3 in the aging rat, compared with the rats
in the control group (Fig. 5).
This increase was attenuated by ginsenoside Rg1 (Fig. 5).
Neuroprotective effect of ginsenoside Rg1
on p-AKT/AKT in isoflurane anesthesia-exposed aging rats
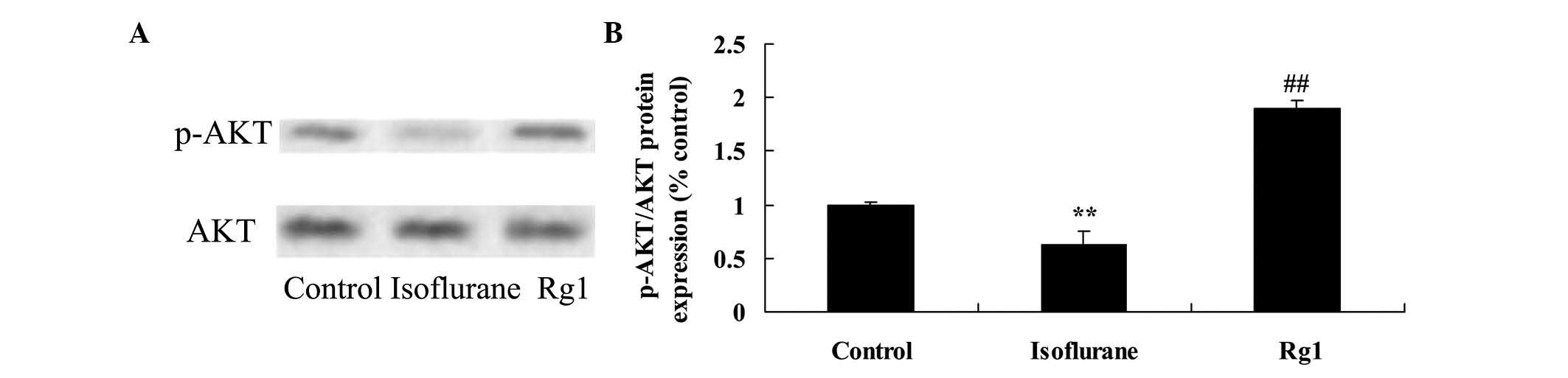
Compared with the rats in the control group, there
was a significant decrease in the level of p-AKT/AKT of the aging
rat following isoflurane exposure (Fig. 6). However, treatment with
ginsenoside Rg1 effectively increased the levels of p-AKT/AKT in
the aging rats exposed to isoflurane (Fig. 6).
Neuroprotective effect of ginsenoside Rg1
on GSK-3β in aging rats exposed to isoflurane anesthesia
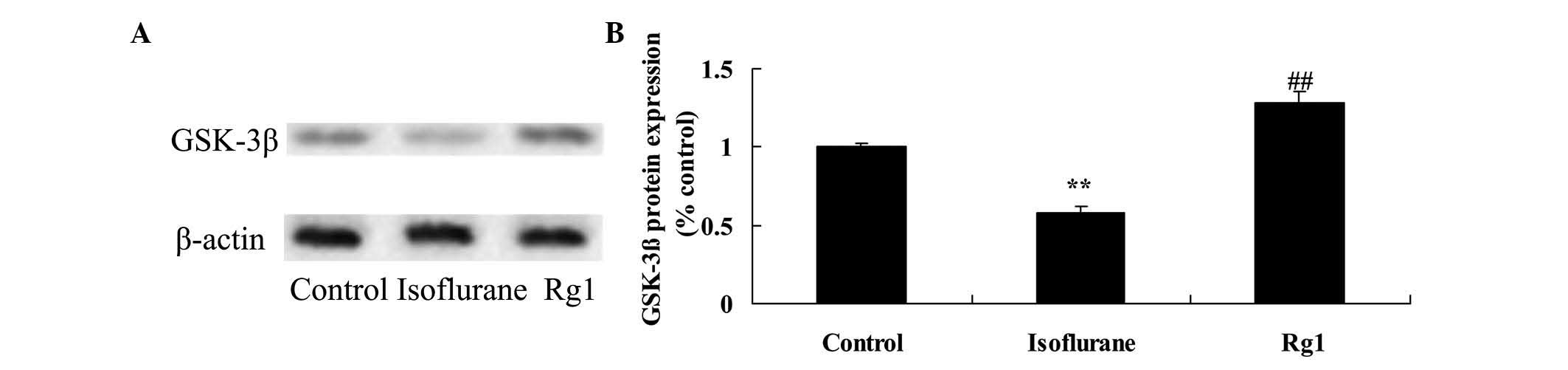
Compared with the rats in the control group, the
protein expression of GSK-3β was reduced in the aging rats
following exposure to isoflurane (Fig.
7). This inhibited protein expression of GSK-3β was elevated by
treatment with ginsenoside Rg1 (Fig.
7).
Neuroprotective effect of ginsenoside Rg1
on p21WAF1/CIP1 in isoflurane anesthesia-exposed aging rats
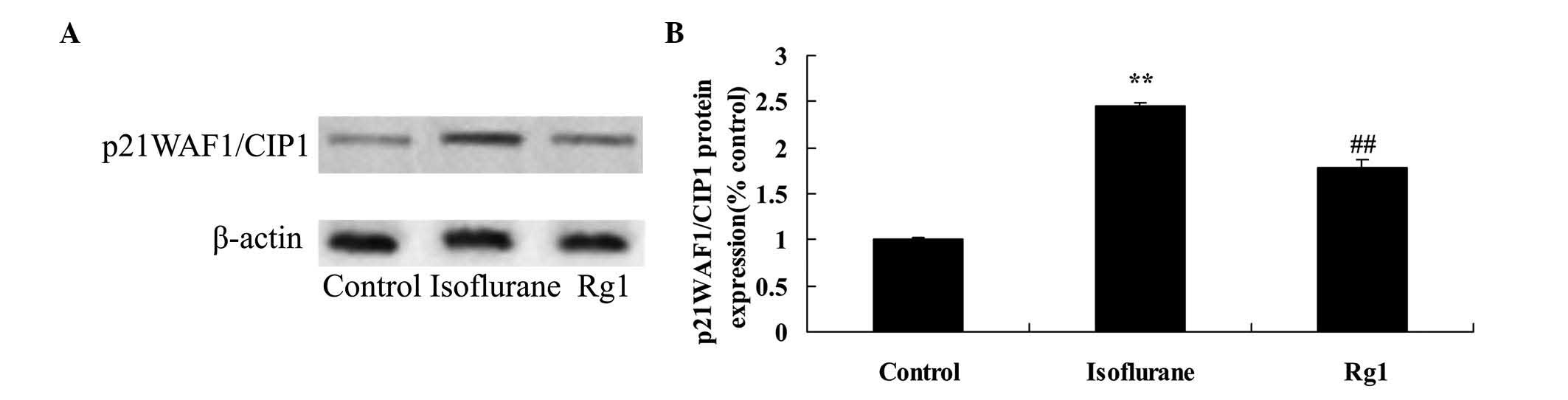
As shown in Fig. 8,
the protein expression of p21WAF1/CIP1 in the isoflurane anesthesia
group was higher, compared with that of the control group.
Ginsenoside Rg1 treatment significantly inhibited the promotion in
the protein expression of p21WAF1/CIP1 in the aging rat exposed to
isoflurane anesthesia (Fig.
8).
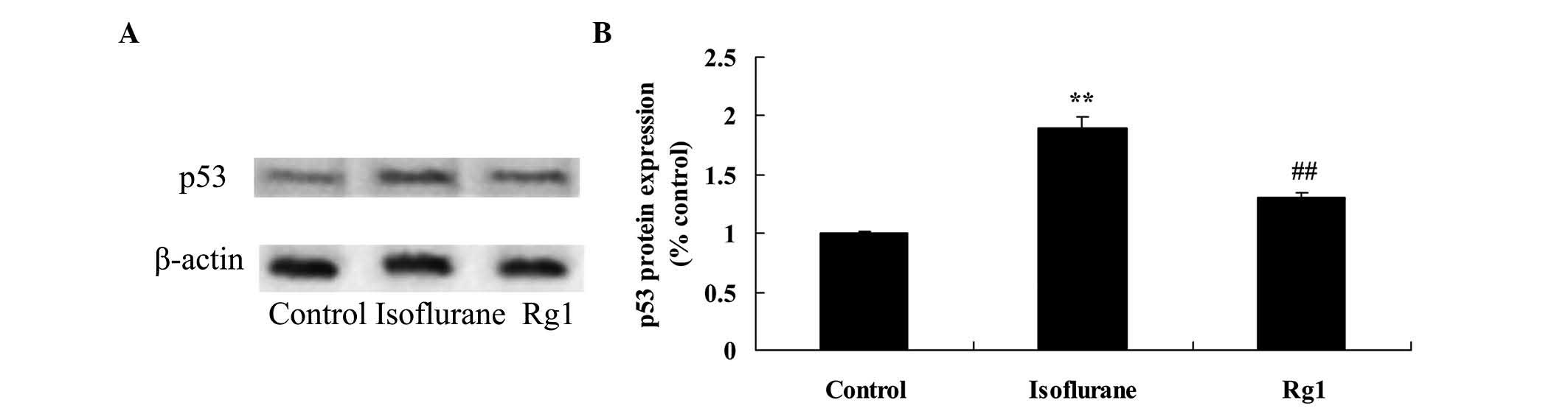
Neuroprotective effect of ginsenoside Rg1
on p53 in isoflurane anesthesia-exposed aging rats
Compared with the control group, the protein
expression of p53 was activated by isoflurane (Fig. 9). However, the isoflurane-induced
protein expression of p53 was suppressed by pretreatment with
ginsenoside Rg1 in the aging rats exposed to isoflurane anesthesia
(Fig. 9).
Discussion
POCD is an acute reversible abalienation syndrome
that occurs following surgery with anesthesia (15). It often occurs in the elderly
several days or several weeks following surgery, with a decline in
memory, comprehension and concentration, and a decrease in social
adaptation ability, possibly leading to the loss of independent
living and a permanent cognitive disorder (16). POCD was originally identified in
gerontal patients following cardiac surgery (17), however, it has been found in
patients who have not had cardiac surgery with higher rates of
morbidity. There are several risk factors for POCD during the early
post-operative period, including history of surgery with
anesthesia, low levels of education, secondary surgery,
postoperative infection and respiratory system complications,
whereas advanced age is the predominant risk factor during the
later post-operative period (18).
Compared with younger adults, the brain cells of the elderly are
atrophied, with seriously decreased cranial capacity, enlarged
ventricles, and alterations in cranial nerve distribution and
neurotransmitter types (19). As a
result, gerontal patients are more sensitive to nerve cell damage
caused by surgery and anesthesia (20). Furthermore, postoperative pain can
affect cognitive function during early period, however, chronic
pain prior to surgery has no effect on the results of cognitive
function assessments (21). The
results of the present study suggested that the neuroprotective
effects of ginsenoside Rg1 improved cognitive function in the aging
rats exposed to isoflurane anesthesia.
In previous years, a number of studies have
indicated that the inflammatory response inside the brain and
oxidative stress are of significant importance in the pathogenesis
of cognitive impairment (22).
Several reports have confirmed that inflammation contributes to
pathological injury in various ways. For example, inflammation
damages the integrity of vascular function and contributes to the
pathogenesis of cognitive impairment diseases, including POCD
(23). Treatment of this disease
through targeting this pathomechanism can significantly improve
neuronal injury and modify nerve fiberdamage to relieve the
inflammatory response within the brain, improving learning and
memory abilities (23,24). Therefore, there has been
substantial investigation on the association between oxidative
stress and cognitive impairment (25). Free radicals have high levels of
activity and potent oxidative effects, which can injure
biomacromolecules and multiple components of cells. In addition,
they are harmful to nerve cells, causing alterations, including
lipofuscin deposition, age pigment increase and vacuolar
degeneration within the cells (23). The present study found that
ginsenoside Rg1 exhibited antioxidant and anti-inflammatory effects
in the treatment of isoflurane-induced cognitive impairment in
aging rats. Yang et al also reported that ginsenoside Rg1
exerts anti-inflammatory and anti-apoptotic properties in rats with
ischemia-reperfusion injury (26),
and Yu et al (9) suggested
that ginsenoside Rg1 ameliorates oxidative stress in
streptozotocin-induced diabetic rats.
Caspase-3 is the most important protease during
apoptosis and is widely used for detecting cell apoptosis (27). Used as an apoptotic detection
indicator, caspase-3 has confirmed that sevoflurane and isoflurane
exposure in juvenile rats may lead to increased brain cell
apoptosis, and that prolonging the duration of anesthesia increases
the number of apoptotic cells (28). These findings indicate that there
is causal association between the severity of brain cell apoptosis
and the duration of drug treatment under these experimental
conditions. Of note, ginsenoside Rg1 exerted anti-apoptotic effects
and inhibited the expression of caspase-3 in the aging rats exposed
to isoflurane anesthesia in the present study. Yang et al
also showed that ginsenoside Rg1 suppresses the inflammation and
level of caspase-3 in neuron cells in rats with cerebral
ischemia-reperfusion injury (26).
The PI3K/AKT signal transduction pathway is
important for membrane receptor signal transduction into cells, and
it reduces cell apoptosis due to ischemia-reperfusion injury in
organs, including the heart, kidney and liver (29). GSK-3 is a multifunctional
serine/threonine protein kinase involved in cell differentiation,
proliferation and apoptosis, with the exception of glycometabolism
(30). The PI3K/AKT pathway is
involved in regulating the gene expression of myeloid cell
leukemia-1 (Mcl-1) in multiple types of tumor and cell line
(30). AKT can phosphorylate B
cell lymphoma (Bcl)-associated X protein and forms a heterodimer
with Bcl-extra large and Mcl-1 in the cytoplasm, leading to
decreased mitochondrial membrane translocation and cell apoptosis.
GSK-3β can phosphorylate Mcl-1 to increase the ubiquitination of
Mcl-1 and decrease the induction of apoptosis. In addition, AKT
affects the stability of Mcl-1 by negatively regulating GSK-3β to
inhibit apoptosis (31). In the
present study, ginsenoside Rg1 activated the PI3K/AKT/GSK-3β
pathway in aging rats exposed to isoflurane anesthesia. Wang et
al also found that ginsenoside Rg1 regulates innate immune
responses via the PI3K/AKT pathway (32), and Song et al reported that
ginsenoside Rg1 attenuates spatial memory impairment through the
GSK3β signaling pathway in rats (33).
The p53 gene is a reactive gene located upstream and
affected by cell DNA damage. If cells are exposed to external
injury, the p53 gene is activated, and the protein expression of
p53 mediates cell cycle arrest by activating a series of downstream
cells, or by immediately combining with single or double stranded
DNA to induce self-repair of DNA in cells (34). As the direct downstream mediator of
p53, p21WAF1/CIP1 is important in adjusting the cell cycle by
combining and restraining cyclin dependent kinase (35). In addition to cell cycle arrest,
p21WAF1/CIP1 also inhibits p53 mediation and the apoptosis mediated
by p53, and under certain specific conditions, it also promote cell
apoptosis (36). On analyzing the
expression and behavior changes of nerve cell apoptosis and
p21WAF1/CIP1, p53 protein, the results indicate that it presents
negative correlation about the expression and behavior changes of
the former two, otherwise, cell apoptosis and p21WAF1/CIP1, p53
protein express more, the injury of cognitive function is more
serious (37). In the present
study, treatment with ginsenoside Rg1 suppressed the protein
expression of p21WAF1/CIP1 in aging rats exposed to isoflurane
anesthesia. Zhu et al also reported that ginsenoside Rg1
prevented cognitive impairment through the inhibition of
p21WAF1/CIP1 in D-galactose-induced aging rat (11).
In conclusion, the present study demonstrated the
neuroprotective effects of ginsenoside Rg1 in preventing the
cognitive impairment induced by isoflurane anesthesia via
antioxidant, anti-inflammatory and anti-apoptotic effects mediated
by the PI3K/AKT/GSK-3β pathway in aging rats.
References
|
1
|
Lin D and Zuo Z: Isoflurane induces
hippocampal cell injury and cognitive impairments in adult rats.
Neuropharmacology. 61:1354–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spitzer WJ and Davidson KW: Future trends
in health and health care: Implications for social work practice in
an aging society. Soc Work Health Care. 52:959–986. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tachibana S, Hayase T, Osuda M, Kazuma S
and Yamakage M: Recovery of postoperative cognitive function in
elderly patients after a long duration of desflurane anesthesia: A
pilot study. J Anesth. 29:627–630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang W, Wang Y, Wu H, Lei L, Xu S, Shen X,
Guo X, Shen R, Xia X, Liu Y and Wang F: Postoperative cognitive
dysfunction: Current developments in mechanism and prevention. Med
Sci Monit. 20:1908–1912. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Wang P, Zhang X, Zhang W and Gu G:
Effects of different concentration and duration time of isoflurane
on acute and long-term neurocognitive function of young adult
C57BL/6 mouse. Int J Clin Exp Pathol. 7:5828–5836. 2014.PubMed/NCBI
|
|
6
|
Velly L, Mantz J and Bruder N: Dual
effects of isoflurane on neuronal proliferation/differentiation: A
substrate to impaired cognitive function? Anesthesiology.
118:487–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stratmann G, Sall JW, May LD, Loepke AW
and Lee MT: Beyond anesthetic properties: The effects of isoflurane
on brain cell death, neurogenesis and long-term neurocognitive
function. Anesth Analg. 110:431–437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Culley DJ, Xie Z and Crosby G: General
anesthetic-induced neurotoxicity: An emerging problem for the young
and old? Curr Opin Anaesthesiol. 20:408–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu HT, Zhen J, Pang B, Gu JN and Wu SS:
Ginsenoside Rg1 ameliorates oxidative stress and myocardial
apoptosis in streptozotocin-induced diabetic rats. J Zhejiang Univ
Sci B. 16:344–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu D, Zhu LH, Shu XM, Zhang CJ, Zhao JY,
Qi RB, Wang HD and Lu DX: Ginsenoside Rg1 relieves tert-Butyl
hydroperoxide-induced cell impairment in mouse microglial BV2
cells. J Asian Nat Prod Res. 17:930–945. 2015. View Article : Google Scholar
|
|
11
|
Zhu J, Mu X, Zeng J, Xu C, Liu J, Zhang M,
Li C, Chen J, Li T and Wang Y: Ginsenoside Rg1 prevents cognitive
impairment and hippocampus senescence in a rat model of
D-galactose-induced aging. PLoS One. 9:e1012912014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang F, Chen X, Huang T, Lue LF, Luddy JS
and Yan SS: Multi-faced neuroprotective effects of Ginsenoside Rg1
in an Alzheimer mouse model. Biochim Biophys Acta. 1822:286–292.
2012. View Article : Google Scholar :
|
|
13
|
Wang Y, Kan H, Yin Y, Wu W, Hu W, Wang M
and Li W and Li W: Protective effects of ginsenoside Rg1 on chronic
restraint stress induced learning and memory impairments in male
mice. Pharmacol Biochem Behav. 120:73–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee B, Shim I, Lee H and Hahm DH: Effect
of ginsenoside Re on depression- and anxiety-like behaviors and
cognition memory deficit induced by repeated immobilization in
rats. J Microbiol Biotechnol. 22:708–720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rossi A, Burkhart C, Dell-Kuster S,
Pollock BG, Strebel SP, Monsch AU, Kern C and Steiner LA: Serum
anticholinergic activity and postoperative cognitive dysfunction in
elderly patients. Anesth Analg. 119:947–955. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Harten AE, Scheeren TW and Absalom AR:
A review of postoperative cognitive dysfunction and
neuroinflammation associated with cardiac surgery and anaesthesia.
Anaesthesia. 67:280–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Riedel B, Browne K and Silbert B: Cerebral
protection: Inflammation, endothelial dysfunction and postoperative
cognitive dysfunction. Curr Opin Anaesthesiol. 27:89–97. 2014.
View Article : Google Scholar
|
|
18
|
Zheng XU, Ma Z and Gu X: Plasma levels of
tumor necrosis factor-α in adolescent idiopathic scoliosis patients
serve as a predictor for the incidence of early postoperative
cognitive dysfunction following orthopedic surgery. Exp Ther Med.
9:1443–1447. 2015.PubMed/NCBI
|
|
19
|
Marshall JW and Ridley RM: Assessment of
cognitive and motor deficits in a marmoset model of stroke. ILAR J.
44:153–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Wang S, Ran K, Hu Z, Liu Z and Duan
K: Differential hippocampal protein expression between normal aged
rats and aged rats with postoperative cognitive dysfunction: A
proteomic analysis. Mol Med Rep. 12:2953–2960. 2015.PubMed/NCBI
|
|
21
|
Jo YY, Kim JY, Lee MG, Lee SG and Kwak HJ:
Changes in cerebral oxygen saturation and early postoperative
cognitive function after laparoscopic gastrectomy: A comparison
with conventional open surgery. Korean J Anesthesiol. 69:44–50.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu C and Han JG: Advances in the
mechanisms and early warning indicators of the postoperative
cognitive dysfunction after the extracorporeal circulation.
Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 37:101–107. 2015.PubMed/NCBI
|
|
23
|
Ogasawara K, Yamadate K, Kobayashi M, Endo
H, Fukuda T, Yoshida K, Terasaki K, Inoue T and Ogawa A: Effects of
the free radical scavenger, edaravone, on the development of
postoperative cognitive impairment in patients undergoing carotid
endarterectomy. Surg Neurol. 64:309–313; discussion 313–314. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan H, Cao J, Zhang J and Zuo Z: Critical
role of inflammatory cytokines in impairing biochemical processes
for learning and memory after surgery in rats. J Neuroinflammation.
11:932014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
An LN, Yue Y, Guo WZ, Miao YL, Mi WD,
Zhang H, Lei ZL, Han SJ and Dong L: Surgical trauma induces iron
accumulation and oxidative stress in a rodent model of
postoperative cognitive dysfunction. Biol Trace Elem Res.
151:277–283. 2013. View Article : Google Scholar
|
|
26
|
Yang Y, Li X, Zhang L, Liu L, Jing G and
Cai H: Ginsenoside Rg1 suppressed inflammation and neuron apoptosis
by activating PPARγ/HO-1 in hippocampus in rat model of cerebral
ischemia-reperfusion injury. Int J Clin Exp Pathol. 8:2484–2494.
2015.
|
|
27
|
Miyamoto A, Miyauchi H, Kogure T, Miyawaki
A, Michikawa T and Mikoshiba K: Apoptosis induction-related
cytosolic calcium responses revealed by the dual FRET imaging of
calcium signals and caspase-3 activation in a single cell. Biochem
Biophys Res Commun. 460:82–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Liu C, Zhao Y, Hu K, Zhang J, Zeng
M, Luo T, Jiang W and Wang H: Sevoflurane induces short-term
changes in proteins in the cerebral cortices of developing rats.
Acta Anaesthesiol Scand. 57:380–390. 2013. View Article : Google Scholar
|
|
29
|
Chen L, Xiang Y, Kong L, Zhang X, Sun B,
Wei X and Liu H: Hydroxysafflor yellow A protects against cerebral
ischemia-reperfusion injury by anti-apoptotic effect through
PI3K/Akt/GSK3β pathway in rat. Neurochem Res. 38:2268–2275. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Son YO, Pratheeshkumar P, Wang L, Wang X,
Fan J, Kim DH, Lee JY, Zhang Z, Lee JC and Shi X: Reactive oxygen
species mediate Cr(VI)-induced carcinogenesis through
PI3K/AKT-dependent activation of GSK-3β/β-catenin signaling.
Toxicol Appl Pharmacol. 271:239–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamaguchi K, Lee SH, Eling TE and Baek SJ:
Identification of nonsteroidal anti-inflammatory drug-activated
gene (NAG-1) as a novel downstream target of phosphatidylinositol
3-kinase/AKT/GSK-3beta pathway. J Biol Chem. 279:49617–49623. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Liu Y, Zhang XY, Xu LH, Ouyang DY,
Liu KP, Pan H, He J and He XH: Ginsenoside Rg1 regulates innate
immune responses in macrophages through differentially modulating
the NF-kappaB and PI3K/Akt/mTOR pathways. Int Immunopharmacol.
23:77–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song XY, Hu JF, Chu SF, Zhang Z, Xu S,
Yuan YH, Han N, Liu Y, Niu F, He X and Chen NH: Ginsenoside Rg1
attenuates okadaic acid induced spatial memory impairment by the
GSK3β/tau signaling pathway and the Aβ formation prevention in
rats. Eur J Pharmacol. 710:29–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hill R, Madureira PA, Waisman DM and Lee
PW: DNA-PKCS binding to p53 on the p21WAF1/CIP1 promoter blocks
transcription resulting in cell death. Oncotarget. 2:1094–1108.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aliouat-Denis CM, Dendouga N, Van den
Wyngaert I, Goehlmann H, Steller U, van de Weyer I, Van Slycken N,
Andries L, Kass S, Luyten W, et al: p53-independent regulation of
p21Waf1/Cip1 expression and senescence by Chk2. Mol Cancer Res.
3:627–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang L, Sowa Y, Sakai T and Pardee AB:
Activation of the p21WAF1/CIP1 promoter independent of p53 by the
histone deacetylase inhibitor suberoylanilide hydroxamic acid
(SAHA) through the Sp1 sites. Oncogene. 19:5712–5719. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alpan RS and Pardee AB: p21WAF1/CIP1/SDI1
is elevated through a p53-independent pathway by mimosine. Cell
Growth Differ. 7:893–901. 1996.PubMed/NCBI
|